Microstructure and Mechanical Properties of CoWB Based Composites Produced by Crystallization of Ni-Co-Zr-Ta-W-B Bulk Metallic Glass
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Thermal Behaviour
3.2. XRD Results
3.3. SEM Analyses
3.4. Microhardness
3.5. Indentation Fracture Toughness
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakhariev, Z.; Zlateva, R.; Petrov, K. Microhardness and high-temperature oxidation stability of CoWB. J. Less-Common Met. 1986, 117, 129–133. [Google Scholar] [CrossRef]
- Karimi, H.; Hadi, M.; Ebrahimzadeh, I.; Farhang, M.R.; Sadeghi, M. High-temperature oxidation behaviour of WC-FeAl composite fabricated by spark plasma sintering. Ceram. Int. 2018, 44, 17147–17153. [Google Scholar] [CrossRef]
- Cao, Z.; Jian, Y.; Huang, Z.; Xu, L.; Fan, J. High-temperature cyclic oxidation behavior and mechanism of Mo2FeB2-based cermets with various boron contents. Corros. Sci. 2021, 190, 109665. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Azcona, I.; Castro, F. Mechanical properties of titanium diboride based cermets. J. Mater. Sci. 2000, 35, 9–14. [Google Scholar] [CrossRef]
- Venkateswaran, T.; Basu, B.; Raju, G.B.; Kim, D.Y. Densification and properties of transition metal borides-based cermets via spark plasma sintering. J. Eur. Ceram. Soc. 2006, 26, 2431–2440. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, J.; Wang, W.; Wang, H.; Zhang, F.; Wang, Y.; Fu, Z. Fabrication and properties of TiB2-based cermets by spark plasma sintering with CoCrFeNiTiAl high-entropy alloy as sintering aid. J. Eur. Ceram. Soc. 2015, 35, 879–886. [Google Scholar] [CrossRef]
