Influence of Surface Pretreatments on the Anticorrosion of Polypyrrole Electro-Polymerized Coatings for Copper in Artificial Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Solutions
2.2. Electrosynthesis of Different PPy Coatings
2.3. Characterization of PPy Coatings
2.4. Anticorrosive Evaluation for PPy Coatings
2.5. Theoretical Study
3. Results and Discussion
3.1. Preparation and Characterization of PPy Coatings
3.1.1. Preparation
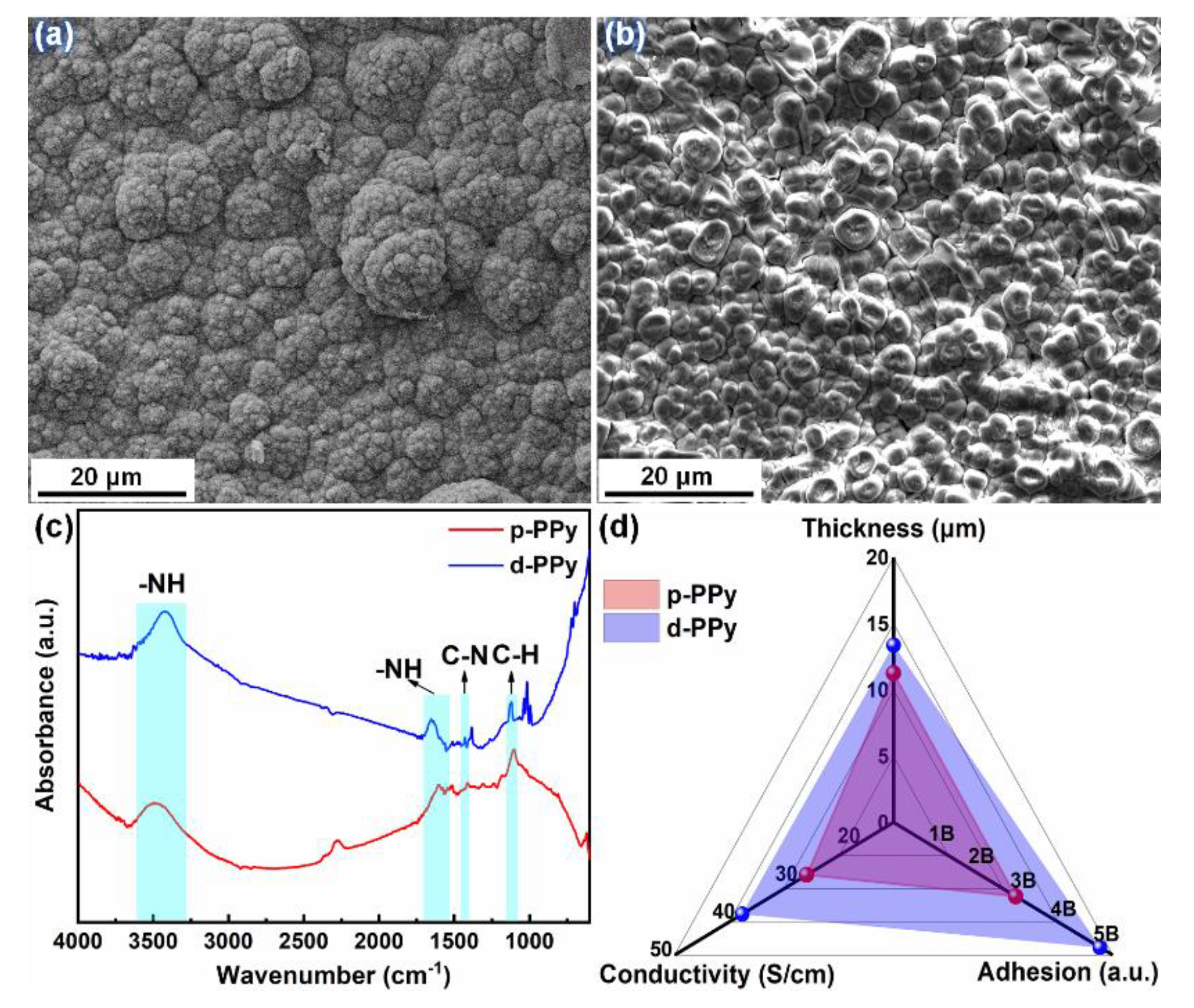
3.1.2. Characterization
3.2. Anticorrosive Evaluations of Different PPy Coatings
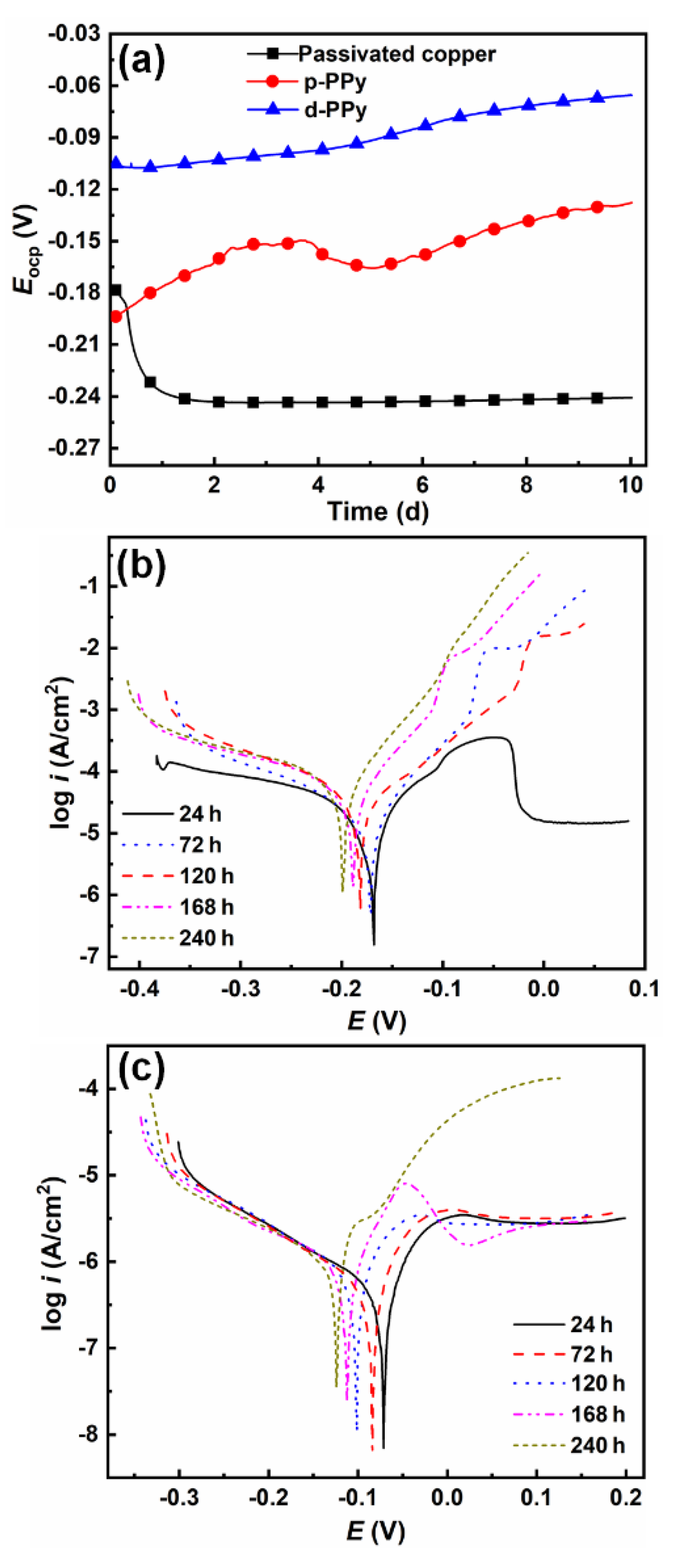
3.2.1. Electrochemical Thermodynamics and Kinetics
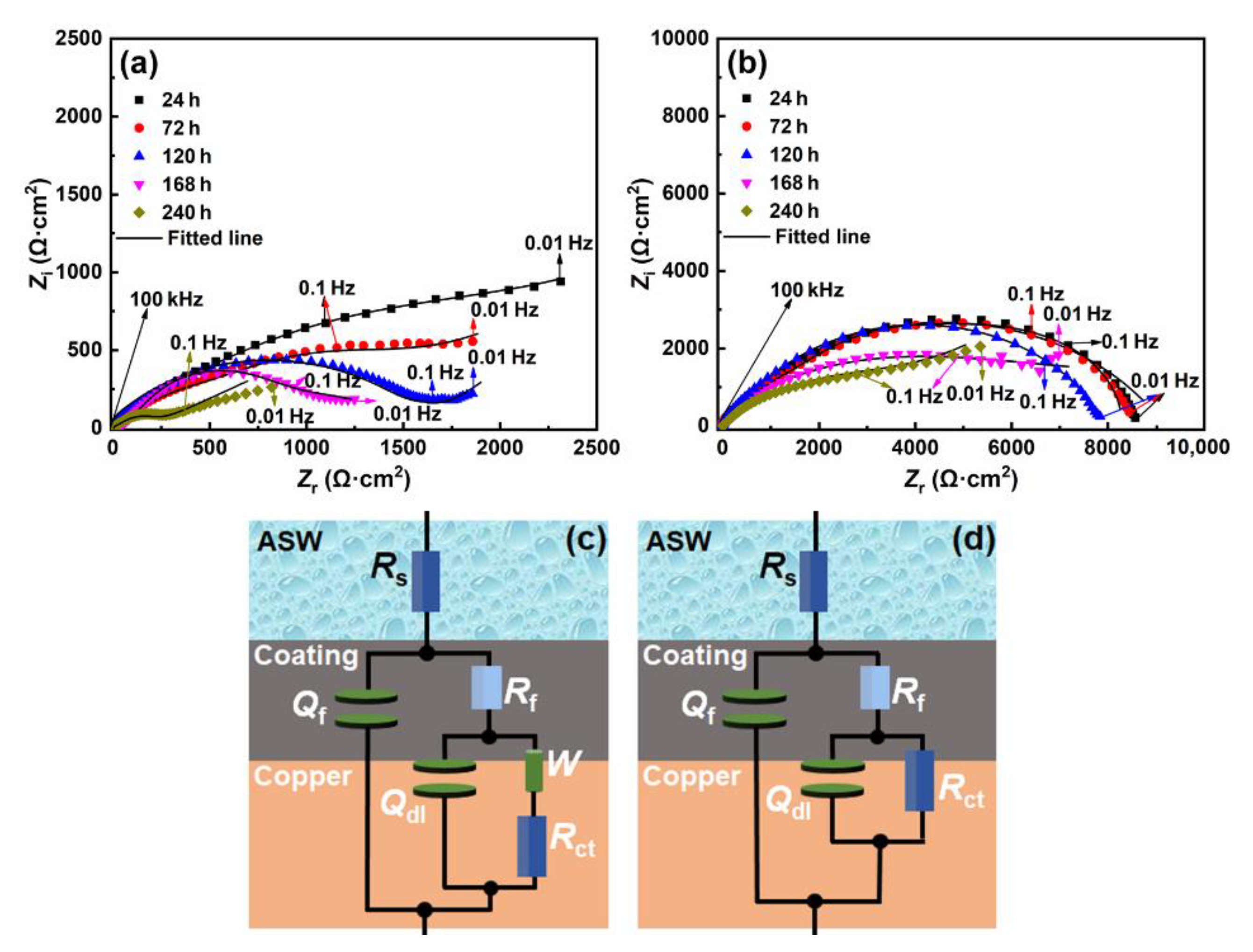
3.2.2. EIS
3.3. Surface and Interface Characterizations
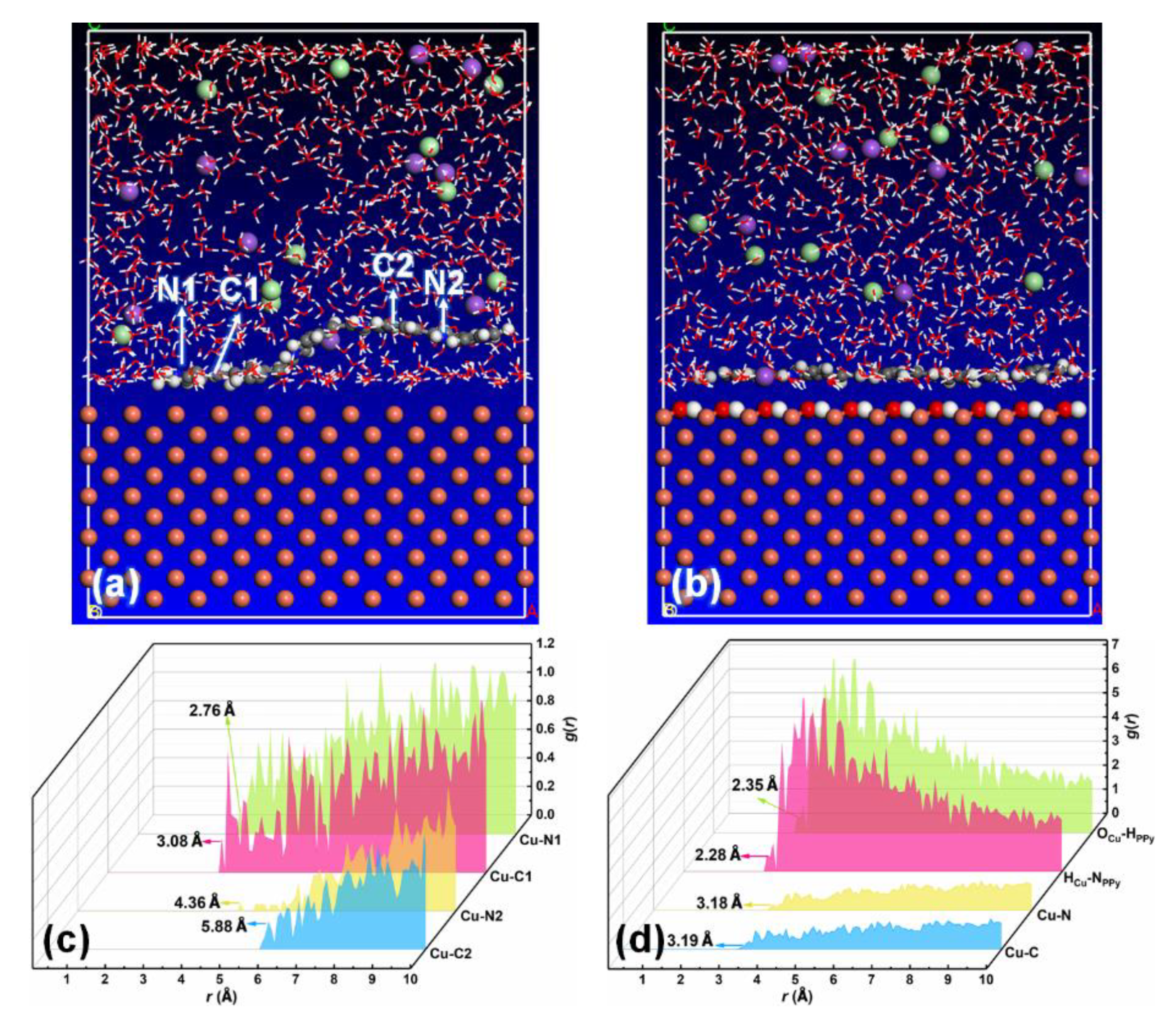
3.4. Molecular Dynamics Simulation
4. Conclusions
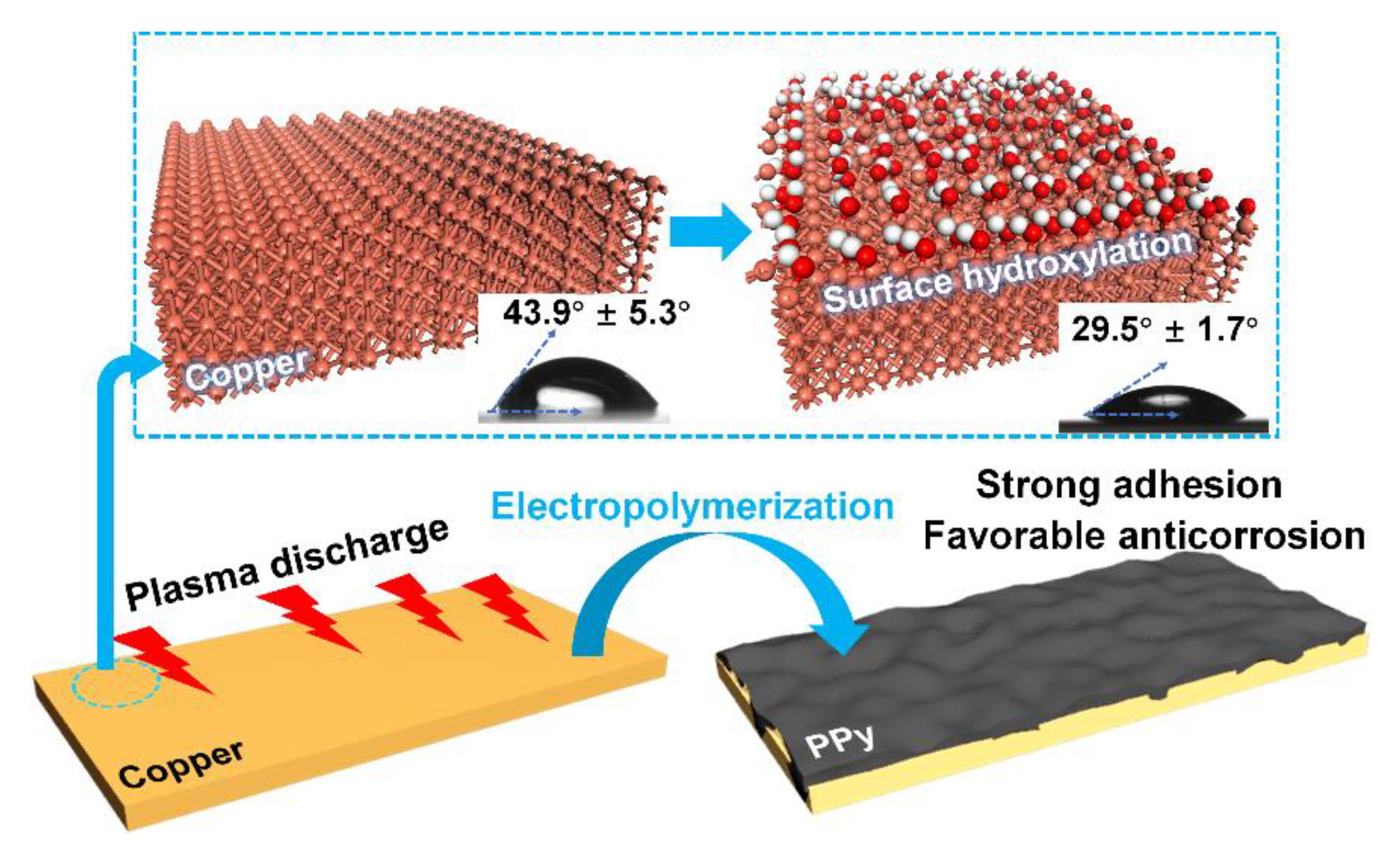
- The hydrophilic copper surface could be established after the surface treatment of plasma discharging (hydroxylation); and the electro-polymerized PPy deposited on the hydroxylated surface (i.e., d-PPy) faster than that formed over the passivated counterpart. Due to the preferential deposition, d-PPy possessed tight coating architecture with a thickness of 13.37 μm, an adhesive grade of 5B and a conductivity of 38.73 S/cm.
- D-Ppy coating effectively isolated the substrate from corrosive species in ASW, and maintained the passivated state for underlying copper. After 240 h of immersion in ASW, icorr merely increased to 3.09 μA/cm2 for the d-PPy coated sample, which also maintained the relatively high Rct value of 4.38 kΩ·cm2. Compact coating architecture was still observed for the d-PPy protected sample.
