The Effect of Aluminum Addition on the Evolution of Inclusions in an Aluminum-Killed Calcium-Treated Steel
Abstract
:1. Introduction
2. Experimental Procedure
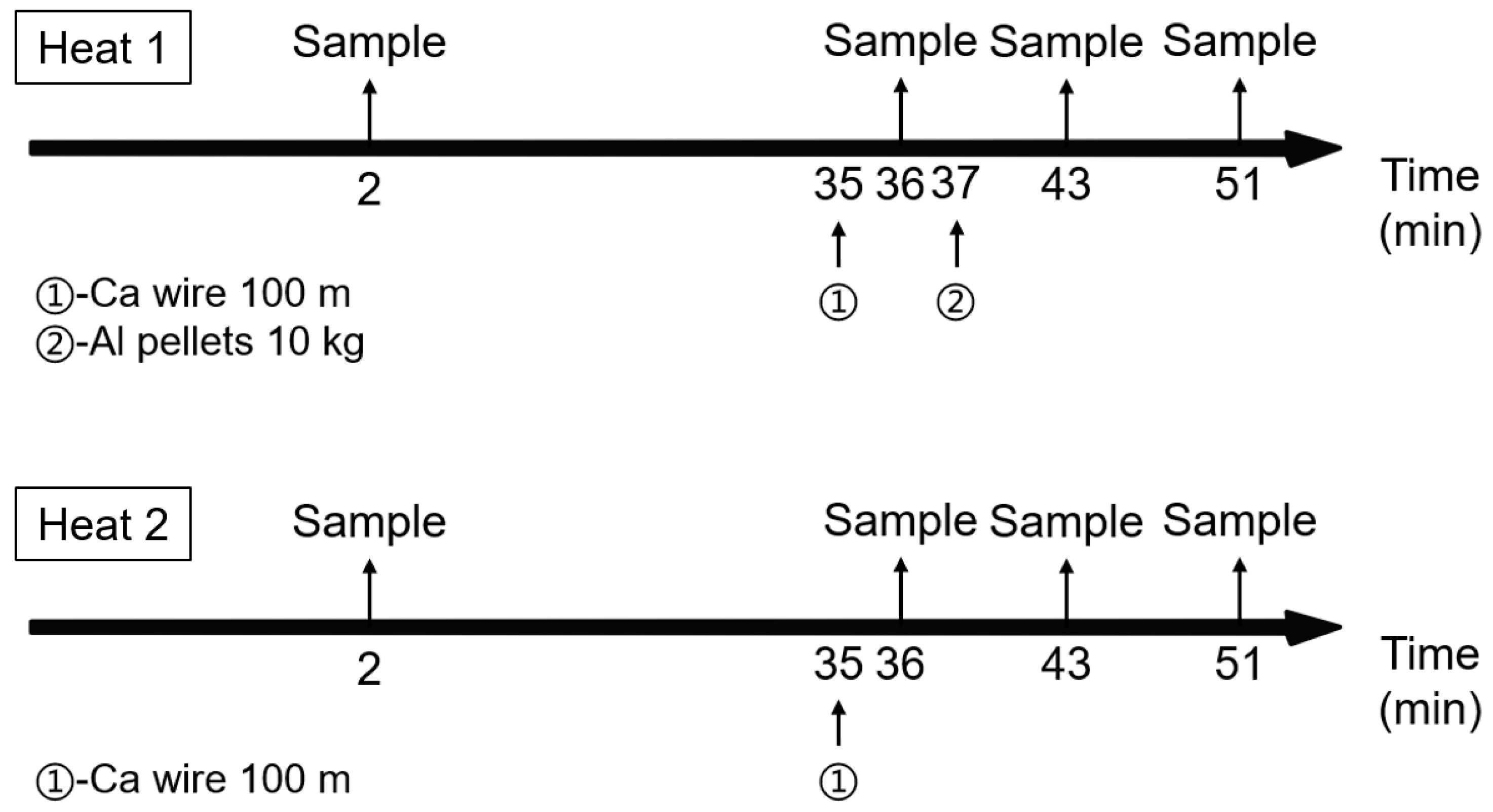
2.1. Industrial Trials
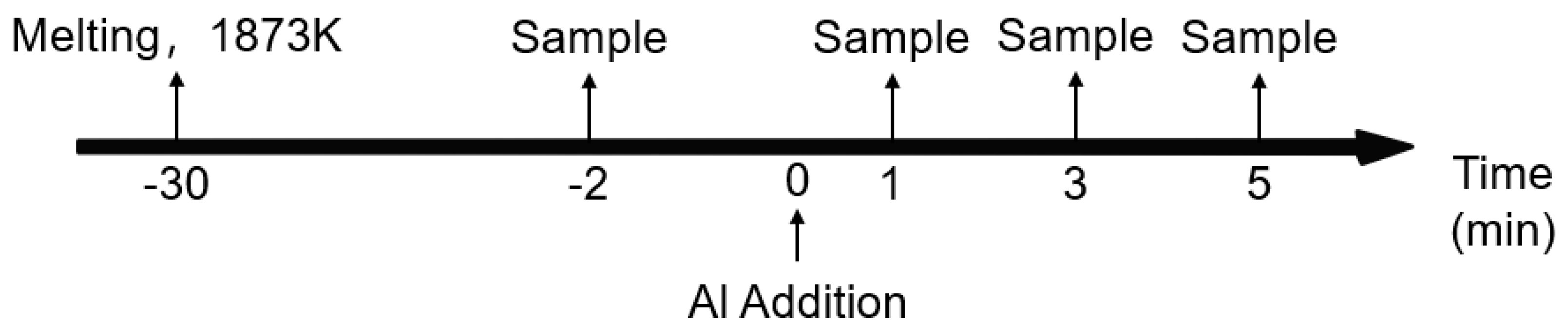
2.2. Laboratory Experiments
2.3. Sample Analysis
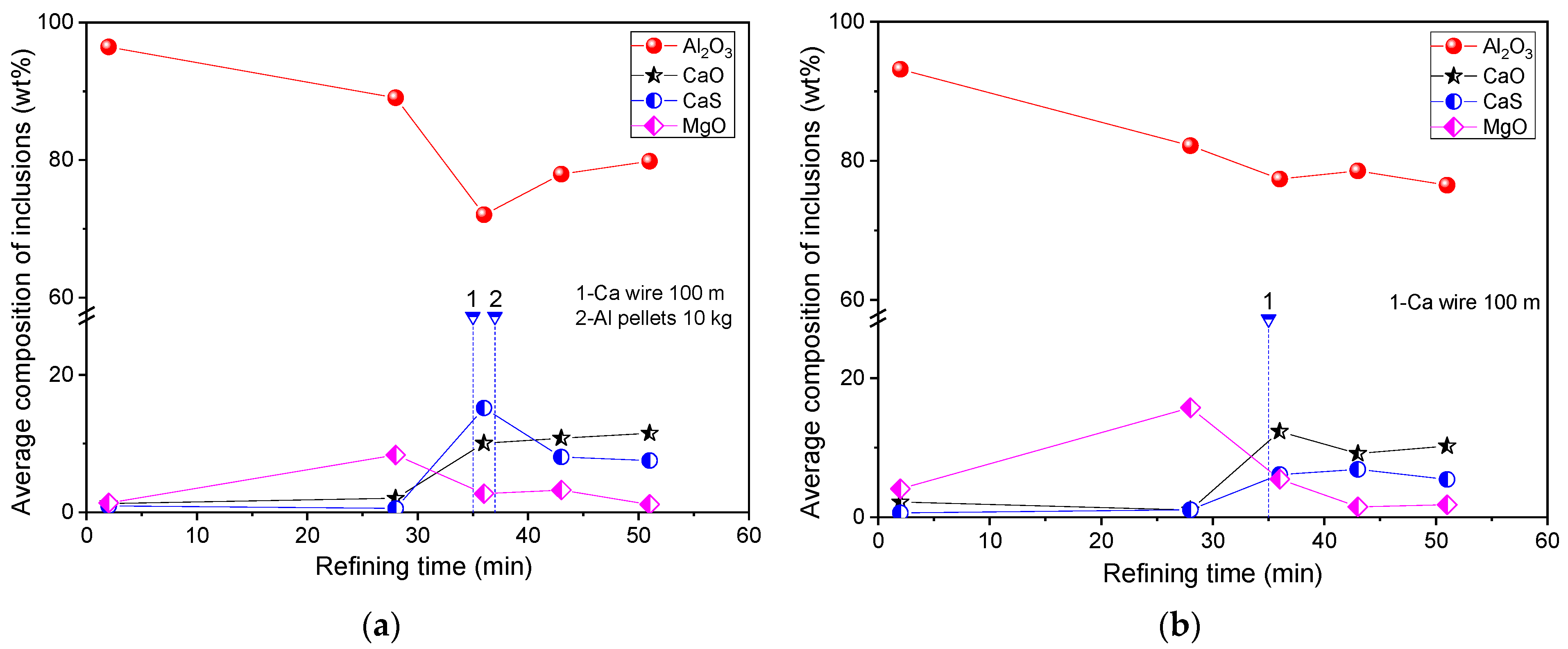
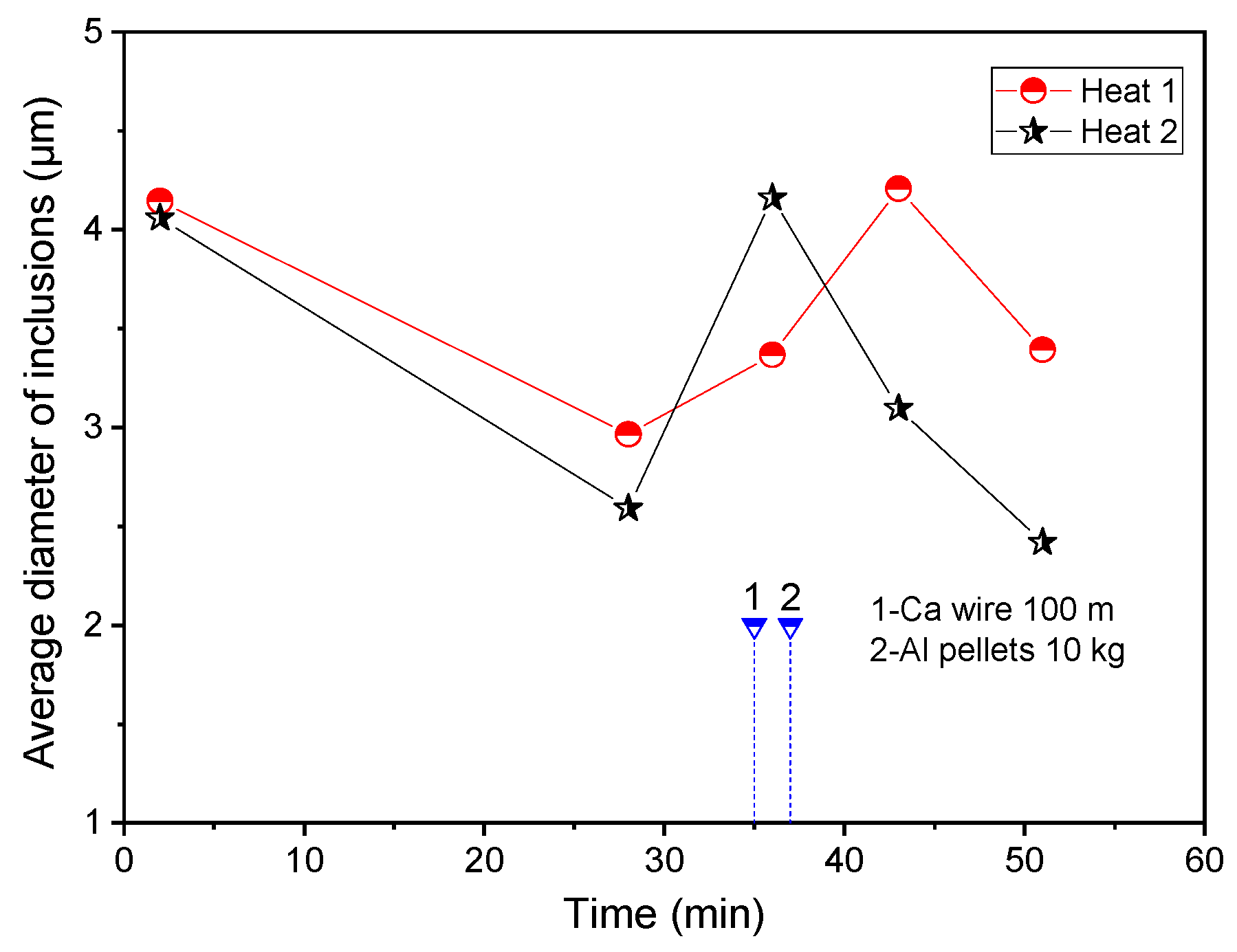