- Lark, A.; Du, J.; Ravi Chandran, K.S. Material design and processing of a new class of titanium boride cermets with tough metallic phases and mechanical properties. J. Mater. Res. 2018, 33, 4296–4306. [Google Scholar] [CrossRef]
- Takagi, K. High tough boride base cermets produced by reaction sintering. Mater. Chem. Phys. 2001, 67, 214–219. [Google Scholar] [CrossRef]
- Takagi, K. Development and application of high strength ternary boride base cermets. J. Solid State Chem. 2006, 179, 2809–2818. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, G.J.; Kan, Y.M.; Wang, P.L. Reactive synthesis and mechanical properties of Mo2NiB2 based hard alloy. Int. J. Refract. Met. Hard Mater. 2010, 28, 291–296. [Google Scholar] [CrossRef]
- Lee, D.R.; Lee, W.J. Fabrication of W2NiB2-Ni borides cermets. J. Jpn. Soc. Powder Metall. 2003, 50, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, Y.; Fujima, T.; Momozawa, A.; Takagi, K. Mechanical properties of W2NiB2-Ni boride base cermets. J. Jpn. Soc. Powder Metall. 2013, 60, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Liu, W.; Zheng, Y. Microstructure and mechanical properties of liquid phase sintered Mo2FeB2 based cermets. Mater. Des. 2011, 32, 3521–3525. [Google Scholar] [CrossRef]