- The bonding tendency of N and H on the PPy backbone toward the hydroxylation layer on the copper surface might be responsible for the strong adhesion and the subsequent anticorrosion efficacy for copper substrate. Overall, this work may supply a reliable method for the targeted preparation of robust electro-polymerized coatings with favorable interfacial adhesion and anticorrosion properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.G.; Zhang, D.W.; Liu, Z.Y.; Li, Z.; Du, C.W.; Dong, C.F. Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.X.; Xiang, L.; Tao, J.Q.; Liu, J.; Chen, Q.; Zhong, Y. Effects of actual marine atmospheric pre-corrosion and pre-fatigue on the fatigue property of 7085 aluminum alloy. Metals 2022, 12, 81. [Google Scholar] [CrossRef]
- Fan, B.M.; Liu, Z.N.; Zhao, X.Q.; Liu, H.; Fan, G.H.; Hao, H. Fabrication, characterization and efficient surface protection mechanism of poly(trans-cinnamaldehyde) electropolymerized coatings for EH36 steel in simulated seawater. Colloid Surf. A 2021, 629, 127434. [Google Scholar] [CrossRef]
- Li, D.J.; Zhao, X.Q.; Liu, Z.N.; Liu, H.; Fan, B.M.; Yang, B.; Zheng, X.W.; Li, W.Z.; Zou, H.J. Synergetic anticorrosion mechanism of main constituents in Chinese Yam peel for copper in artificial seawater. ACS Omega 2021, 6, 29965–29981. [Google Scholar] [CrossRef] [PubMed]
- Khademian, E.; Salehi, E.; Sanaeepur, H.; Galiano, F.; Figoli, A. A Systematic Review on Carbohydrate biopolymers for adsorptive remediation of copper ions from aqueous environments-part A: Classification and modification strategies. Sci. Total Environ. 2020, 738, 139829. [Google Scholar] [CrossRef]
- Fan, B.M.; Wei, G.; Hao, H.; Guo, A.; Li, J. Preparation of a ceramic membrane from prevalent natural clay for the purification of phosphate wastewater. Desalin. Water Treat. 2016, 57, 17308–17321. [Google Scholar] [CrossRef]
