Structural and Tribological Studies of “(TiC + WC)/Hardened Steel” PMMC Coating Deposited by Air Pulsed Plasma
Abstract
:1. Introduction
2. Materials and Methods
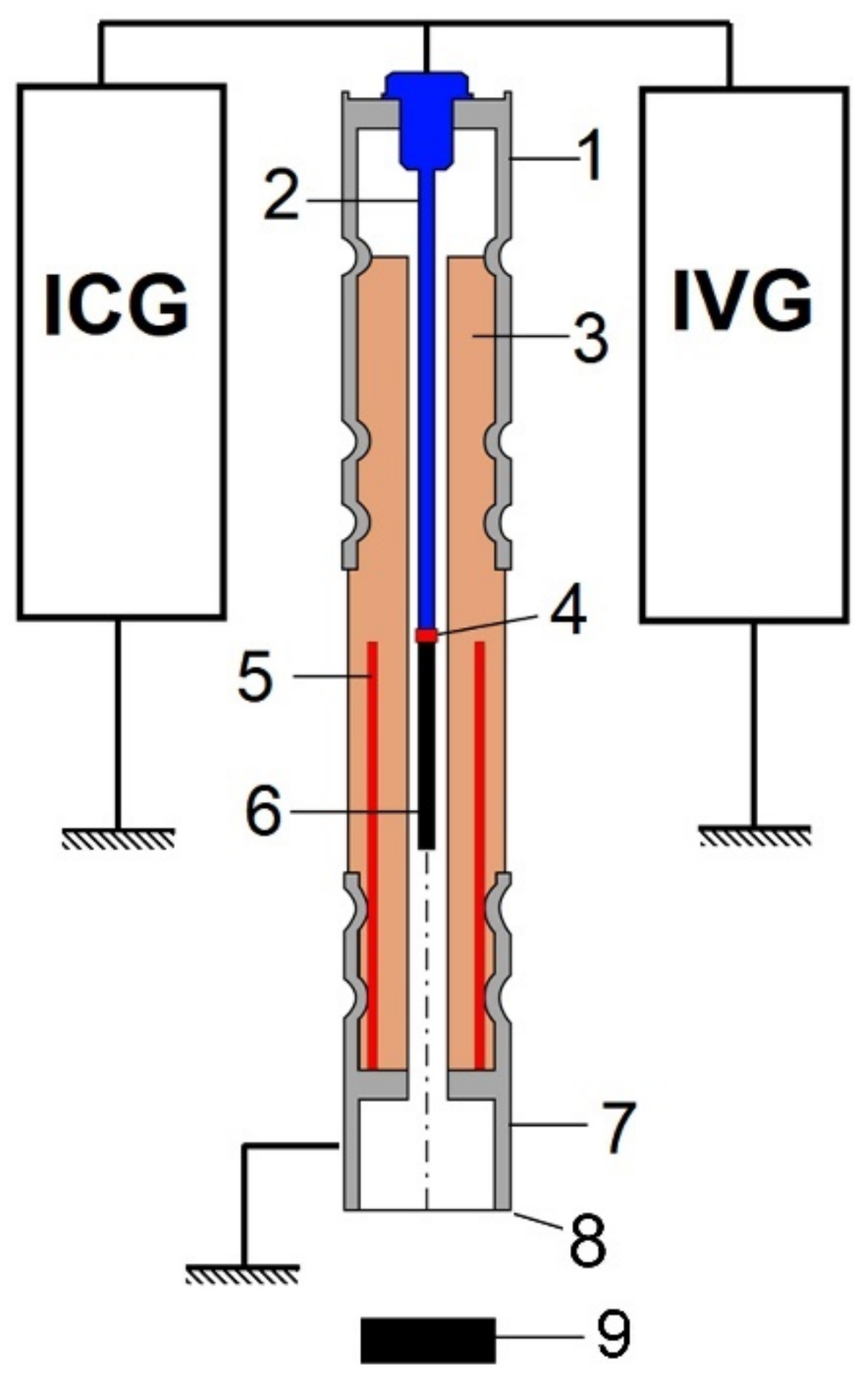
2.1. EAPA Cathode Design and PPD Parameters
2.2. Coating Characterization and Dry-Sliding Testing
3. Results
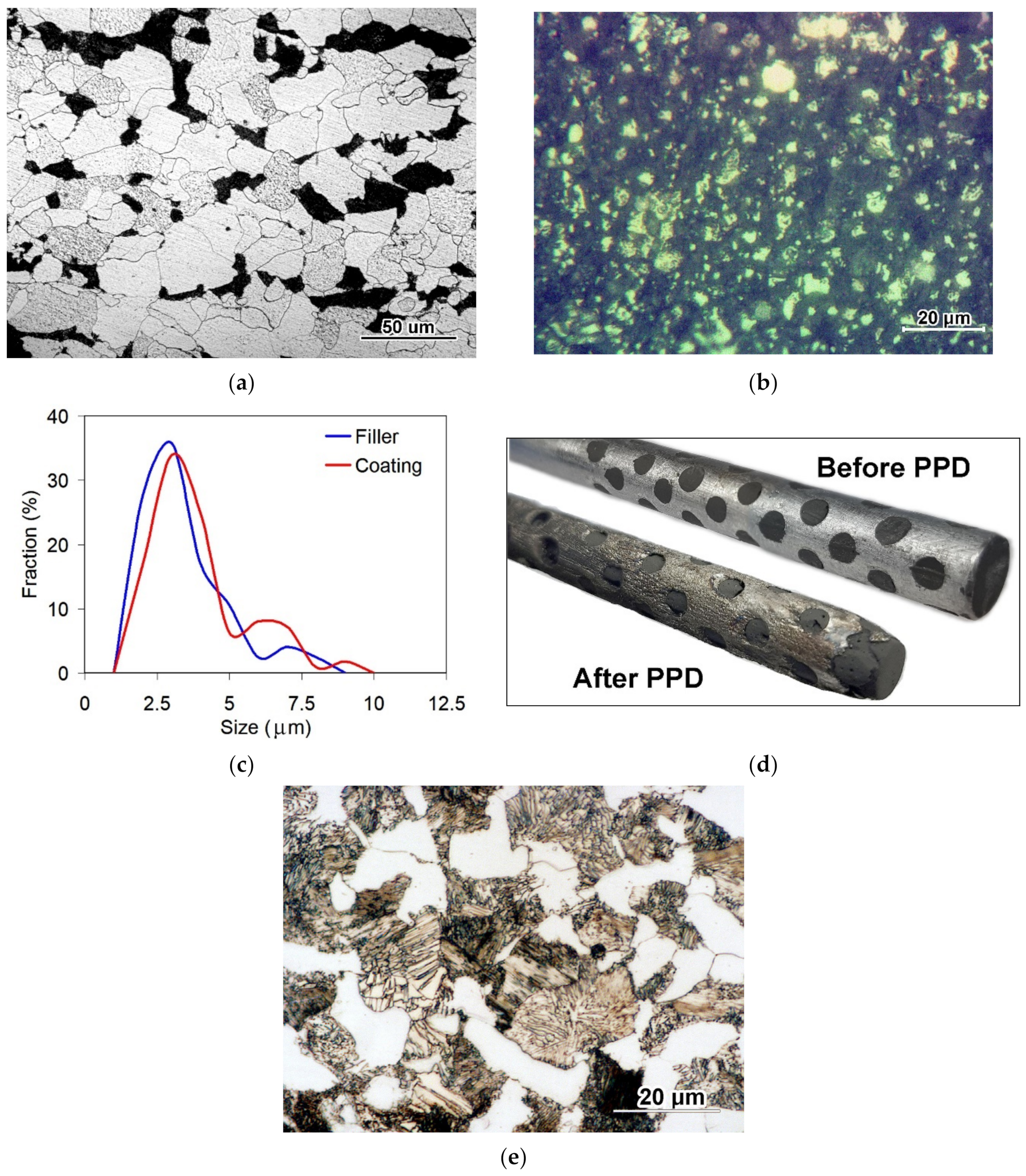
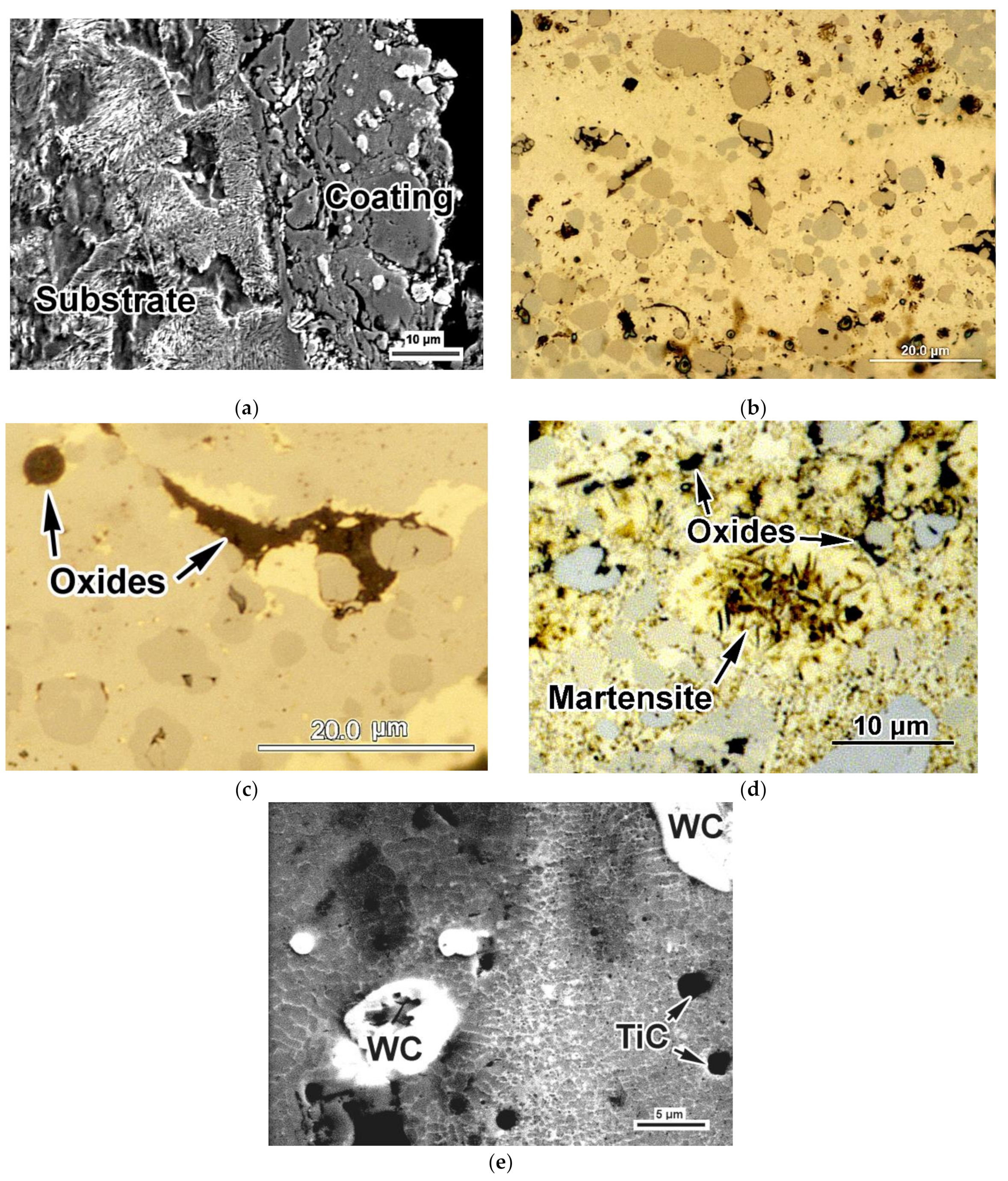
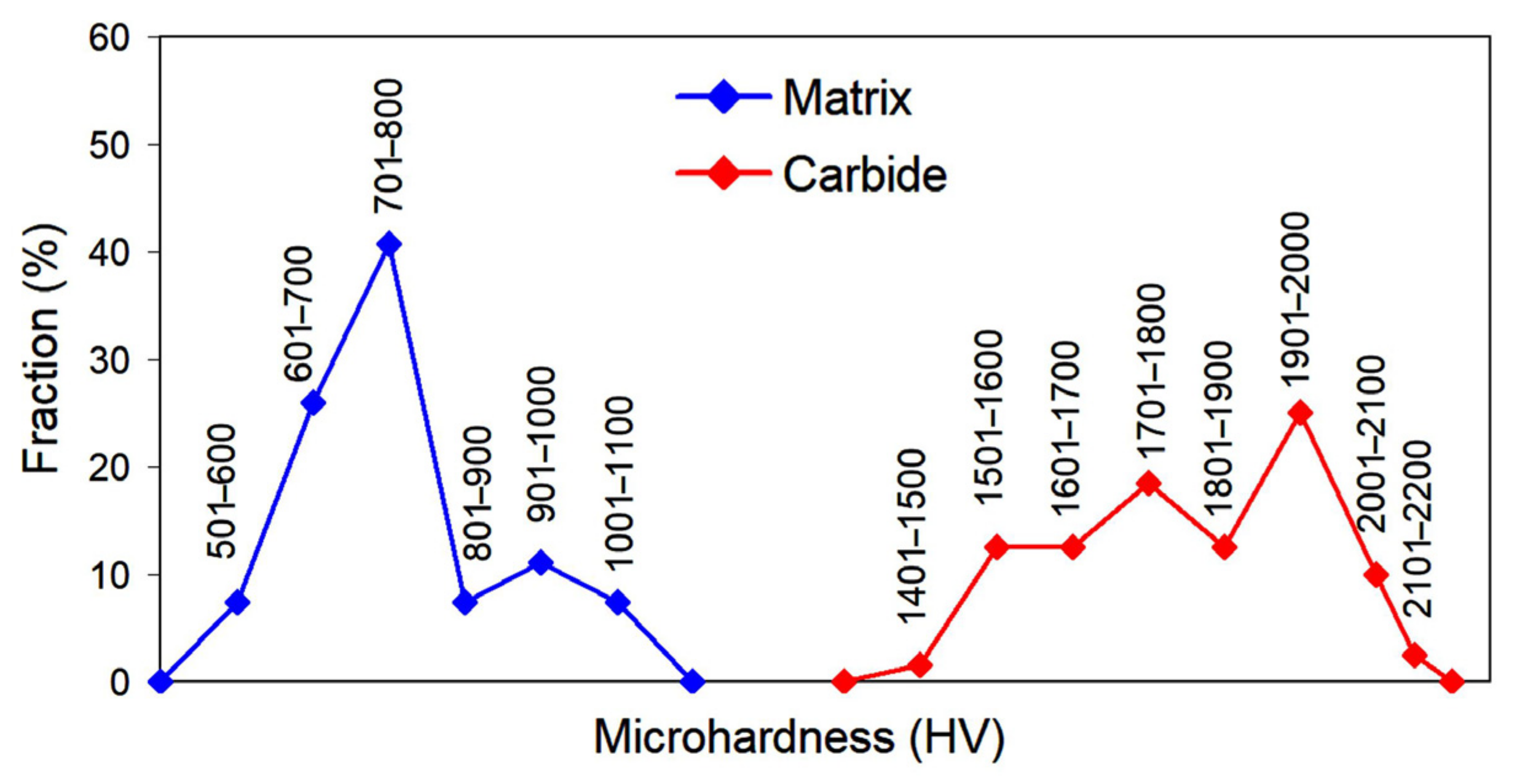
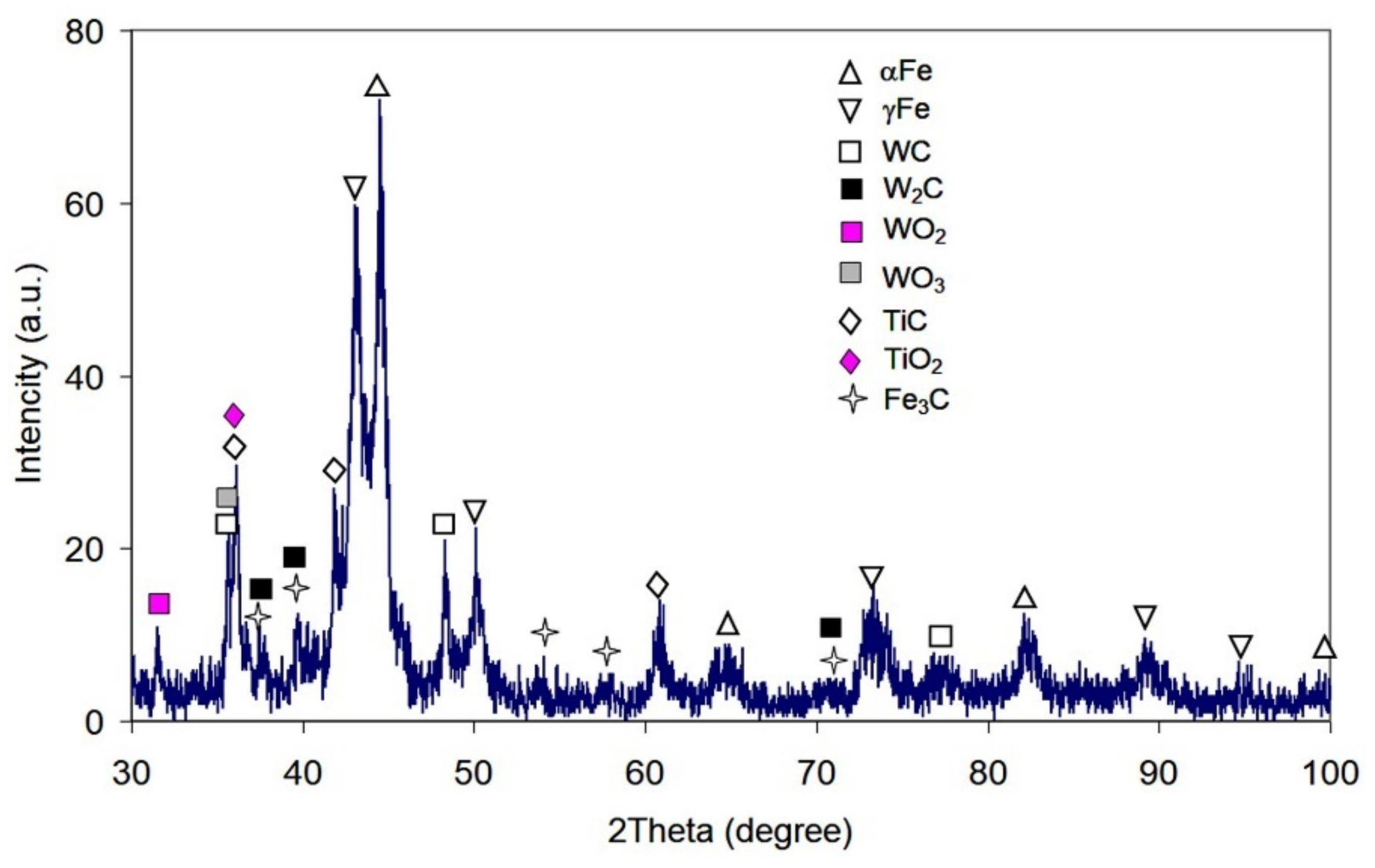
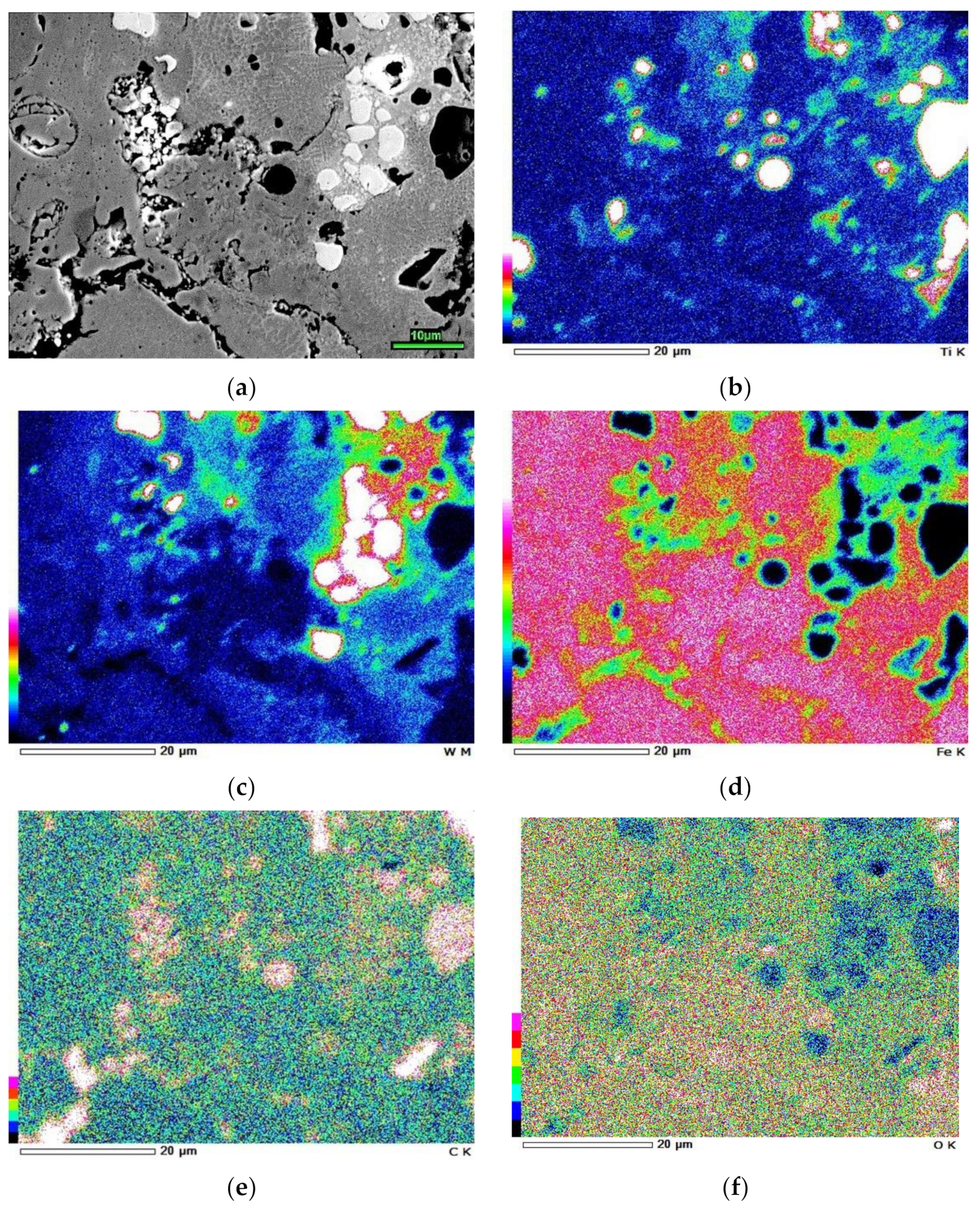
