Surface Hardening Behavior of Advanced Gear Steel C61 by a Novel Solid-Solution Carburizing Process
Abstract
:1. Introduction
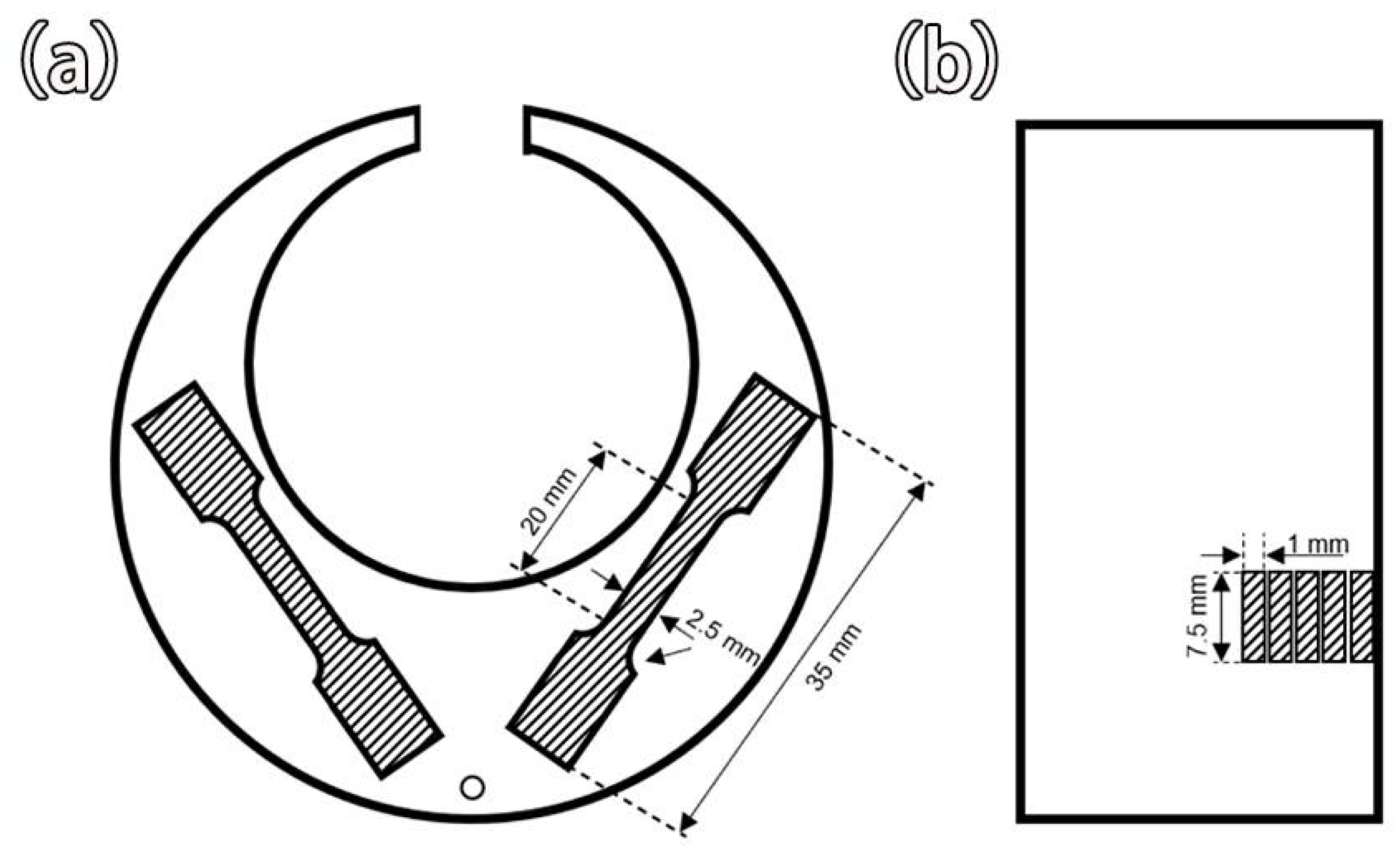
2. Materials and Methods
2.1. Experimental Materials and Methods
2.2. Performance Testing and Observation
