Effect of La Addition on Microstructure and Properties of Al-0.2Fe-0.06Cu Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
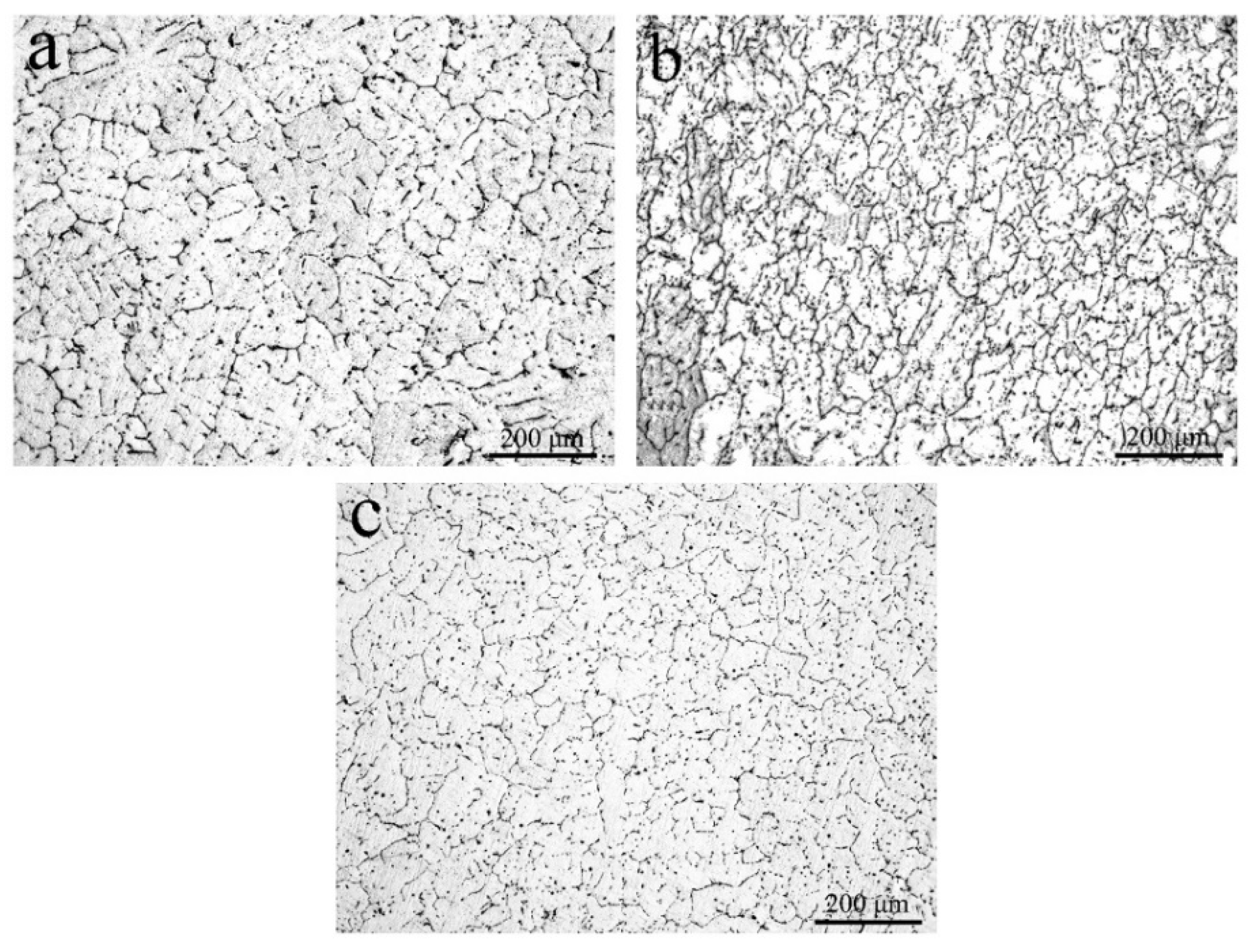
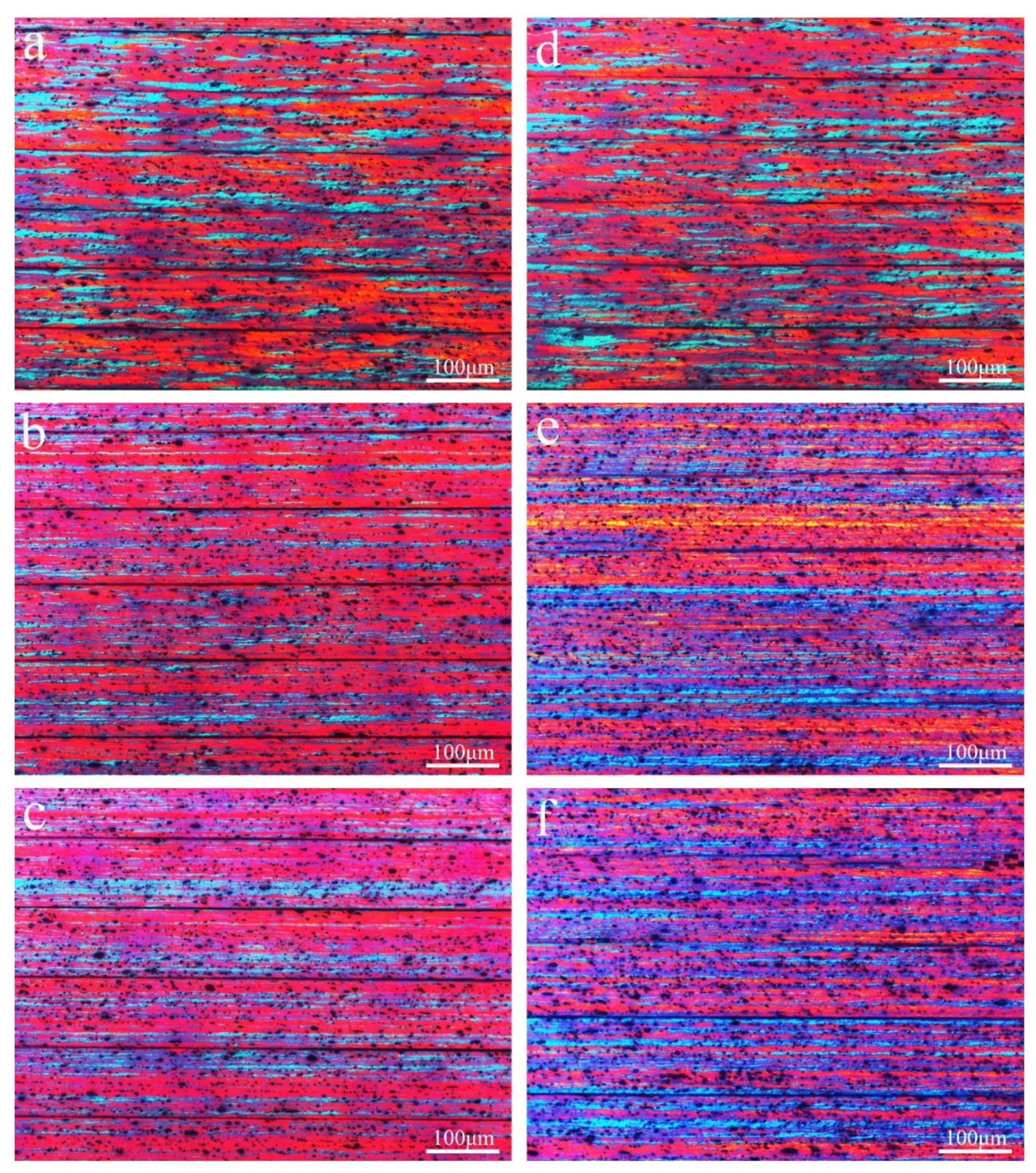
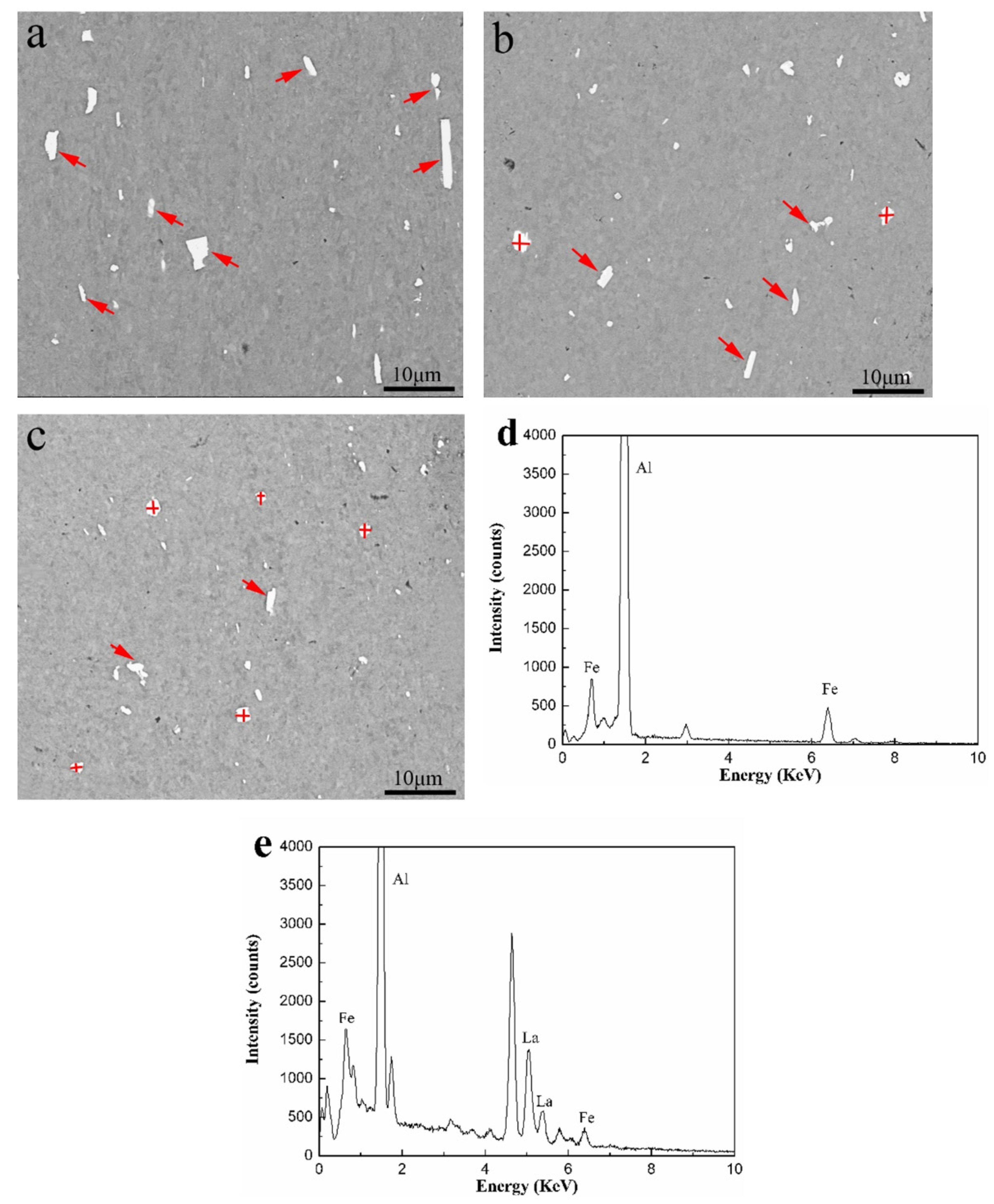
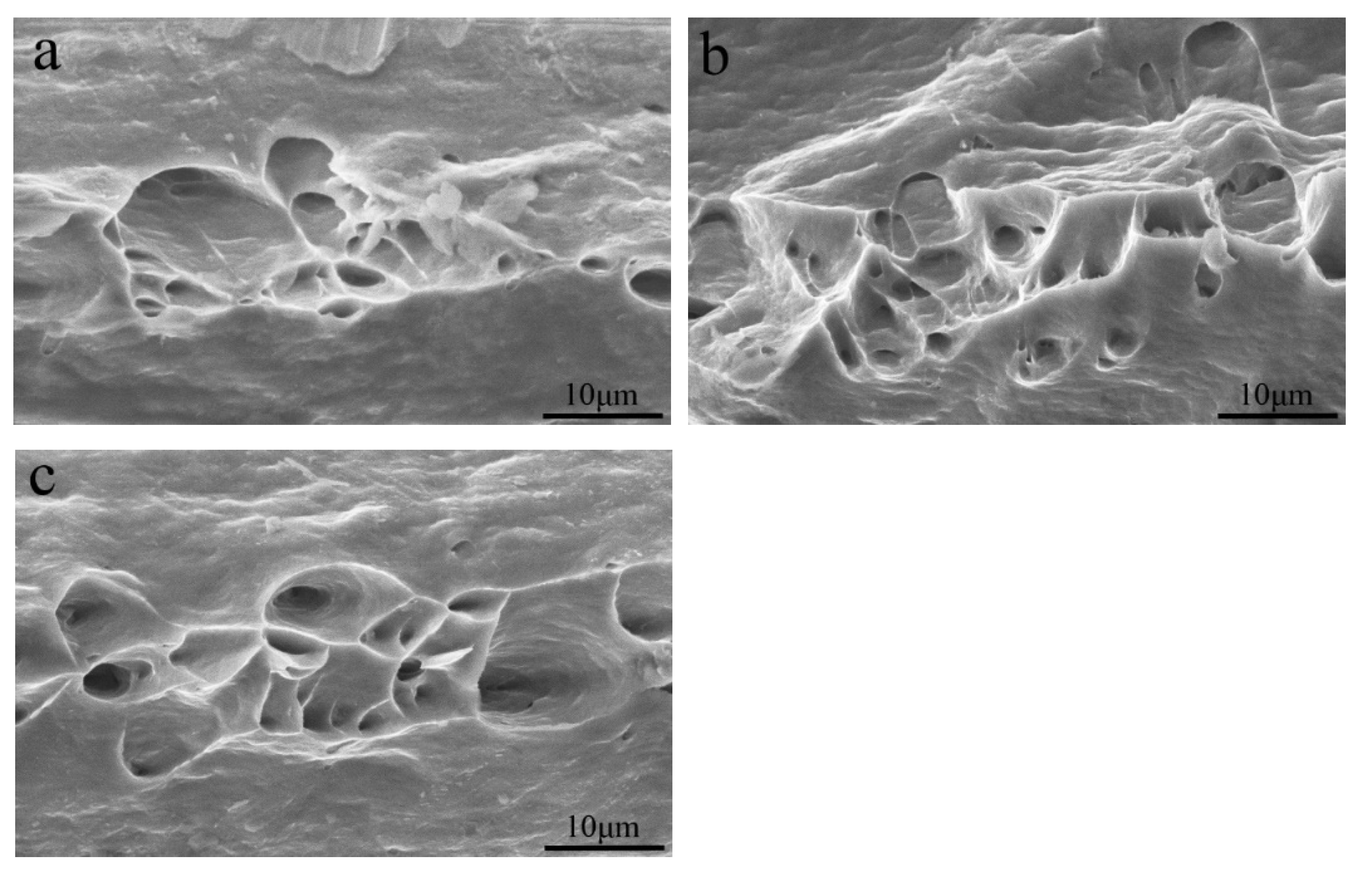
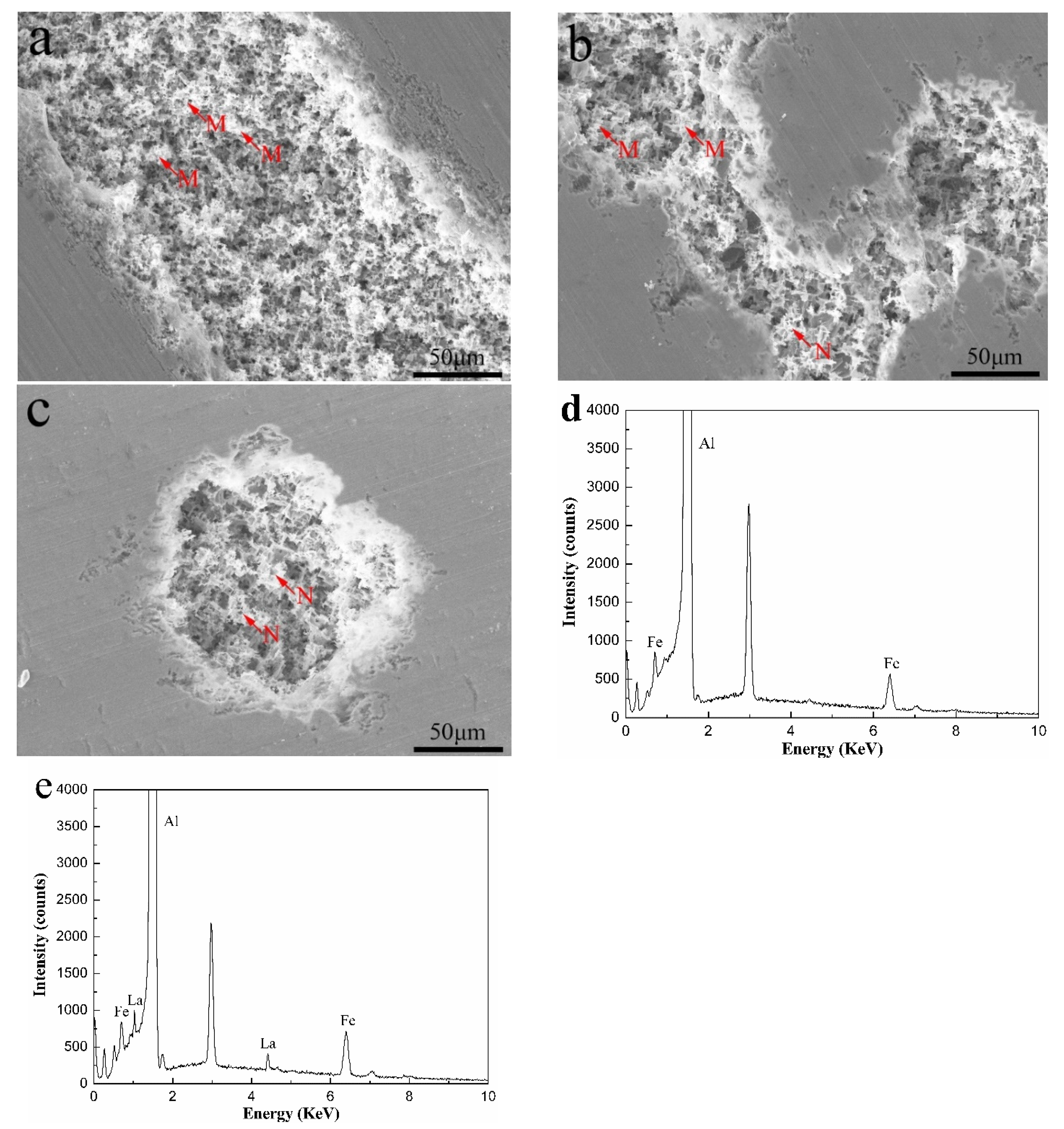
3.1. Microstructure
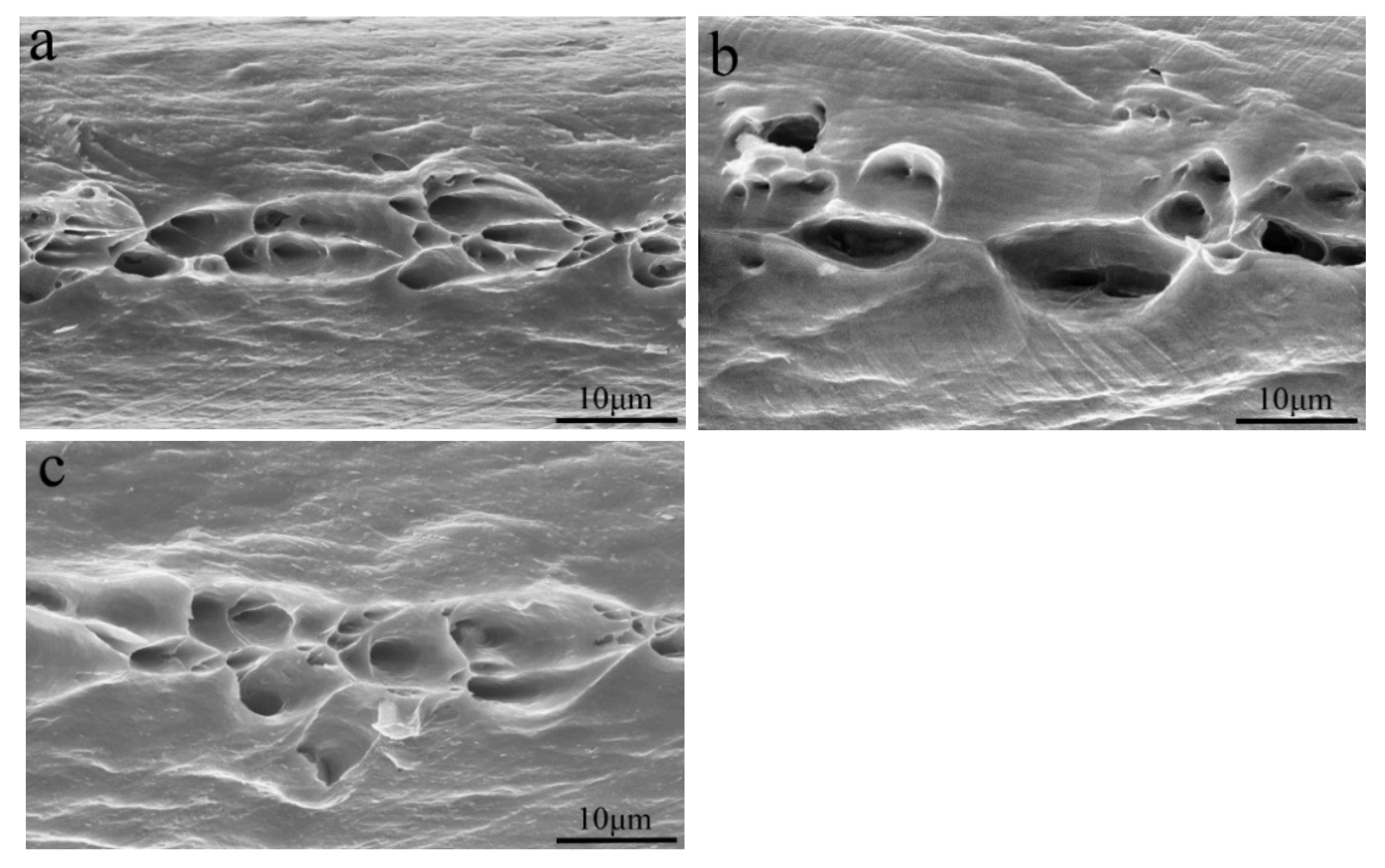
3.2. Mechanical Properties
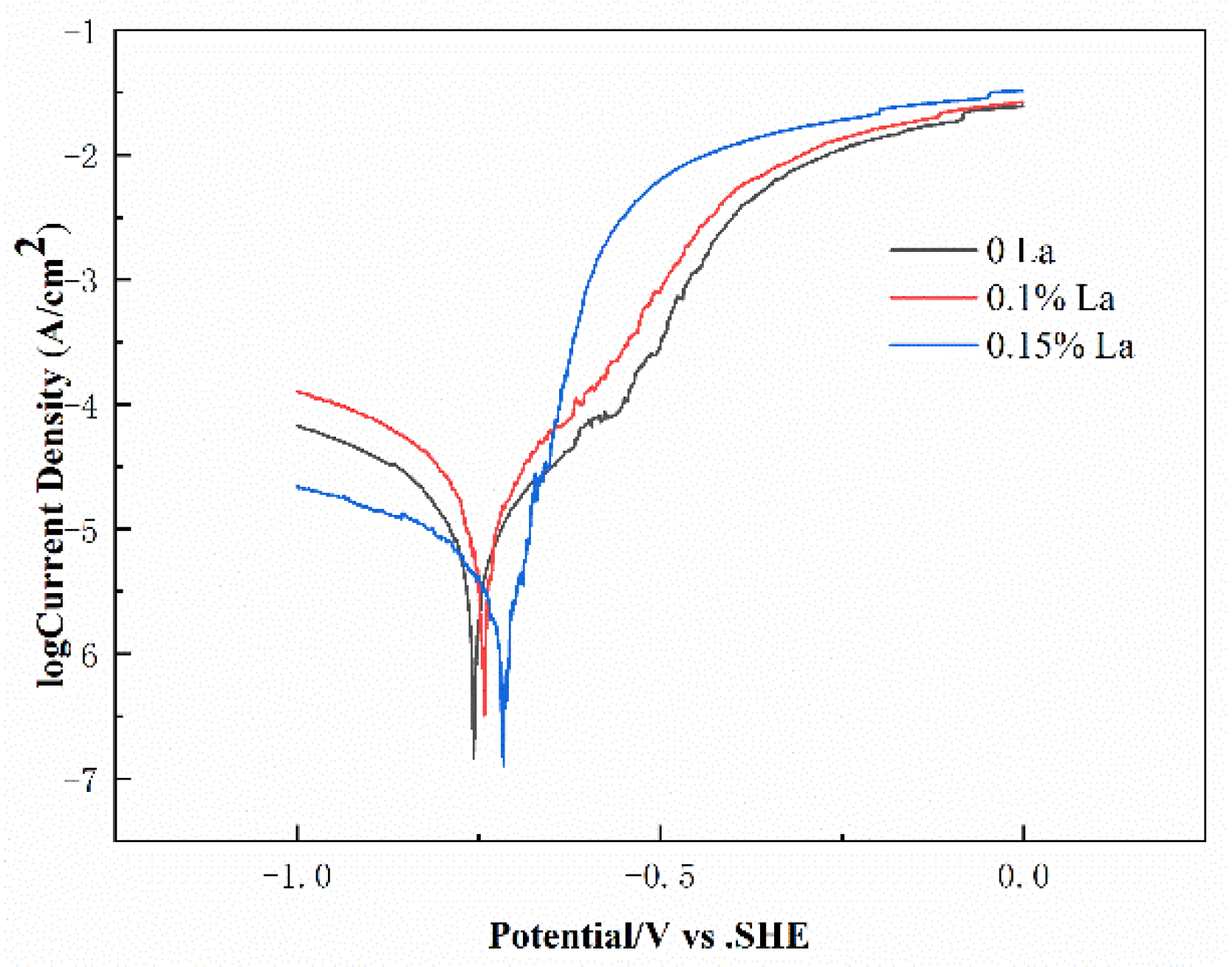
3.3. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Köster, U.; Liu, W.; Liebertz, H.; Michel, M. Mechanical properties of quasicrystalline and crystalline phases in Al-Cu-Fe alloys. J. Non-Cryst. Solids 1993, 153–154, 446–452. [Google Scholar] [CrossRef]
- Köster, U.; Liebertz, H.; Liu, W. Plastic deformation of quasi-crystalline and crystalline phases in Al-Cu-Fe alloys. Mater. Sci. Eng. A 1994, 181–182, 777–780. [Google Scholar] [CrossRef]
- Bilušić, A.; Pavuna, D.; Smontara, A. Figure of merit of quasicrystals: The case of Al–Cu–Fe. Vacuum 2001, 61, 345–348. [Google Scholar] [CrossRef]