- Takagi, K.; Koike, W.; Momozawa, A.; Fujima, T. Effects of Cr on the properties of Mo2NiB2 ternary boride. Solid State Sci. 2012, 14, 1643–1647. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Z.; Shen, Y.; Guo, L. Bulk Mo2FeB2 based cermets fabricated by mechanical ball milling and reaction boronizing sintering. Powder Metall. Met. Ceram. 2017, 55, 665–675. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, Y.; Zhang, J.; Zhang, G.; Ke, Z.; Xu, X.; Lu, X.; Zhou, W. Preparation of Mo2FeB2-based cermets with a core/rim structure by multistep sintering approach. Ceram. Int. 2019, 45, 22371–22375. [Google Scholar] [CrossRef]
- Ivanov, M.B.; Vershinina, T.N.; Ivanisenko, V.V. The effect of composition and microstructure on hardness and toughness of Mo2FeB2 based cermets. Mater. Sci. Eng. A 2019, 763, 138117. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, Y.; Zhang, J.; Zhang, G.; Ke, Z.; Xu, X.; Lu, X. Influence of Cr and W addition on microstructure and mechanical properties of multi-step sintered Mo2FeB2-based cermets. Ceram. Int. 2020, 46, 10963–10970. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Li, C.; Liu, Y. Reactive synthesis of FeWB powders and preparation of bulk materials. Int. J. Refract. Met. Hard Mater. 2014, 46, 80–83. [Google Scholar] [CrossRef]
- Hongwei, Y.; Jun, L.; Cheng, L.; Ying, L. Microstructure evolution and phase transformation of FeWB based cermets during the vacuum sintering. Rare Metal Mat. Eng. 2018, 47, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Yin, H.; Zhang, C.; Zhang, R.; Yang, Z.; Elder, S.; Jiang, X.; Deng, Z.; Yang, G.; Zheng, Q.; et al. Synthesis and microstructure evolution of WCoB based cermets during spark plasma sintering. Ceram. Int. 2019, 45, 17536–17544. [Google Scholar] [CrossRef]