3. Industrial Trials on the Comparison of Inclusions in Steel with and without Al Addition after Ca Treatment
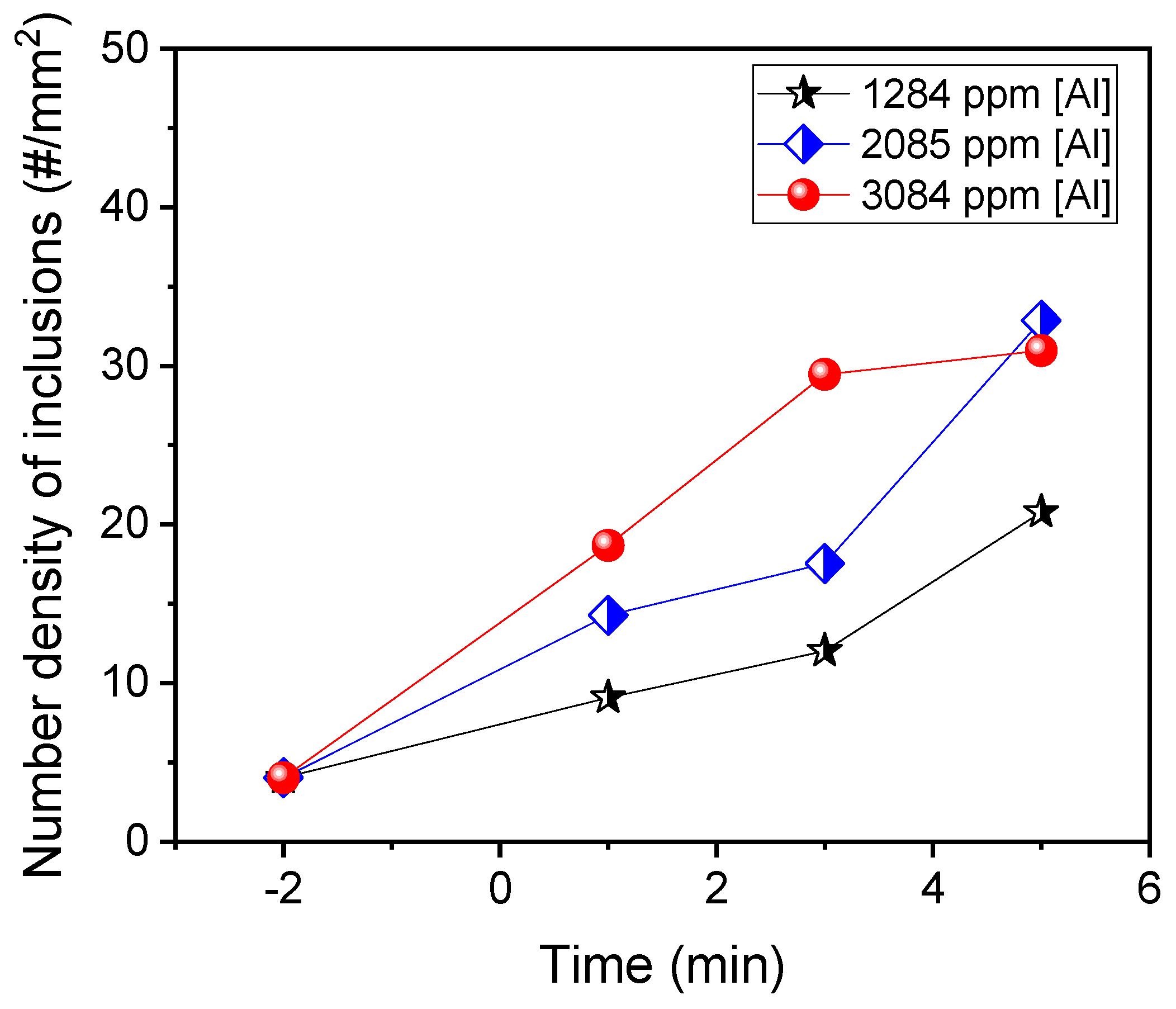
4. Effect of Al Content on the Inclusions after Ca Treatment
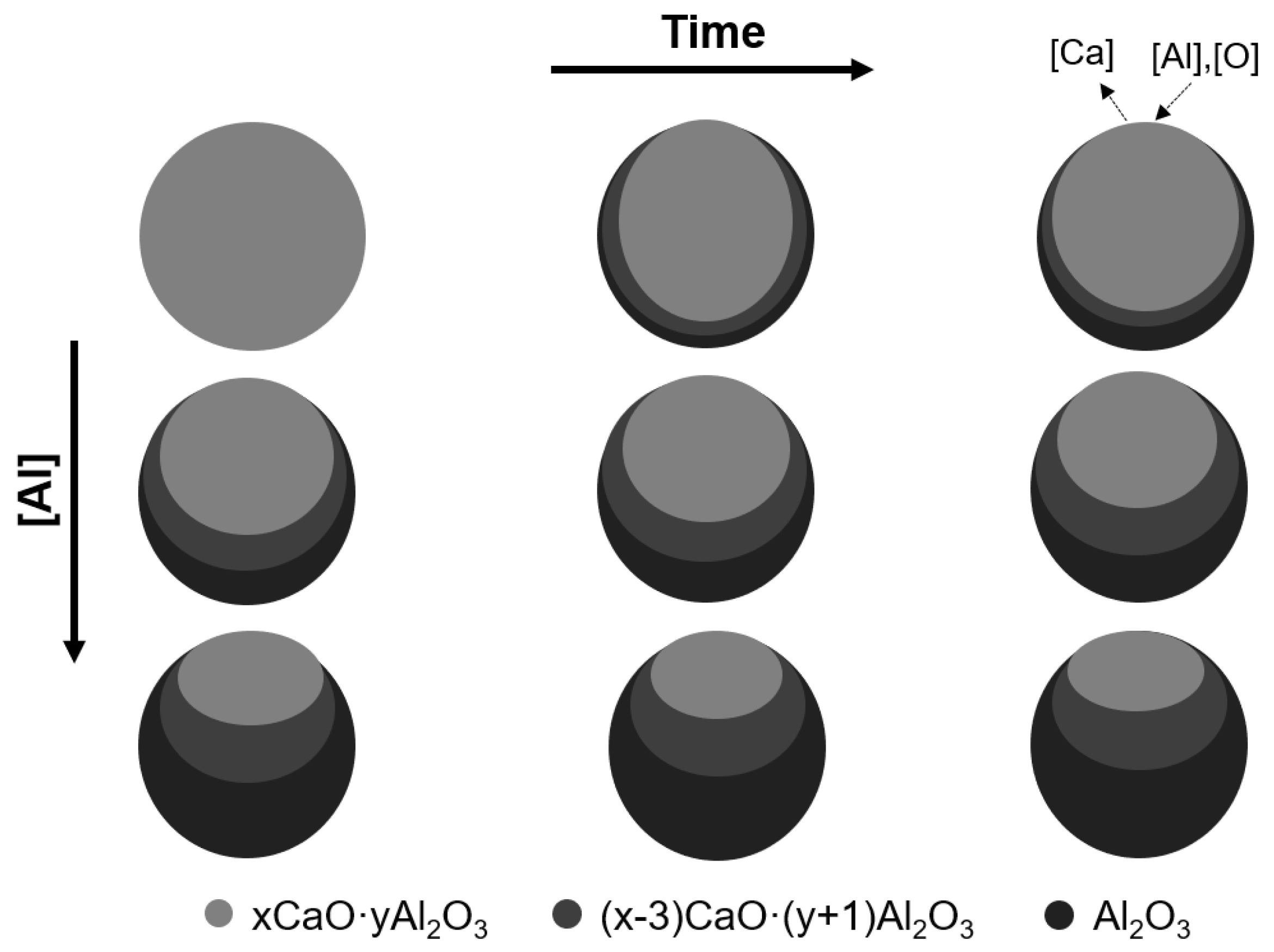
5. Evolution Mechanism of Inclusions after Al Addition
6. Conclusions
- (1)
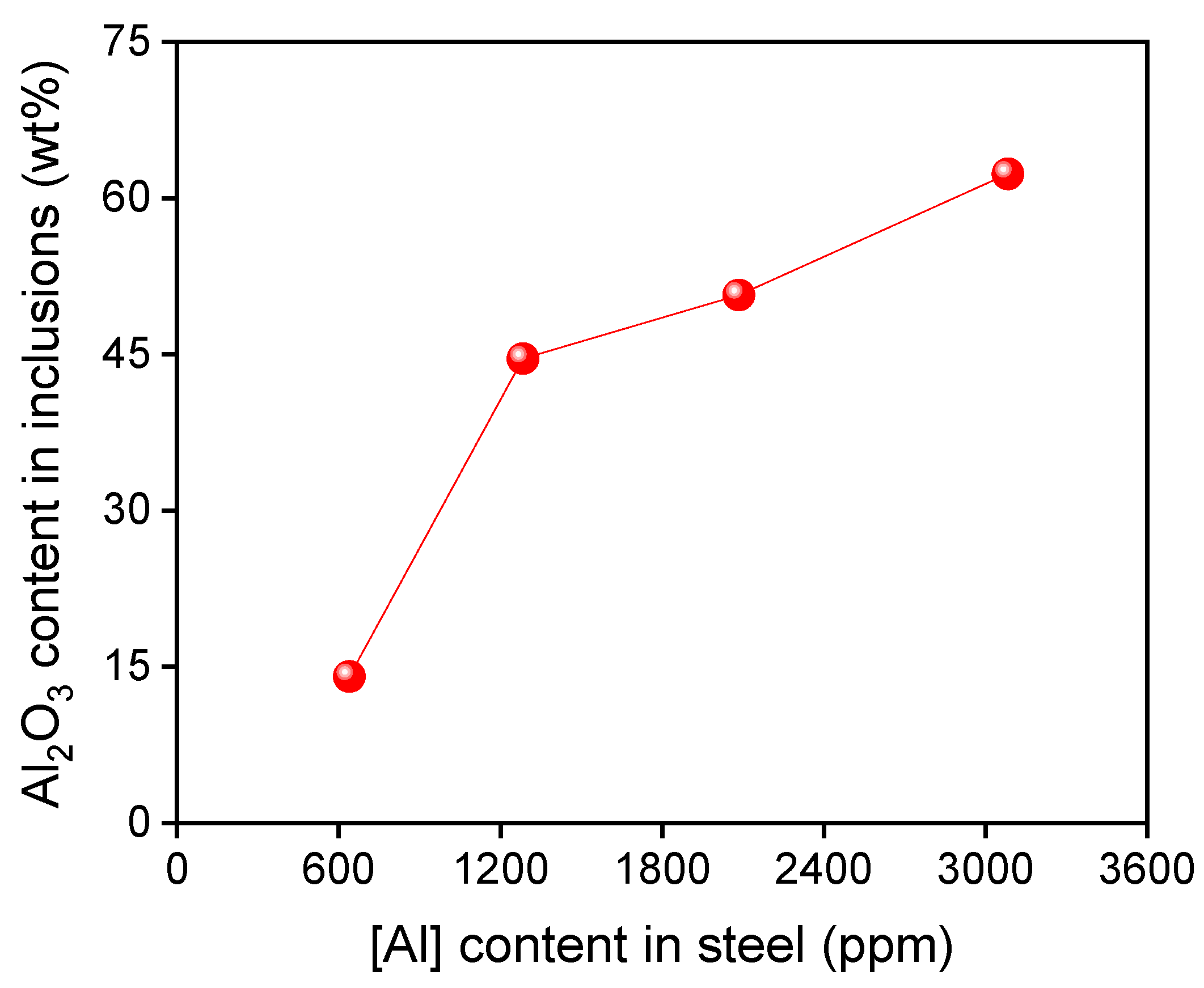
