Interfacial Polarization-Dominated Dielectric Loss in SnO2@rGO Electromagnetic Wave Absorbers
Abstract
:1. Introduction
2. Materials and Methods
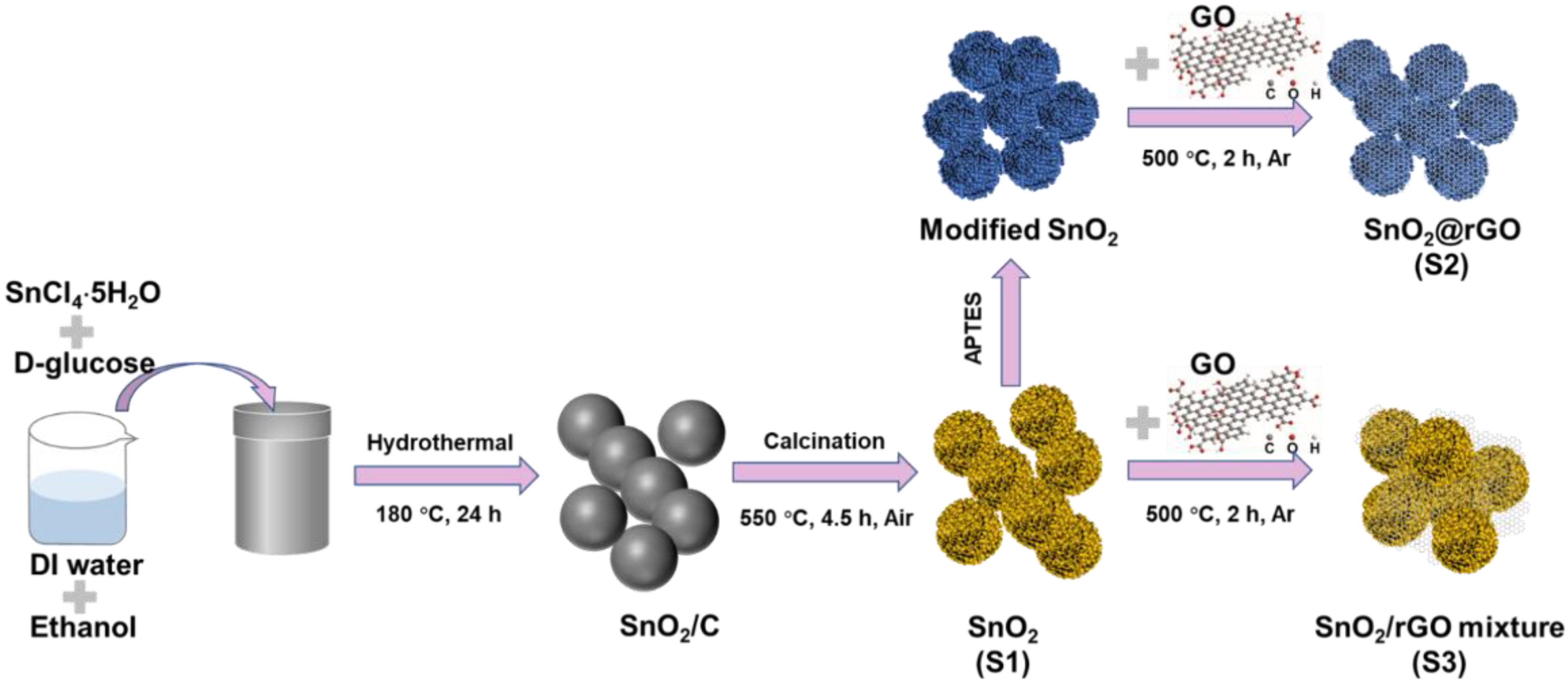
2.1. Synthesis of SnO2 Microspheres (Marked as S1)
2.2. Synthesis of SnO2@rGO Composites (Marked as S2)
2.3. Synthesis of SnO2/rGO Mixture (Marked as S3)
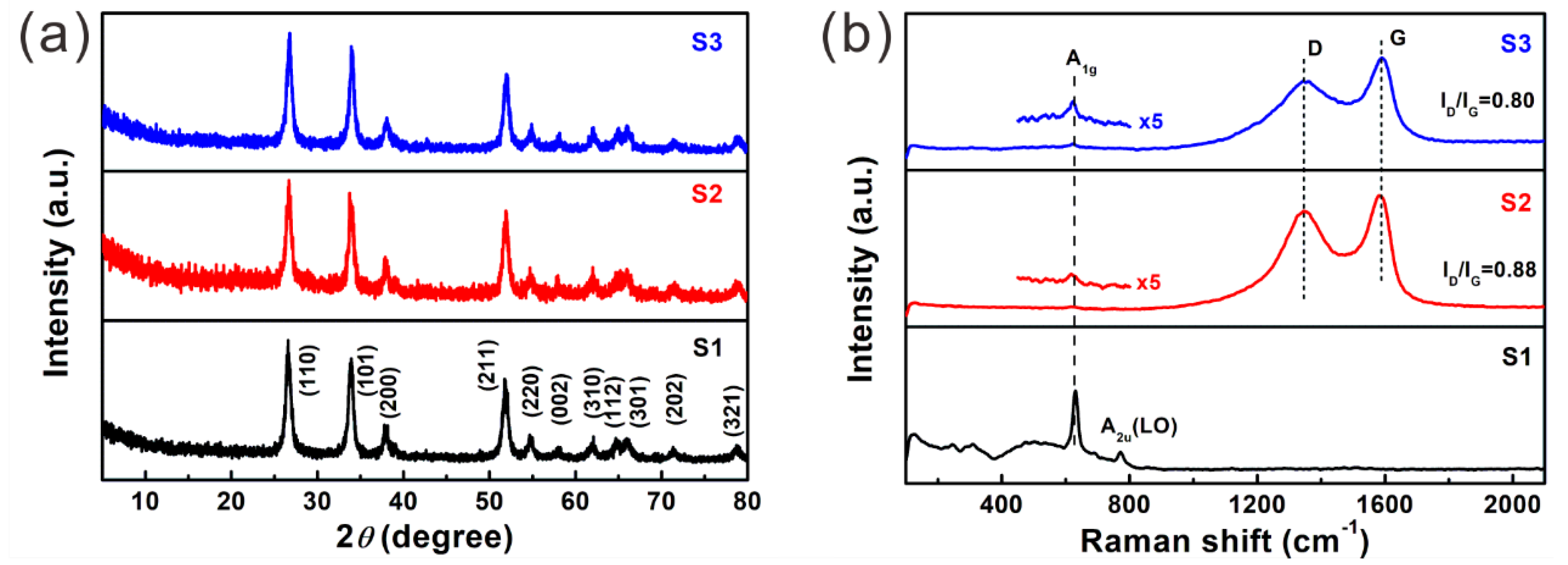
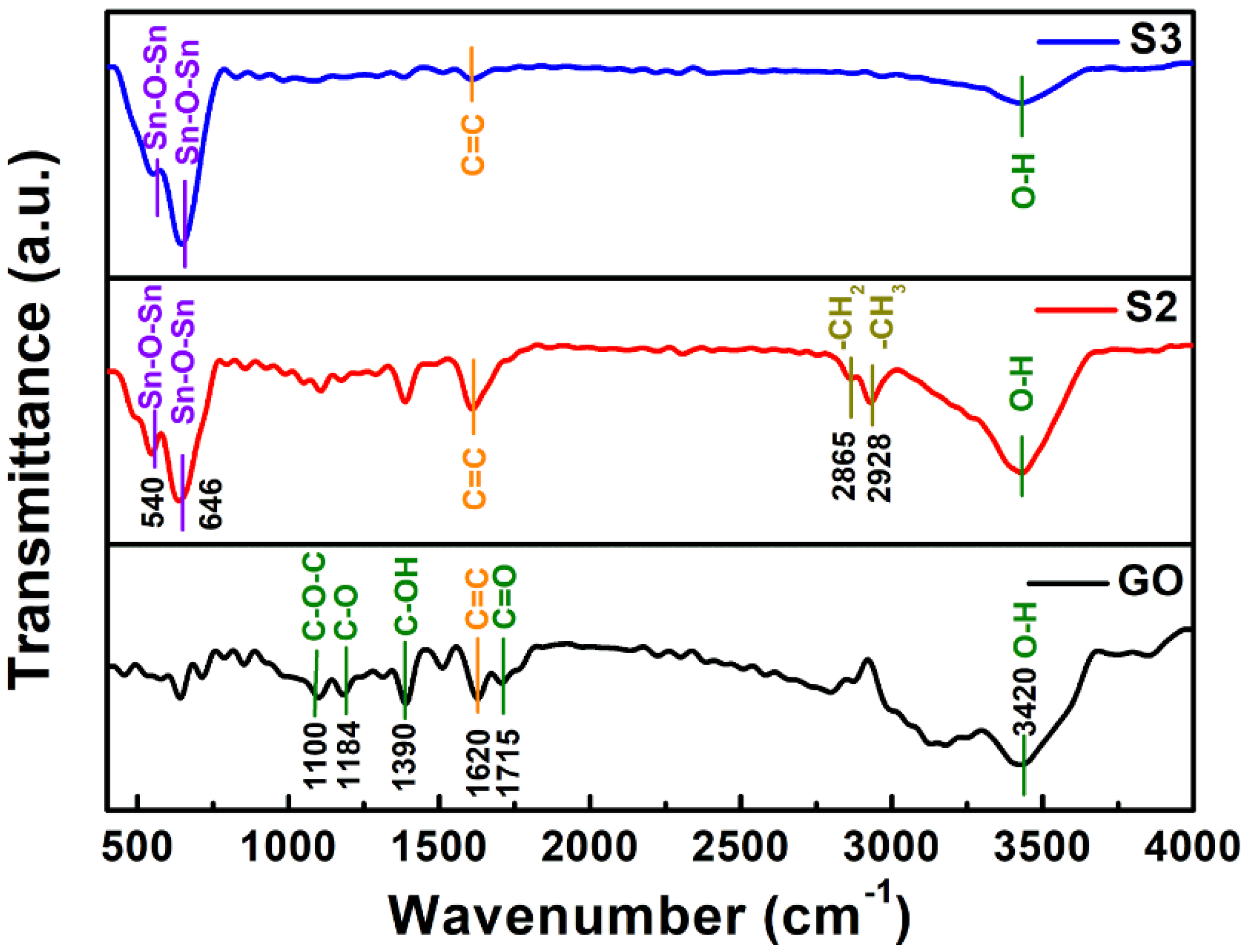
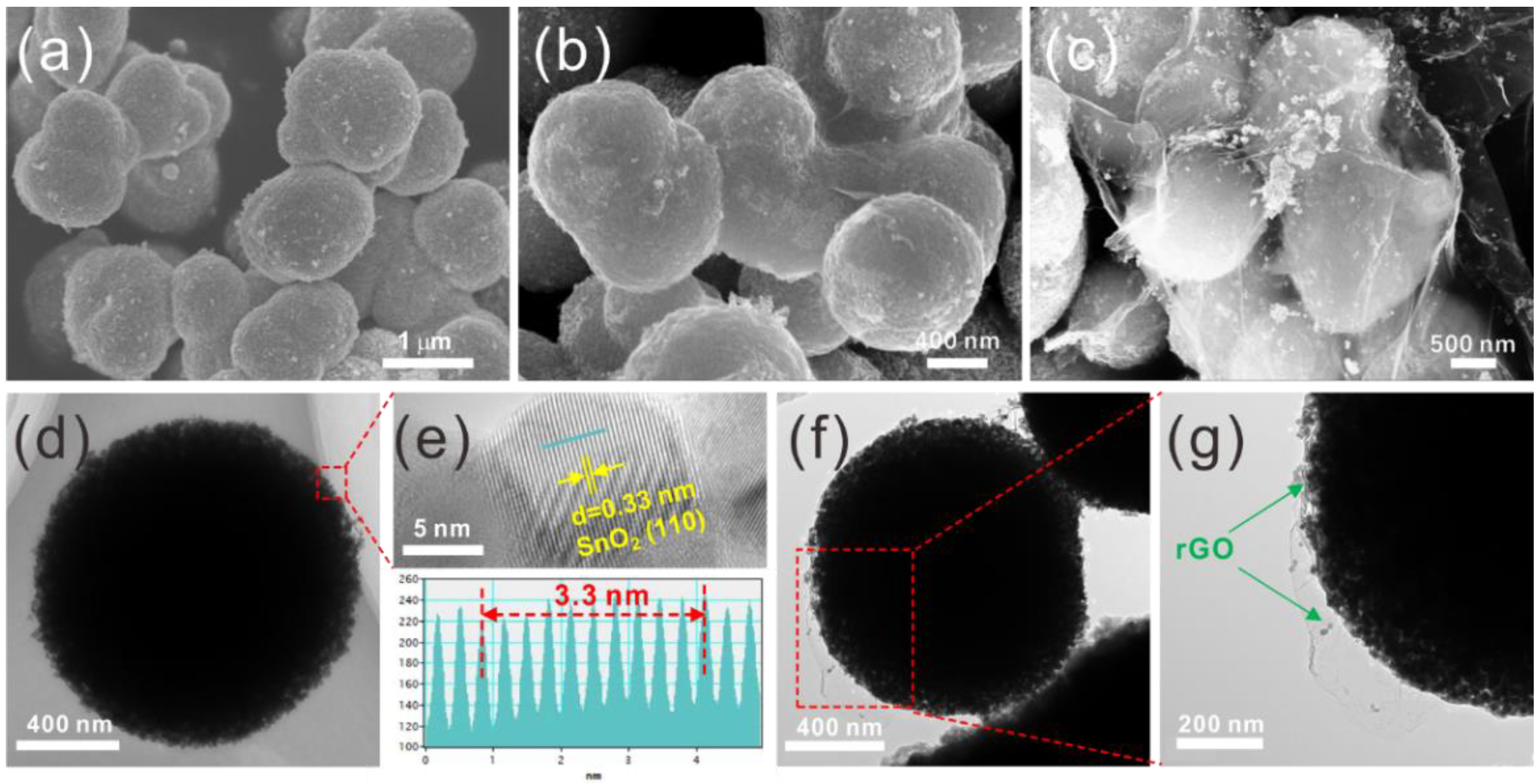
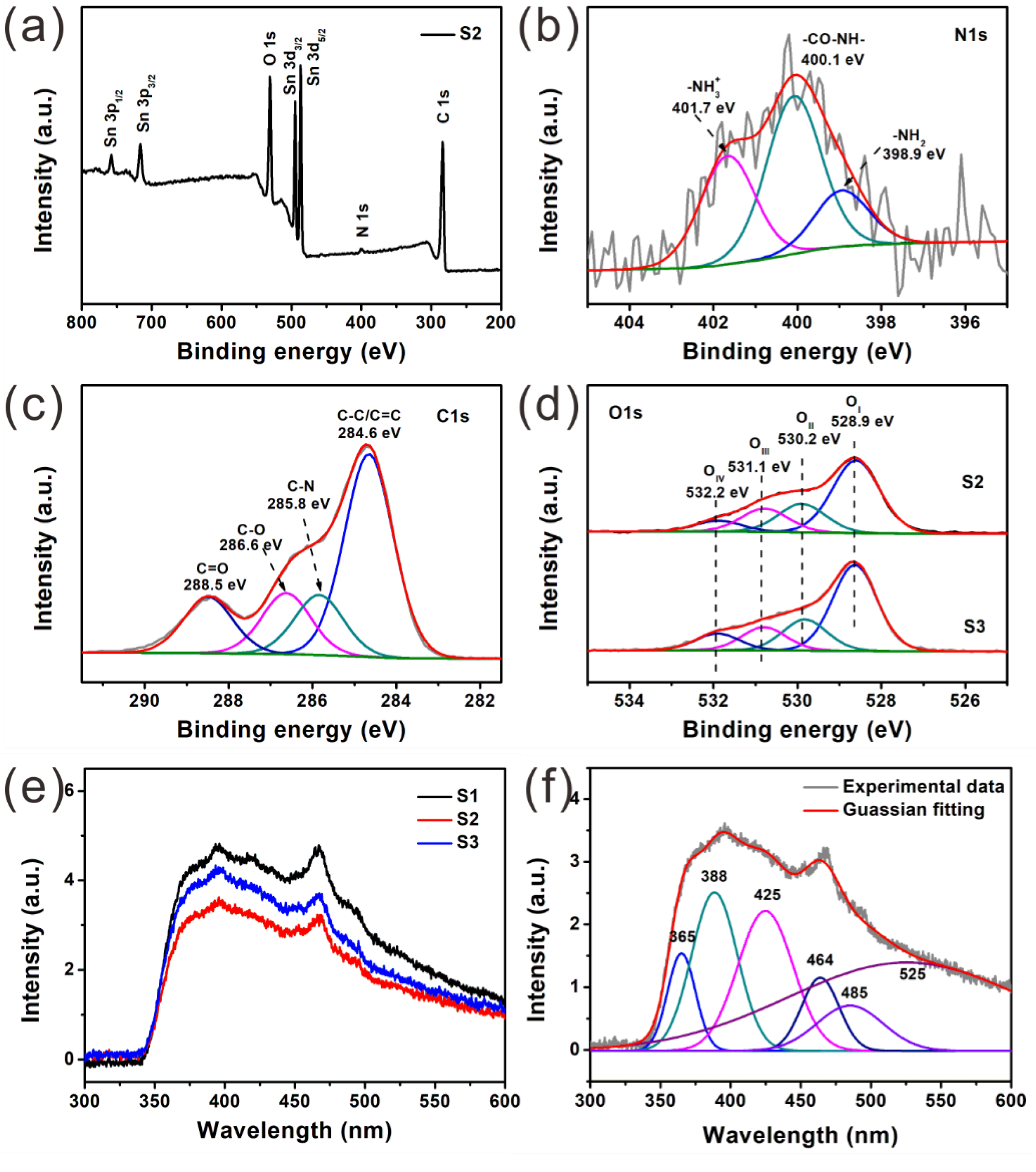
2.4. Material Characterization
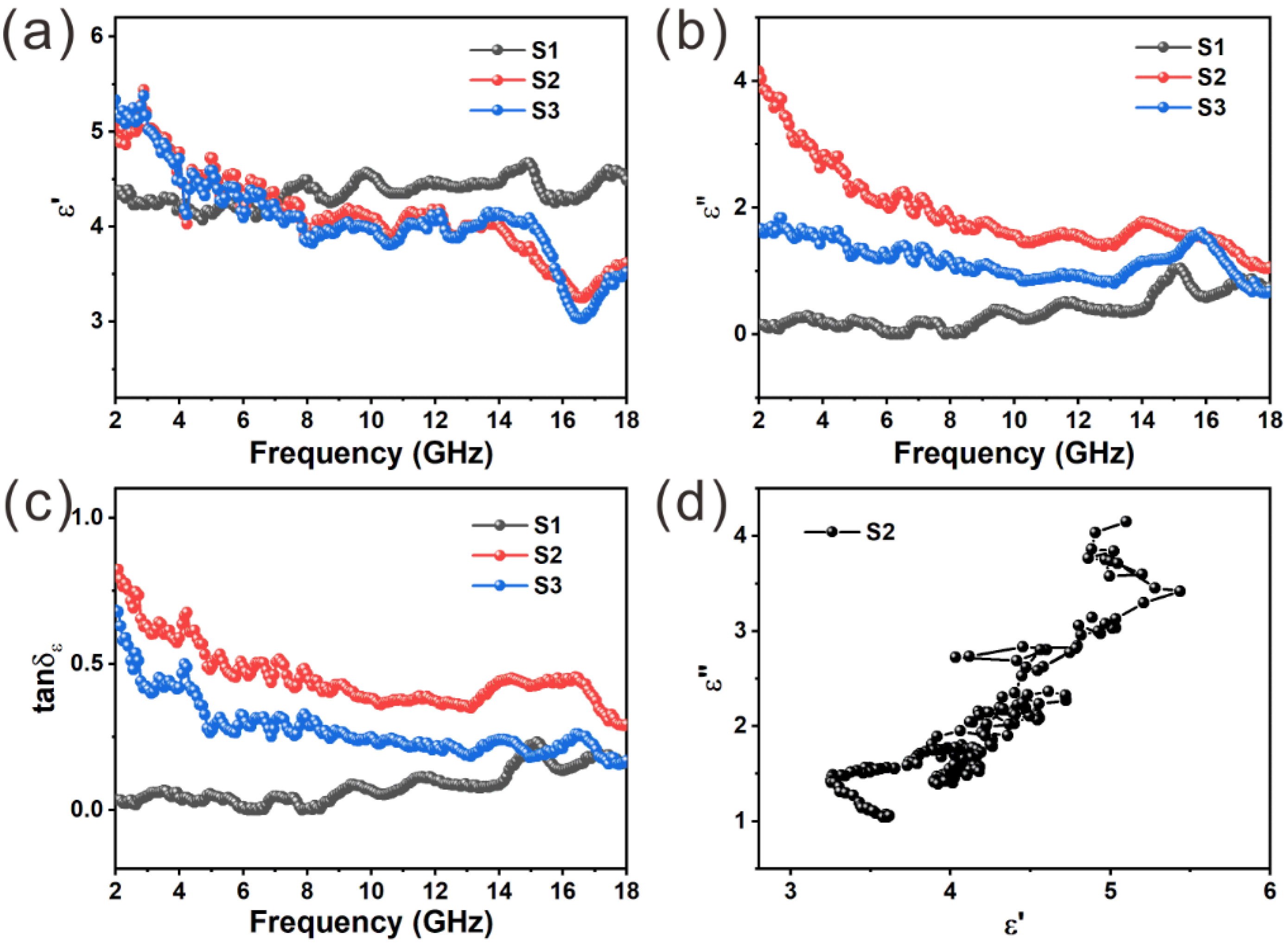
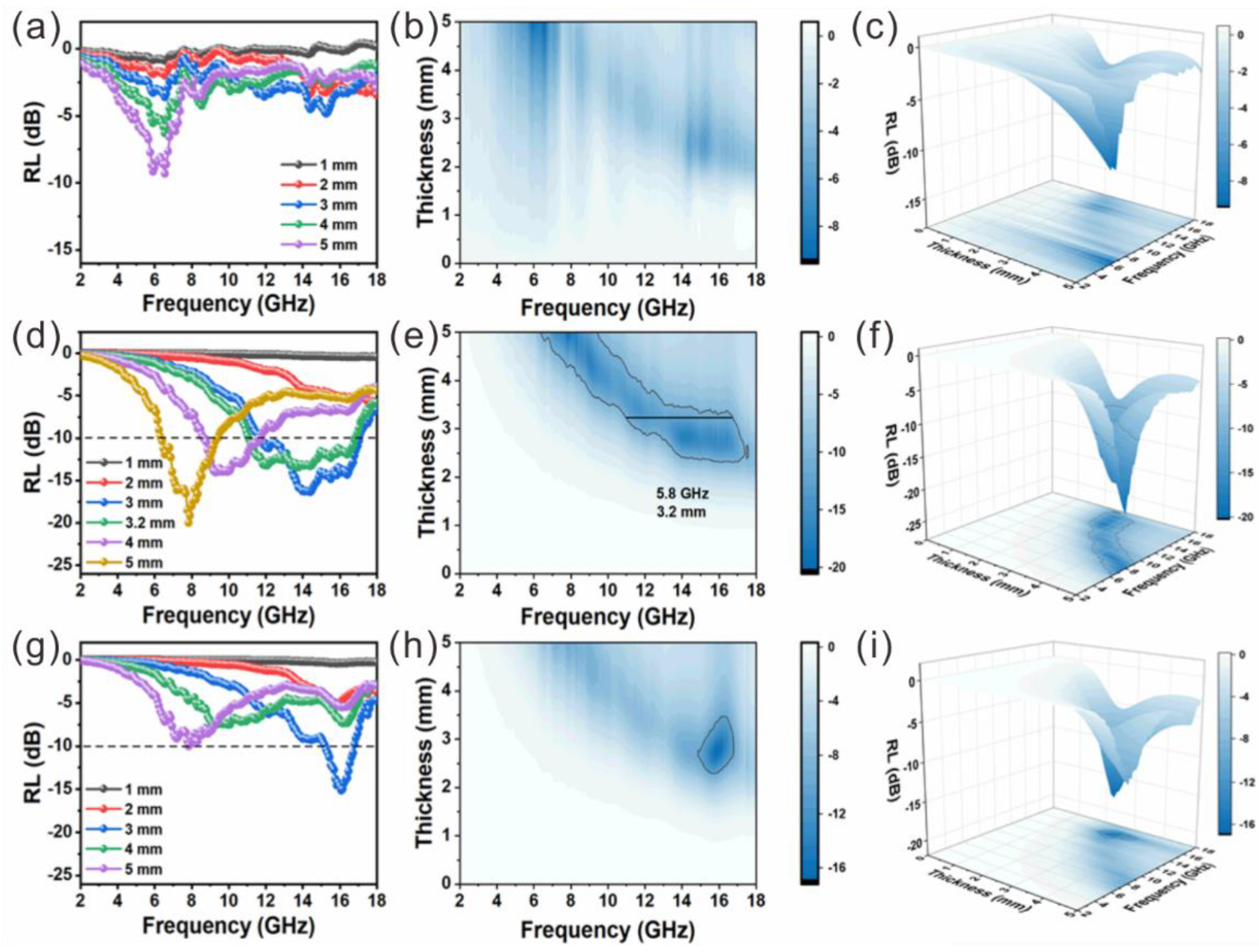
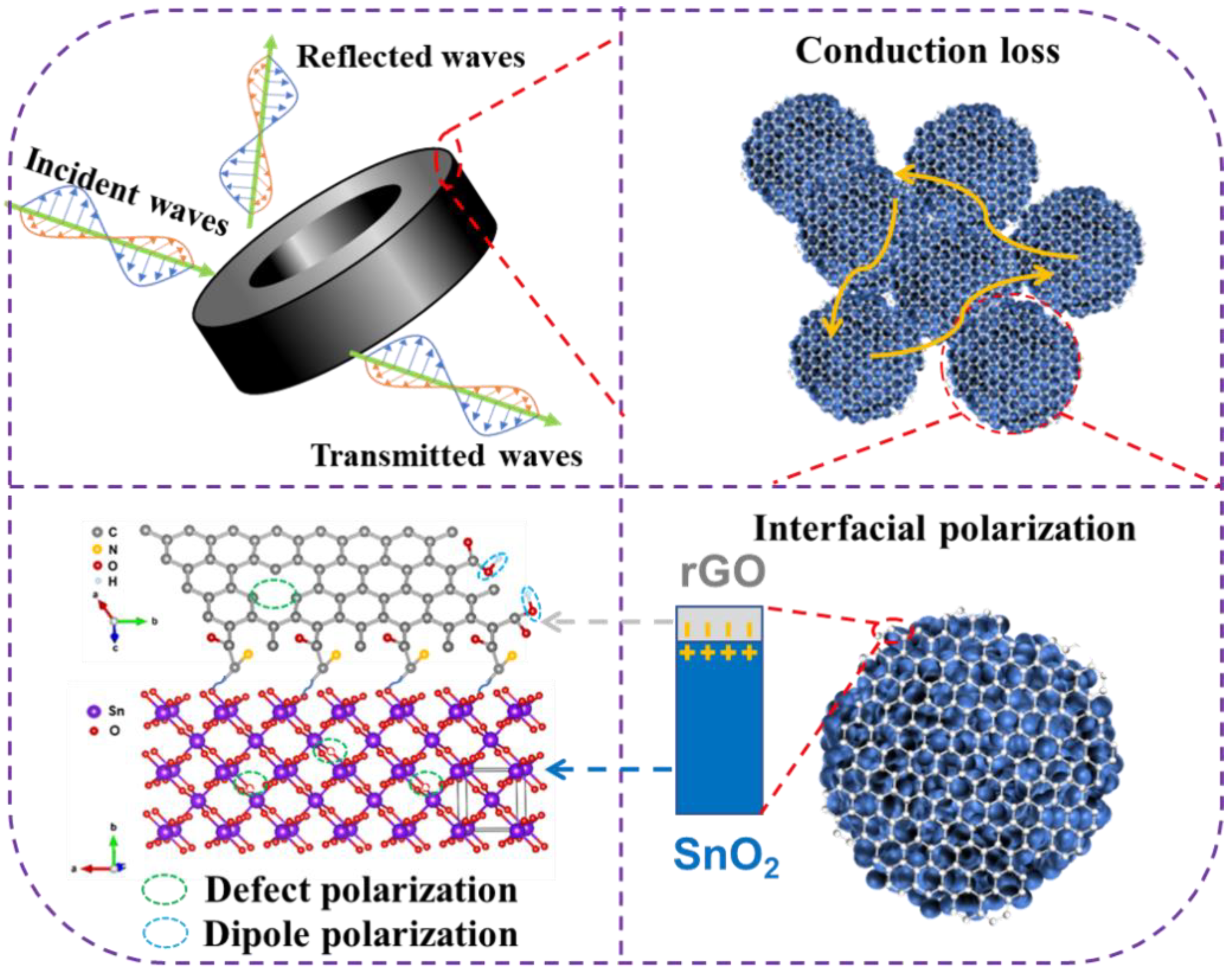
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younes, H.; Shoaib, N.; Rahman, M.M.; Abu Al-Rub, R.; Hong, H.; Christensen, G.; Chen, H.; Younes, A.B.; Al Ghaferi, A. Thin carbon nanostructure mat with high electromagnetic interference shielding performance. Synth. Met. 2019, 253, 48–56. [Google Scholar] [CrossRef]
- Miller, A.B.; Sears, M.E.; Morgan, L.L.; Davis, D.L.; Hardell, L.; Oremus, M.; Soskolne, C.L. Risks to health and well-being from radio-frequency radiation emitted by cell phones and other wireless devices. Front Public Health 2019, 7, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.L.; Zhang, L.M.; Wu, H.J. Anion-doping-induced vacancy engineering of cobalt sulfoselenide for boosting electromagnetic wave absorption. Adv. Funct. Mater. 