- Zhang, T.; Yin, H.; Zhang, C.; Qu, X. Microstructure and mechanical properties of Cr doped WCoB based cermets by spark plasma sintering and first principle calculation. Prog. Nat. Sci. 2020, 30, 417–423. [Google Scholar] [CrossRef]
- Yang, G.; Yin, H.; Deng, Z.; Zhang, C.; Zhang, R.; Qu, X. Effect of chemical composition on the microstructure and mechanical properties of MoCoB based cermets. Ceram. Int. 2020, 46, 18046–18055. [Google Scholar] [CrossRef]
- Fornell, J.; Gonzales, S.; Rossinyol, E.; Surinach, E.; Baro, M.D.; Louzguine-Luzgin, D.V.; Perepezko, J.H.; Sort, J.; Inoue, A. Enhanced mechanical properties due to structural changes induced by devitrification in Fe–Co–B–Si–Nb bulk metallic glass. Acta Mater. 2010, 58, 6256–6266. [Google Scholar] [CrossRef]
- Han, J.J.; Wang, C.P.; Kou, S.Z.; Liu, X.J. Thermal stability, crystallization behavior, Vickers hardness and magnetic properties of Fe−Co−Ni−Cr−Mo−C−B−Y bulk metallic glasses. Trans. Nonferrous Met. Soc. China 2013, 23, 148–155. [Google Scholar] [CrossRef]
- Hitit, A.; Geçgin, M.; Öztürk, P. Effect of annealing on microstructure and microhardness of Co-Fe-Ni-Ta-B-Si bulk metallic glass. J. Mater. Sci. Technol. 2015, 31, 148–152. [Google Scholar] [CrossRef]
- Hitit, A.; Yazici, Z.O.; Öztürk, P.; Şahin, H.; Aşgın, A.M.; Hitit, B. A Ni–CoWB composite developed by devitrification of Ni-Co-W-B bulk metallic glass. Mater. Sci. Eng. A 2021, 803, 140479. [Google Scholar] [CrossRef]