- Yang, X.; Ding, D.; Xu, Y.; Zhang, W. Al–Fe–Si–La alloys for current collectors of positive electrodes in lithium ion batteries. Metals 2020, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Ding, D.; Zhang, W. Effect of La addition on the microstructure and properties of Al-Fe-Mn alloys for lithium battery current collectors. J. Electron. Mater. 2021, 50, 1032–1043. [Google Scholar] [CrossRef]
- Couture, A. Iron in aluminium casting alloys-a literature survey. AFS Inter. Cast. Met. 1981, 12, 9–17. [Google Scholar]
- Stefaniay, V.; Criger, A.; Turmezey, T. Intermetallic phases in the aluminum-side corner of the AlFeSi alloy system. J. Mater. Sci. 1987, 23, 539–546. [Google Scholar] [CrossRef]
- Nie, Z.R.; Jin, T.; Fu, J.; Xu, G.; Yang, J.; Zhou, J.X.; Zuo, T. Research on rare earth in aluminum. Mater. Sci. Forum 2002, 396, 1731–1735. [Google Scholar] [CrossRef]
- Yao, D.; Xia, Y.; Qiu, F.; Jiang, Q. Effects of La addition on the elevated temperature properties of the casting Al–Cu alloy. Mater. Sci. Eng. A 2011, 528, 1463–1466. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, J.; Zhao, G.; Lai, R.; Wu, Y. Effect of rare earth La on microstructures and ageing characteristics of 6063 aluminum alloy. Trans. Nonferrous Met. Soc. China 2020, 62, 1–5. [Google Scholar]