2022, 32, 2200544. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, L.; Zhao, X.; Wu, H. Defect induced polarization loss in multi-shelled spinel hollow spheres for electromagnetic wave absorption application. Adv. Sci. 2021, 8, 2004640. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress on carbon-based composite materials for microwave electromagnetic interference shielding. Carbon 2021, 177, 304–331. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, X.; Yu, R.; Stucky, G.D. Electromagnetic microwave absorption theory and recent achievements in microwave absorbers. Carbon 2020, 168, 606–623. [Google Scholar] [CrossRef]
- Houbi, A.; Aldashevich, Z.A.; Atassi, Y.; Telmanovna, Z.B.; Saule, M.; Kubanych, K. Microwave absorbing properties of ferrites and their composites: A review. J. Magn. Magn. Mater. 2021, 529, 167839. [Google Scholar] [CrossRef]
- Bao, W.; Chen, C.; Si, Z. An easy method of synthesis CoxOy@C composite with enhanced microwave absorption performance. Nanomaterials 2020, 10, 902. [Google Scholar] [CrossRef]
- Lv, H.; Yang, Z.; Pan, H.; Wu, R. Electromagnetic absorption materials: Current progress and new frontiers. Prog. Mater. Sci. 2022, 127, 100946. [Google Scholar] [CrossRef]