- Hitit, A.; Yazici, Z.O.; Öztürk, P.; Eryeşil, B.; Barut, N.; Şahin, H. The effects of tantalum addition on the glass forming ability, thermal stability, and mechanical properties of Ni-Co-W-B bulk metallic glasses. J. Non-Cryst. Solids 2021, 572, 121089. [Google Scholar] [CrossRef]
- Massalski, T. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1992; Volume 3. [Google Scholar]
- Lonsdale, K. International Tables for X-ray Crystallography; Dordrecht u.a.: Kluwer, UK, 1968. [Google Scholar]
- Evans, A.G.; Wilshaw, T.R. Quasi-static solid particle damage in brittle solids–I. Observations, analysis and implications. Acta Metall. 1976, 24, 939–956. [Google Scholar] [CrossRef]
- Evans, A.G.; Charles, E.A. Fracture toughness determinations by indentation. J. Am. Ceram. Soc. 1976, 59, 371–372. [Google Scholar] [CrossRef]
- Aning, A.O.; Wang, Z.; Courtney, T.H. Tungsten solution kinetics and amorphization of nickel in mechanically alloyed Ni-W alloys. Acta Metall. 1993, 41, 165–174. [Google Scholar] [CrossRef]
- Yamasaki, T.; Schloβmacher, P.; Ehrlich, K.; Ogino, Y. Formation of amorphous electrodeposited Ni-W alloys and their nanocrystallization. Nanostruct. Mater. 1998, 10, 375–388. [Google Scholar] [CrossRef]
- Rupert, T.J.; Schuh, C.A. Sliding wear of nanocrystalline Ni–W: Structural evolution and the apparent breakdown of Archard scaling. Acta Metall. 2010, 58, 4137–4148. [Google Scholar] [CrossRef]
- Yıldız, K. Investigation of Structural Properties and Martensitic Phase Transformations in Heat-treated Ni-25.5 at. %Ta High Temperature Shape Memory Alloys. ADYU J. Sci. 2020, 10, 391–402. [Google Scholar] [CrossRef]