- Gschneidner, K.A. Rare earth alloys: A critical review of the alloy systems of the rare earth, scandium, and yttrium metals. J. Am. Chem. Soc. 1962, 84, 123. [Google Scholar]
- Chen, Y.; Pan, Y.; Lu, T.; Tao, S.; Wu, J. Effects of combinative addition of lanthanum and boron on grain refinement of Al-Si casting alloys. Mater. Des. 2014, 64, 423–426. [Google Scholar] [CrossRef]
- Chang, J.Y.; Kim, G.H.; Moon, I.G.; Choi, C.S. Rare earth concentration in the primary Si crystal in rare earth added Al-21wt.%Si alloy. Scr. Mater. 1998, 39, 307–314. [Google Scholar] [CrossRef]
- Chang, J.; Moon, I.; Choi, C. Refinement of cast microstructure of hypereutectic Al-Si alloys through the addition of rare earth metals. J. Mater. Sci. 1998, 33, 5015–5023. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Dai, Y.; Li, C. Effects of rare earth elements addition on microstructures, tensile properties and fractography of A357 alloy. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2014, 597, 237–244. [Google Scholar] [CrossRef]
- Yao, D.; Zhao, W.; Zhao, H.; Qiu, F.; Jiang, Q. High creep resistance behavior of the casting Al–Cu alloy modified by La. Scr. Mater. 2009, 61, 1153–1155. [Google Scholar] [CrossRef]