- Wang, L.; Huang, M.; Yu, X.; You, W.; Zhao, B.; Liang, C.; Liu, X.; Zhang, X.; Che, R. Engineering polarization surface of hierarchical ZnO microspheres via spray-annealing strategy for wide-frequency electromagnetic wave absorption. J. Mater. Sci. Technol. 2022, 131, 231–239. [Google Scholar] [CrossRef]
- Lv, H.; Wu, C.; Tang, J.; Du, H.; Qin, F.; Peng, H.; Yan, M. Two-dimensional SnO/SnO2 heterojunctions for electromagnetic wave absorption. Chem. Eng. J. 2021, 411, 128445. [Google Scholar] [CrossRef]
- Ojha, G.P.; Pant, B.; Park, S.J.; Park, M.; Kim, H.Y. Synthesis and characterization of reduced graphene oxide decorated with CeO2-doped MnO2 nanorods for supercapacitor applications. J. Colloid Interface Sci. 2017, 494, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Li, T.; Li, J.; Ren, H.; Meng, F. A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption. Compos. Part B Eng. 2021, 211, 108642. [Google Scholar] [CrossRef]
- Song, Q.; Ye, F.; Kong, L.; Shen, Q.; Han, L.; Feng, L.; Yu, G.; Pan, Y.; Li, H. Graphene and MXene nanomaterials: Toward high-performance electromagnetic wave absorption in gigahertz band range. Adv. Funct. Mater. 2020, 30, 2105190. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Dong, H.; Yu, M.; Zhang, B.; Wang, W.; Dong, L. Synthesis of nanostructured MnO2, SnO2, and Co3O4: Graphene composites with enhanced microwave absorption properties. Appl. Phys. A 2015, 119, 1483–1490. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Wu, C.; Liu, Y.; Liu, Y.; Zhao, G. Reduced graphene oxide decorated with ZnO microrods for efficient electromagnetic wave absorption performance. J. Mater. Sci. Mater. Electron. 2020, 31, 8637–8648. [Google Scholar] [CrossRef]
- Shi, S.; Hao, S.; Yang, C.; Chen, Y.; Chu, H.; Dai, S. Enhanced microwave absorption properties of reduced graphene oxide/TiO2 nanowire composites synthesized via simultaneous carbonation and hydrogenation. J. Mater. Chem. C 2022, 10, 9586–9595. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, L.; Wu, H. Dielectric loss mechanism in electromagnetic wave absorbing materials. Adv. Sci. 2022, 9, 2105553. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, N.; An, D.; Li, Y.; Zou, Y.; Lian, X.; Tong, X. Enhanced gas sensing properties of hierarchical SnO2 nanoflower assembled from nanorods via a one-pot template-free hydrothermal method. Ceram. Int. 2016, 42, 15889–15896. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, Y.; Zhang, J.; Liu, X.; Wang, N.; Yang, S. The study of graphene oxide on the regulations and controls of the sol-gel film structure and its performance. Metals 2021, 12, 20. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Z.; Wang, C.; Zou, P.; Hou, Z.; Xu, C.; Wu, H. Defect- and interface-induced dielectric loss in ZnFe2O4/ZnO/C electromagnetic wave absorber. Nanomaterials 2022, 12, 2871. [Google Scholar] [CrossRef] [PubMed]