- Garcia-Cabezon, C.; Garcia-Hernandez, C.; Rodriguez-Mendez, M.L.; Martin-Pedrosa, F. A new strategy for corrosion protection of porous stainless steel using polypyrrole films. J. Mater. Sci. Technol. 2020, 37, 85–95. [Google Scholar] [CrossRef]
- Saady, A.; Rais, Z.; Benhiba, F.; Salim, R.; Ismaily Alaoui, K.; Arrousse, N.; Elhajjaji, F.; Taleb, M.; Jarmoni, K.; Kandri Rodi, Y.; et al. Chemical, electrochemical, quantum, and surface analysis evaluation on the inhibition performance of novel imidazo[4,5-b] pyridine derivatives against mild steel corrosion. Corros. Sci. 2021, 189, 109621. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Chen, Z.H.; Villani, E.; Inagi, S. Recent progress in bipolar electropolymerization methods toward one-dimensional conducting polymer structures. Curr. Opin. Electrochem. 2021, 28, 100702. [Google Scholar] [CrossRef]
- Zadeh, M.K.; Yeganeh, M.; Shoushtari, M.T.; Esmaeilkhanian, A. Corrosion performance of polypyrrole-coated metals: A review of perspectives and recent advances. Synth. Metals 2021, 274, 116723. [Google Scholar] [CrossRef]
- Menkuer, M.; Ozkazanc, H. Anticorrosive Polypyrrole/zirconium-oxide composite film prepared in oxalic acid and dodecylbenzene sulfonic acid mix electrolyte. Prog. Org. Coat. 2020, 147, 105815. [Google Scholar] [CrossRef]
- Nascimento, C.B.; dos Santos, S.L.; Venancio, E.C.; Antunes, R.A. Exploring the relationship between the surface chemistry and the corrosion behavior of electropolymerized polypyrrole films deposited on the surgical ISO 5832-1 stainless steel. Surf. Interface Anal. 2020, 52, 635–644. [Google Scholar] [CrossRef]
- Chen, Z.H.; Yang, W.Z.; Xu, B.; Chen, Y.; Qian, M.Q.; Su, X.; Li, Z.H.; Yin, X.S.; Liu, Y. Corrosion protection of carbon steels by electrochemically synthesized V-TiO2/polypyrrole composite coatings in 0.1 M HCl solution. J. Alloys Compd. 2019, 771, 857–868. [Google Scholar] [CrossRef]