3. Results and Discussion
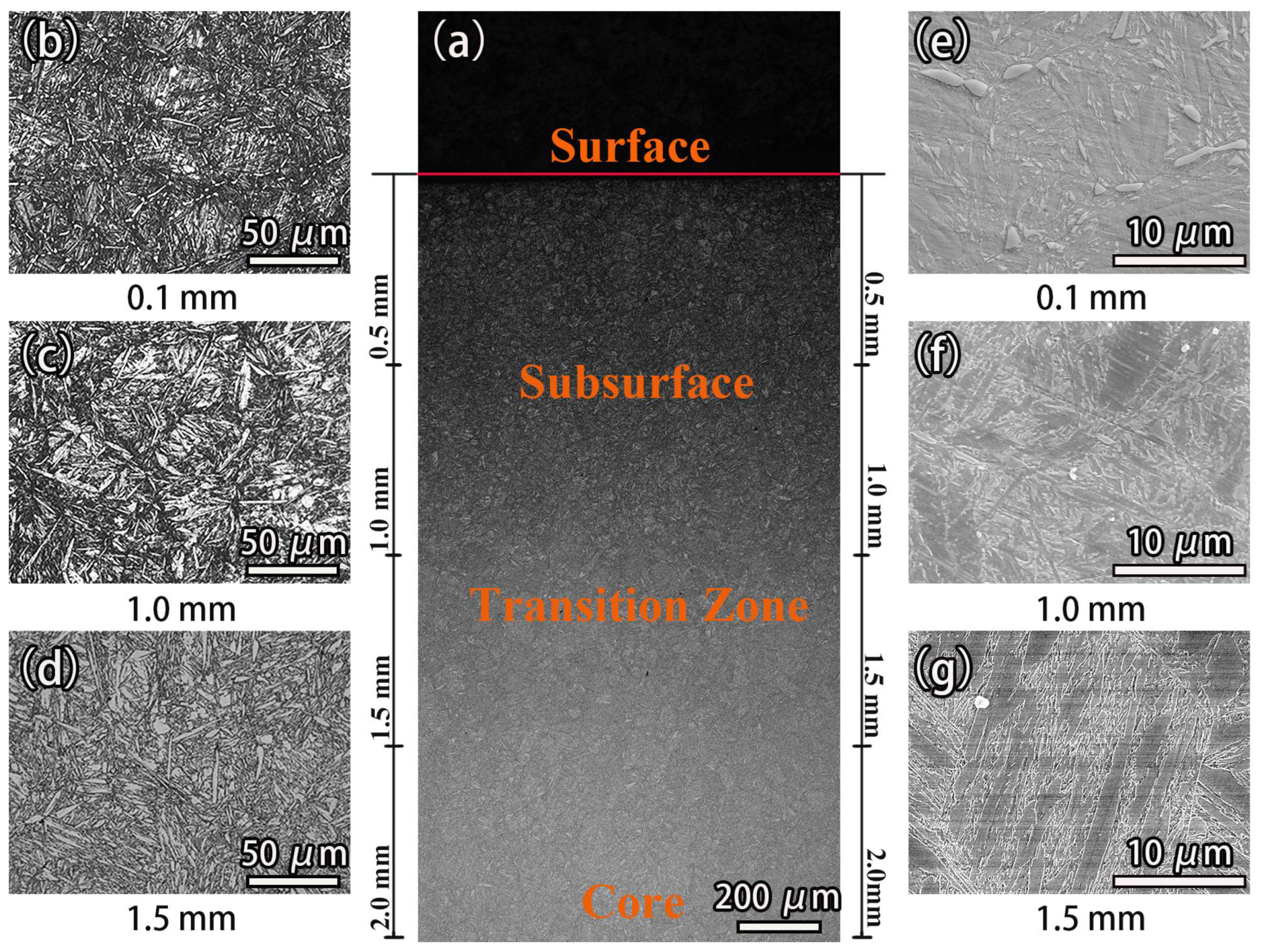
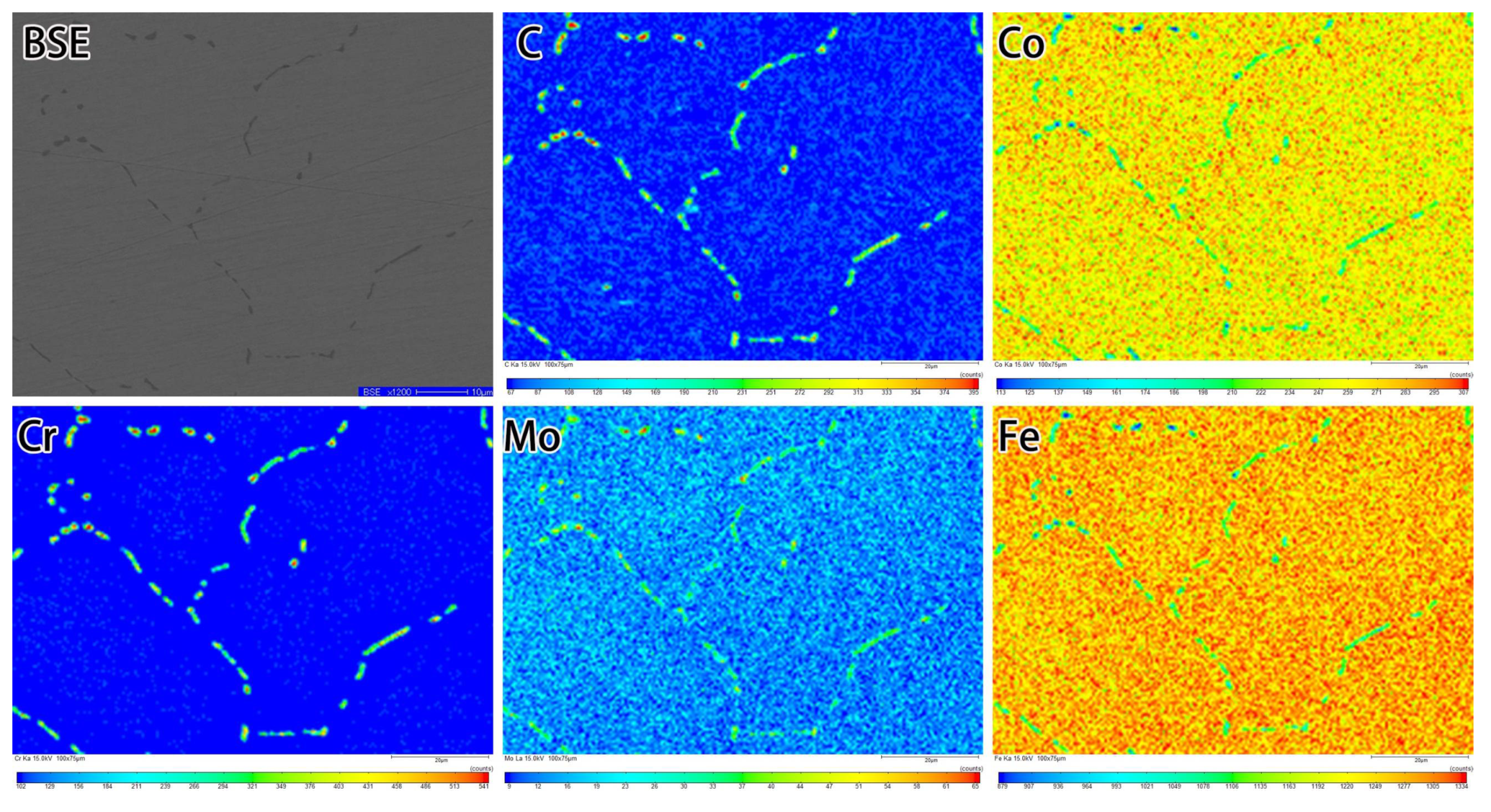
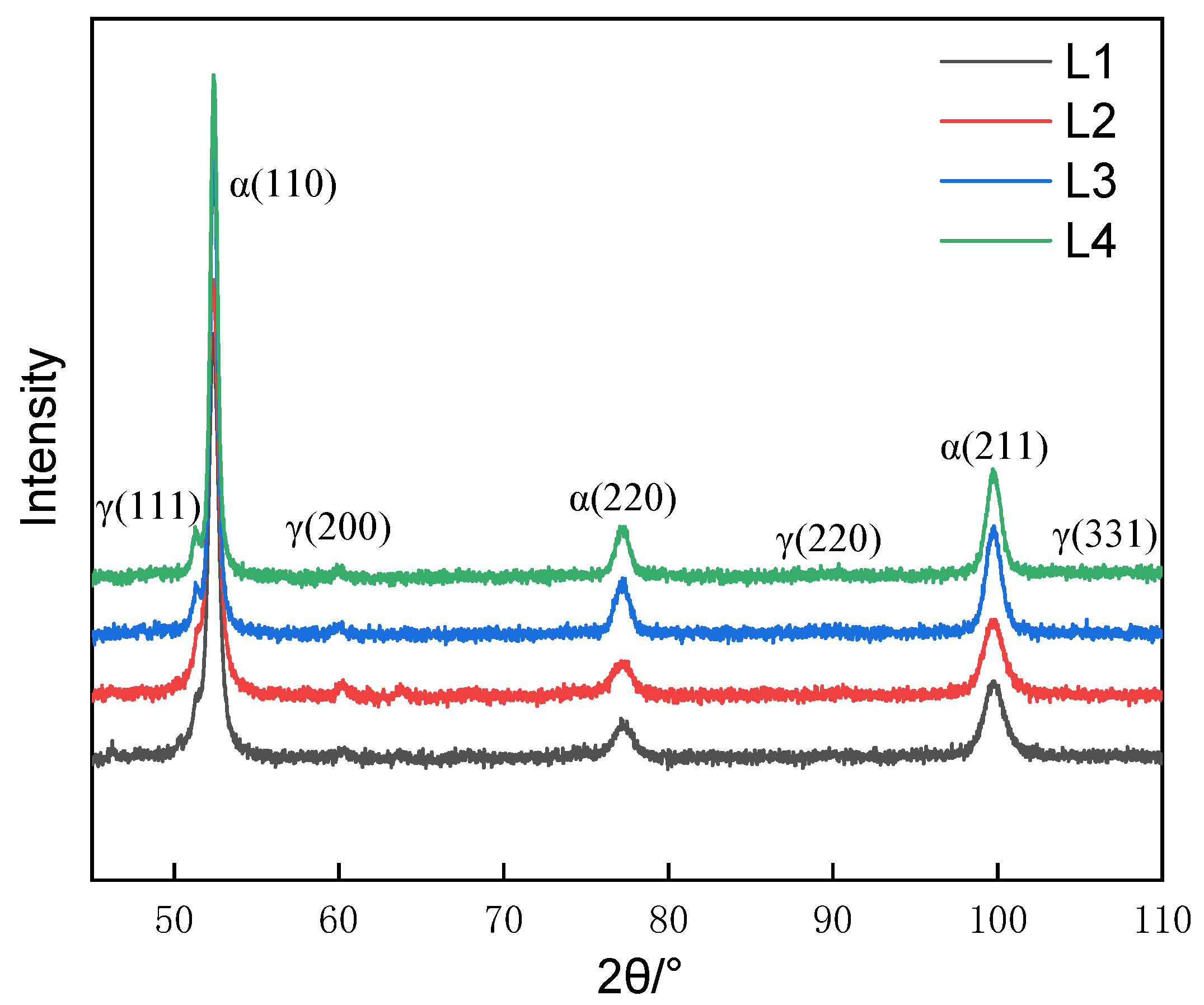
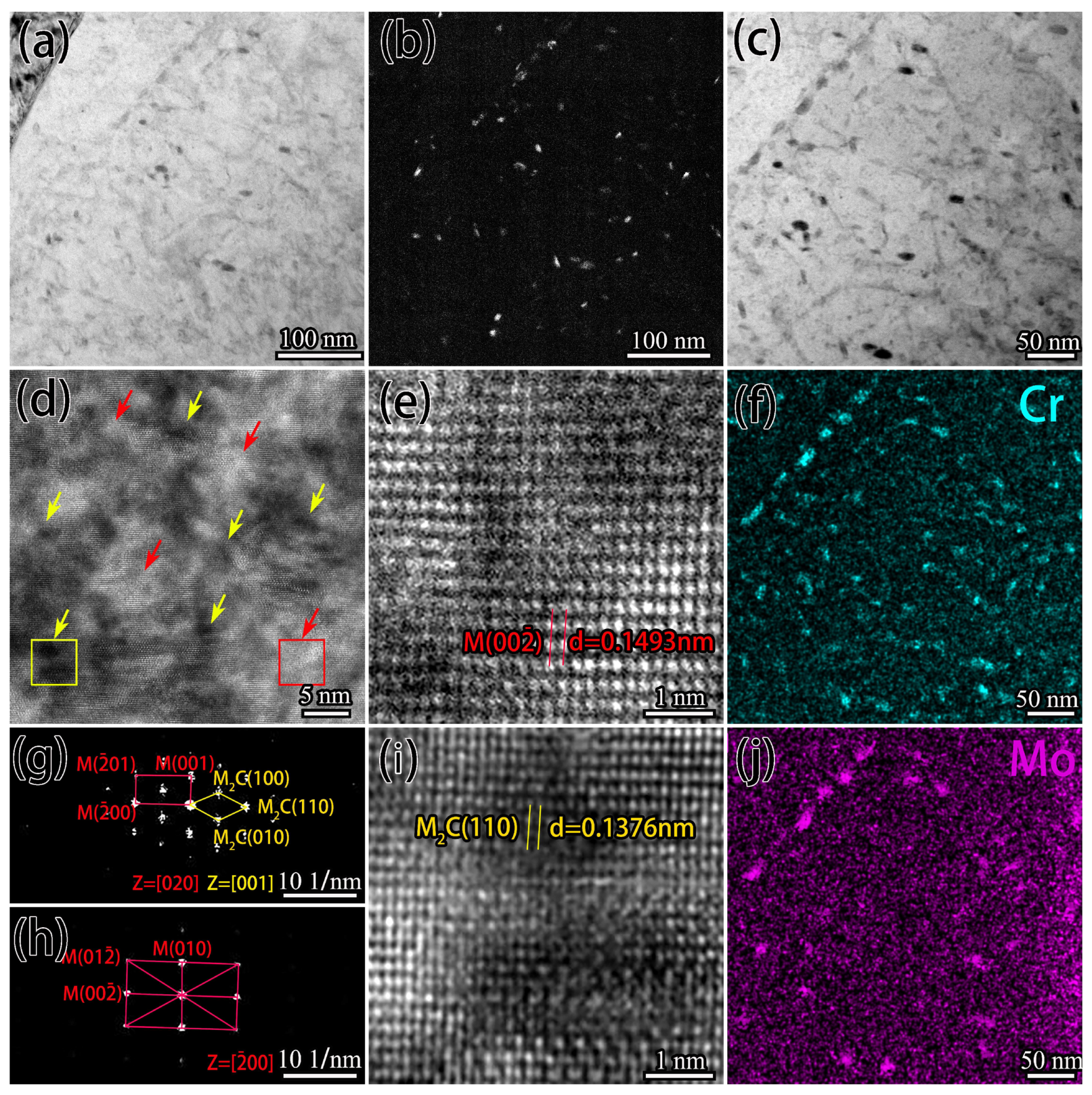
3.1. Microstructure of the Carburized Layer