- Rosoiu, S.P.; Pantazi, A.G.; Petica, A.; Cojocaru, A.; Costovici, S.; Zanella, C.; Visan, T.; Anicai, L.; Enachescu, M. Electrodeposition of NiSn-rGO composite coatings from deep eutectic solvents and their physicochemical characterization. Metals 2020, 10, 1455. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, P.; Li, G.; Lu, D.; Sun, R.; Wong, C. Core–shell SiO2@RGO hybrids for epoxy composites with low percolation threshold and enhanced thermo-mechanical properties. J. Mater. Chem. A 2014, 2, 18246–18255. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, X.R.; Duan, L.B.; Liu, R.D.; Li, H. Enhanced visible light photocatalytic activity in SnO2@g-C3N4 core-shell structures. Mater. Sci. Eng. B 2017, 218, 23–30. [Google Scholar] [CrossRef]
- Elci, A.; Demirtas, O.; Ozturk, I.M.; Bek, A.; Nalbant Esenturk, E. Synthesis of tin oxide-coated gold nanostars and evaluation of their surface-enhanced Raman scattering activities. J. Mater. Sci. 2018, 53, 16345–16356. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.W.; Yin, L.C.; Li, N.; Li, F.; Cheng, H.M. Oxygen bridges between NiO nanosheets and graphene for improvement of lithium storage. ACS Nano 2012, 6, 3214–3223. [Google Scholar] [CrossRef]