- Ma, Y.C.; Fan, B.M.; Liu, H.; Fan, G.F.; Hao, H.; Yang, B. Enhanced corrosion inhibition of aniline derivatives electropolymerized coatings on copper: Preparation, characterization and mechanism modeling. Appl. Surf. Sci. 2020, 514, 146086. [Google Scholar] [CrossRef]
- Tharchanaa, S.B.; Priyanka, K.; Preethi, K.; Shanmugavelayutham, G. Facile synthesis of Cu and CuO nanoparticles from copper scrap using plasma arc discharge method and evaluation of antibacterial activity. Mater. Technol. 2021, 36, 97–104. [Google Scholar] [CrossRef]
- Wang, M.M.; Fan, B.M.; Wen, B.Y.; Jiang, C. Experimental and theoretical studies on the removal mechanism of formaldehyde from water by mesoporous calcium silicate. Sci. China Technol. Sci. 2020, 63, 2098–2112. [Google Scholar] [CrossRef]
- Ma, Y.C.; Zhou, T.T.; Zhu, W.Q.; Fan, B.M.; Liu, H.; Fan, G.F.; Hao, H.; Sun, H.; Yang, B. Understanding the anticorrosive mechanism of a cross-linked supramolecular polymer for mild steel in the condensate water: Comprehensive experimental, molecular docking, and molecular dynamics investigations. J. Mol. Model. 2020, 26, 81. [Google Scholar] [CrossRef]
- Liu, H.; Fan, B.M.; Fan, G.F.; Zhao, X.Q.; Liu, Z.N.; Hao, H.; Yang, B. Long-term protective mechanism of poly(N-methylaniline)/phosphate one-step electropolymerized coatings for copper in 3.5% NaCl solution. J. Alloys Compd. 2021, 872, 159752. [Google Scholar] [CrossRef]
- Herlem, G.; Picaud, F. Breaking the controversy of the electropolymerization of pyrrole mechanisms by the effective screening medium quantum charged model interface. J. Phys. Chem. A 2021, 125, 1860–1869. [Google Scholar] [CrossRef]