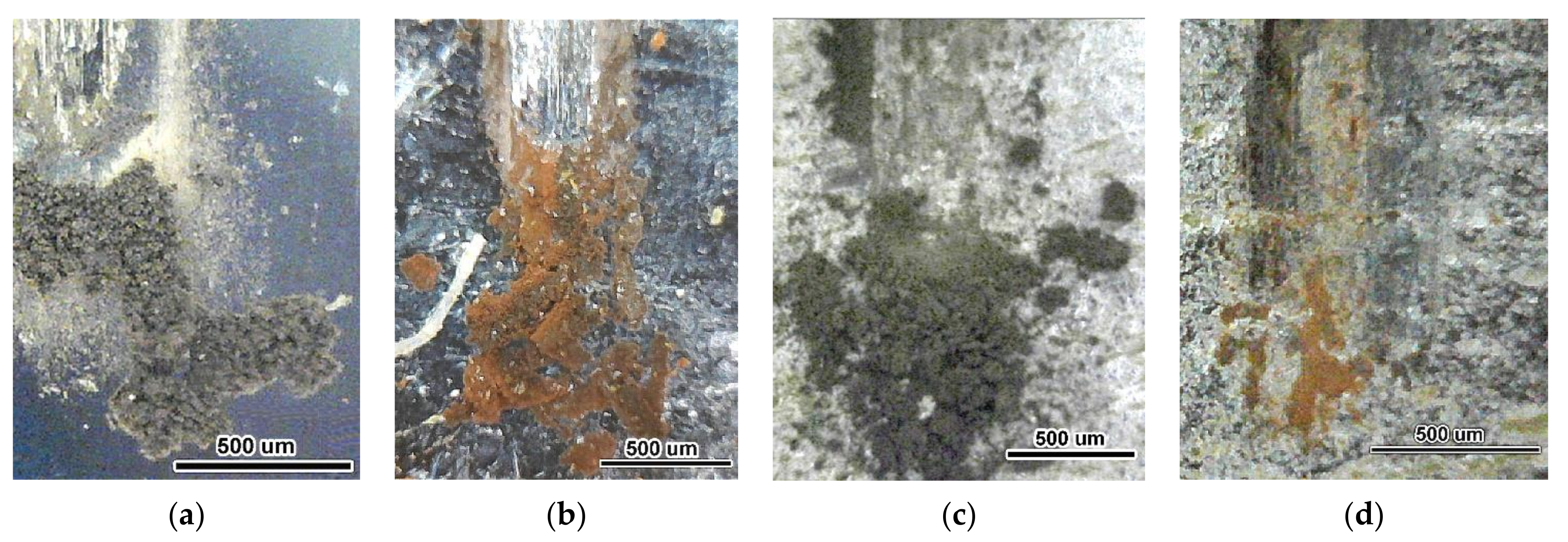
3.1. Coatings Microstructure and Phase Composition Characterization
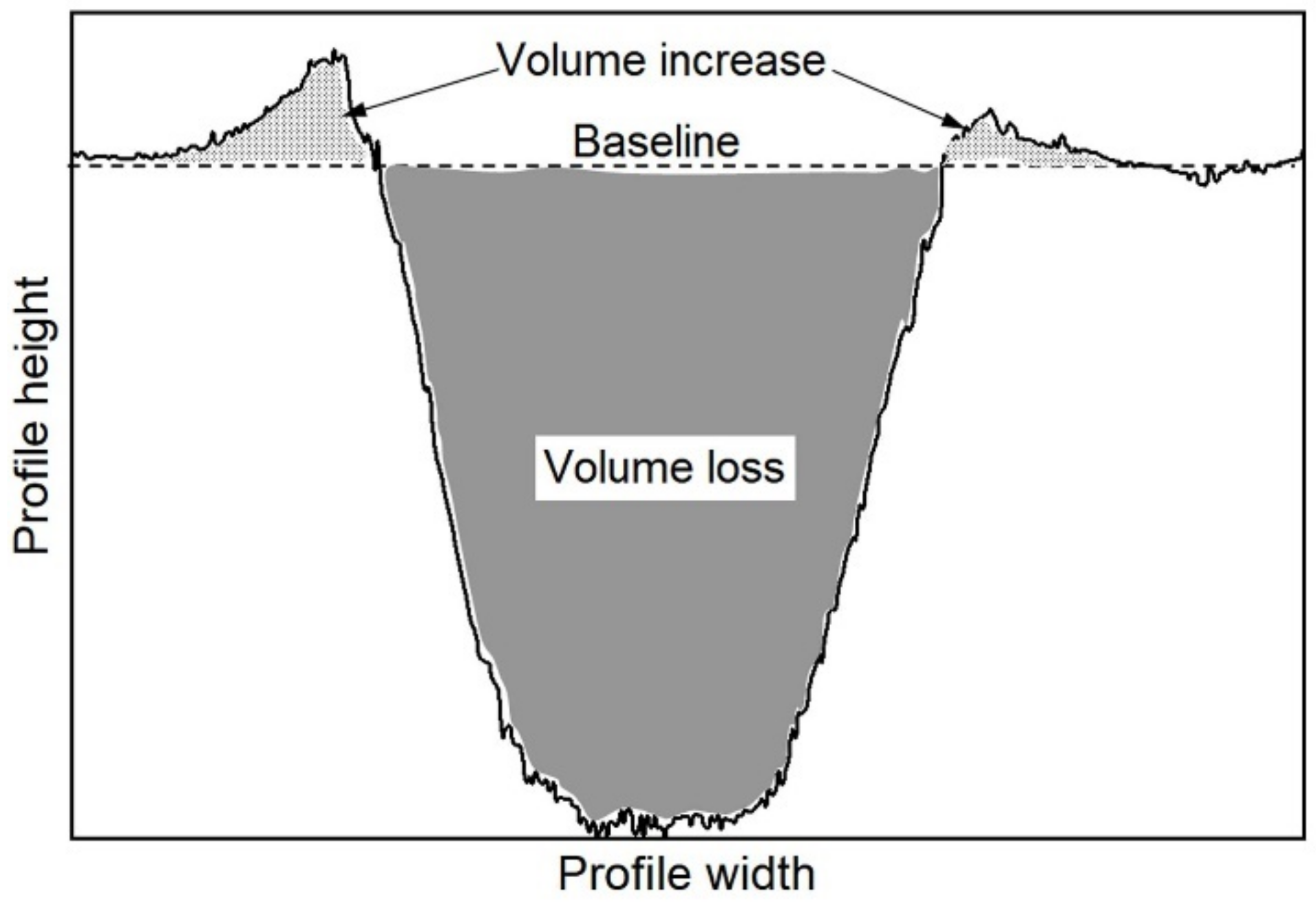
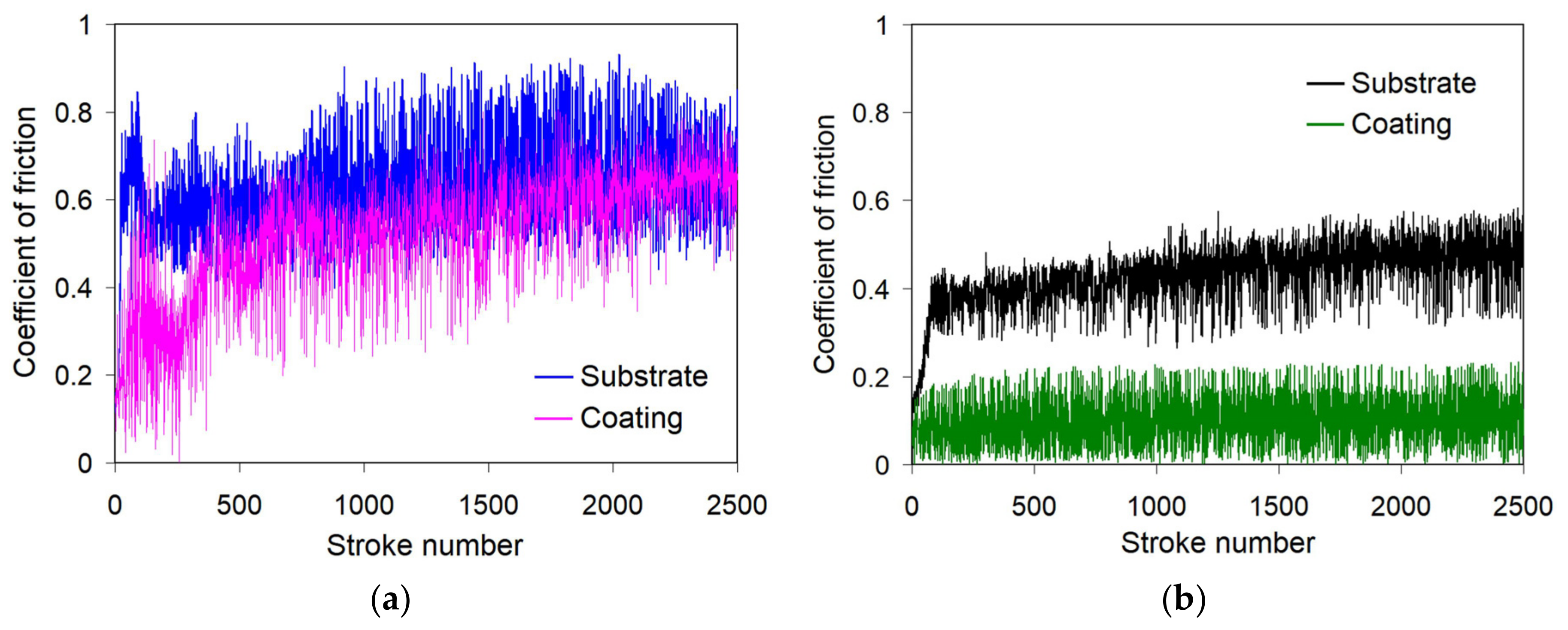
3.2. Tribological Behaviour Evaluation
4. Discussion
5. Conclusions
- A composite coating “(TiC + WC)/Hardened matrix” of 24–31 µm thick was consolidated on the steel surface after 10 plasma pulsed with the discharge voltage of 4.0 kV and each discharge duration of 1 ms. Using the consumable