- Serodre, T.; Oliveira, N.; Miquita, D.; Ferreira, M.; Santos, A.; Resende, V.; Furtado, C. Surface silanization of graphene oxide under mild reaction conditions. J. Braz. Chem. Soc. 2019, 30, 2488–2499. [Google Scholar] [CrossRef]
- Min, H.; Girard-Lauriault, P.L.; Gross, T.; Lippitz, A.; Dietrich, P.; Unger, W.E. Ambient-ageing processes in amine self-assembled monolayers on microarray slides as studied by ToF-SIMS with principal component analysis, XPS, and NEXAFS spectroscopy. Anal. Bioanal. Chem. 2012, 403, 613–623. [Google Scholar] [CrossRef]
- Cong, R.; Park, H.H.; Jo, M.; Lee, H.; Lee, C.S. Synthesis and electrochemical performance of electrostatic self-assembled nano-silicon@N-doped reduced graphene oxide/carbon nanofibers composite as anode material for lithium-ion batteries. Molecules 2021, 26, 4831. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Wu, H. Hierarchical flower-like Fe3O4/MoS2 composites for selective broadband electromagnetic wave absorption performance. Compos. Part A-Appl. Sci. Manuf. 2020, 130, 105760. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Jia, Z.; Wu, H.; Wu, G. Oxygen vacancy-induced dielectric polarization prevails in the electromagnetic wave-absorbing mechanism for Mn-based MOFs-derived composites. Adv. Funct. Mater. 2022, 32, 2204499. [Google Scholar] [CrossRef]
