Interfacial Phenomena and Reaction Kinetics between High Al Molten Steel and CaO-SiO2-Type Flux
Abstract
:1. Introduction
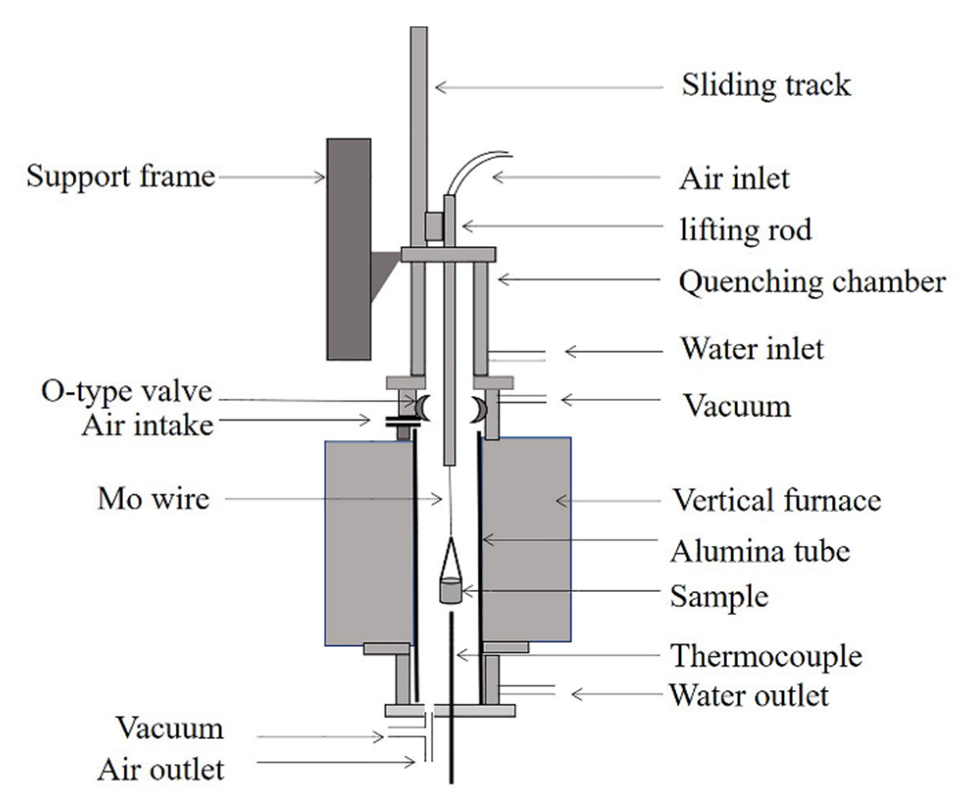
2. Materials and Methods
3. Results and Discussion
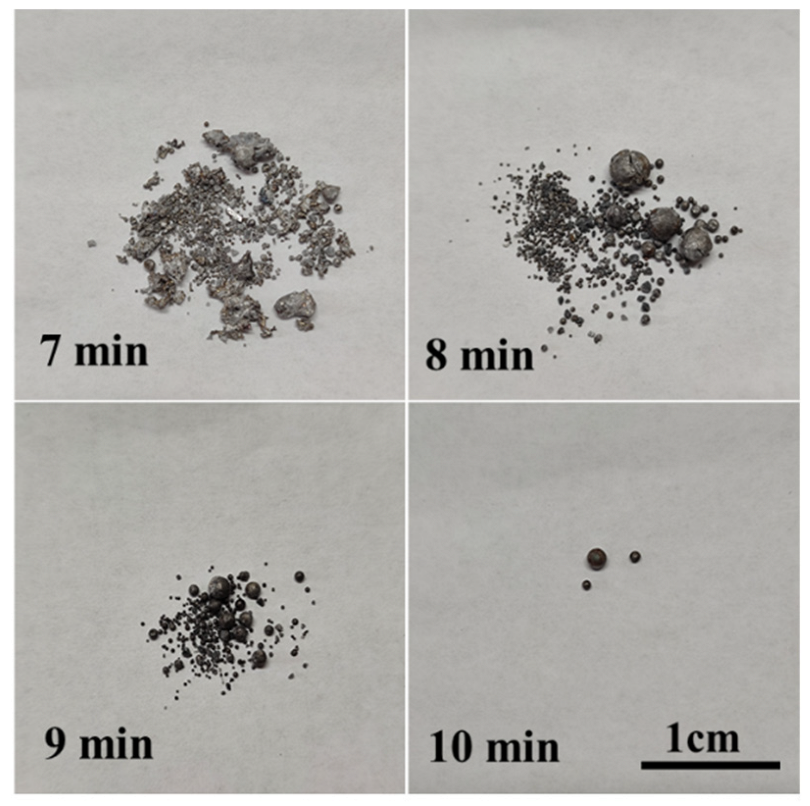