cathode of a novel complex design (low-carbon plane steel tube filled with “TiC/WC + Epoxy resin” mixer) enables the direct formation of PMMC coating via plasma-transfer of carbides from the cathode to the substrate (steel of AISI 4145H grade)surface without their substantial melting.
- It is shown that the formation of the coating’s matrix occurred due to melt enrichment with carbon released upon epoxy resin burnout and EAPA inner walls (bakelite) evaporation under high-current arc discharge. This resulted in the PMMC matrix consisting of 43 vol.% of plate martensite and 57 vol.% of retained austenite (RA) with high carbon content in the latter (1.43 wt.%). In addition, partial melting/oxidation of TiC and WC particles occurred under the plasma transfer leading to oxide films (TiO2, WO2, WO3) formation and matrix enrichment with Ti (up to 3.3 wt.%) and W (up to 17 wt.%). The latter caused the precipitation of (Fe,W,Ti)3C carbide network under the coating crystallization.
- The high hardness of the matrix (500–1044 HV) and its heterogeneous character supported by the protective oxide films presence enabled the improved wear behaviour of pulsed-plasma PMMC under dry-sliding conditions. It performed a lower coefficient of friction and increased wear characteristics compared with the substrate (steel of AISI 4145H grade):
- -
- by 4.4 times lower volumetric wear under sliding against hardened 100Cr6 steel ball;
- -
- by 16 times lower volumetric wear under sliding against SiC ball.