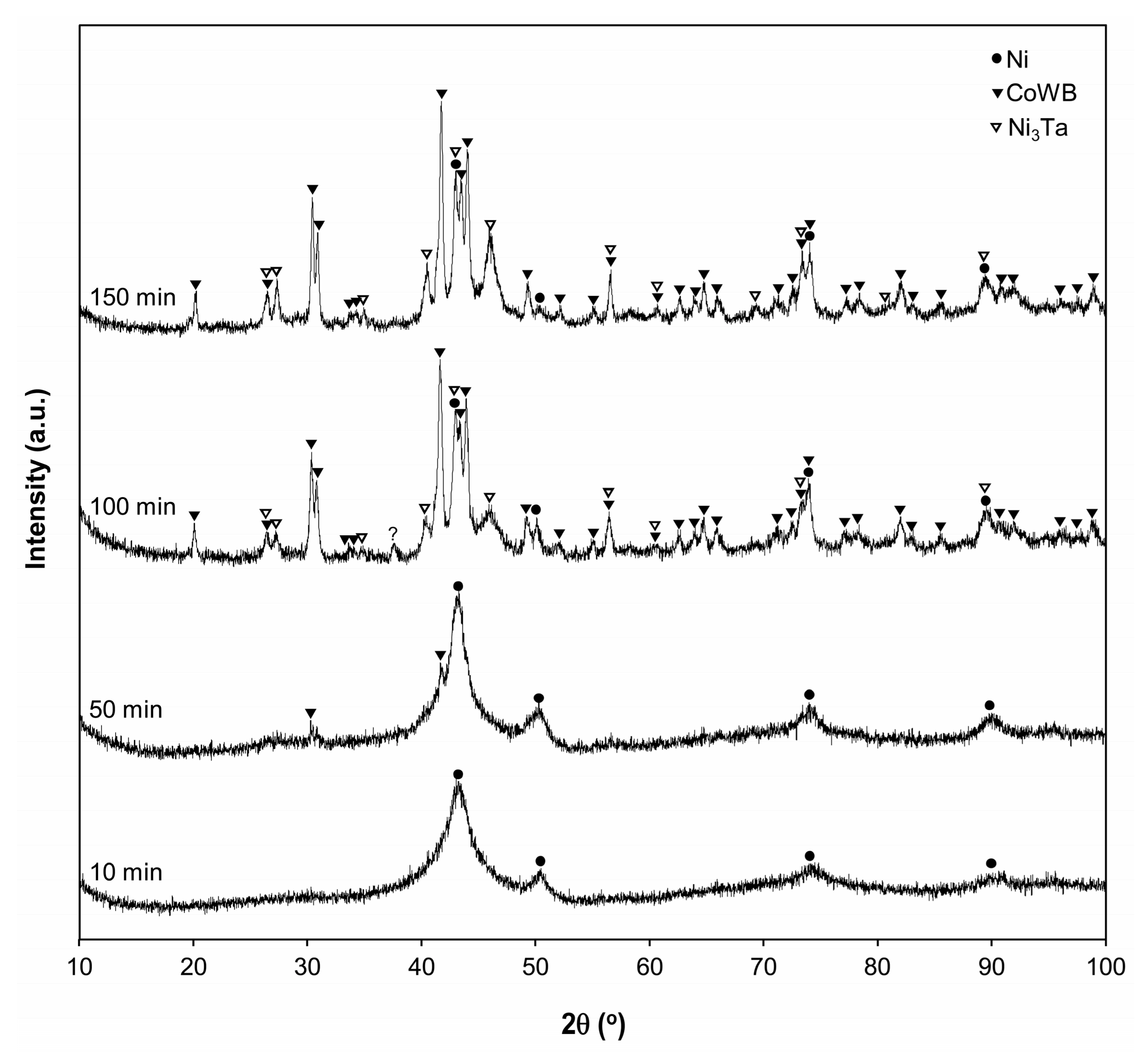

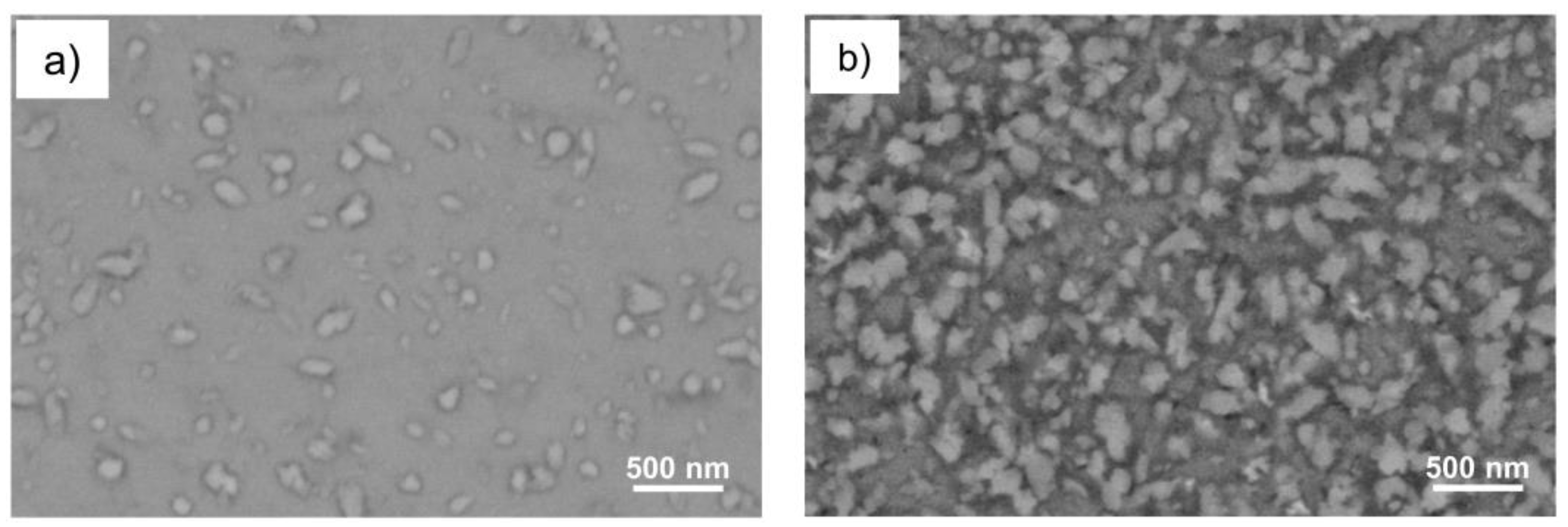
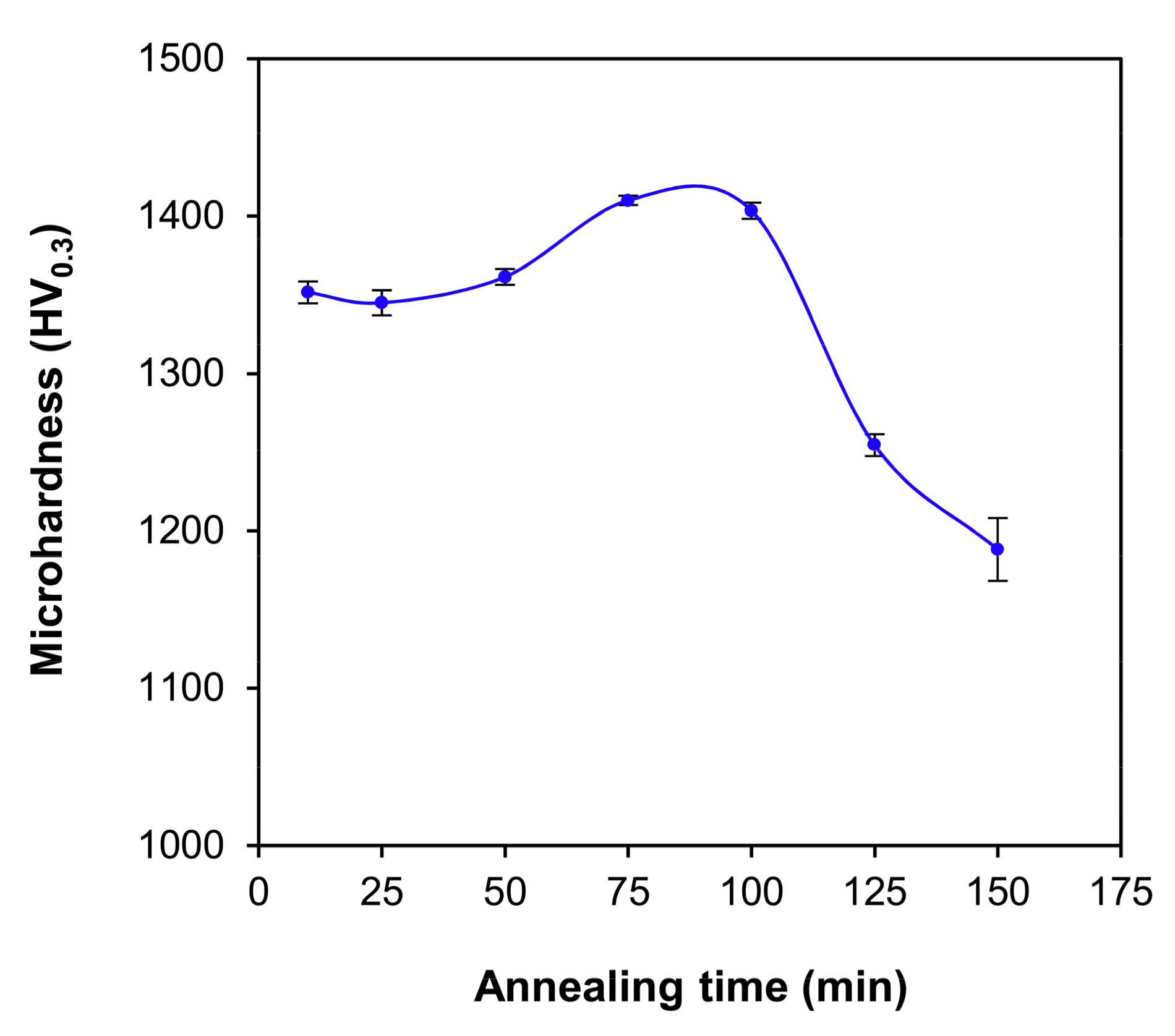
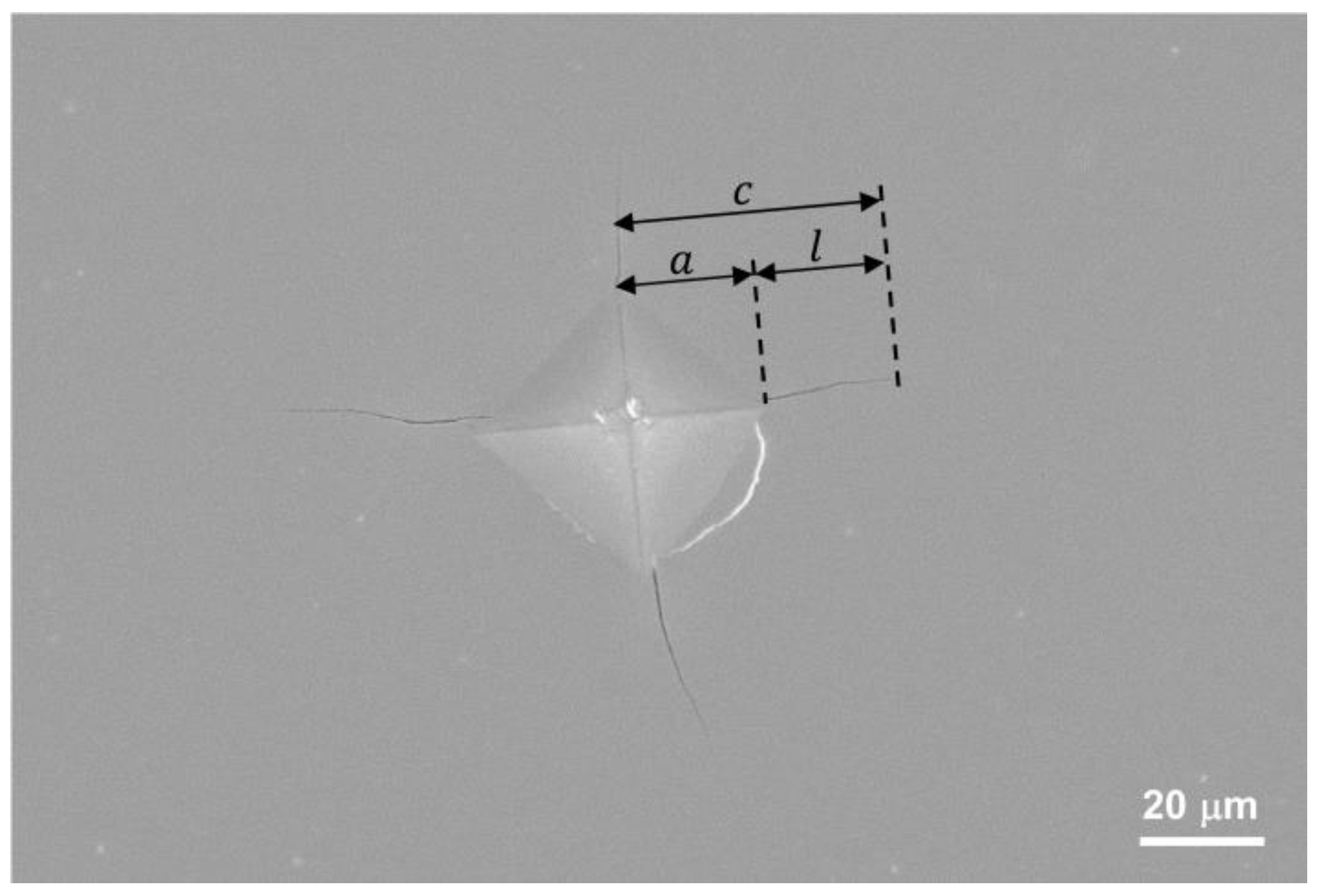
| Phase | a | b | c |
|---|---|---|---|
| (nm) | (nm) | (nm) | |
| Ni (Fm3m) | 0.3623 ± 0.0001 | - | - |
| CoWB (Pnma) | 0.5745 ± 0.0001 | 0.3244 ± 0.0001 | 0.6663 ± 0.0001 |
| Ni3Ta (Pmmn) | 0.5073 ± 0.0004 | 0.4183 ± 0.0004 | 0.4507 ± 0.0004 |
| Annealing Time | Ni-Phase | CoWB | Ni3Ta |
|---|---|---|---|
| (min) | |||
| 100 | 0.18 | 0.42 | 0.40 |
| 150 | 0.12 | 0.38 | 0.50 |
| Phase | Average Atomic Number |
|---|---|
| (Z/Å3) | |
| Ni | 2.901 |
| Ni3Ta | 2.893 |
| CoWB | 3.415 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitit, A.; Yazici, Z.O.; Şahin, H.; Öztürk, P.; Eryeşil, B.; Barut, N. Microstructure and Mechanical Properties of CoWB Based Composites Produced by Crystallization of Ni-Co-Zr-Ta-W-B Bulk Metallic Glass. Metals 2022, 12, 251. https://doi.org/10.3390/met12020251
Hitit A, Yazici ZO, Şahin H, Öztürk P, Eryeşil B, Barut N. Microstructure and Mechanical Properties of CoWB Based Composites Produced by Crystallization of Ni-Co-Zr-Ta-W-B Bulk Metallic Glass. Metals. 2022; 12(2):251. https://doi.org/10.3390/met12020251
Chicago/Turabian StyleHitit, Aytekin, Ziya Ozgur Yazici, Hakan Şahin, Pelin Öztürk, Buğrahan Eryeşil, and Nusrettin Barut. 2022. "Microstructure and Mechanical Properties of CoWB Based Composites Produced by Crystallization of Ni-Co-Zr-Ta-W-B Bulk Metallic Glass" Metals 12, no. 2: 251. https://doi.org/10.3390/met12020251






