Atmospheric Corrosion Evolution of Carbon Steel AISI 1020 along a Longitude Transect in the Atacama Desert
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Corrosion Density Rate Measurements
2.3. Morphological and Patterns Characterization
3. Results and Discussion
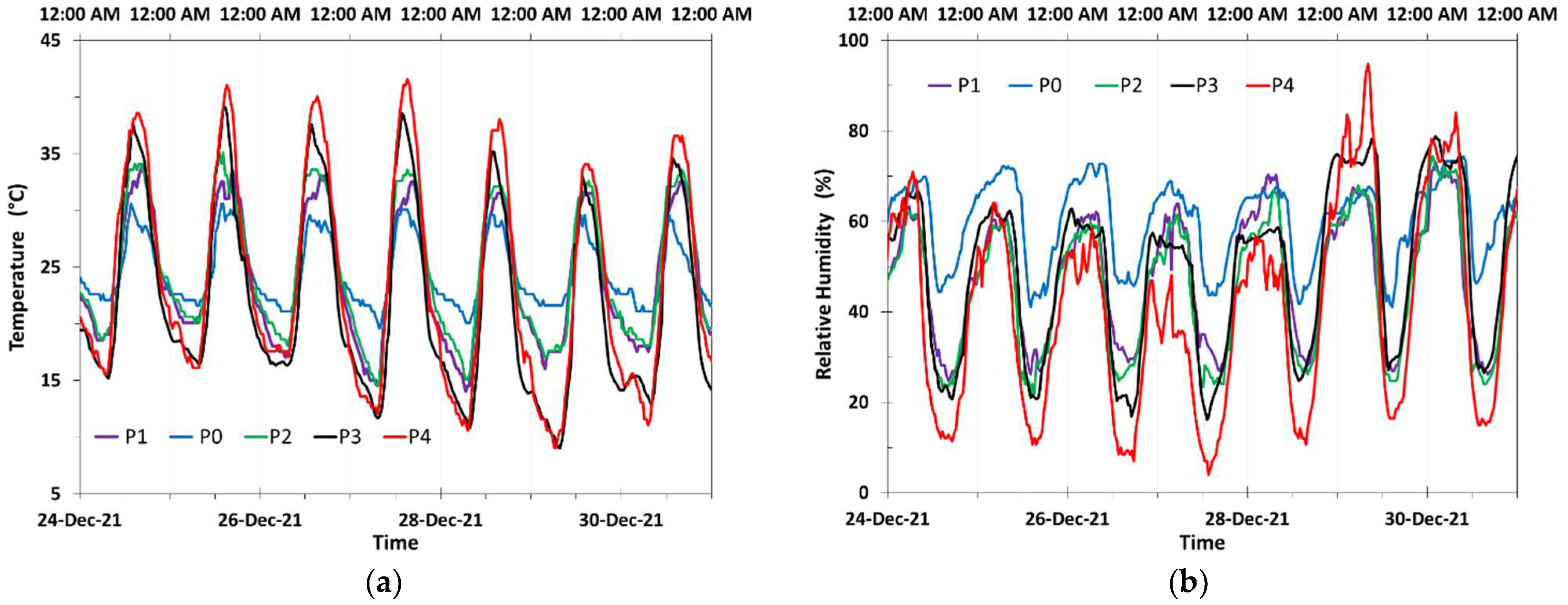
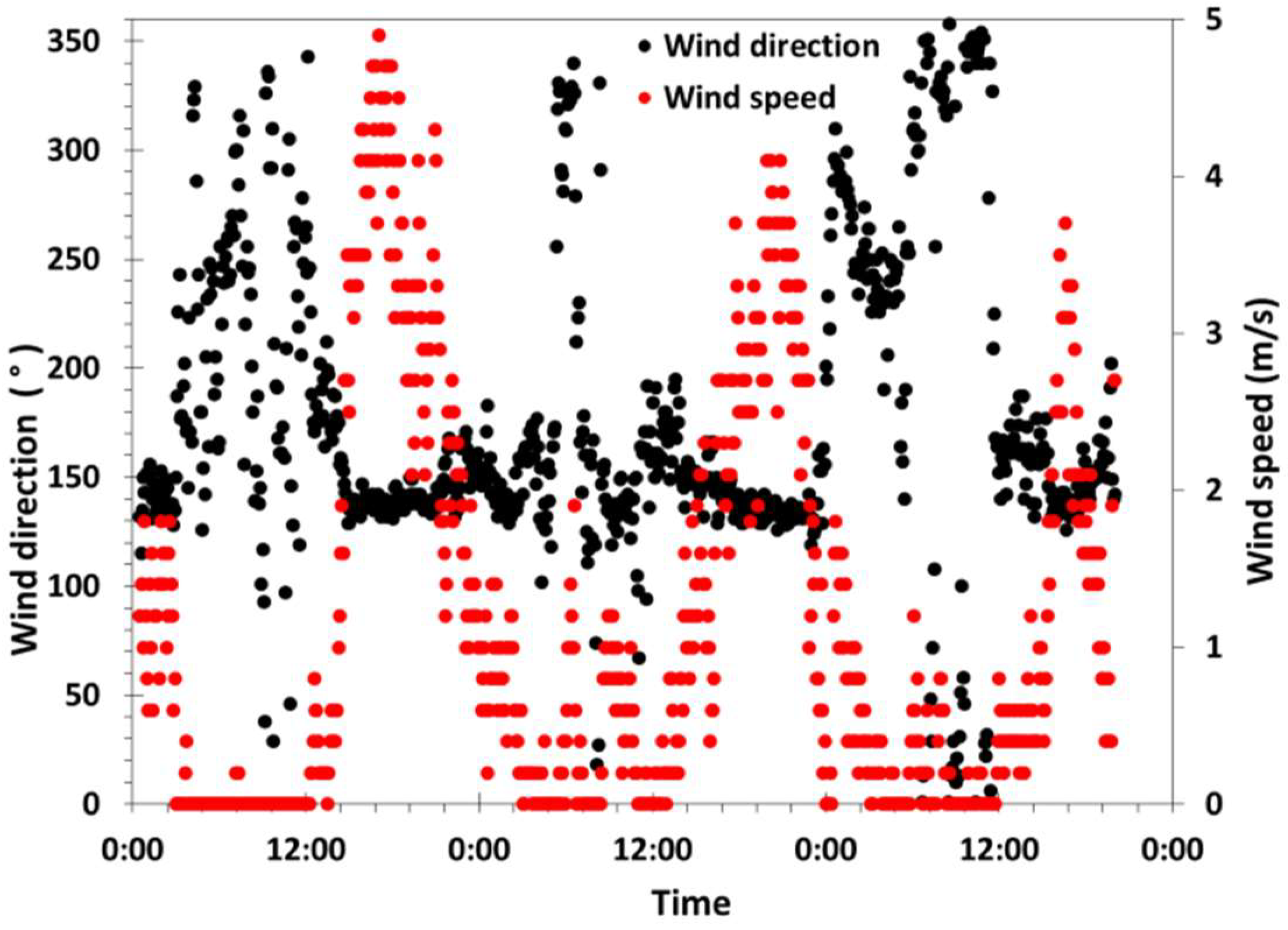
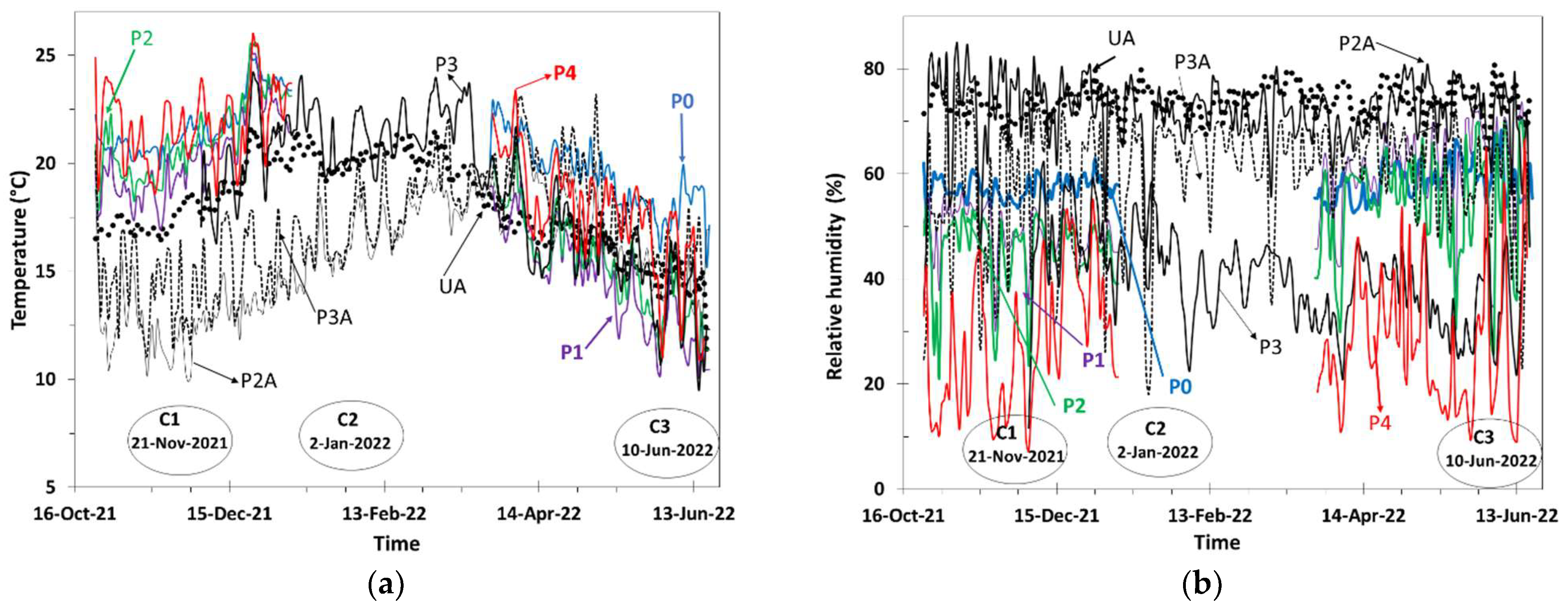