- The wear mechanisms of pulsed-plasma PMMC coating depended on the counter-body material to be: (a) dry oxidative wear and galling when sliding against 100Cr steel counter ball (proceeded through the formation/destruction of thick opaque discontinuous oxide scale on the coating surface), (b) dry oxidative wear when sliding against SiC ball (accompanied with the formation of thin transparent oxide scale entirely covering the wear track surface).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauri, R.; Yadav, D. Metal Matrix Composites by Friction Stir Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–119. [Google Scholar] [CrossRef]
- Samal, P.; Vundavilli, P.R.; Meher, A.; Mahapatra, M.M. Recent progress in aluminum metal matrix composites: A review on processing, mechanical and wear properties. J. Manuf. Process. 2020, 59, 131–152. [Google Scholar] [CrossRef]
- Mechnik, V.A.; Bondarenko, N.A.; Kolodnitskyi, V.M.; Zakiev, V.I.; Zakiev, I.M.; Ignatovich, S.R.; Dub, S.N.; Kuzin, N.O. Effect of vacuum hot pressing temperature on the mechanical and tribological properties of the Fe-Cu-Ni-Sn-Vn composites. Powder Metall. Met. Ceram. 2020, 58, 679–691. [Google Scholar] [CrossRef]
- Efremenko, V.G.; Shimizu, K.; Cheiliakh, A.P.; Kozarevs’ka, T.V.; Chabak, Y.G.; Hara, H.; Kusumoto, K. Abrasive wear resistance of spheroidal vanadium carbide cast irons. J. Frict. Wear 2013, 34, 466–474. [Google Scholar] [CrossRef]
- Alpas, A.T.; Bhattacharya, S.; Hutchings, I.M. Wear of particulate metal matrix composites. In Comprehensive Composite Materials II; Beaumont, P.W.R., Zweben, C.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 4, pp. 137–172. [Google Scholar] [CrossRef]
- Bakkar, A.; Ahmed, M.M.Z.; Alsaleh, N.A.; Seleman, M.M.E.-S.; Ataya, S. Microstructure, wear, and corrosion characterization of high TiC content Inconel 625 matrix composites. J. Mater. Res. Technol. 2019, 8, 1102–1110. [Google Scholar] [CrossRef]
- Sukhova, O.V.; Syrovatko, Y.V. New metallic materials and synthetic metals. Metallofiz. Noveishie Tekhnologii 2019, 41, 1171–1185. [Google Scholar] [CrossRef]
- Pramanik, S.; Kar, K.K. Coating Technologies for Metal Matrix Composites. In Encyclopedia of Materials: Composites; Brabazon, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 454–473. [Google Scholar] [CrossRef]
- Kılıç, F.; Gül, H.; Aslan, S.; Alp, A.; Akbulut, H. Effect of CTAB concentration in the electrolyte on the tribological properties of nanoparticle SiC reinforced Ni metal matrix composite (MMC) coatings produced by electrodeposition. Colloids Surf. A 2013, 419, 53–60. [Google Scholar] [CrossRef]
- Lee, Y.T.R.; Ashrafizadeh, H.; Fisher, G.; McDonald, A. Effect of type of reinforcing particles on the deposition efficiency and wear resistance of low-pressure cold-sprayed metal matrix composite coatings. Surf. Coat. Technol. 2017, 324, 190–200. [Google Scholar] [CrossRef]
- Winnicki, M.; Łapa, W.; Znamirowski, Z. Field electron emission experiments with cold-sprayed Cu-SiC composite coatings. Coatings 2021, 11, 134. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Chen, Z.; Addad, A.; Xie, Y.; Wu, X.; Verdy, C.; Wang, Y.; Wang, J.; Ren, Z.; et al. Effect of annealing treatment on microstructure and mechanical properties of cold sprayed TiB2/AlSi10Mg composites. Surf. Interfaces 2021, 26, 101341. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, X.; Li, Y.; Wang, D.; Liu, Y.; Yamaguchi, T.; Nishio, K.; Cao, J. Fabrication, microstructure, and wear performance of WC-Fe composite/metal coating fabricated by resistance seam welding. Mater. Charact. 2017, 134, 182–193. [Google Scholar] [CrossRef]
- Farías, I.; Olmos, L.; Jiménez, O.; Flores, M.; Braem, A.; Vleugels, J. Wear modes in open porosity titanium matrix composites with TiC addition processed by spark plasma sintering. Trans. Nonferrous Met. Soc. China 2019, 29, 1653–1664. [Google Scholar] [CrossRef]
- Cabezas-Villa, J.L.; Olmos, L.; Vergara-Hernández, H.J.; Jiménez, O.; Garnica, P.; Bouvard, D.; Flores, M. Constrained sintering and wear properties of Cu-WC composite coatings. Trans. Nonferrous Met. Soc. China 2017, 27, 2214–2224. [Google Scholar] [CrossRef]
- Ziejewska, C.; Marczyk, J.; Szewczyk-Nykiel, A.; Nykiel, M.; Hebda, M. Influence of size and volume share of WC particles on the properties of sintered metal matrix composites. Adv. Powder Technol. 2019, 30, 835–842. [Google Scholar] [CrossRef]
- Xu, J.; Zou, B.; Zhao, S.; Hui, Y.; Huang, W.; Zhou, X.; Wang, Y.; Cai, X.; Cao, X. Fabrication and properties of ZrC–ZrB2/Ni cermet coatings on a magnesium alloy by atmospheric plasma spraying of SHS powders. Ceram. Int. 2014, 40, 15537–15544. [Google Scholar] [CrossRef]
- Xu, L.; Song, J.; Zhang, X.; Deng, C.; Liu, M.; Zhou, K. Microstructure and corrosion resistance of WC-based cermet/Fe-based amorphous alloy composite coatings. Coatings 2018, 8, 393. [Google Scholar] [CrossRef] [Green Version]
- Mordyuk, B.N.; Voloshko, S.M.; Zakiev, V.I.; Burmak, A.P.; Mohylko, V.V. Enhanced resistance of Ti6Al4V alloy to high-temperature oxidation and corrosion by forming alumina composite coating. J. Mater. Eng. Perform. 2021, 30, 1780–1795. [Google Scholar] [CrossRef]
- Ostolaza, M.; Arrizubieta, J.I.; Cortina, M.; Lamikiz, A. Study of the reinforcement phase dilution into the metal matrix in functionally graded Stellite 6 and WC metal matrix composite by laser metal deposition. Proc. CIRP 2020, 94, 330–335. [Google Scholar] [CrossRef]
- Duryagina, Z.A.; Bespalov, S.A.; Borysyuk, A.K.; Pidkova, V.Y. Magnetometric analysis of surface layers of 12X18H10T steel after ion-beam nitriding. Metallofiz. Noveishie Tekhnologii 2011, 33, 615–622. [Google Scholar]
- Tang, B.; Tan, Y.; Xu, T.; Sun, Z.; Li, X. Effects of TiB2 particles content on microstructure, mechanical properties and tribological properties of Ni-based composite coatings reinforced with TiB2 particles by laser cladding. Coatings 2020, 10, 813. [Google Scholar] [CrossRef]
- Kotarska, A.; Poloczek, T.; Janicki, D. Characterization of the structure, mechanical properties and erosive resistance of the laser cladded Inconel 625-based coatings reinforced by TiC particles. Materials 2021, 14, 2225. [Google Scholar] [CrossRef]
- Li, Z.; Yan, H.; Zhang, P.; Guo, J.; Yu, Z.; Ringsberg, J.W. Improving surface resistance to wear and corrosion of nickel-aluminum bronze by laser-clad TaC/Co-based alloy composite coatings. Surf. Coat. Technol. 2021, 405, 126592. [Google Scholar] [CrossRef]