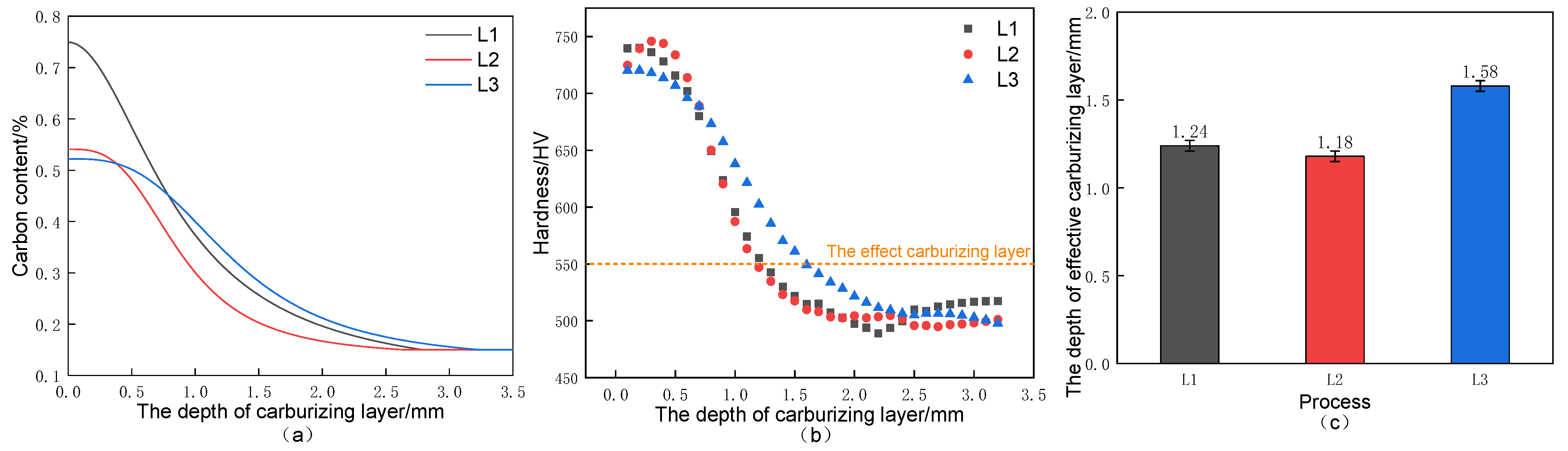
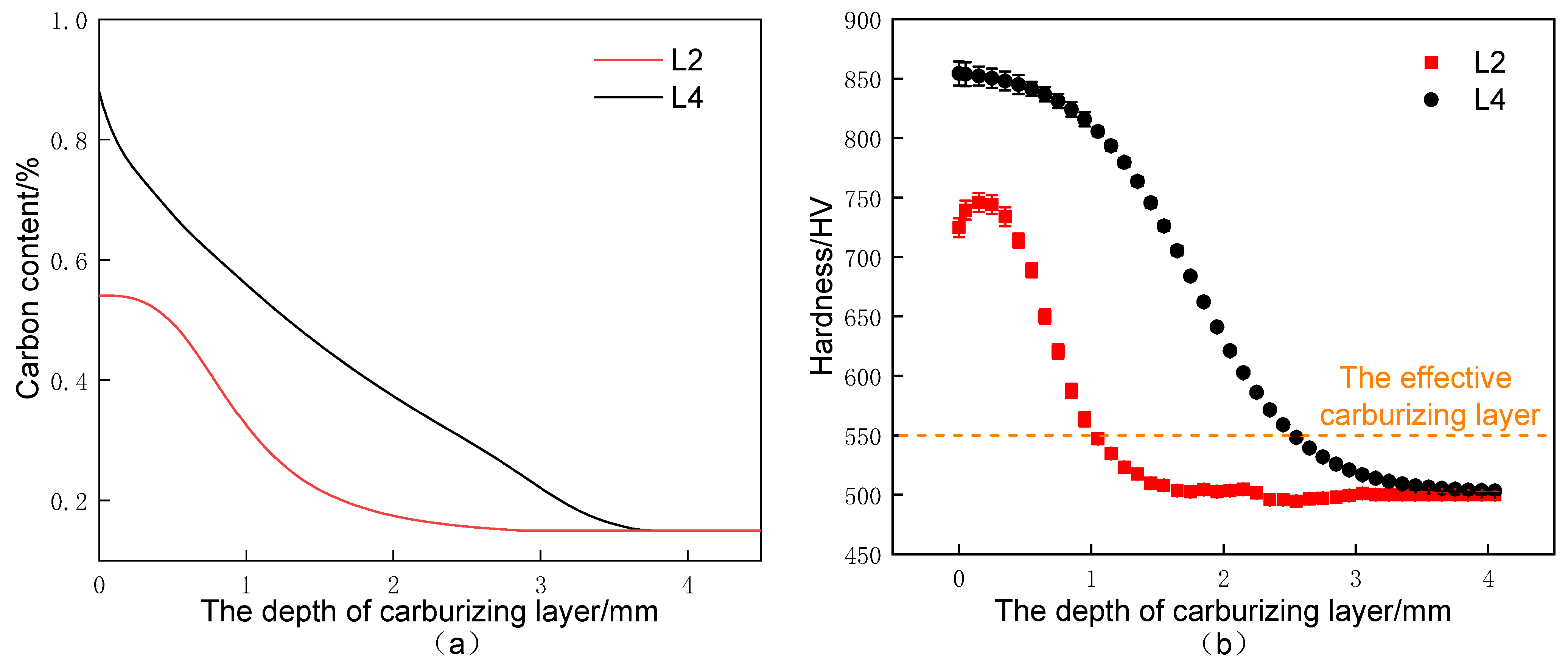
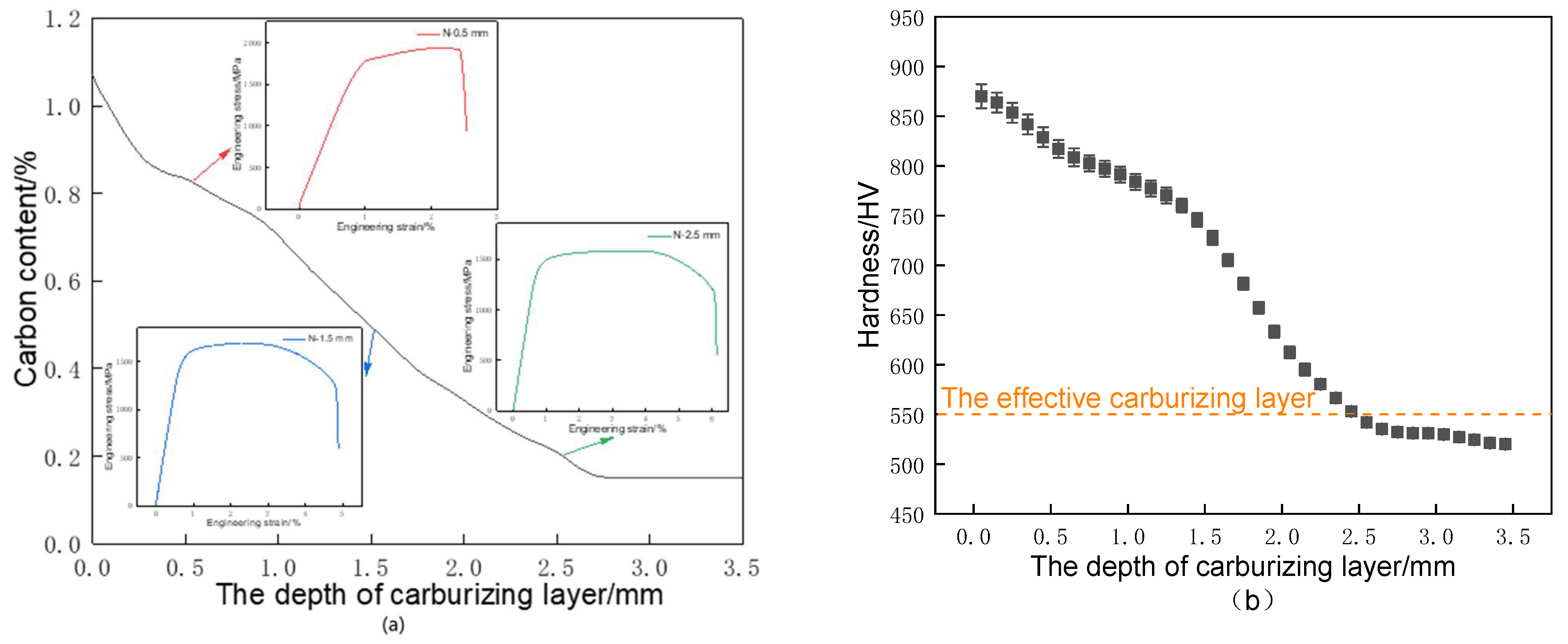
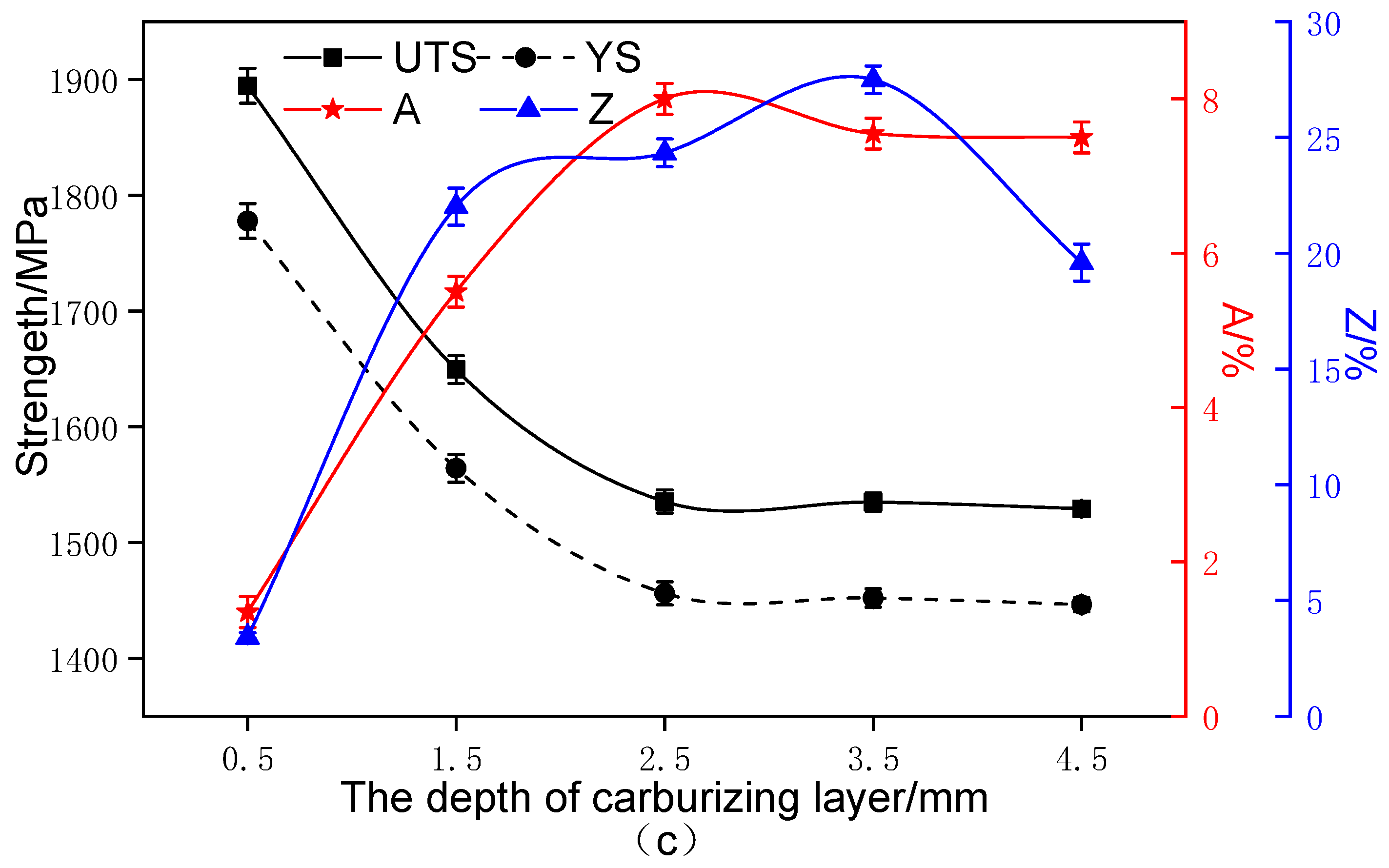
3.2. Carbon Concentration Gradient and Hardness Distribution
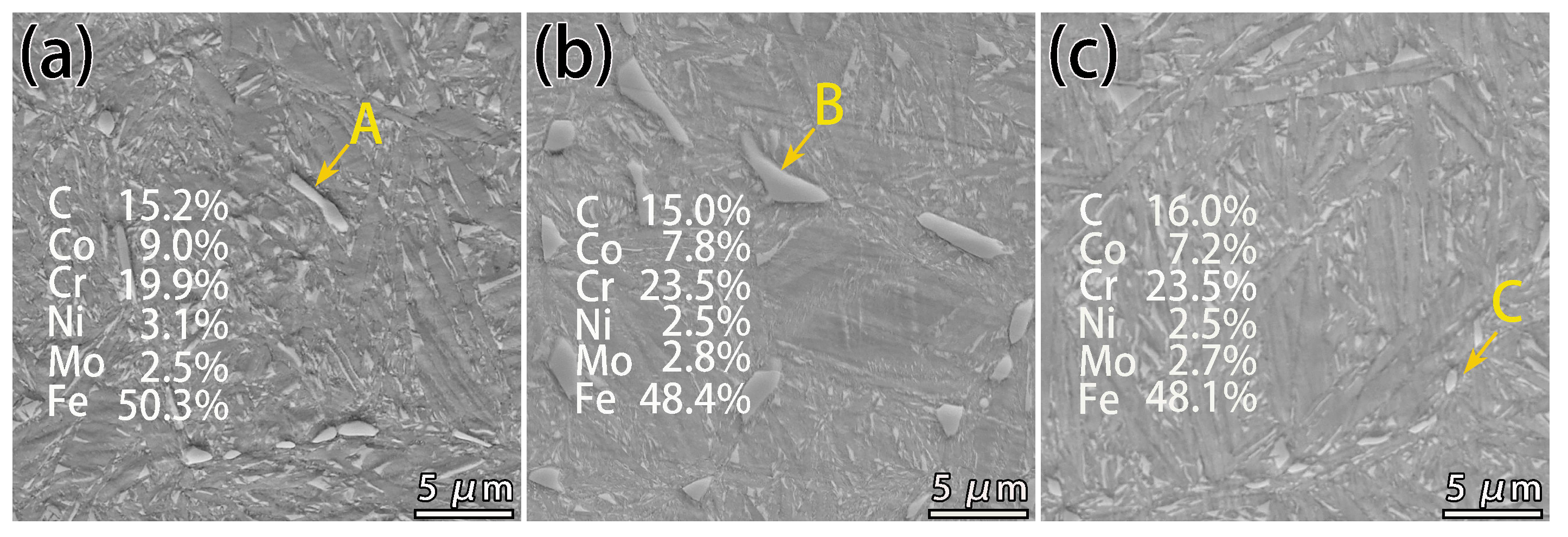