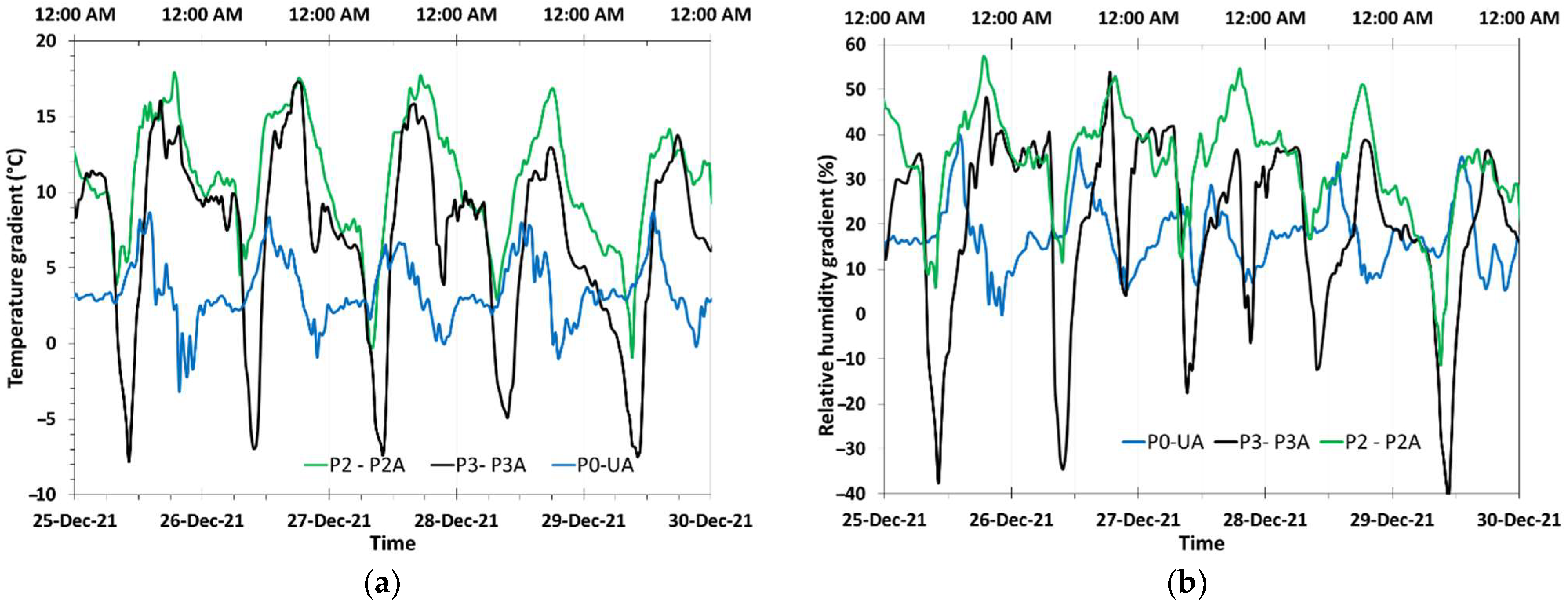
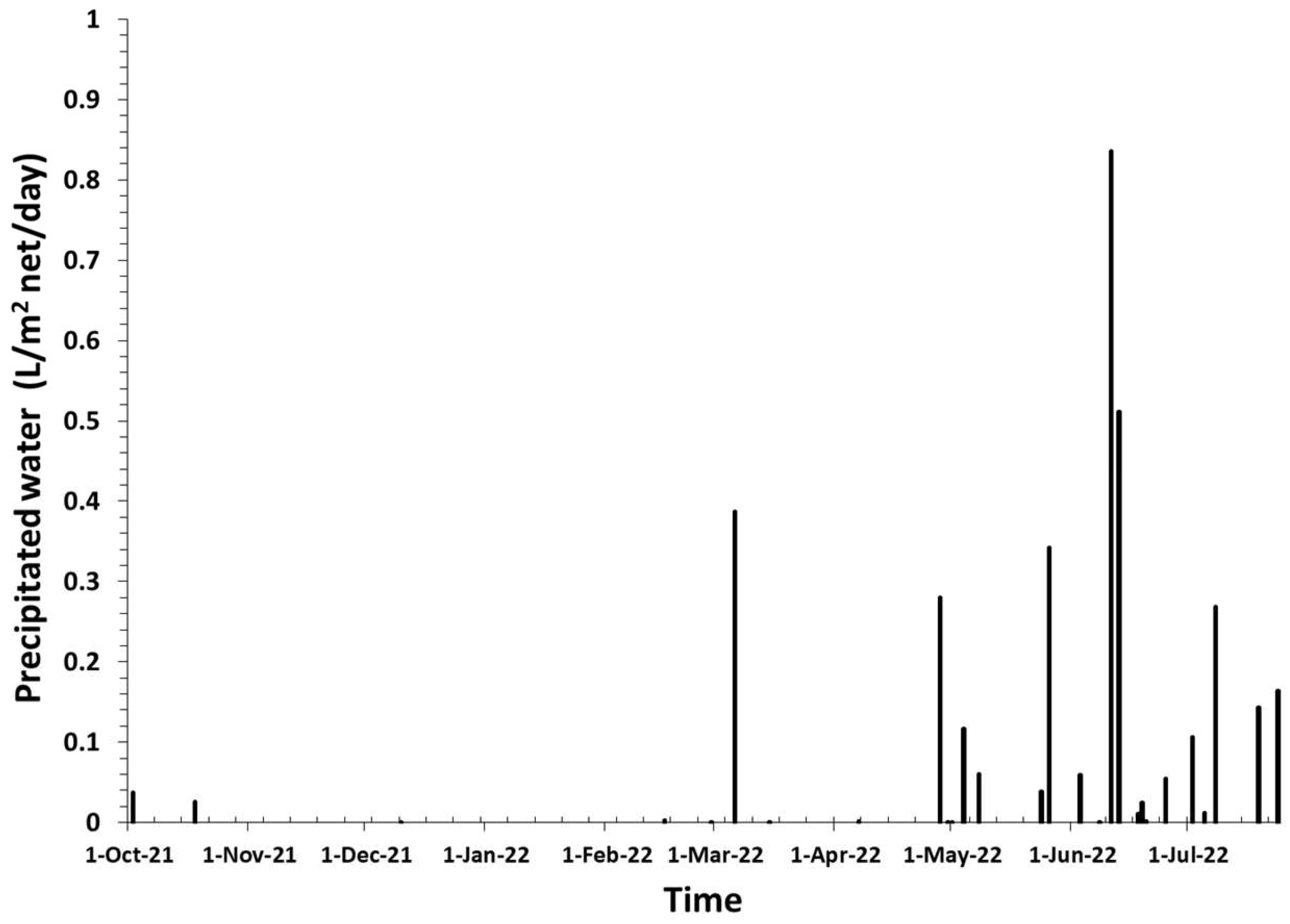
3.1. Meteorological Characterization and Daily Cyclic Precipitation of Water
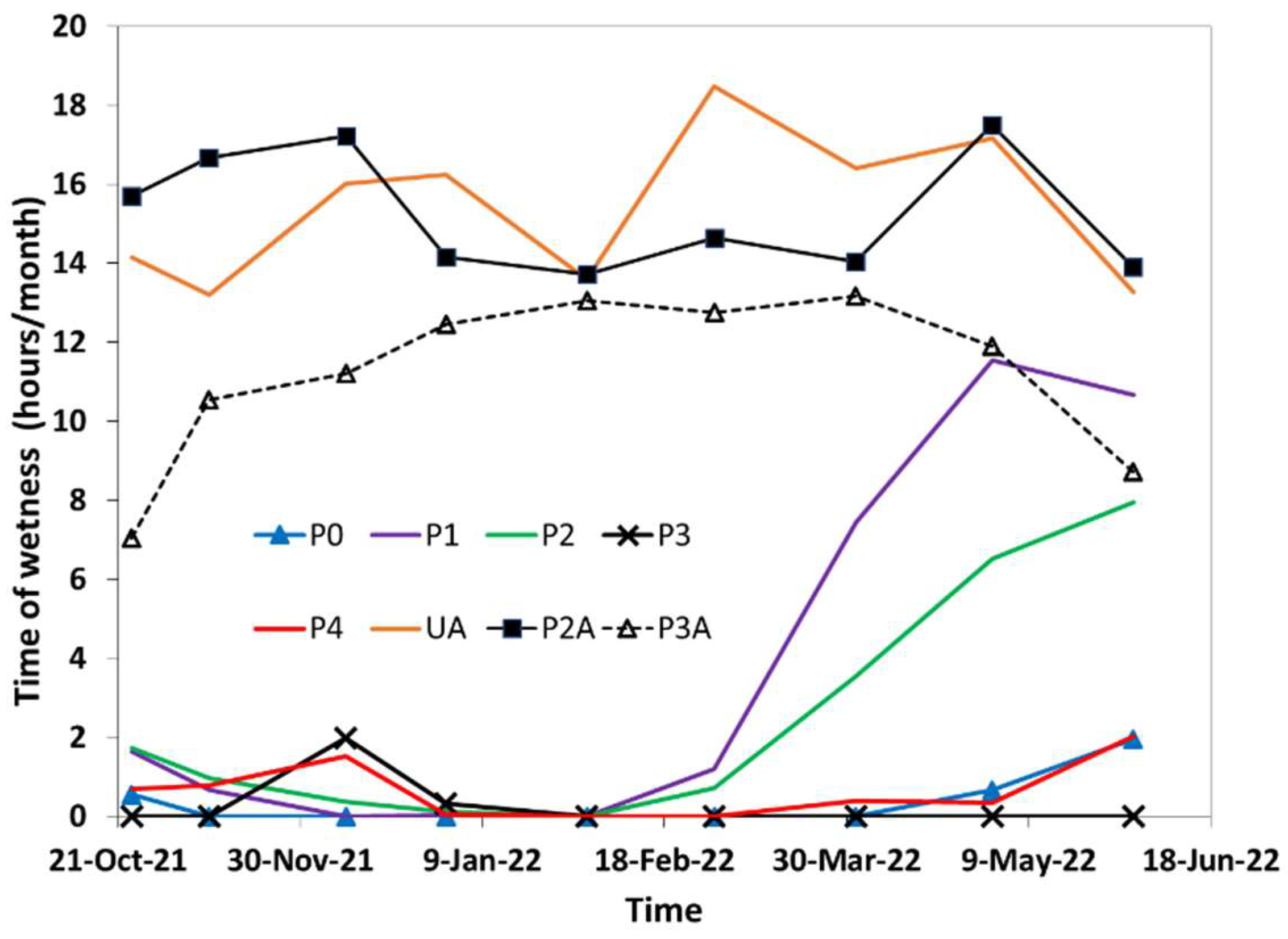
3.2. Time of Wetness (TOW)
3.3. Corrosion Distribution and Patterns
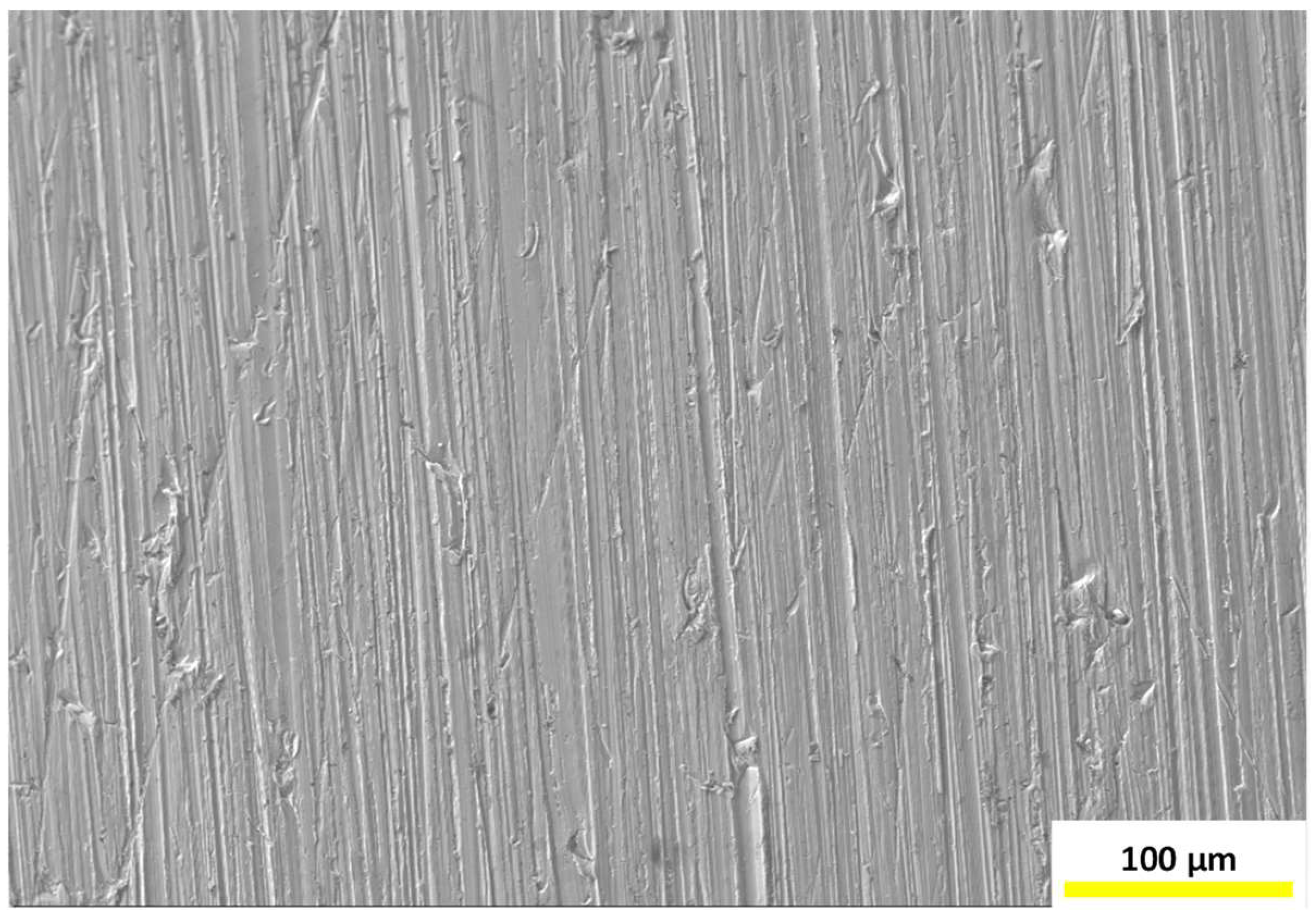
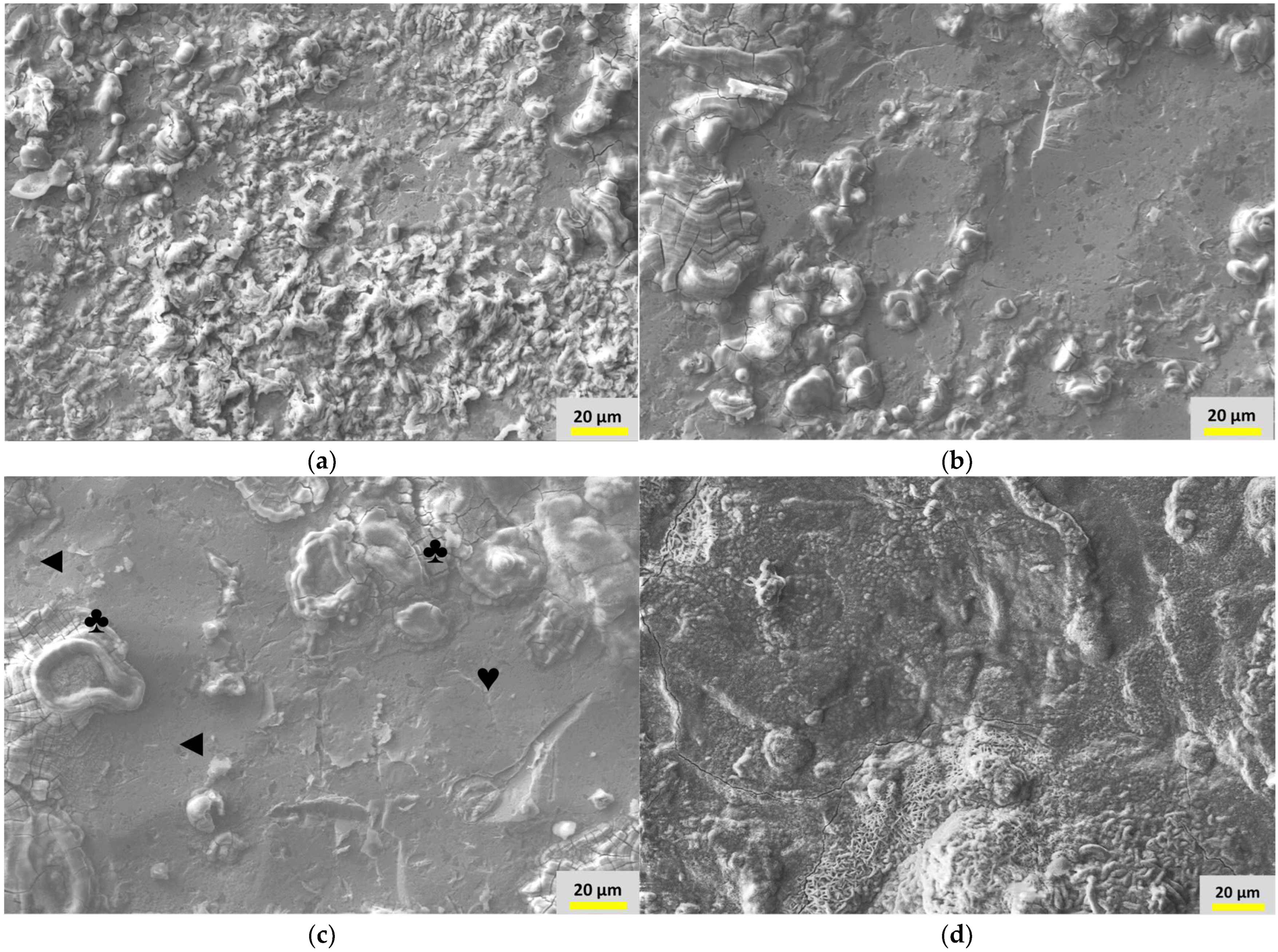
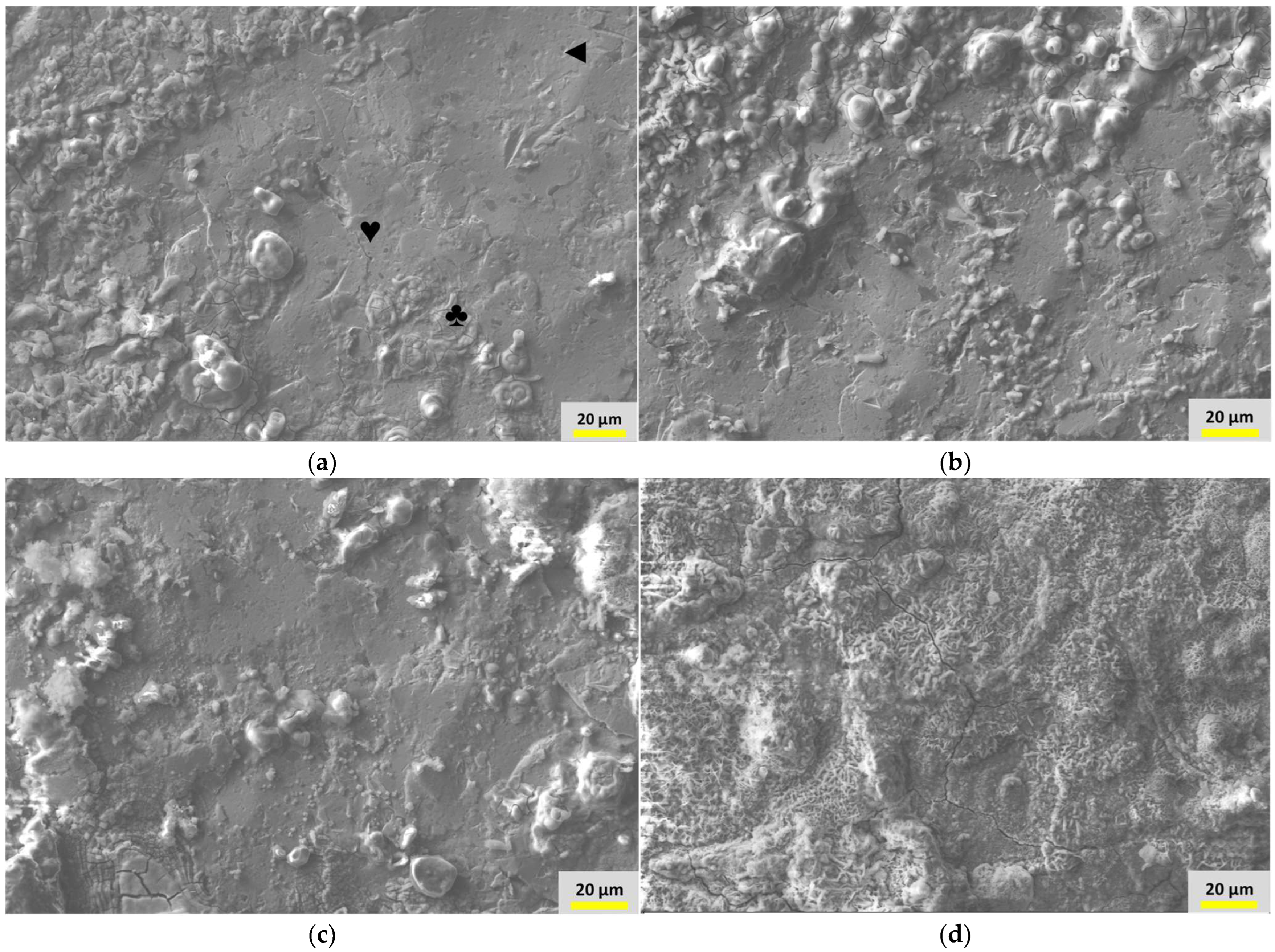