- Bonechi, M.; Innocenti, M.; Vanossi, D.; Fontanesi, C. The fundamental and underrated role of the base electrolyte in the polymerization mechanism. The resorcinol case study. J. Phys. Chem. A 2021, 125, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fan, B.M.; Fan, G.F.; Ma, Y.C.; Hao, H.; Zhang, W. Anti-corrosive mechanism of poly (N-ethylaniline)/sodium silicate electrochemical composites for copper: Correlated experimental and in-silico studies. J. Mater. Sci. Technol. 2021, 72, 202–216. [Google Scholar] [CrossRef]
- Golgovici, F.; Cârlan, M.S.; Popescu, A.G.; Anicai, L. Electrochemical Synthesis of Polypyrrole and Polypyrrole-Indomethacin Coatings on Nicr Alloys Involving Deep Eutectic Solvents. Metals 2020, 10, 1130. [Google Scholar] [CrossRef]
- Jothi, V.; Adesina, A.Y.; Madhan Kumar, A.; Rahman, M.M.; Nirmal Ram, J.S. Enhancing the biodegradability and surface protective performance of AZ31 mg alloy using polypyrrole/gelatin composite coatings with anodized Mg surface. Surf. Coat. Technol. 2020, 381, 125139. [Google Scholar] [CrossRef]
- Garcia-Cabezón, C.; Rodriguez-Mendez, M.L.; Borrás, V.A.; Raquel, B.; Cabello, J.C.R.; Fonseca, A.I.; Martin-Pedrosa, F. Application of plasma electrolytic oxidation coating on powder metallurgy Ti-6Al-4V for dental implants. Metals 2020, 10, 1167. [Google Scholar] [CrossRef]
- Ma, Y.C.; Fan, B.M.; Hao, H.; Lu, J.Y.; Feng, Y.H.; Yang, B. Experimental and theoretical studies of action mechanism of an octadecylamine-based molecular assembly on mild steel. Chem. J. Chin. Univ. 2019, 40, 96–107. [Google Scholar]
- Fan, B.M.; Ma, Y.C.; Wang, M.M.; Hao, H.; Yang, B.; Lv, J.Y.; Sun, H. Revealing the assembly mechanism of an octadecylamine based supramolecular complex on mild steel in condensate water: Correlative experimental and theoretical studies. J. Mol. Liq. 2019, 292, 111446. [Google Scholar] [CrossRef]