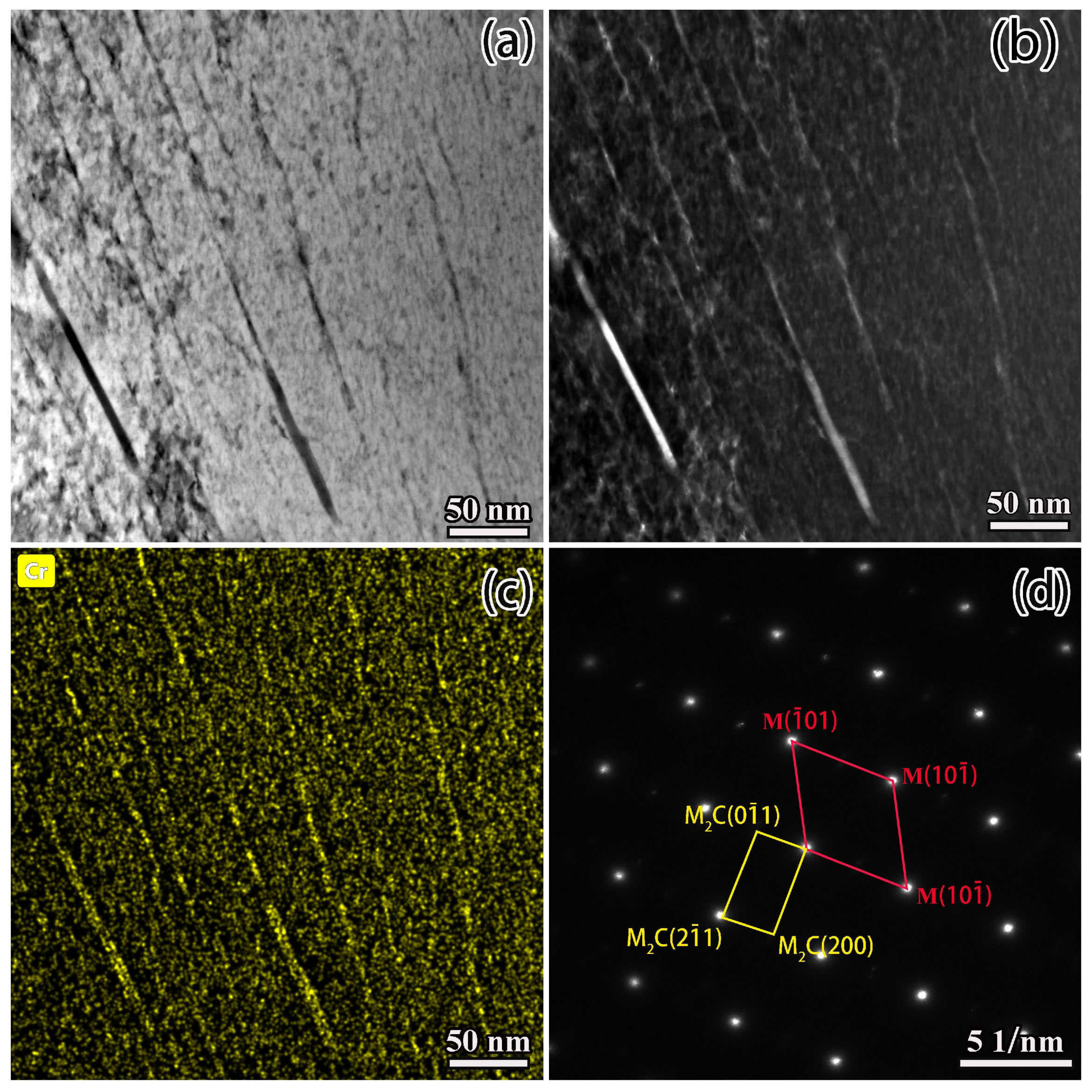
3.3. Formation of Network Carbides during the Conventional Carburizing Process
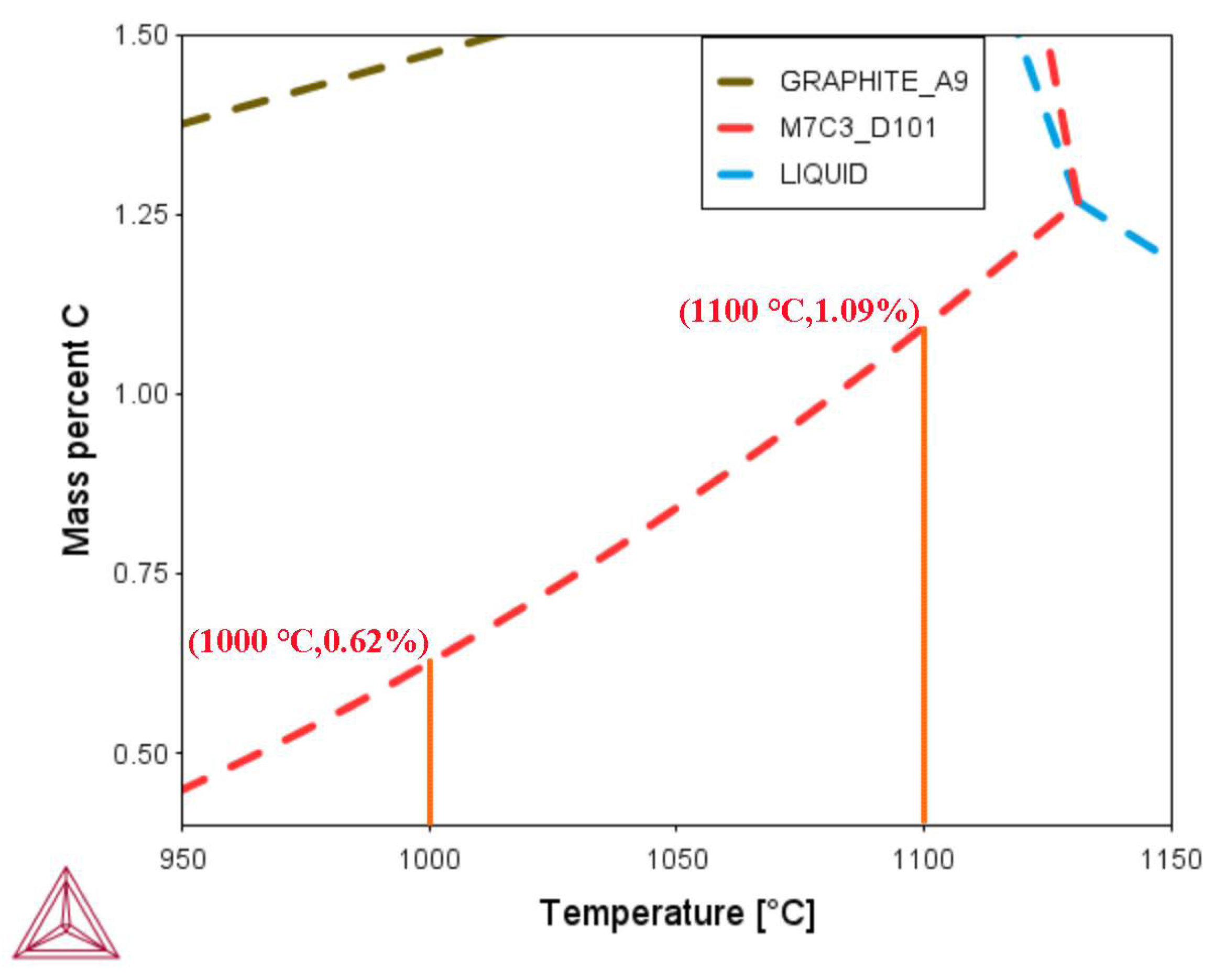
3.4. Effect of Carburizing Temperature during the Conventional Carburizing Process
3.5. Advantages of the Novel Solid-Solution Carburizing Process
4. Conclusions
- (1)
- After high-temperature vacuum carburizing heat treatment of C61 steel, the carburized surface layer was mainly high-carbon needle martensite with carbide, while the carbide gradually decreased with the depth of the carburized layer, and the core was mainly low-carbon lath-martensite. Both the carbon concentration and hardness decreased progressively with the depth of the carburized layer and then stabilized.