- During the industrial production process of the Al-killed Ca-treated steel, the Al2O3 content in CaO–Al2O3 inclusions increased from 72.05% to 79.83% after the aluminum addition. In laboratory experiments, with the increase of the [Al] content from 639 ppm to 1284 ppm, 2085 ppm, and 3084 ppm, the Al2O3 content in the inclusions increased from 14.07% to 45.61%, 56.21%, and 62.65%, respectively.
- (2)
- The addition of Al promoted the modification of liquid calcium aluminates and the formation of small Al2O3 inclusions, resulting in the decrease in the average diameter and the increase in the number density of the inclusions. At 5 min after aluminum addition, the average value of the average diameter of inclusions in the laboratory experiments was 3.3 μm.
- (3)
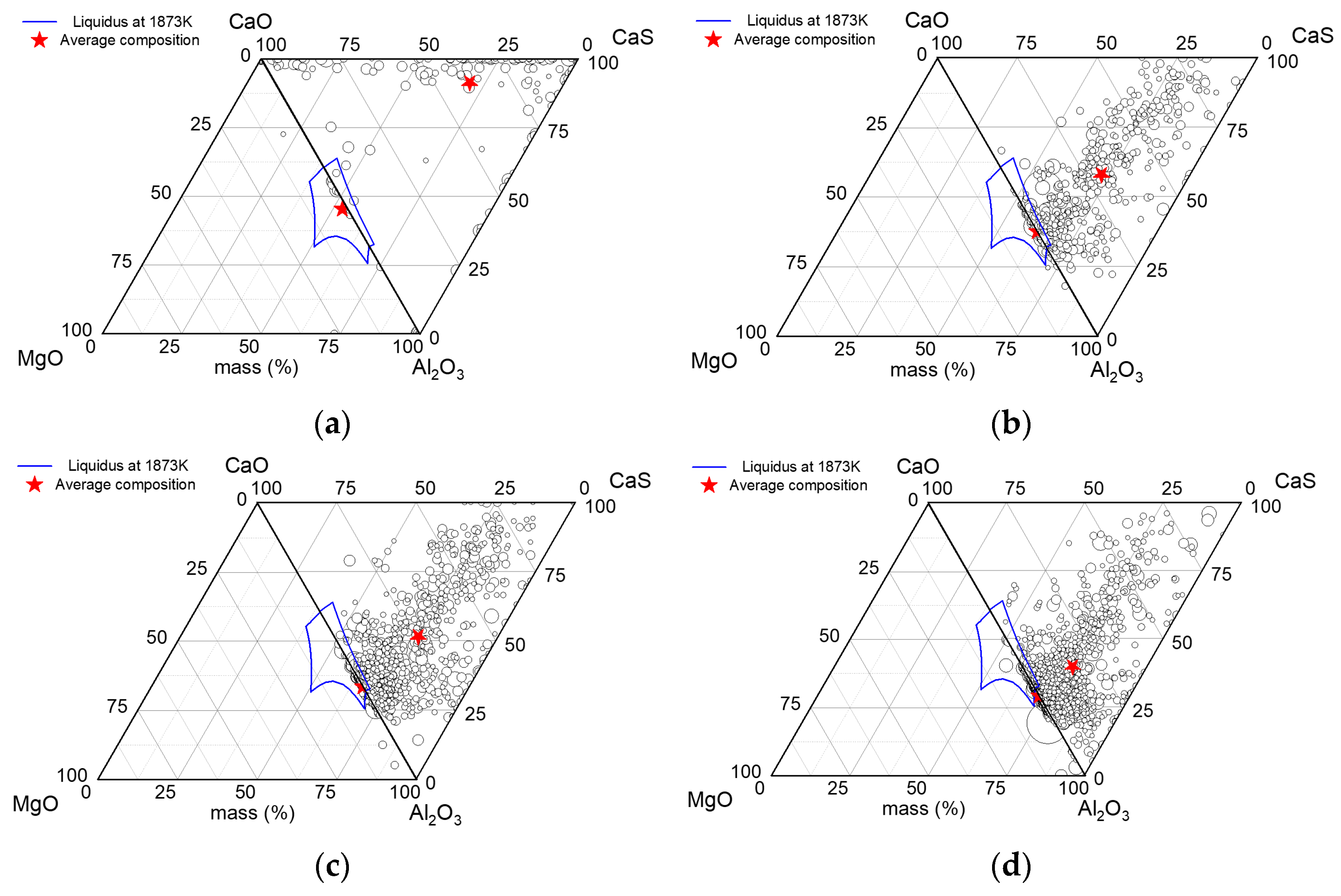
- As the content of T.O increased from 24 ppm to 27 ppm, the height of the “liquid window” remained unchanged. Under the initial steel composition, liquid inclusions could be formed when the Ca content was 12–25 ppm, which was conducive to the castability of molten steel during the continuous casting.