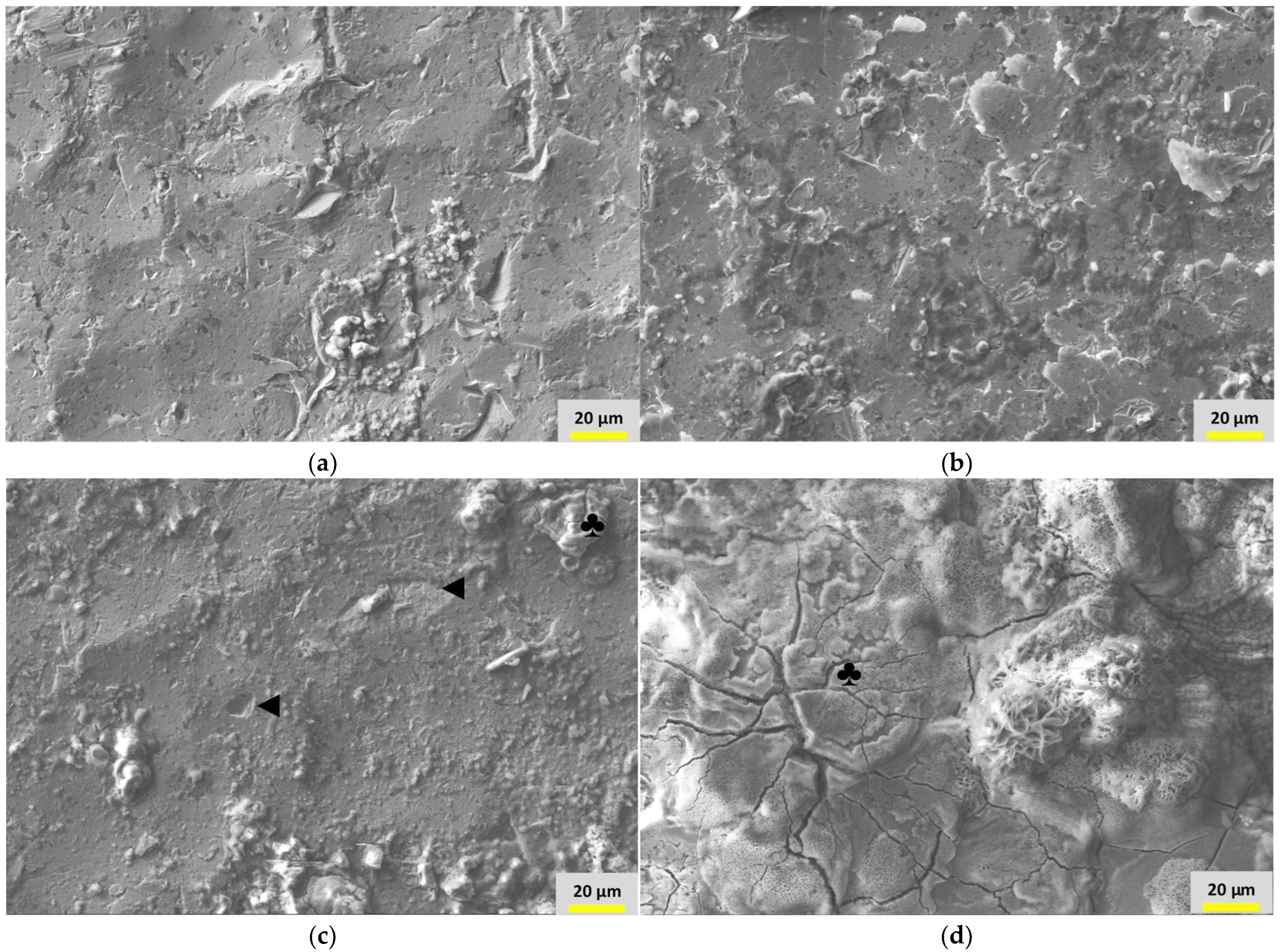
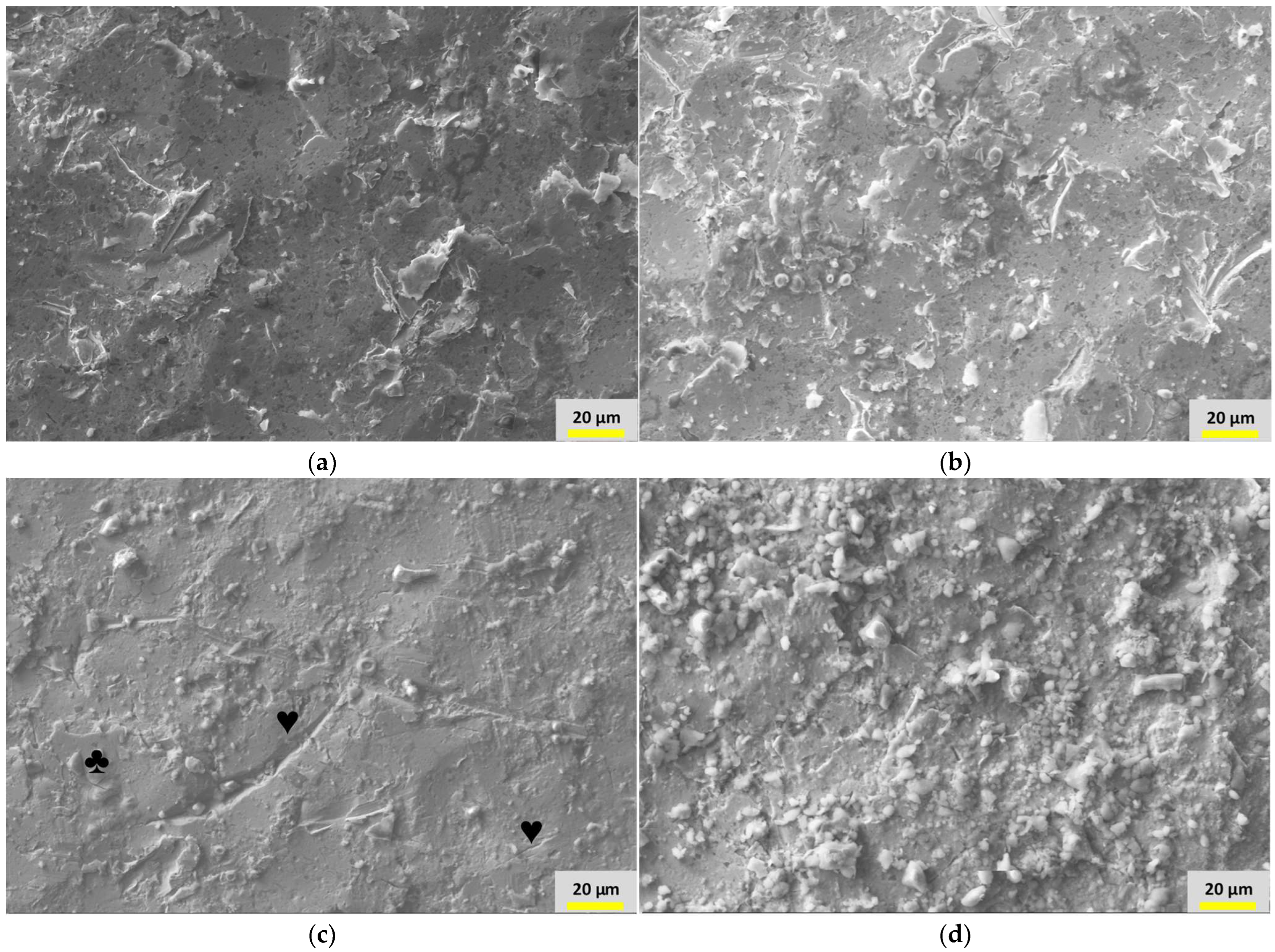
3.4. Surface Morphology and Elemental Analyses
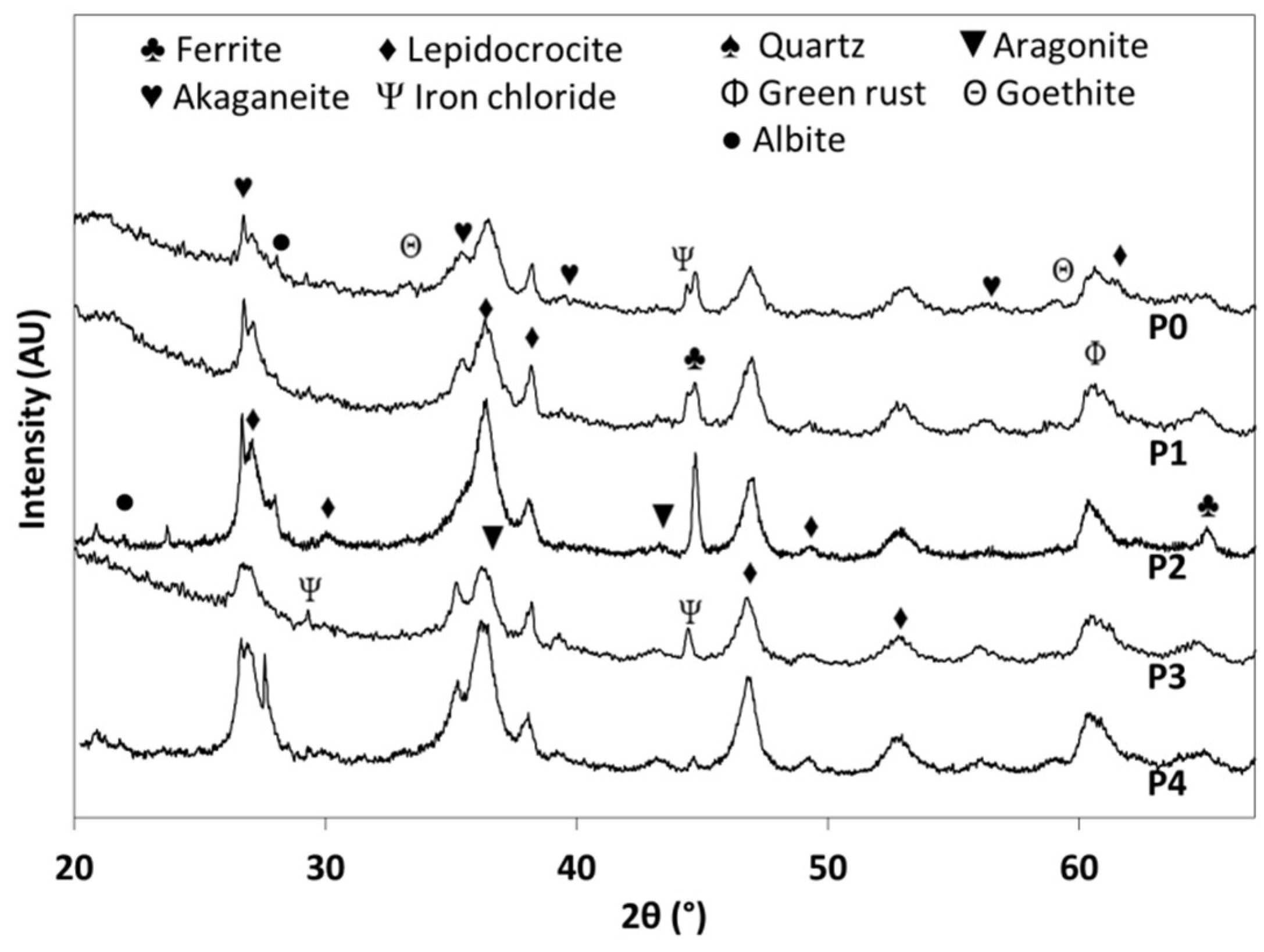
3.5. Oxide Evolution
3.6. Remarks on Corrosion Prevention and Pending Issues
4. Conclusions
- Vertical temperatures and RH gradients starting with low RH values at the ground level were observed in all sites;