- Fan, G.F.; Liu, H.; Fan, B.M.; Ma, Y.C.; Hao, H.; Yang, B. Trazodone as an efficient corrosion inhibitor for carbon steel in acidic and neutral chloride-containing media: Facile synthesis, experimental and theoretical evaluations. J. Mol. Liq. 2020, 311, 113302. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Li, X.; Guo, D.; Shinde, D.B.; Lu, D.W.; Chen, L.; Liu, X.W.; Cao, L.; Aboalsaud, A.M.; Hu, Y.X. Electropolymerization of robust conjugated microporous polymer membranes for rapid solvent transport and narrow molecular sieving. Nat. Commun. 2020, 11, 5323. [Google Scholar] [CrossRef]
- Ma, Y.C.; Fan, B.M.; Wang, M.M.; Yang, B.; Hao, H.; Sun, H.; Zhang, H.J. Two-step preparation of trazodone and its corrosion inhibition mechanism for carbon steel. Chem. J. Chin. Univ. 2019, 40, 1706–1716. [Google Scholar]
- Asan, G.; Asan, A.; Çelikkan, H. The effect of 2D-MoS2 doped polypyrrole coatings on brass corrosion. J. Mol. Struct. 2020, 1203, 127318. [Google Scholar] [CrossRef]
- Liu, H.; Fan, B.M.; Liu, Z.N.; Zhao, X.Q.; Yang, B.; Zheng, X.W.; Hao, H. Electronic effects on protective mechanism of electropolymerized coatings based on N-substituted aniline derivatives for mild steel in saline solution. J. Ind. Eng. Chem. 2022, 106, 297–310. [Google Scholar] [CrossRef]
- Meng, S.G.; Liu, Z.N.; Zhao, X.Q.; Fan, B.M.; Liu, H.; Guo, M.; Hao, H. Efficient corrosion inhibition by sugarcane purple rind extract for carbon steel in HCl solution: Mechanism analyses by experimental and in silico insights. RSC Adv. 2021, 11, 31693–31711. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B. Electrochemical frequency modulation (EFM) technique: Theory and recent practical applications in corrosion research. J. Mol. Liq. 2018, 249, 83–96. [Google Scholar] [CrossRef]