- (2)
- Compared with the conventional carburizing process, the carburized layer’s surface hardness and carbon concentration were significantly increased by the novel solid-solution carburizing process, reaching 875 HV and 1.07%, respectively. The tensile strength of the carburized surface layer was 1900 MPa, and the strength gradually decreased with the depth of the carburized layer, but the plastic toughness gradually increased, and the tensile strength was 1550 MPa in the core region to maintain a better match of strength and toughness.
- (3)
- During the conditions of the conventional carburizing process, there was usually a large amount of precipitation of carbide along the grain due to the long boost carburizing time or high carbon potential. This reticulated carbide was eliminated in the final diffusion process with the novel solid-solution carburizing process. However, a large number of carbon atoms improved to diffuse inside the lattice, and the carbon content in the carburized surface layer improved compared with the conventional carburizing process.
- (4)
- The rise in carburizing temperature could eliminate the reticulated carbides, but grain growth was noticeable, and the strength and toughness match was poor. On the other hand, the novel solid-solution carburizing process could effectively transform micron-carbides along the grain to nano-carbides, achieving full nanosized carbides on the surface with a size of 50–100 nm and good dispersion. Modifying the size, morphology, and distribution of M2C was found to be the main factor in improving the comprehensive mechanical properties such as microhardness and wear resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teng, B. New Material for Aeroengine—16Cr3NiWMoVNbE Gear Steel. Aeroengine 2003, 2, 34–37. [Google Scholar]
- Sebastian, J.; Llc, Q.; Krantz, T.; Center, N.; Shen, T.; Company, T.B. Advanced Gear Alloys for Ultra High Strength Applications. 2011. Available online: https://www.semanticscholar.org/paper/Advanced-Gear-Alloys-for-Ultra-High-Strength-Shen-Krantz/a92917b4ad23e2209e9e8cd04dc32ecb26bc741b (accessed on 1 January 2022).
- Morris, J.W., Jr. Making steel strong and cheap. Nat. Mater. 2017, 16, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, J.; Olson, G.; Snyder, D. QuesTek’s Integrated Computational Materials Engineering (ICME) Approach to the Design and Development of New Materials for Aerospace and Additive Manufacturing Applications. Available online: https://drc.uc.edu/bitstream/handle/2374.UC/745759/ISABE2015_CS%26A_Jason%20Sebastian%20Ph.D._227_MANUSCRIPT_20181.pdf?sequence=2 (accessed on 1 January 2022).
- Sebastian, J. New Highly-Processable, High-Strength, High-Durability, Temperature-Resistant Gear Steels. 2011. Available online: https://www.researchgate.net/publication/267900798_New_Highly-Processable_High-Strength_High-Durability_Temperature-Resistant_Gear_Steels (accessed on 1 January 2022).
- Wang, Y.; Yang, Z.; Zhang, F.; Wu, D. Microstructures and mechanical properties of surface and center of carburizing 23Cr2Ni2Si1Mo steel subjected to low-temperature austempering. Mater. Sci. Eng. A 2016, 670, 166–177. [Google Scholar] [CrossRef]
- Gorockiewicz, R.; Adamek, A.; Korecki, M. Steels for Vacuum Carburizing and Structure of the Carburizing Layer after Low Pressure Carburizing. Ind. Heat. Int. J. Therm. Technol. 2007, 1–16. [Google Scholar]
- Gorockiewicz, R. The Benefits of Using 3 Gas Mixture Low Pressure Carburizing (LPC) for High Alloy Steels. 2007. Available online: https://www.researchgate.net/profile/Maciej-Korecki/publication/268376948_The_benefits_of_using_3_Gas_Mixture_Low_Pressure_Carburizing_LPC_for_high_alloy_steels/links/56b08a3b08ae8e37214f8abf/The-benefits-of-using-3-Gas-Mixture-Low-Pressure-Carburizing-LPC-for-high-alloy-steels.pdf (accessed on 1 January 2022).