- Hosseinifar, M.; Malakhov, D.V. Effect of Ce and La on microstructure and properties of a 6xxx series type aluminum alloy. J. Mater. Sci. 2008, 43, 7157–7164. [Google Scholar] [CrossRef]
- Westphal, B.; Bockholt, H.; Günther, T.; Haselrieder, W.; Kwade, A. Influence of convective drying parameters on electrode performance and physical electrode properties. ECS Trans. 2015, 64, 57–68. [Google Scholar] [CrossRef]
- Gu, J.; Gu, S.; Xue, L. Microstructure and mechanical properties of in-situ Al13Fe4/Al composites prepared by mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A 2012, 558, 684–691. [Google Scholar] [CrossRef]
- Kim, D.H.; Cantor, R.; Lee, H.I. Structure and decomposition behavior of rapidly solidified AI-Cu-Li-Mg-Zr alloys. J. Mater. Sci. 1988, 23, 1695–1708. [Google Scholar] [CrossRef]
- Kim, D.H.; Cantor, B. Quasicrystalline and related crystalline phases in rapidly solidified Al-Fe alloys. Philos. Mag. A 1993, 69, 45–55. [Google Scholar] [CrossRef]
- Väyrynen, A.; Salminen, J. Lithium ion battery production. J. Chem. Thermodyn. 2012, 46, 80–85. [Google Scholar] [CrossRef]