- Shewale, P.S.; Ung Sim, K.; Kim, Y.-b.; Kim, J.H.; Moholkar, A.V.; Uplane, M.D. Structural and photoluminescence characterization of SnO2:F thin films deposited by advanced spray pyrolysis technique at low substrate temperature. J. Lumin. 2013, 139, 113–118. [Google Scholar] [CrossRef]
- Deshmukh, S.M.; Patil, S.S.; Babar, S.B.; Alshehri, S.; Ghoneim, M.M.; Tamboli, A.M.; Lam, N.H.; Truong, N.T.N.; Kim, C.D.; Tamboli, M.S.; et al. TiO2–SnO2 nanocomposites for photocatalytic environmental remediation under UV-light. Metals 2022, 12, 733. [Google Scholar] [CrossRef]
- Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Ahamed, M. One-pot synthesis of SnO2-rGO nanocomposite for enhanced photocatalytic and anticancer activity. Polymers 2022, 14, 2036. [Google Scholar] [CrossRef]
- Chang, Q.; Liang, H.; Shi, B.; Li, X.; Zhang, Y.; Zhang, L.; Wu, H. Ethylenediamine-assisted hydrothermal synthesis of NiCo2O4 absorber with controlled morphology and excellent absorbing performance. J. Colloid Interface Sci. 2021, 588, 336–345. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Zhou, N.; Du, Y.; Zeng, X.; Zhou, S.; He, Q.; Wu, G. In-situ growth of core-shell ZnFe2O4@ porous hollow carbon microspheres as an efficient microwave absorber. J. Colloid Interface Sci. 2021, 581, 475–484. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, L.; Luo, B.; Wu, H. Optimal control of the compositions, interfaces, and defects of hollow sulfide for electromagnetic wave absorption. J. Colloid Interface Sci. 2022, 607, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, K.; Zhou, Y.; Wu, H.; Xie, H. In situ construction of Fe3Al@Al2O3 core-shell particles with excellent electromagnetic absorption. J. Colloid Interface Sci. 2022, 611, 306–316. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Shi, X.; Wang, Z.; Zou, P.; Hou, Z.; Xu, C.; Zhang, L.; Wu, H. Interfacial Polarization-Dominated Dielectric Loss in SnO2@rGO Electromagnetic Wave Absorbers. Metals 2022, 12, 2154. https://doi.org/10.3390/met12122154
Shen H, Shi X, Wang Z, Zou P, Hou Z, Xu C, Zhang L, Wu H. Interfacial Polarization-Dominated Dielectric Loss in SnO2@rGO Electromagnetic Wave Absorbers. Metals. 2022; 12(12):2154. https://doi.org/10.3390/met12122154
Chicago/Turabian StyleShen, Hao, Xinyu Shi, Zhen Wang, Pengfei Zou, Zhaoyang Hou, Chunlong Xu, Limin Zhang, and Hongjing Wu. 2022. "Interfacial Polarization-Dominated Dielectric Loss in SnO2@rGO Electromagnetic Wave Absorbers" Metals 12, no. 12: 2154. https://doi.org/10.3390/met12122154
APA StyleShen, H., Shi, X., Wang, Z., Zou, P., Hou, Z., Xu, C., Zhang, L., & Wu, H. (2022). Interfacial Polarization-Dominated Dielectric Loss in SnO2@rGO Electromagnetic Wave Absorbers. Metals, 12(12), 2154. https://doi.org/10.3390/met12122154