- Tan, H.; Luo, Z.; Li, Y.; Yan, F.; Duan, R.; Huang, Y. Effect of strengthening particles on the dry sliding wear behavior of Al2O3–M7C3/Fe metal matrix composite coatings produced by laser cladding. Wear 2015, 324–325, 36–44. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, W.; Li, T.; Zhang, M.; Liu, B.; Liu, Y.; Wang, L.; Hu, S. Effect of WC content on microstructures and mechanical properties of FeCoCrNi high-entropy alloy/WC composite coatings by plasma cladding. Surf. Coat. Technol. 2020, 385, 125326. [Google Scholar] [CrossRef]
- Mashovets, N.S.; Pastukh, I.M.; Voloshko, S.M. Aspects of the practical application of titanium alloys after low temperature nitriding glow discharge in hydrogen-free-gas media. Appl. Surf. Sci. 2017, 392, 356–361. [Google Scholar] [CrossRef]
- Oukach, S.; Pateyron, B.; Pawłowski, L. Physical and chemical phenomena occurring between solid ceramics and liquid metals and alloys at laser and plasma composite coatings formation: A review. Surf. Sci. Rep. 2019, 74, 213–241. [Google Scholar] [CrossRef]
- Czupryński, A. Microstructure and abrasive wear resistance of metal matrix composite coatings deposited on steel grade AISI 4715 by powder plasma transferred arc welding part 1. Mechanical and structural properties of a cobalt-based alloy surface layer reinforced with particles of titanium carbide and synthetic metal–diamond composite. Materials 2021, 14, 2382. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.Z.; Wang, J.F.; Zhang, G.S.; Zhao, Y.Q.; Sun, K. Effects of TiB2 + TiC content on microstructure and wear resistance of Ni55-based composite coatings produced by plasma cladding. Trans. Nonferrous Met. Soc. China 2019, 29, 132–142. [Google Scholar] [CrossRef]
- Rokanopoulou, A.; Skarvelis, P.; Papadimitriou, G.D. Improvement of the tribological properties of Al2O3 reinforced duplex stainless steel MMC coating by the addition of TiS2 powder. Surf. Coat. Technol. 2016, 289, 144–149. [Google Scholar] [CrossRef]
- Huang, Z.; Hou, Q.; Wang, P. Microstructure and properties of Cr3C2-modified nickel-based alloy coating deposited by plasma transferred arc process. Surf. Coat. Technol. 2008, 202, 2993–2999. [Google Scholar] [CrossRef]
- Sivkov, A.; Shanenkov, I.; Pak, A.; Gerasimov, D.; Shanenkova, Y. Deposition of a TiC/Ti coating with a strong substrate adhesion using a high-speed plasma jet. Surf. Coat. Technol. 2016, 291, 1–6. [Google Scholar] [CrossRef]
- Kovaleva, M.; Tyurin, Y.; Vasilik, N.; Kolisnichenko, O.; Prozorova, M.; Arseenko, M.; Yapryntsev, M.; Sirota, V.; Pavlenko, I. Effect of processing parameters on the microstructure and properties of WC–10Co–4Cr coatings formed by a new multi-chamber gas-dynamic accelerator. Ceram. Int. 2015, 41, 15067–15074. [Google Scholar] [CrossRef]
- Kovaleva, M.G.; Goncharov, I.Y.; Novikov, V.Y.; Pavlenko, I.A.; Yapryntsev, M.N.; Vagina, O.N.; Sirota, V.V.; Tyurin, Y.N.; Kolisnichenko, O.V. Dense ZrB2-MoSi2 composite coating fabricated by a new multi- chamber detonation accelerator on carbon/carbon composites. Mater. Sci. Forum 2020, 987, 53–58. [Google Scholar] [CrossRef]
- Rosinski, M.; Fortuna, E.; Michalski, A.; Pakiela, Z.; Kurzydlowski, K.J. W/Cu composites produced by pulse plasma sintering technique (PPS). Fusion Eng. Des. 2007, 82, 2621–2626. [Google Scholar] [CrossRef]
- Darabara, M.; Papadimitriou, G.D.; Bourithis, L. Production of Fe–B–TiB2 metal matrix composites on steel surface. Surf. Coat. Technol. 2006, 201, 3518–3523. [Google Scholar] [CrossRef]
- Efremenko, B.; Belik, A.; Chabak, Y.; Halfa, H. Simulation of structure formation in the Fe–C–Cr–Ni–Si surfacing materials. East.-Eur. J. Enterp. Technol. 2018, 2, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Salloom, R.; Joshi, S.S.; Dahotre, N.B.; Srinivasan, S.G. Laser surface engineering of B4C/Fe nano composite coating on low carbon steel: Experimental coupled with computational approach. Mater. Des. 2020, 190, 108576. [Google Scholar] [CrossRef]
- Zhang, Z.; Kovacevic, R. Laser cladding of iron-based erosion resistant metal matrix composites. J. Manuf. Process. 2019, 38, 63–75. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Y.; Sun, Y.; Wei, Y.; Wang, X.; Liu, D. Coarse TiC particles reinforced H13 steel matrix composites produced by laser cladding. Surf. Coat. Technol. 2020, 395, 125867. [Google Scholar] [CrossRef]
- Jiang, W.H.; Kovacevic, R. Laser deposited TiC/H13 tool steel composite coatings and their erosion resistance. J. Mater. Process. Technol. 2007, 186, 331–338. [Google Scholar] [CrossRef]
- Gordo, E.; Velasco, F.; Antón, N.; Torralba, J.M. Wear mechanisms in high speed steel reinforced with (NbC)p and (TaC)p MMCs. Wear 2000, 239, 251–259. [Google Scholar] [CrossRef]
- Kolyada, Y.E.; Fedun, V.I.; Tyutyunnikov, V.I.; Savinkov, N.A.; Kapustin, A.E. Formation mechanism of the metallic nanostructures using pulsed axial electrothermal plasma accelerator. Probl. At. Sci. Technol. 2013, 86, 297–300. [Google Scholar]
- Kolyada, Y.E.; Bizyukov, A.A.; Bulanchuk, O.N.; Fedun, V.I. Pulse electrothermal plasma accelerators and its application in the technologies. Probl. At. Sci. Technol. 2015, 98, 319–324. [Google Scholar]
- Efremenko, V.G.; Chabak, Y.G.; Lekatou, A.; Karantzalis, A.E.; Shimizu, K.; Fedun, V.I.; Azarkhov, A.Y.; Efremenko, A.V. Pulsed plasma deposition of Fe-C-Cr-W coating on high-Cr-cast iron: Effect of layered morphology and heat treatment on the microstructure and hardness. Surf. Coat. Technol. 2016, 304, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Chabak, Y.G.; Fedun, V.I.; Shimizu, K.; Efremenko, V.G.; Zurnadzhy, V.I. Phase-structural composition of coating obtained by pulsed plasma treatment using eroded cathode of T1 high speed steel. Probl. At. Sci. Technol. 2016, 4, 100–106. [Google Scholar]
- Efremenko, V.G.; Chabak, Y.G.; Fedun, V.I.; Shimizu, K.; Pastukhova, T.V.; Petryshynets, I.; Zusin, A.M.; Kudinova, E.V.; Efremenko, B.V. Formation mechanism, microstructural features and dry-sliding behaviour of “Bronze/WC carbide” composite synthesised by atmospheric pulsed-plasma deposition. Vacuum 2021, 185, 110031. [Google Scholar] [CrossRef]
- Kolyada, Y.E.; Fedun, V.I.; Onishchenko, I.N.; Kornilov, E.A. The use of a magnetic switch for commutation of high-current pulse circuits. Instrum. Exp. Tech. 2001, 44, 213–214. [Google Scholar] [CrossRef]
- Chabak, Y.G.; Efremenko, V.G.; Shimizu, K.; Lekatou, A.; Pastukhova, T.V.; Azarkhov, A.Y.; Zurnadzhy, V.I. Comparative analysis of the microstructural features of 28 wt.% Cr cast iron fabricated by pulsed plasma deposition and conventional casting. J. Mater. Eng. Perform. 2018, 27, 379–388. [Google Scholar] [CrossRef]
- Vander Voort, G.F. Metallography, Principles and Practice; ASTM International: West Conshohocken, PA, USA, 1999; pp. 1–752. [Google Scholar]