- Heintzberger, P.J. Influence of the Temperature of Vacuum Carburizing on the Thickness of the Carburized Layer and Properties of Steel Parts. Met. Sci. Heat Treat. 2020, 62, 279–284. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, Y.; Zhou, L.; Liu, B.; Hao, G. Study on the Process of Vacuum Low Pressure Carburizing and High Pressure Gas Quenching for Carburizing Steels. J. Phys. Conf. Ser. 2020, 1624, 042076. [Google Scholar] [CrossRef]
- Kula, P.; Pietrasik, R.; Dybowski, K. Vacuum carburizing—Process optimization. J. Mater. Process. Technol. 2005, 164, 876–881. [Google Scholar] [CrossRef]
- Di, C.; Yan, X.; Lv, X.; Yan, C.; Li, D. Effect of Vacuum Carburizing Time on Microstructure and Mechanical Properties of Tantalum Carbide Layer. Met. Mater. Int. 2021, 27, 5008–5016. [Google Scholar] [CrossRef]
- Asi, O.; Can, A.C.; Pineault, J.; Belassel, M. The effect of high temperature gas carburizing on bending fatigue strength of SAE 8620 steel. Mater. Des. 2009, 30, 1792–1797. [Google Scholar] [CrossRef]
- Tibbetts, G.G. Diffusivity of carbon in iron and steels at high temperatures. J. Appl. Phys. 1980, 51, 4813–4816. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Moren, A.E. Moren Diffusion modeling of the carburization process. Metall. Trans. A 1978, 9, 1515–1525. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Greben’kov, S.K.; Bogdanova, M.V. Bogdanova Optimization of the Process of Carburizing and Heat Treatment of Low-Carbon Martensitic Steels. Met. Sci. Heat Treat. 2016, 58, 116–119. [Google Scholar] [CrossRef]
- Wang, B.; He, Y.; Liu, Y.; Tian, Y.; You, J.; Wang, Z.; Wang, G. Mechanism of the Microstructural Evolution of 18Cr2Ni4WA Steel during Vacuum Low-Pressure Carburizing Heat Treatment and Its Effect on Case Hardness. Materials 2020, 13, 2352. [Google Scholar] [CrossRef]
- Trillo, E.A.; Murr, L.E. A TEM investigation of M23C6 carbide precipitation behaviour on varying grain boundary misorientations in 304 stainless steels. J. Mater. Sci. 1998, 33, 1263–1271. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, M.; Xiao, J.; Dong, J.; Du, C. Investigations on carburizing mechanisms of Cr35Ni45Nb subjected to different service conditions in a high-temperature vacuum environment. J. Mater. Res. 2015, 30, 841–851. [Google Scholar] [CrossRef]
- Huo, X.; Ning, Y.; LI, L.; Lv, Z.; Chen, S. Research and control of network carbide in GCr15 bearing steel. Mater. Res. Express 2019, 7, 016559. [Google Scholar] [CrossRef]
- An, X.; Tian, Y.; Wang, B.; Jia, T.; Wang, Z. Prediction of the formation of carbide network on Grain boundaries in Carburizing of 18CrNiMo7-6 steel alloys. Surf. Coat. Technol. 2021, 421, 127348. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, H.; Zhu, J.H.; Xu, B.Q.; Wu, J.L. Uniformly Dispersed Carbide Reinforcements in the Medium-Entropy High-Speed Steel Coatings by Wide-Band Laser Cladding. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 1145–1150. [Google Scholar] [CrossRef]
- Zheng, C.; Lv, B.; Zhang, F.; Yang, Z.; Kang, J.; She, L.; Wang, T. A novel microstructure of carbide-free bainitic medium carbon steel observed during rolling contact fatigue. Scr. Mater. 2016, 114, 13–16. [Google Scholar] [CrossRef]
- Zheng, C.; Rui, D.; Zhang, F.; Bo, L.; Yan, Z.; Shan, J.; Long, X. Effects of retained austenite and hydrogen on the rolling contact fatigue behaviours of carbide-free bainitic steel. Mater. Sci. Eng. A 2014, 594, 364–371. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Yang, Z.; Bo, L.; Zheng, C. Rolling Contact Fatigue Performances of Carburized and High-C Nanostructured Bainitic Steels. Materials 2016, 9, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zheng, F.; Zhou, J. Effect of the Morphology of Carbides in the Carburizing Case on the Wear Resistance and the Contact Fatigue of Gears. J. Mater. Eng. China 1989, 6, 35–40. [Google Scholar]
- Wang, H.; Liu, J.; Tian, Y.; Wang, Z.; An, X. Mathematical Modeling of Carbon Flux Parameters for Low-Pressure Vacuum Carburizing with Medium-High Alloy Steel. Coatings 2020, 10, 1075. [Google Scholar] [CrossRef]
- Gregory, N.W. Elements of X-Ray Diffraction. Contemp. Phys. 1957, 20, 87–88. [Google Scholar] [CrossRef]
- Ishida, K. Calculation of the effect of alloying elements on the M s temperature in steels. J. Alloys Compd. 1995, 220, 126–131. [Google Scholar] [CrossRef]
- Su, S.; Wang, L.; Chen, C.; Wang, Y.; Li, J. Gradient microstructure evolution and hardening mechanism of carburized steel under novel heat treatment. Mater. Lett. 2020, 280, 128486. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, D.; Qi, X.; Jiang, Z. Effect of tempering temperature on the microstructure and properties of ultrahigh-strength stainless steel. J. Mater. Sci. Technol. 2019, 35, 1240–1249. [Google Scholar] [CrossRef]





| Element | C | Cr | Ni | Co | Mo | V | Fe |
|---|---|---|---|---|---|---|---|
| Content | 0.15 | 3.5 | 9.5 | 18 | 1.1 | 0.08 | bal |
| Process | Carburizing (Divided into Three-Stage) | Quenching | Sub-Zero Cooling | Tempering | ||
|---|---|---|---|---|---|---|
| Temperature | Boosting (C = 1.3%) | Diffusing (C = 0.9%) | ||||
| L1 | 1000 °C | 30 min | 210 min | OQ | −196 °C × 1 h | 482 °C × 16 h |
| L2 | 1000 °C | 60 min | 180 min | |||
| L3 | 1000 °C | 60 min | 420 min | |||
| L4 | 1100 °C | 60 min | 180 min | |||
| N | ①1050 °C | 60 min | 180 min | |||
| ②1100 °C | ||||||
| Process | Carbon Content | Ms Point | Martensite | Austenite | M7C3 Carbide | Surface Hardness |
|---|---|---|---|---|---|---|
| L1 | 0.75% | 245.05 °C | 93.98% | 4.65% | 1.37% | 740 HV |
| L2 | 0.54% | 314.35 °C | 90.69% | 4.58% | 4.73% | 725 HV |
| L3 | 0.52% | 320.95 °C | 98.23% | 4.31% | 1.71% | 720 HV |
| L4 | 0.88% | 202.15 °C | 98.92% | 1.08% | - | 854 HV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Kang, L.; Han, S.; Li, Y.; Liu, Y.; Lei, S.; Wang, C. Surface Hardening Behavior of Advanced Gear Steel C61 by a Novel Solid-Solution Carburizing Process. Metals 2022, 12, 379. https://doi.org/10.3390/met12030379
Dai Y, Kang L, Han S, Li Y, Liu Y, Lei S, Wang C. Surface Hardening Behavior of Advanced Gear Steel C61 by a Novel Solid-Solution Carburizing Process. Metals. 2022; 12(3):379. https://doi.org/10.3390/met12030379
Chicago/Turabian StyleDai, Yanzhang, Lixia Kang, Shun Han, Yong Li, Yu Liu, Simin Lei, and Chunxu Wang. 2022. "Surface Hardening Behavior of Advanced Gear Steel C61 by a Novel Solid-Solution Carburizing Process" Metals 12, no. 3: 379. https://doi.org/10.3390/met12030379
APA StyleDai, Y., Kang, L., Han, S., Li, Y., Liu, Y., Lei, S., & Wang, C. (2022). Surface Hardening Behavior of Advanced Gear Steel C61 by a Novel Solid-Solution Carburizing Process. Metals, 12(3), 379. https://doi.org/10.3390/met12030379