- Han, L.; Sui, Y.; Wang, Q.; Wang, K.; Jiang, Y. Effects of Nd on microstructure and mechanical properties of cast Al-Si-Cu-Ni-Mg piston alloys. J. Alloys Compd. 2016, 695, 1566–1572. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Marcos, M.; Sánchez-Amaya, J.M. Influence of the degree of polishing of alloy AA 5083 on its behavior against localised alkaline corrosion. Corros. Sci. 2004, 46, 1909–1920. [Google Scholar] [CrossRef]
- Zaid, B.; Saidi, D.; Benzaid, A.; Hadji, S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corros. Sci. 2008, 50, 1841–1847. [Google Scholar] [CrossRef]
- Alavi, A.; Cottis, R.A. The determination of pH, potential and chloride concentration in corroding crevices on 304 stainless steel and 7475 aluminium alloy. Corros. Sci. 1987, 27, 443–451. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Investigation and discussion of characteristics for intermetallic phases common to aluminum alloys as a function of solution pH. J. Electrochem. Soc. 2008, 155, C117–C126. [Google Scholar] [CrossRef] [Green Version]
- Tabrizi, M.R.; Lyon, S.B.; Thompson, G.E. The long-term corrosion of aluminium in alkaline media. Corros. Sci. 1991, 32, 733–742. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. Localized alkaline corrosion of alloy AA5083 in neutral 3.5% NaCl solution. Corros. Sci. 2001, 43, 1657–1674. [Google Scholar] [CrossRef]
- Reboul, M.C.; Warner, T.J.; Mayer, H. A ten step mechanism for the pitting corrosion of aluminium alloys. Corros. Rev. 1997, 15, 471–496. [Google Scholar] [CrossRef]
- Bogar, F.D.; Foley, R.T. The influence of chloride ion on the pitting of aluminum. J. Electrochem. Soc. 1972, 119, 462–464. [Google Scholar] [CrossRef]
- Brown, R.H.; Mears, R.B. The electrochemistry of corrosion. Trans. Electrochem. Soc. 1938, 74, 495. [Google Scholar] [CrossRef] [Green Version]
- Godard, H.P. The corrosion behavior of aluminum in natural waters. Can. J. Chem. Eng. 1960, 38, 167–173. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Chai, Z.; Jiang, C.; Zhu, K.; Zhao, Y.; Wang, C.; Cai, F.; Chen, M.; Wang, L. Pretreatment behaviors and improved corrosion resistance for Cu/Co-Ni-Cu coating electrodeposition on magnesium alloy. J. Electrochem. Soc. 2016, 163, D493–D499. [Google Scholar] [CrossRef]
