Multi-Compound H2, CH4, and N2 Adsorption Analysis
Abstract
1. Introduction
2. Modelling
2.1. Extended Langmuir Equation
2.2. Extended Freundlich Equation
2.3. Extended Sips (Langmuir–Freundlich Equation)
2.4. Extended Jovanovic–Freundlich Equation
2.5. Potential Theory for Gas Mixtures
2.6. Ideal Adsorbed Solution Theory (IAS)
2.7. Fast-IAS Theory
3. Results and Discussion
3.1. Peculiarities in the Practical Application of Some Models
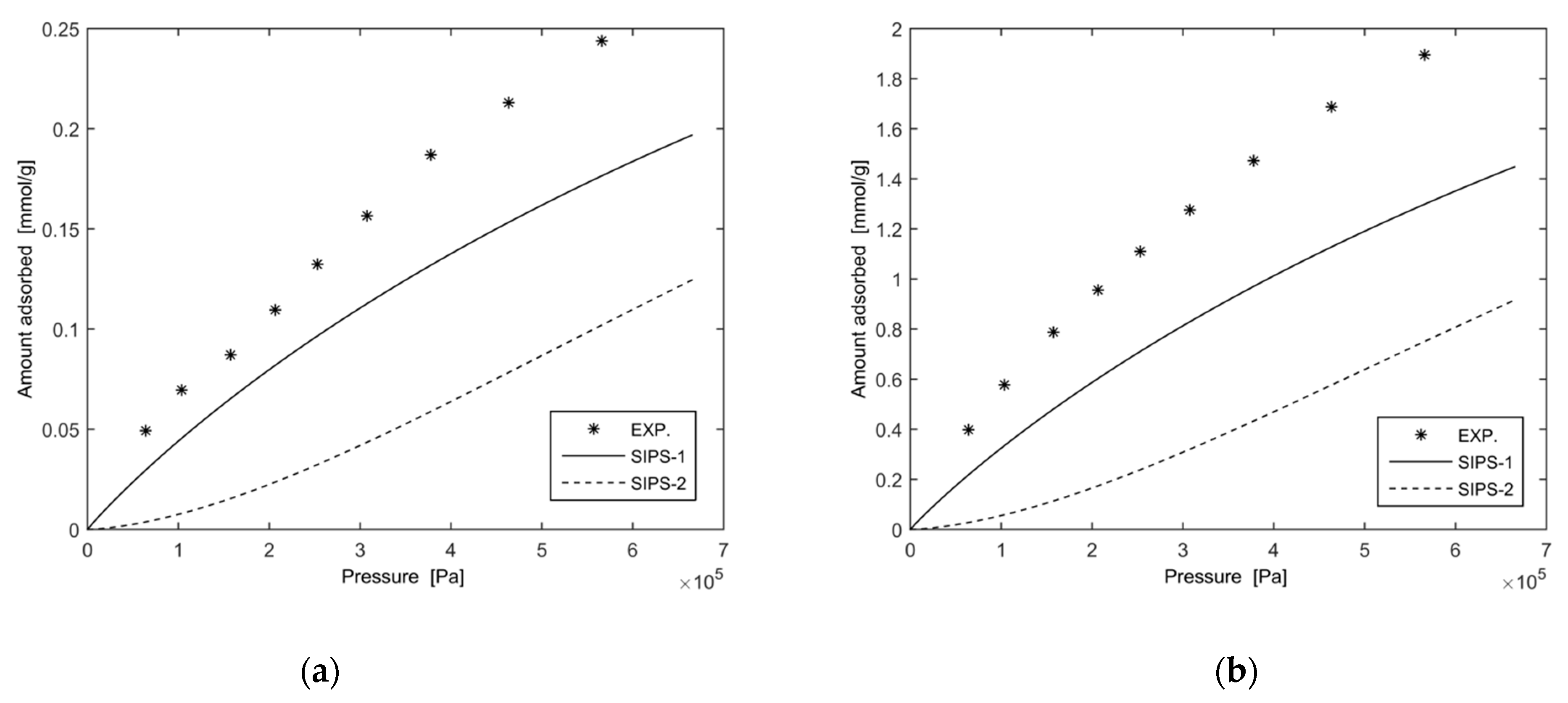
3.1.1. Results and Discussion as Regards the Application of the Extended Sips Equation
3.1.2. Results and Discussion as Regards the Applicability of the IAS Theory
3.1.3. Results and Discussion as Regards the Applicability of the Fast-IAS Theory
3.2. Results for Gas Mixtures
3.2.1. Modelling Results for Case N° 2
3.2.2. Modelling Results for Case N° 5
4. Conclusions
- the extended Sips equation always gives lower values than those of the experimental data and describes the adsorption equilibrium of the modelled gas mixture with insufficient accuracy;
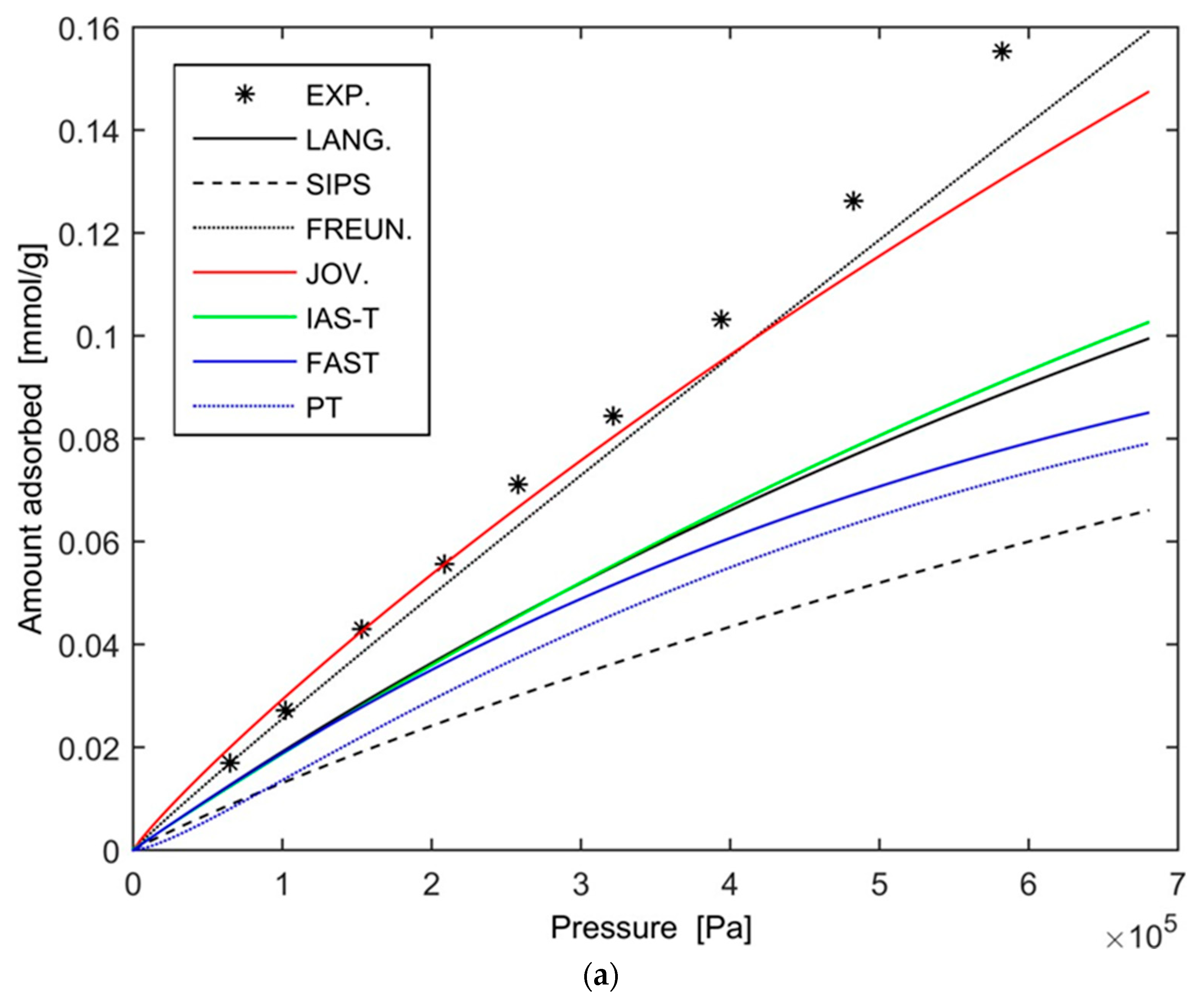
- the extended Langmuir equation along with the IAS, Fast-IAS, and PT models give almost identical values and describe well the adsorption equilibrium of nitrogen and methane. However, when using these models to describe the adsorption equilibrium of hydrogen, unrealistically low values are obtained;
- the extended Freundlich equation gives unrealistically high values for the adsorption of nitrogen and of methane, but quite precise results for the adsorption of hydrogen;
- the extended Jovanovic model gives very accurate results for the adsorption of nitrogen whereas, in the case of methane and of hydrogen, the results are less precise.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicoleti, G. The hydrogen option for energy: A review of technical, environmental and economic aspects. Int. J. Hydrogen. Energ. 1995, 20, 759–765. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.D.; Kim, J.T.; Seo, K.J. Thermal-Structural Characteristics of Multi-Layer Vacuum-Insulated Pipe for the Transfer of Cryogenic Liquid Hydrogen. Metals 2022, 12, 549. [Google Scholar] [CrossRef]