- The vertical gradient generated a significant distortion in the TOW calculation when using RH values measured at the ground level. The use of RH values measured at 5 m above the ground level was found adequate for TOW determination;
- Water precipitation as small water droplets were demonstrated by using a fog water collector;
- The use of a limiting absolute humidity value is suggested instead of a limiting RH for the TOW determination;
- Metal corrosion in the Atacama Desert takes place as a sequence of wet−dry periods. The duration of wet periods is of a few hours during nighttime;
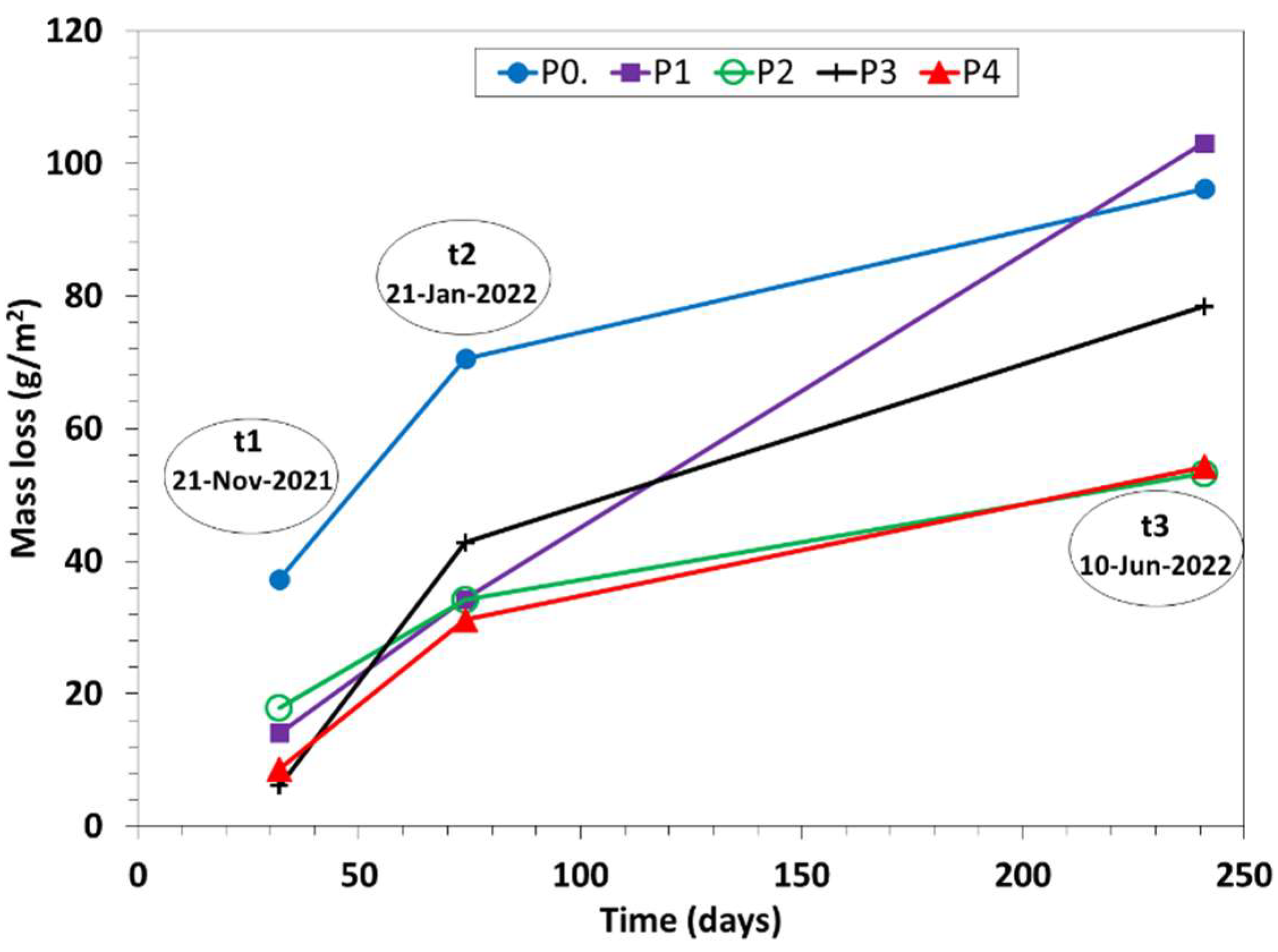
- Morphological examination showed that the corrosion was initiated by pits that gradually evolved to cover the complete metal surface in a maximum period of 250 days.
- It was found after 32 days of exposure a significant oxygen uptake in coupons oxide layer that was highest in the coastal site P0 and gradually decreased with the increasing distance from the coast. Similarly, but in lower extent, the chloride content in coupons decreased with the increasing distance from the coast. This is attributed to both relative humidity and saline marine fog intrusion from the coast.