- Adeosun, W.A.; Asiri, A.M.; Marwani, H.M. Sensitive determination of 2-nitrophenol using electrochemically deposited polymethyl red film for healthcare and environmental safety. Synth. Metals 2020, 261, 116321. [Google Scholar] [CrossRef]
- Martinez, A.L.; Brugnoni, L.I.; Flamini, D.O.; Saidman, S.B. Immobilization of Zn species in a polypyrrole matrix to prevent corrosion and microbial growth on Ti-6Al-4V alloy for biomedical applications. Prog. Org. Coat. 2020, 144, 105650. [Google Scholar] [CrossRef]
- Fan, B.M.; Hao, H.; Yang, B.; Li, Y. Insights into the inhibition mechanism of a novel supramolecular complex towards the corrosion of mild steel in the condensate water: Experimental and theoretical studies. Res. Chem. Intermed. 2018, 44, 5711–5736. [Google Scholar] [CrossRef]
- Ma, Y.C.; Fan, B.M.; Zhou, T.T.; Hao, H.; Yang, B.; Sun, H. Molecular assembly between weak crosslinking cyclodextrin polymer and trans-cinnamaldehyde for corrosion inhibition towards mild steel in 3.5% NaCl solution: Experimental and theoretical studies. Polymers 2019, 11, 635. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.M.; Hao, H.; Guo, A.R.; Yang, R.P. Fabrication and evaluation of an attapulgite membrane as the filter for recycling blowdown water from industrial boilers. J. Water Reuse Desal. 2016, 6, 399–412. [Google Scholar] [CrossRef] [Green Version]



| Compound | NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 | KBr | H3BO3 | SrCl2 | NaF |
| Content (g/L) | 24.53 | 5.20 | 4.09 | 1.16 | 0.695 | 0.201 | 0.101 | 0.027 | 0.025 | 0.003 |
| Sample | Time (h) | Ecorr (mV) | icorr (μA/cm2) | βa (mV/dec) | −βc (mV/dec) |
|---|---|---|---|---|---|
| p-PPy | 24 | −168 | 25.41 | / | 128.19 |
| 72 | −171 | 26.25 | 114.26 | 109.61 | |
| 120 | −181 | 33.08 | 111.49 | 110.23 | |
| 168 | −189 | 60.13 | 95.60 | 104.58 | |
| 240 | −199 | 63.79 | 98.73 | 99.89 | |