- (4)
- The CaO in the liquid calcium aluminate was reduced by the aluminum in steel as the reaction of 2[Al] + xCaO·yAl2O3 → 3[Ca] + (x − 3)CaO·(y + 1)Al2O3. The higher the [Al] content in steel, the larger the Al2O3 content in the inclusions. The thermodynamic calculations were consistent with the experimental results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zhang, L. Several Important Scientific Research Points of Non-Metallic Inclusions in Steel. Steelmaking 2016, 32, 1–16. [Google Scholar]
- Zhang, L. Non-Metallic Inclusions in Steel: Fundamentals; Metallurgical Industry Press: Beijing, China, 2019. [Google Scholar]
- Zhang, L. Non-Metallic Inclusions in Steel: Industrial Practice; Metallurgical Industry Press: Beijing, China, 2019. [Google Scholar]
- Zhang, A. Effect of Non-Metallic Inclusion on Property of Steel. Phys. Exam. Test. 2006, 24, 42–44. [Google Scholar]
- Heon, Y.H.; Chan, J.P.; Hyuk, S.K. Effects of Non-Metallic Inclusions on the Initiation of Pitting Corrosion in 11% Cr Ferritic Stainless Steel Examined by Micro-Droplet Cell. Corros. Sci. 2007, 49, 1266–1275. [Google Scholar]
- Park, J.H.; Todoroki, H. Control of MgO·Al2O3 Spinel Inclusions in Stainless Steels. ISIJ Int. 2010, 50, 1333–1346. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L. State of the Art in the Control of Inclusions in Tire Cord Steels—A Review. Steel Res. Int. 2006, 77, 158–169. [Google Scholar] [CrossRef]
- Zhang, L.; Thomas, B.G. State of the Art in Evaluation and Control of Steel Cleanliness. ISIJ Int. 2003, 43, 271–291. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Thomas, B.G. State of the Art in the Control of Inclusions during Steel Ingot Casting. Metall. Mater. Trans. B 2006, 37, 733–761. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X. Clean Steel and Inclusions. In Proceedings of the 13th National Steelmaking Conference, Kunming, China, 8–9 December 2004; pp. 12–51. [Google Scholar]
- Cai, X.; Bao, Y.; Lin, L. Evolution of Inclusions During Calcium Treatment in Liquid Steel and its Thermodynamic Analysis. Chin. J. Eng. 2016, 38, 32–36. [Google Scholar]
- Ito, Y.; Suda, M.; Kato, Y.; Nakato, H.; Sorimachi, K. Kinetics of Shape Control of Alumina Inclusions with Calcium Treatment in Line Pipe Steel for Sour Service. ISIJ Int. 1996, 36, 148–150. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Zhang, L.; Li, S. Transient Evolution of Inclusions during Calcium Modification in Linepipe Steels. ISIJ Int. 2014, 54, 2772–2779. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jing, L.; Shi, C.; Yu, W. Evolution of Inclusions in Calcium-Treated H13 Die Steel. Chin. J. Eng. 2018, 40, 11–18. [Google Scholar]