- Das, L.M. On-board hydrogen storage systems for automotive application. Int. J. Hydrogen. Energ. 1996, 21, 789–800. [Google Scholar] [CrossRef]
- Pullumbi, P.; Brandani, F.; Brandani, S. Gas separation by adsorption: Technological drivers and opportunities for improvement. Curr. Opin. Chem. Eng. 2019, 24, 131–142. [Google Scholar] [CrossRef]
- Sayılgan, Ş.Ç.; Mobedi, M.; Ülkü, S. Effect of regeneration temperature on adsorption equilibria and mass diffusivity of zeolite 13x-water pair. Micropor. Mesopor. Mat. 2016, 224, 9–16. [Google Scholar] [CrossRef]
- Hamid, U.; Vyawahare, P.; Tun, H.; Chen, C.C. Generalization of thermodynamic Langmuir isotherm for mixed-gas adsorption equilibria. AIChE J. 2022, 68, e17663. [Google Scholar] [CrossRef]
- Lamari, F.D.; Weinberger, B.P.; Kunowsky, M.; Levesque, D. Material design using molecular modeling for hydrogen storage. AIChE J. 2009, 55, 538–547. [Google Scholar] [CrossRef]
- Dicko, M.; Seydou, M.; Darkrim Lamari, F.; Langlois, P.; Maurel, F.; Levesque, D. Hydrogen adsorption on graphane: An estimate using ab-initio interaction. Int. J. Hydrogen. Energ. 2017, 42, 10057–10063. [Google Scholar] [CrossRef]
- Hu, X.; Do, D.D. Multicomponent adsorption kinetics of hydrocarbons onto activated carbon: Effect of adsorption equilibrium equations. Chem. Eng. Sci. 1992, 47, 1715–1725. [Google Scholar] [CrossRef]
- Guiochon, G.; Golshan-Shirazi, S.; Katti, A.M. Fundamentals of Preparative and Nonlinear Chromatography; Academic Press: Boston, MA, USA, 1994. [Google Scholar]
- Chilev, C.; Dicko, M.; Langlois, P.; Lamari, F. Modelling of single-gas adsorption isotherms. Metals 2022, 12, 1698. [Google Scholar] [CrossRef]
- Martinez, G.M.; Basmadjian, D. Towards a general gas adsorption isotherm. Chem. Eng. Sci. 1996, 51, 1043–1054. [Google Scholar] [CrossRef]
- Beyaz, S.; Lamari, F.D.; Weinberger, B.; Langlois, P. Nanoscale carbon material porosity effect on gas adsorption. Int. J. Hydrogen. Energ. 2010, 35, 217–224. [Google Scholar] [CrossRef]
- Jakšić, O.; Spasenović, M.; Jakšić, Z.; Vasiljević-Radović, D. Monolayer Gas Adsorption on Graphene-Based Materials: Surface Density of Adsorption Sites and Adsorption Capacity. Surfaces 2020, 3, 423–432. [Google Scholar] [CrossRef]