- The oxide layer evolved in all sites to form a compact layer whose main constituents were lepidocrocite, goethite, and small amounts of akageneite. The corrosion depth can be modelled by a simple power function with B < 1, indicating a deceleration process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweitzer, A.P. Atmospheric Corrosion. In Fundamentals of Metallic Corrosion. Atmospheric and Media Corrosion of Metals, 2nd ed.; Schweitzer, P.A., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 39–66. [Google Scholar]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Atmospheric corrosion of different steels in marine, rural and industrial environments. Corr. Sci. 1999, 41, 1687–1702. [Google Scholar] [CrossRef]
- Di Sarno, L.; Majidian, A.; Karagiannakis, G. The effect of atmospheric corrosion on steel structures: A state-of-the-art and case-study. Buildings 2021, 11, 571. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, Y.; Zhao, Y.; Ma, X. Atmospheric corrosion prediction: A review. Corros. Rev. 2020, 38, 299–321. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Díaz, I.; Cano, H.; de la Fuente, D. Atmospheric corrosion data of weathering steels. A review. Corr. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef]
- Pei, Z.; Cheng, X.; Yang, X.; Li, Q.; Xia, C.; Zhang, D.; Li, X. Understanding environmental impacts on initial atmospheric corrosion based on corrosion monitoring sensors. J. Mater. Sci. Technol. 2021, 64, 214–221. [Google Scholar] [CrossRef]
- Popova, K.; Prošek, T. Corrosion monitoring in atmospheric conditions: A review. Metals 2022, 12, 171. [Google Scholar] [CrossRef]
- Rodríguez, J.J.S.; Hernández, F.J.S.; González, J.E.G. The effect of environmental and meteorological variables on atmospheric corrosion of carbon steel, copper, zinc and aluminium in a limited geographic zone with different types of environment. Corros. Sci. 2003, 45, 799–815. [Google Scholar] [CrossRef]
- Almeida, E.; Morcillo, M.; Rosales, B. Atmospheric corrosion of zinc Part 1: Rural and urban atmospheres. Br. Corros. J. 2000, 35, 284–288. [Google Scholar] [CrossRef]
- Morales, J.; Martín-Krijer, S.; Díaz, F.; Hernández-Borges, J.; González, S. Atmospheric corrosion in subtropical areas: Influences of time of wetness and deficiency of the ISO 9223 norm. Corros. Sci. 2005, 47, 2005–2019. [Google Scholar] [CrossRef]
- Chico, B.; De la Fuente, D.; Díaz, I.; Simancas, J.; Morcillo, M. Annual atmospheric corrosion of carbon steel worldwide. An integration of ISOCORRAG, ICP/UNECE and MICAT databases. Materials 2017, 10, 601. [Google Scholar] [CrossRef]
- de la Fuente, D.; Díaz, I.; Simancas, J.; Chico, B.; Morcillo, M. Long-term atmospheric corrosion of mild steel. Corros. Sci. 2011, 53, 604–617. [Google Scholar] [CrossRef]
- Cereceda, P.; Osses, P.; Larrain, H.; Farías, M.; Lagos, M.; Pinto, R.; Schemenauer, R. Advective, orographic and radiation fog in the Tarapacá region, Chile. Atmos. Res. 2002, 64, 261–271. [Google Scholar] [CrossRef]
- Klemm, O.; Schemenauer, R.S.; Lummerich, A.; Cereceda, P.; Marzol, V.; Corell, D.; van Heerden, J.; Reinhard, D.; Gherezghiher, T.; Olivier, J.; et al. Fog as a fresh-water resource: Overview and perspectives. Ambio 2012, 41, 221–234. [Google Scholar] [CrossRef]
- Cereceda, P.; Larrain, H.; Osses, P.; Farías, M.; Egaña, I. The spatial and temporal variability of fog and its relation to fog oases in the Atacama Desert, Chile. Atmos. Res. 2008, 87, 312–323. [Google Scholar] [CrossRef]
- McKay, C.P.; Friedmann, E.I.; Gómez-Silva, B.; Cáceres-Villanueva, L.; Andersen, D.T.; Landheim, R. Temperature and moisture conditions for life in the extreme arid region of the Atacama Desert: Four years of observations including the El Niño of 1997–1998. Astrobiology 2003, 3, 393–406. [Google Scholar] [CrossRef]
- Beiderwieden, E.; Wrzesinsky, T.; Klemm, O. Chemical characterization of fog and rain water collected at the eastern Andes cordillera. Hydrol. Earth Syst. Sci. 2005, 9, 185–191. [Google Scholar] [CrossRef]
- Straub, D.J.; Hutchings, J.W.; Herckes, P. Measurements of fog composition at a rural site. Atmos. Environ. 2012, 47, 195–205. [Google Scholar] [CrossRef]
- Polkowska, Z.; Górecki, T.; Namieśnik, J. Determination of atmospheric pollutants in wet deposition. Environ. Rev. 2011, 19, 185–213. [Google Scholar] [CrossRef]
- Navarro-González, R.; Rainey, F.A.; Molina, P.; Bagaley, D.R.; Hollen, B.J.; de la Rosa, J.; Small, A.M.; Quinn, R.C.; Grunthaner, F.J.; Cáceres, L.; et al. Mars-Like Soils in the Atacama Desert, Chile, and the Dry Limit of Microbial Life. Science 2003, 302, 1018–1021. [Google Scholar] [CrossRef]
- Quinn, R.C.; Zent, A.P.; Grunthaner, F.J.; Ehrenfreund, P.; Taylor, C.L.; Garry, J.R.C. Detection and characterization of oxidizing acids in the Atacama Desert using the Mars Oxidation Instrument, Planet. Space Sci. 2005, 53, 1376–1388. [Google Scholar] [CrossRef]
- Bull, A.T.; Asenjo, J.A. Microbiology of hyper-arid environments: Recent insights from the Atacama Desert, Chile. Antonie van Leeuwenhoek 2013, 103, 1173–1179. [Google Scholar] [CrossRef]
- Cáceres, L.; Gómez-Silva, B.; Garró, X.; Rodríguez, V.; Monardes, V.; McKay, C.P. Relative humidity patterns and fog water precipitation in the Atacama Desert and biological implications. J. Geophys. Res. Biogeosci. 2007, 112, 1–11. [Google Scholar] [CrossRef]
- ASTM G1-90; ASTM, 1999; p. 8. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/G1-03R11.htm (accessed on 5 August 2022).
- Vargas, G.; Ortlieb, L.; Rutllant, J. Aluviones históricos en Antofagasta y su relación con eventos El Niño/Oscilación del Sur. Rev. Geol. Chile 2000, 27, 157–176. [Google Scholar]
- Rutllant, J.A.; Fuenzalida, H.; Aceituno, P. Climate dynamics along the arid northern coast of Chile: The 1997–1998 dinámica del clima de la Región de Antofagasta (DICLIMA) experiment. J. Geophys. Res. Atmos. 2003, 108, 1–13. [Google Scholar] [CrossRef]
- Wierzchos, J.; DiRuggiero, J.; Vítek, P.; Artieda, O.; Souza-Egipsy, V.; Škaloud, P.; Tisza, M.; Davila, A.F.; Vílchez, C.; Garbayo, I.; et al. Adaptation strategies of endolithic chlorophototrophs to survive the hyperarid and extreme solar radiation environment of the Atacama Desert. Front. Microbiol. 2015, 6, 934. [Google Scholar] [CrossRef]
- Cordero, R.R.; Damiani, A.; Seckmeyer, G.; Jorquera, J.; Caballero, M.; Rowe, P.; Ferrer, J.; Mubarak, R.; Carrasco, J.; Rondanelli, R.; et al. The solar spectrum in the Atacama Desert. Sci. Rep. 2016, 6, 22457. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Díaz, F.; Fuentes, B.; Reyers, M.; Fiedler, S.; Böhm, C.; Campos, E.; Shao, Y.; Bol, R. Dust and aerosols in the Atacama Desert. Earth-Sci. Rev. 2022, 226, 103925. [Google Scholar] [CrossRef]
- Pinto, R.; Barría, I.; Marquet, P.A. Geographical distribution of Tillandsia lomas in the Atacama Desert, northern Chile. J. Arid Environ. 2006, 65, 543–552. [Google Scholar] [CrossRef]
- Qadir, M.; Jiménez, G.C.; Farnum, R.L.; Dodson, L.L.; Smakhtin, V. Fog Water Collection: Challenges beyond Technology. Water 2018, 10, 372. [Google Scholar] [CrossRef]
- Schindelholz, E.; Kelly, R.G.; Cole, I.S.; Ganther, W.D.; Muster, T.H. Comparability and accuracy of time of wetness sensing methods relevant for atmospheric corrosion. Corros. Sci. 2013, 67, 233–241. [Google Scholar] [CrossRef]
- Larson, J.W. General Corrosion. In ASM Handbook. Corrosion Materials, 1st ed.; Crammer, S.D., Covino, B.S., Eds.; ASM International: Novelty, OH, USA, 2005; p. 181. [Google Scholar]
- Hoseinpoor, M.; Prošek, T.; Babusiaux, L.; Mallégol, J. Toward more realistic time of wetness measurement by means of surface relative humidity. Corros. Sci. 2020, 177, 33. [Google Scholar] [CrossRef]
- ISO 9223; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. ISO: Geneva, Switzerland, 2012.