- Buketov, A.; Brailo, M.; Yakushchenko, S.; Sapronova, A. Development of epoxy-polyester composite with improved thermophysical properties for restoration of details of sea and river transport. Adv. Mater. Sci. Eng. 2018, 2018, 6378782. [Google Scholar] [CrossRef] [Green Version]
- Atta, A.M.; Ahmed, M.A.; El-Saeed, A.M.; Abo-Elenien, O.M.; El-Sockary, M.A. Hybrid ZrO2/Cr2O3 epoxy nanocomposites as organic coatings for steel. Coatings 2020, 10, 997. [Google Scholar] [CrossRef]
- Chabak, Y.G.; Fedun, V.I.; Pastukhova, T.V.; Zurnadzhy, V.I.; Berezhnyy, S.P.; Efremenko, V.G. Modification of steel surface by pulsed plasma heating. Probl. At. Sci. Technol. 2017, 110, 97–102. [Google Scholar]
- Sun, J.; Hao, Y. Microstructure development and mechanical properties of quenching and partitioning (Q&P) steel and an incorporation of hot-dipping galvanization during Q&P process. Mater. Sci. Eng. A. 2013, 586, 100–107. [Google Scholar] [CrossRef]
- Van Dijk, N.H.; Butt, A.M.; Zhao, L.; Sietsma, J.; Offerman, S.E.; Wright, J.P.; van der Zwaag, S. Thermal stability of retained austenite in TRIP steels studied by synchrotron X-ray diffraction during cooling. Acta Mater. 2005, 53, 5439–5447. [Google Scholar] [CrossRef]
- Berglund, K.; Rodiouchkina, M.; Hardell, J.; Kalliorinne, K.; Johansson, J. A Novel reciprocating tribometer for friction and wear measurements with high contact pressure and large area contact configurations. Lubricants 2021, 9, 123. [Google Scholar] [CrossRef]
- Stormvinter, A.; Borgenstam, A.; Hedström, P. Investigation of lath and plate martensite in a carbon steel. Solid State Phenom. 2011, 172–174, 61–66. [Google Scholar] [CrossRef]
- Kurlov, A.S.; Gusev, A.I. Tungsten Carbides: Structure, Properties and Application in Hardmetal; Springer Science & Business Media: Cham, Switzerland, 2013; pp. 1–242. [Google Scholar] [CrossRef]
- Rolland, P.; Carlino, V.L.; Vane, R. Improved carbon analysis with evactron plasma cleaning. Microsc. Microanal. 2004, 10, 964–965. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhan, Q.; Huang, J.; Ding, S.; Li, H. Decarburization mechanisms of WC–Co during thermal spraying: Insights from controlled carbon loss and microstructure characterization. Mater. Chem. Phys. 2013, 142, 165–171. [Google Scholar] [CrossRef]
- Tansir, A.; Alshehri, S. Thermal degradation and evolved gas analysis of epoxy (DGEBA)/novolac resin blends (ENB) during pyrolysis and combustion. J. Therm. Anal. Calorim. 2012, 111, 445–451. [Google Scholar] [CrossRef]
- Payson, P.; Savage, C.H. Martensite reactions in alloy steels. Trans. ASM 1944, 33, 261–280. [Google Scholar]
- Barbier, D. Extension of the martensite transformation temperature relation to larger alloying elements and contents. Adv. Eng. Mater. 2014, 1, 122–127. [Google Scholar] [CrossRef]
- Mendiratta, M.G.; Krauss, G. The development of martensitic microstructure and microcracking in an Fe-1.86 C alloy. Metall. Mater. Trans. B 1972, 3, 1755–1760. [Google Scholar] [CrossRef]
- Malinov, L.S.; Malysheva, I.E.; Klimov, E.S.; Kukhar, V.V.; Balalayeva, E. Effect of particular combinations of quenching, tempering and carburization on abrasive wear of low-carbon manganese steels with metastable austenite. Mater. Sci. Forum 2019, 945, 574–578. [Google Scholar] [CrossRef]
- Golyshev, A.A.; Malikov, A.G.; Orishich, A.M. The formation of heterogeneous wear-resistant coatings by the additive technology method. J. Phys. Conf. Ser. 2019, 1404, 012019. [Google Scholar] [CrossRef]
- Efremenko, V.G.; Shimizu, K.; Cheiliakh, A.P.; Pastukhova, T.V.; Chabak, Y.G.; Kusumoto, K. Abrasive resistance of metastable V–Cr–Mn–Ni spheroidal carbide cast irons using the factorial design method. Int. J. Miner. Metall. Mater. 2016, 23, 645–657. [Google Scholar] [CrossRef]
- Hutchings, I.; Shipway, P. Tribology: Friction and Wear of Engineering Materials, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 107–132. [Google Scholar]
- Dohda, K.; Yamamoto, M.; Hu, C.; Dubar, L.; Ehmann, K.F. Galling phenomena in metal forming. Friction 2021, 9, 665–685. [Google Scholar] [CrossRef]
- Ostash, O.P.; Kulyk, V.V.; Poznyakov, V.D.; Duriagina, Z.A.; Tepla, T.L. Fatigue crack growth resistance of welded joints simulating the weld-repaired railway wheels metal. Arch. Mater. Sci. Eng. 2017, 86, 49–55. [Google Scholar] [CrossRef]
- Ilie, F.; Covaliu, C. Tribological properties of the lubricant containing titanium dioxide nanoparticles as an additive. Lubricants 2016, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Cura, M.E.; Trebala, M.; Ge, Y.; Klimczyk, P.; Hannula, S.-P. Mechanical and tribological properties of WO2.9 and ZrO2 + WO2.9 composites studied by nanoindentation and reciprocating wear tests. Wear 2021, 478–479, 203920. [Google Scholar] [CrossRef]






| Phase | Elements (wt.%) | ||||
|---|---|---|---|---|---|
| C | Ti | W | Cu | Fe | |
| WC | 7.79 ± 1.91 | - | 89.53 ± 0.82 | - | 2.67 ± 0.79 |
| TiC | 17.46 ± 3.65 | 77.57 ± 6.92 | 0.72 ± 0.43 | - | 4.25 ± 3.02 |
| Carbide-free matrix | 5.95 ± 0.89 | 0.37 ± 0.06 | 11.26 ± 1.28 | 0.77 ± 0.24 | 81.25 ± 1.93 |
| “Carbide network” matrix | 8.76 ± 0.70 | 3.32 ± 1.07 | 16.98 ± 2.02 | 1.06 ± 0.05 | 74.72 ± 2.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chabak, Y.; Efremenko, V.; Zurnadzhy, V.; Puchý, V.; Petryshynets, I.; Efremenko, B.; Fedun, V.; Shimizu, K.; Bogomol, I.; Kulyk, V.; et al. Structural and Tribological Studies of “(TiC + WC)/Hardened Steel” PMMC Coating Deposited by Air Pulsed Plasma. Metals 2022, 12, 218. https://doi.org/10.3390/met12020218
Chabak Y, Efremenko V, Zurnadzhy V, Puchý V, Petryshynets I, Efremenko B, Fedun V, Shimizu K, Bogomol I, Kulyk V, et al. Structural and Tribological Studies of “(TiC + WC)/Hardened Steel” PMMC Coating Deposited by Air Pulsed Plasma. Metals. 2022; 12(2):218. https://doi.org/10.3390/met12020218
Chicago/Turabian StyleChabak, Yuliia, Vasily Efremenko, Vadym Zurnadzhy, Viktor Puchý, Ivan Petryshynets, Bohdan Efremenko, Victor Fedun, Kazumichi Shimizu, Iurii Bogomol, Volodymyr Kulyk, and et al. 2022. "Structural and Tribological Studies of “(TiC + WC)/Hardened Steel” PMMC Coating Deposited by Air Pulsed Plasma" Metals 12, no. 2: 218. https://doi.org/10.3390/met12020218
APA StyleChabak, Y., Efremenko, V., Zurnadzhy, V., Puchý, V., Petryshynets, I., Efremenko, B., Fedun, V., Shimizu, K., Bogomol, I., Kulyk, V., & Jakubéczyová, D. (2022). Structural and Tribological Studies of “(TiC + WC)/Hardened Steel” PMMC Coating Deposited by Air Pulsed Plasma. Metals, 12(2), 218. https://doi.org/10.3390/met12020218