- Dragan, G.; Kutarov, V.; Schieferstein, E.; Iorgov, A. Adsorption Hysteresis in Open Slit-like Micropores. Molecules 2021, 26, 5074. [Google Scholar] [CrossRef] [PubMed]
- Markham, E.C.; Benton, A.F. The adsorption of gas mixtures by silica. J. Am. Chem. Soc. 1931, 53, 497–507. [Google Scholar] [CrossRef]
- Hu, X.; Do, D.D. Comparing various multicomponent adsorption equilibrium models. AIChE J. 1995, 41, 1585–1592. [Google Scholar] [CrossRef]
- Digiano, F.A.; Baldauf, G.; Frick, B.; Sontheimer, H. A simplified competitive equilibrium adsorption model. Chem. Eng. Sci. 1978, 33, 1667–1673. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principle of Adsorption and Adsorption Processes; John Wiley-& Sons: New York, NY, USA, 1984. [Google Scholar]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Jaroniec, M.; Madey, R. Physical Adsorption on Heterogeneous Solids; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Dabrowski, A.; Jaroniec, M. Effects of surface heterogeneity in adsorption from binary liquid mixtures: III. Analysis of experimental data by using Langmuir—Freundlich type equations. J. Colloid Interface Sci. 1980, 73, 475–482. [Google Scholar] [CrossRef]
- Quinones, I.; Guiochon, G. Extension of a Jovanovic–Freundlich isotherm model to multicomponent adsorption on heterogeneous surfaces. J. Chromatogr. A 1998, 796, 15–40. [Google Scholar] [CrossRef]
- Kapoor, A.; Ritter, J.A.; Yang, R.T. An extended Langmuir model for adsorption of gas mixtures on heterogeneous surfaces. Langmuir 1990, 6, 660–664. [Google Scholar] [CrossRef]
- Bering, B.P.; Myers, A.L.; Serpinsky, V.V. Problemi inertnosti adsorbentov. Dokl. Akad. Nauk. SSSR 1970, 193, 119–122. [Google Scholar]
- Doong, S.J.; Yang, R.T. A simple potential-theory model for predicting mixed-gas adsorption. Ind. Eng. Chem. Res. 1988, 27, 630–635. [Google Scholar] [CrossRef]
- Mehta, S.D.; Dannes, R.P. An improved potential theory method for predicting gas-mixture adsorption equilibria. Ind. Eng. Chem. Fundam. 1985, 24, 325–330. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998. [Google Scholar]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of mixed-gas adsorption. AICHE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Myers, A.L. Rapid calculations of multicomponent adsorption equilibria from pure isotherm data. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 1188–1191. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Myers, A.L. A comprehensive technique for equilibrium calculations in adsorbed mixtures: The generalized FastIAS method. Ind. Eng. Chem. Res. 1988, 27, 2085–2092. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, L.; Wu, J.; Zhou, Y. Adsorption equilibrium of the mixture CH4 + N2 + H2 on activated carbon. J. Chem. Eng. Data 2005, 50, 635–642. [Google Scholar] [CrossRef]
- Chilev, C.; Lamari, F.D. Hydrogen storage at low temperature and high pressure for application in automobile manufacturing. Int. J. Hydrogen. Energ. 2016, 41, 1744–1758. [Google Scholar] [CrossRef]
- Lemmon, E.W.; McLinden, M.O.; Friend, D.G. Thermophysical Properties of Fluid Systems, in the NIST Chemistry Webbook, Database Reference Number 69; National Institute of Standards and Technology: Gaithersburg MD, USA, 1998. [Google Scholar] [CrossRef]
- Dubinin, M.M. Modern state of the theory of gas and vapour adsorption by microporous adsorbents. Pure Appl. Chem. 1965, 10, 309–322. [Google Scholar] [CrossRef]





| Case | T [K] | %yCH4 | %yN2 | %yH2 |
|---|---|---|---|---|
| N° 1a | 283 | 36.48 | 27.75 | 35.77 |
| N° 1b | 298 | 36.48 | 27.75 | 35.77 |
| N° 1c | 313 | 36.48 | 27.75 | 35.77 |
| N° 2 | 298 | 52.78 | 23.61 | 23.61 |
| N° 3 | 298 | 25.27 | 23.80 | 50.93 |
| N° 4 | 298 | 30.19 | 44.93 | 24.89 |
| N° 5 | 298 | 28.47 | 12.03 | 59.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilev, C.; Langlois, P.; Lamari, F. Multi-Compound H2, CH4, and N2 Adsorption Analysis. Metals 2022, 12, 1895. https://doi.org/10.3390/met12111895
Chilev C, Langlois P, Lamari F. Multi-Compound H2, CH4, and N2 Adsorption Analysis. Metals. 2022; 12(11):1895. https://doi.org/10.3390/met12111895
Chicago/Turabian StyleChilev, Chavdar, Patrick Langlois, and Farida Lamari. 2022. "Multi-Compound H2, CH4, and N2 Adsorption Analysis" Metals 12, no. 11: 1895. https://doi.org/10.3390/met12111895
APA StyleChilev, C., Langlois, P., & Lamari, F. (2022). Multi-Compound H2, CH4, and N2 Adsorption Analysis. Metals, 12(11), 1895. https://doi.org/10.3390/met12111895