- Cáceres, L.; De la Torre, J.; Gómez-Silva, B.; Rodríguez, V.; McKay, C.P. Atmospheric moisture collection from a continuous air flow through a refrigerated coil tube. Atmos. Res. 2004, 71, 127–137. [Google Scholar] [CrossRef]
- Houston, J. Evaporation in the Atacama Desert: An empirical study of spatio-temporal variations and their causes. J. Hydrol. 2006, 330, 402–412. [Google Scholar] [CrossRef]
- Treybal, R. Operaciones de Transferencia de Masa, 2nd ed.; McGraw-Hill: Ciudad de México, Mexico, 2000; pp. 254–264. [Google Scholar]
- Morcillo, M.; Flores, S.; Salas, G.; Valencia, M. An extremely low corrosion rate of steel in the atmosphere of Cuzco (Peru). Atmos. Environ. Part A Gen. Top. 1993, 27, 1959–1962. [Google Scholar] [CrossRef]
- Han, W.; Yu, G.; Wang, Z.; Wang, J. Characterization of initial atmospheric corrosion carbon steels by field exposure and laboratory simulation. Corros. Sci. 2007, 49, 2920–2935. [Google Scholar] [CrossRef]
- Xiao, K.; Dong, C.; Li, X.; Wang, F. Corrosion products and formation mechanism during initial stage of atmospheric corrosion of carbon steel. J. Iron Steel Res. Int. 2008, 15, 42–48. [Google Scholar] [CrossRef]
- Liu, J.; Fa, K.; Zhang, Y.; Wu, B.; Qin, S.; Jia, X. Abiotic CO2 uptake from the atmosphere by semiarid desert soil and its partitioning into soil phases. Geophys. Res. Lett. 2015, 42, 5779–5785. [Google Scholar] [CrossRef]
- Stratmann, M.; Bohnenkamp, K.; Engell, H.J. An electrochemical study of phase-transitions in rust layers. Corros. Sci. 1983, 23, 969–985. [Google Scholar] [CrossRef]
- Tamura, H. The role of rusts in corrosion and corrosion protection of iron and steel. Corros. Sci. 2008, 50, 1872–1883. [Google Scholar] [CrossRef]
- Xiao, H.; Ye, W.; Song, X.; Ma, Y.; Li, Y. Formation process of akaganeite in the simulated wet-dry cycles atmospheric environment. J. Mater. Sci. Technol. 2018, 34, 1387–1396. [Google Scholar] [CrossRef]
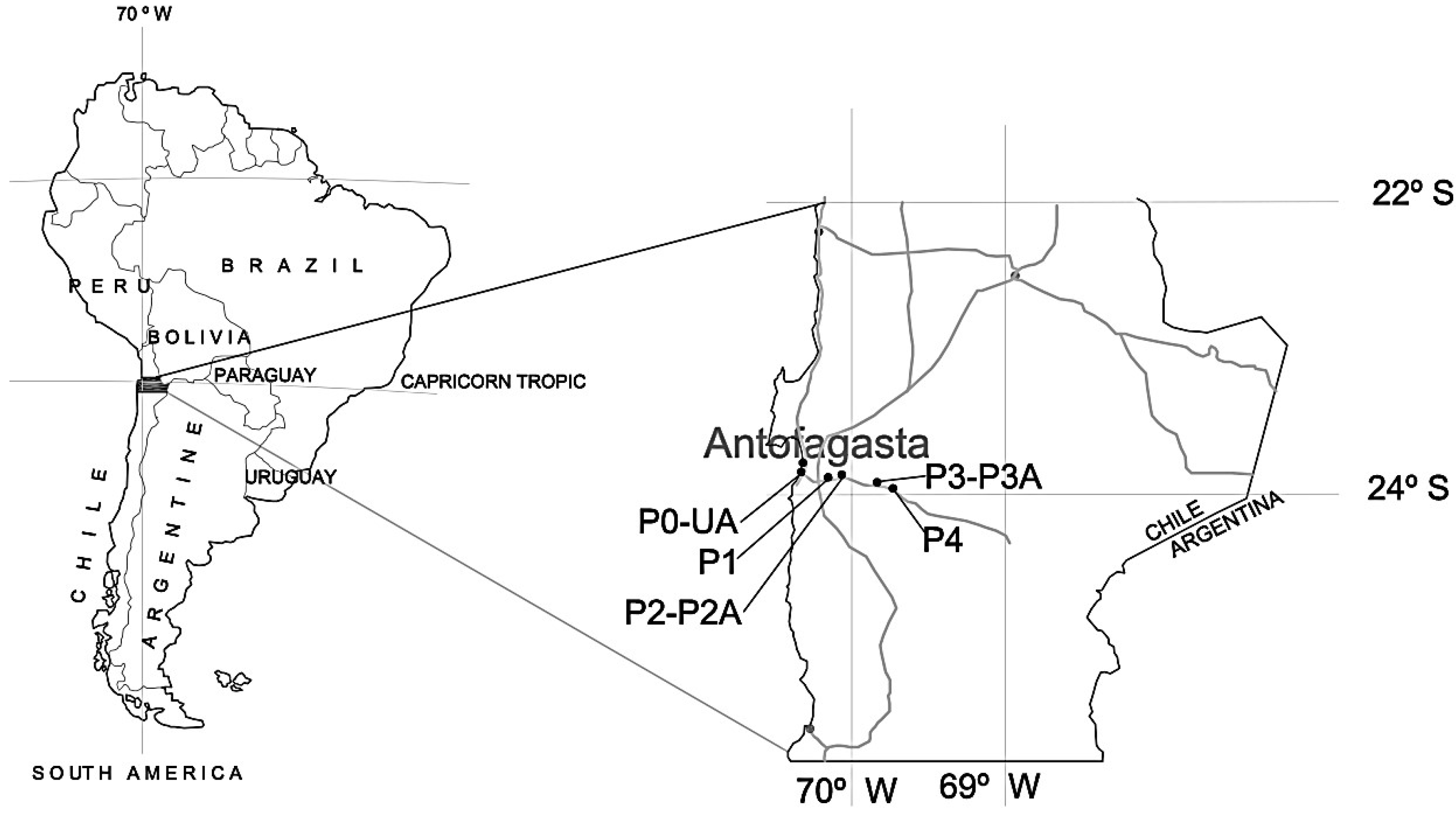



| Site | Coordinates | Coupons and Devices | |
|---|---|---|---|
| Position (WGS84) | Altitude (m) | ||
| P0 | 23°43.020′ S 70′25.187′ W | 20 | 11 coupons on the ground and one T/RH sensor |
| One meteorological station with 9 sensors: University of Antofagasta (UA) | |||
| P1 | 23°45.563′ S 70′15.769′ W | 570 | 11 coupons on the ground and T/RH sensor |
| P2, P2A | 23°44.773′ S 70′11.106′ W | 655 | P2: 11 coupons on the ground and one T/RH sensor |
| P2A: one T/RH sensor above 3 m over the ground | |||
| P3, P3A | 23°51.964′ S 69′50.956′ W | 789 | P3: 11 coupons on the ground and one T/RH sensor |
| P3A: one fog water collector and one T/RH sensor above 3 m over the ground | |||
| P4 | 23°56.606′ S 69′43.781′ W | 915 | 11 coupons on the ground and one T/RH sensor |
| Site | Average Values | Average Daily Oscillations | ||
|---|---|---|---|---|
| Temperature °C | Relative Humidity % | Site | Temperature °C | |
| P0 | 20.6 | 58.0 | P0 | 20.6 |
| UA | 18.1 | 73.9 | UA | 18.1 |
| P1 | 17.3 | 54.9 | P1 | 17.3 |
| P2 | 18.1 | 49.8 | P2 | 18.1 |
| P2A | 18.4 | 63.7 | P2A | 18.4 |
| P3 | 18.8 | 40.2 | P3 | 18.8 |
| P3A | 17.0 | 59.2 | P3A | 17.0 |
| P4 | 19.6 | 27.6 | P4 | 19.6 |
| Element (%) | AISI 1020 Composition | Sites | ||||
|---|---|---|---|---|---|---|
| P0 | P1 | P2 | P3 | P4 | ||
| Fe | 98.5 | 67.3 | 77.6 | 86.2 | 86.4 | 88.3 |
| O | - | 24.4 | 13.8 | 5.8 | 5.6 | 4.3 |
| C | 0.2 | 5.7 | 5.8 | 5.5 | 4.8 | 4.6 |
| Si | Traces | 0.7 | 1.2 | 0.6 | 1.4 | 1.5 |
| Cl | - | 0.5 | 0.2 | 0.1 | 0.1 | - |
| Na | - | 0.4 | 0.3 | 0.1 | 0.3 | 0.2 |
| Mn | 0.6 | 0.3 | 0.5 | 0.6 | 0.5 | 0.6 |
| Al | - | 0.3 | 0.3 | 0.3 | 0.5 | 0.2 |
| Ca | - | 0.1 | 0.3 | 0.2 | 0.2 | 0.3 |
| S | Traces | 0.1 | 0.1 | 0.1 | 0.1 | - |
| Cr | Traces | 0.1 | - | - | - | 0.1 |
| Co | - | - | - | 0.4 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres, L.; Soliz, A.; Galleguillos, F. Atmospheric Corrosion Evolution of Carbon Steel AISI 1020 along a Longitude Transect in the Atacama Desert. Metals 2022, 12, 1980. https://doi.org/10.3390/met12111980
Cáceres L, Soliz A, Galleguillos F. Atmospheric Corrosion Evolution of Carbon Steel AISI 1020 along a Longitude Transect in the Atacama Desert. Metals. 2022; 12(11):1980. https://doi.org/10.3390/met12111980
Chicago/Turabian StyleCáceres, Luis, Alvaro Soliz, and Felipe Galleguillos. 2022. "Atmospheric Corrosion Evolution of Carbon Steel AISI 1020 along a Longitude Transect in the Atacama Desert" Metals 12, no. 11: 1980. https://doi.org/10.3390/met12111980
APA StyleCáceres, L., Soliz, A., & Galleguillos, F. (2022). Atmospheric Corrosion Evolution of Carbon Steel AISI 1020 along a Longitude Transect in the Atacama Desert. Metals, 12(11), 1980. https://doi.org/10.3390/met12111980