- Thiede, V.M.T.; Ebel, T.; Jeitschko, W. Ternary aluminides LnT2Al10 (Ln = Y, La–Nd, Sm, Gd–Lu and T = Fe, Ru, Os) with YbFe2Al10 type structure and magnetic properties of the iron-containing series. J. Mater. Chem. 1998, 8, 125–130. [Google Scholar] [CrossRef]
- Rynders, R.M.; Paik, C.H.; Ke, R. Use of in situ atomic force microscopy to image corrosion at inclusions. J. Electrochem. Soc. 1994, 141, 1439–1445. [Google Scholar] [CrossRef]
- Speckert, L.; Burstein, G.T. Combined anodic/cathodic transient currents within nucleating pits on Al-Fe alloy surfaces. Corros. Sci. 2011, 53, 534–539. [Google Scholar] [CrossRef]



| Alloy Composition | Al-0.2Fe-0.06Cu | ||
|---|---|---|---|
| La content | 0wt.% La | 0.1wt.% La | 0.15wt.% La |
| Average grain size (μm) | 80.80 ± 5.40 | 56.64 ± 5.13 | 60.57 ± 6.41 |
| Alloy Composition | La Content (wt.%) | (MPa) | (MPa) | δ (%) | |||
|---|---|---|---|---|---|---|---|
| 25 °C | 50 °C | 25 °C | 50 °C | 25 °C | 50 °C | ||
| Al-0.2Fe-0.06Cu | 0 | 203 ± 2 | 191 ± 4 | 193 ± 1 | 171 ± 2 | 1.1 ± 0.1 | 1.3 ± 0.1 |
| 0.1 | 221 ± 1 | 209 ± 2 | 208 ± 1 | 181 ± 5 | 1.4 ± 0.1 | 1.6 ± 0.1 | |
| 0.15 | 213 ± 1 | 203 ± 5 | 198 ± 1 | 175 ± 5 | 1.5 ± 0.1 | 1.6 ± 0.1 | |