- Wu, X.; Zhang, D.; Chen, R. Application of Calcium Treatment to Sulfur Gear Steel 20CrMo. Adv. Mater. Res. 2014, 936, 1323–1328. [Google Scholar]
- Yang, J.; Chen, B.; Wei, T.; Zeng, F.; Gang, Y.; Feng, S. Experimental Study to Improve the Castability of Aluminum Killed Cold Heading Steel. Steel Res. Int. 2013, 84, 703–712. [Google Scholar]
- Yang, W.; Zhang, L.; Wang, X.; Ren, Y.; Liu, X.; Shan, Q. Characteristics of Inclusions in Low Carbon Al-Killed Steel during Ladle Heat Refining and Calcium Treatment. ISIJ Int. 2013, 53, 1401–1410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, Y.; Zhang, Y.; Yang, W.; Chen, W. Transient Evolution of Nonmetallic Inclusions during Calcium Treatment of Molten Steel. Metall. Mater. Trans. B 2018, 49, 1841–1859. [Google Scholar] [CrossRef]
- Ren, Q.; Jiang, D.; Zhang, L.; Liu, L.; Wen, Y.B.; Chen, W. Analysis of Evolution Law and Formation Mechanism of Inclusions in Q235 Steel. Iron Steel 2020, 55, 47–52. [Google Scholar]
- Cicutti, C.E.; Madias, J.; Gonzalez, J.C. Control of Microinclusions in Calcium Treated Aluminium Killed Steels. Ironmak. Steelmak. 1997, 24, 155–159. [Google Scholar]
- Zhang, L.; Li, F.; Fang, W. Thermodynamic Investigation for the Accurate Calcium Addition during Calcium Treatment of Molten Steels. Steelmaking 2016, 32, 7–14. [Google Scholar]
- Li, H.G. Principles of Metallurgy; Science Press: Beijing, China, 2005. [Google Scholar]
- Wang, X.; Li, Q.; Huang, F.; Li, H.; Yang, J. Study on the Control of Type B Inclusions in the CaO-Al2O3 System of X80/70 Pipeline Steel Strips. In Proceedings of the 2012 National Steelmaking-Continuous Casting Production Technology Conference, Chongqing, China, 17–20 July 2012; pp. 12–20. [Google Scholar]
- Wang, X.; Li, X.; Li, Q.; Huang, F.; Li, H.; Yang, J. Control of CaO-Al2O3 Series Non-metallic Inclusions in X80 Pipeline Steel Plate. Acta Metall. Sin. 2013, 49, 553–561. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Ren, Y.; Duan, H.; Yang, W. Effect of Sulfur in Steel on Transient Evolution of Inclusions during Calcium Treatment. Metall. Mater. Trans. B 2018, 495, 310–315. [Google Scholar] [CrossRef]
- Ye, G.; Jönsson, P.; Lund, T. Thermodynamics and Kinetics of the Modification of Al2O3 Inclusions. ISIJ Int. 1996, 36, 105–108. [Google Scholar] [CrossRef]
- Prešern, V.; Koroušić, B.; Hastie, J.W. Thermodynamic Conditions for Inclusions Modification in Calcium Treated Steel. Steel Res. Int. 1991, 62, 289–295. [Google Scholar] [CrossRef]
- Blaženko, K. Fundamental Thermodynamic Aspects of the CaO-Al2O3-SiO2 System. Steel Res. Int. 1991, 62, 285–288. [Google Scholar]
- Jung, I.H.; Decterov, S.A.; Pelton, A.D. Computer Applications of Thermodynamic Databases to Inclusion Engineering. ISIJ Int. 2004, 44, 527–536. [Google Scholar] [CrossRef]