| d-PPy | 24 | −71 | 0.68 | / | 93.94 |
| 72 | −84 | 1.07 | / | 98.29 | |
| 120 | −101 | 1.93 | / | 101.76 | |
| 168 | −112 | 2.25 | / | 109.85 | |
| 240 | −124 | 3.09 | 95.87 | 99.04 |
| Sample | Time (h) | Rs (Ω·cm2) | Rf (kΩ·cm2) | Cf (μF/cm2) | nf | Rct (kΩ·cm2) | Cdl (μF/cm2) | ndl | W (mΩ·cm2·s1/2) |
|---|---|---|---|---|---|---|---|---|---|
| p-PPy | 24 | 2.36 | 206.70 | 99.14 | 0.58 | 1.96 | 85.57 | 0.75 | 4.04 |
| 72 | 3.31 | 137.84 | 113.20 | 0.55 | 1.85 | 88.29 | 0.73 | 9.75 | |
| 120 | 2.19 | 135.25 | 120.35 | 0.57 | 1.59 | 93.56 | 0.69 | 2.33 | |
| 168 | 4.02 | 98.68 | 122.84 | 0.53 | 0.96 | 109.79 | 0.68 | 3.97 | |
| 240 | 3.37 | 96.23 | 131.71 | 0.58 | 0.45 | 123.25 | 0.66 | 11.20 | |
| d-PPy | 24 | 1.08 | 435.29 | 68.29 | 0.61 | 8.36 | 57.44 | 0.78 | / |
| 72 | 3.94 | 434.57 | 68.31 | 0.59 | 8.28 | 57.59 | 0.77 | / | |
| 120 | 5.21 | 406.79 | 75.59 | 0.55 | 7.33 | 61.06 | 0.78 | / | |
| 168 | 2.73 | 358.44 | 80.14 | 0.58 | 5.84 | 68.25 | 0.74 | 5.25 | |
| 240 | 5.58 | 321.02 | 83.22 | 0.60 | 4.38 | 71.97 | 7.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Liu, X.; Hao, H.; Zheng, X. Influence of Surface Pretreatments on the Anticorrosion of Polypyrrole Electro-Polymerized Coatings for Copper in Artificial Seawater. Metals 2022, 12, 383. https://doi.org/10.3390/met12030383
Zhu H, Liu X, Hao H, Zheng X. Influence of Surface Pretreatments on the Anticorrosion of Polypyrrole Electro-Polymerized Coatings for Copper in Artificial Seawater. Metals. 2022; 12(3):383. https://doi.org/10.3390/met12030383
Chicago/Turabian StyleZhu, Hetao, Xiaoyan Liu, Hua Hao, and Xingwen Zheng. 2022. "Influence of Surface Pretreatments on the Anticorrosion of Polypyrrole Electro-Polymerized Coatings for Copper in Artificial Seawater" Metals 12, no. 3: 383. https://doi.org/10.3390/met12030383