| Alloys Composition (wt.%) | La Content (wt.%) | Ecorr (mV vs. SCE) | Icorr (10−5 A/cm2) |
|---|---|---|---|
| Al-0.2Fe-0.06Cu | 0 | −761 ± 8 | 1.175 |
| 0.1 | −746 ± 3 | 1.799 | |
| 0.15 | −723 ± 5 | 0.478 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Peng, Z.; Ding, D.; Zhang, W.; Gao, Y.; Chen, G.; Xie, Y.; Liao, Y. Effect of La Addition on Microstructure and Properties of Al-0.2Fe-0.06Cu Alloy. Metals 2022, 12, 211. https://doi.org/10.3390/met12020211
Xu Y, Peng Z, Ding D, Zhang W, Gao Y, Chen G, Xie Y, Liao Y. Effect of La Addition on Microstructure and Properties of Al-0.2Fe-0.06Cu Alloy. Metals. 2022; 12(2):211. https://doi.org/10.3390/met12020211
Chicago/Turabian StyleXu, Yawu, Zixuan Peng, Dongyan Ding, Wenlong Zhang, Yongjin Gao, Guozhen Chen, Yonglin Xie, and Yongqi Liao. 2022. "Effect of La Addition on Microstructure and Properties of Al-0.2Fe-0.06Cu Alloy" Metals 12, no. 2: 211. https://doi.org/10.3390/met12020211
APA StyleXu, Y., Peng, Z., Ding, D., Zhang, W., Gao, Y., Chen, G., Xie, Y., & Liao, Y. (2022). Effect of La Addition on Microstructure and Properties of Al-0.2Fe-0.06Cu Alloy. Metals, 12(2), 211. https://doi.org/10.3390/met12020211