- Zheng, W. Preparation of Aluminum calcium alloy by Thermit Reduction of CaO. Chin. Sci. Bull. 1961, 9, 48–50. [Google Scholar]
- Zhang, T.; Xue, L.; Zhou, L.; Huang, D.; Wang, W. Influence of Al Content on the Ca-treatment Process of the Ti-bearing Steel. In Proceedings of the 2018 (20th) National Steelmaking Conference, Chengdu, China, 17–18 May 2018; pp. 93–114. [Google Scholar]
- Guo, B.; Bao, Y.; Wang, M.; Lin, L. Effect of w(Ca)/w(Al) on Transformation of Inclusions in the Calcium Treatment in 20Mn2 Steel. Steelmaking 2015, 31, 36–40. [Google Scholar]
- Li, Z.; Zheng, S.; Lv, M. Effect of Aluminizing Treatment on Hot Corrosion Behavior of Commercial Steel. Anti-Corros. Eng. 2005, 19, 123–135. [Google Scholar]
- Itoh, H.; Hino, M.; Ban-Ya, S. Thermodynamics on the Formation of Spinel Nonmetallic Inclusion in Liquid Steel. Metall. Mater. Trans. B 1997, 28, 953–956. [Google Scholar] [CrossRef]

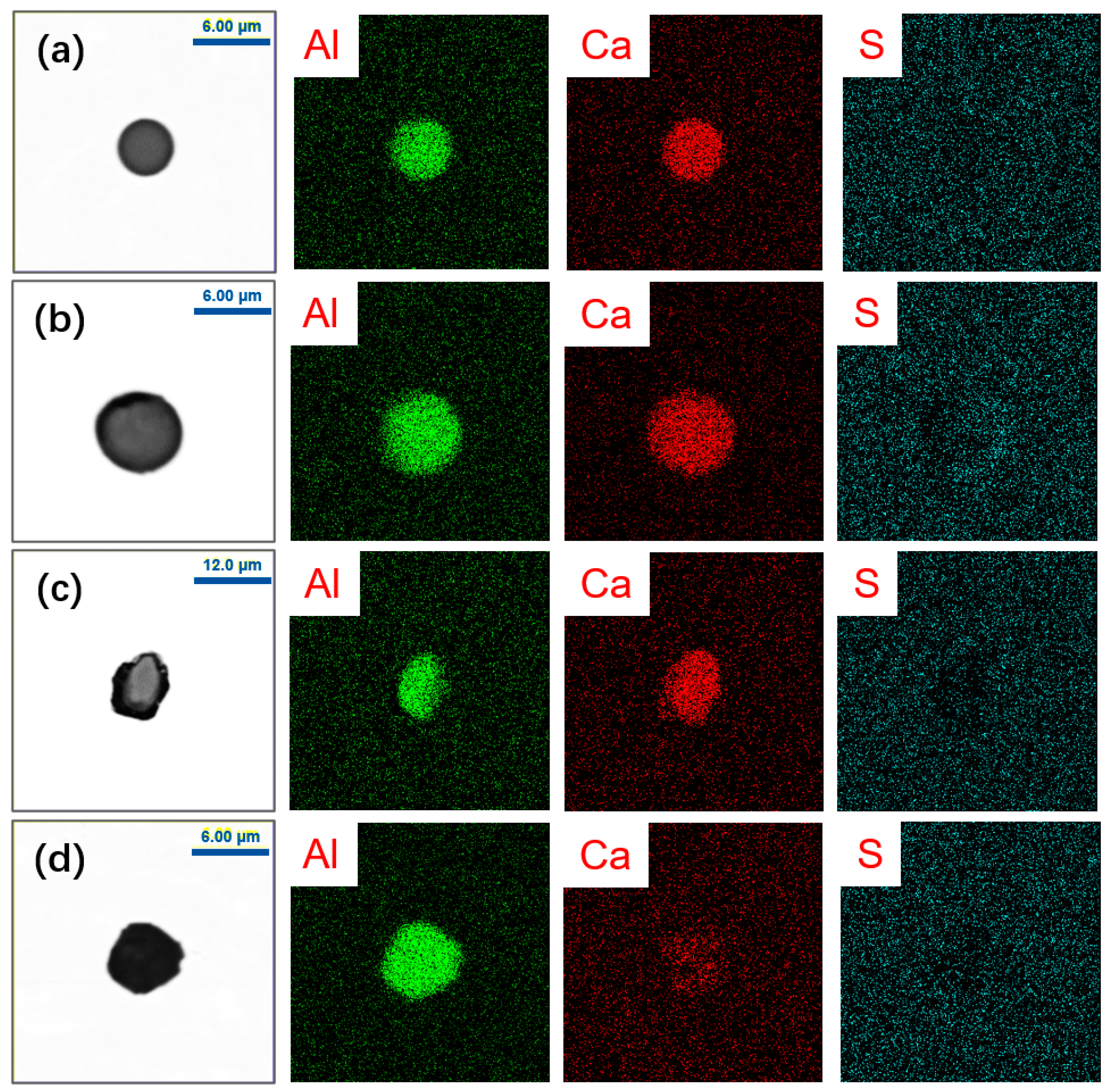
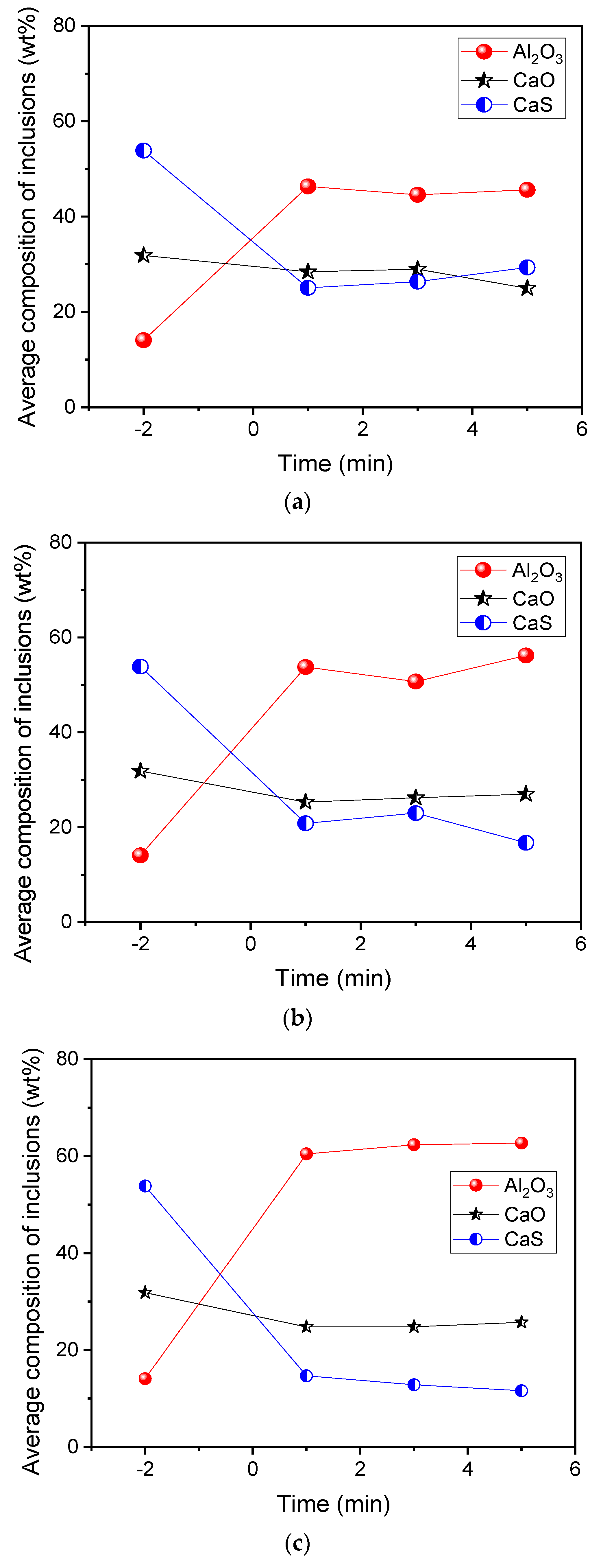



| Element | C | T.O | Si | Mn | P | T.Al | T.Mg | T.Ca | T.S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.113 | 0.0027 | 0.168 | 1.4 | 0.0143 | 0.065 | 0.0004 | 0.0009 | 0.0034 | Bal. |
| No. | Cast Billet | Al Pellets |
|---|---|---|
| Steel 0 | -- | -- |
| Steel 1 | 823 | 0.6 |
| Steel 2 | 825 | 1.4 |
| Steel 3 | 823 | 2.2 |
| No. | T.O | [O] | T.Al | [Al] | T.Ca | [Ca] |
|---|---|---|---|---|---|---|
| Steel 0 | 0.0027 | 0.0006 | 0.0650 | 0.0639 | 0.0073 | 0.0012 |
| Steel 1 | 0.0026 | 0.0004 | 0.1300 | 0.1284 | 0.0039 | 0.0005 |
| Steel 2 | 0.0024 | 0.0004 | 0.2100 | 0.2085 | 0.0036 | 0.0004 |
| Steel 3 | 0.0026 | 0.0005 | 0.3100 | 0.3084 | 0.0033 | 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Zhang, L.; Ren, Y.; Yang, W.; Wu, S. The Effect of Aluminum Addition on the Evolution of Inclusions in an Aluminum-Killed Calcium-Treated Steel. Metals 2022, 12, 181. https://doi.org/10.3390/met12020181
Fan X, Zhang L, Ren Y, Yang W, Wu S. The Effect of Aluminum Addition on the Evolution of Inclusions in an Aluminum-Killed Calcium-Treated Steel. Metals. 2022; 12(2):181. https://doi.org/10.3390/met12020181
Chicago/Turabian StyleFan, Xingle, Lifeng Zhang, Ying Ren, Wen Yang, and Songjie Wu. 2022. "The Effect of Aluminum Addition on the Evolution of Inclusions in an Aluminum-Killed Calcium-Treated Steel" Metals 12, no. 2: 181. https://doi.org/10.3390/met12020181
APA StyleFan, X., Zhang, L., Ren, Y., Yang, W., & Wu, S. (2022). The Effect of Aluminum Addition on the Evolution of Inclusions in an Aluminum-Killed Calcium-Treated Steel. Metals, 12(2), 181. https://doi.org/10.3390/met12020181