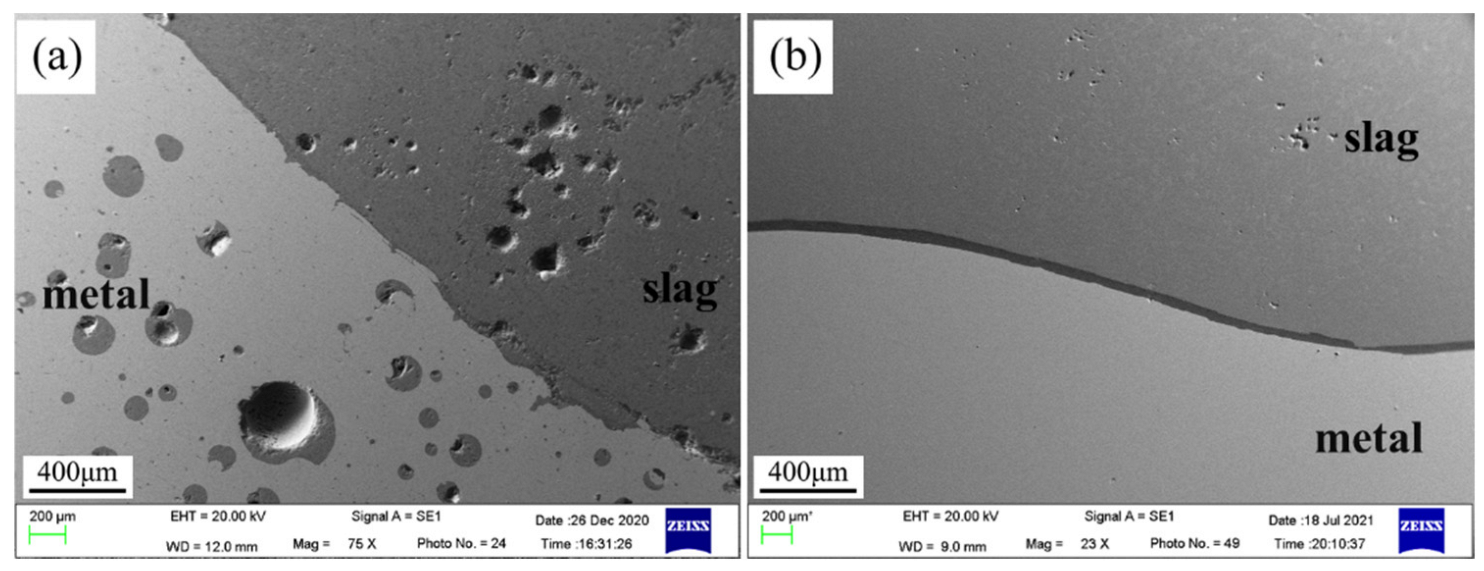
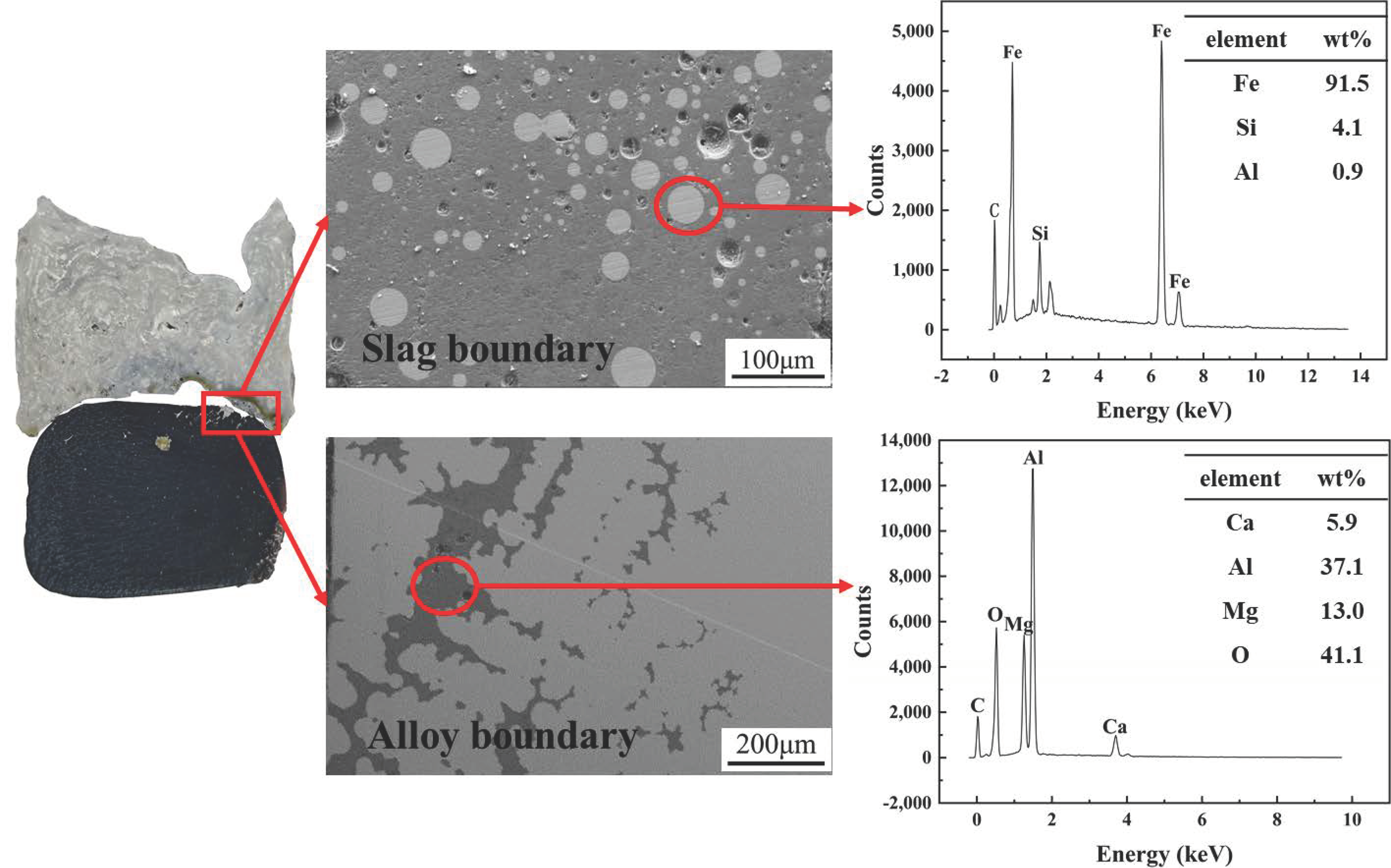
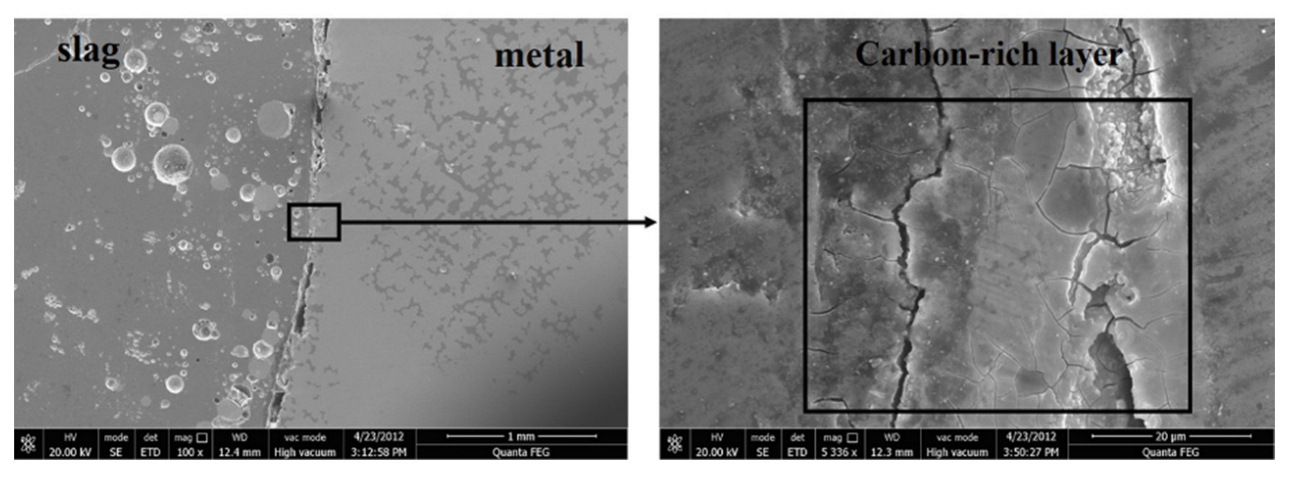
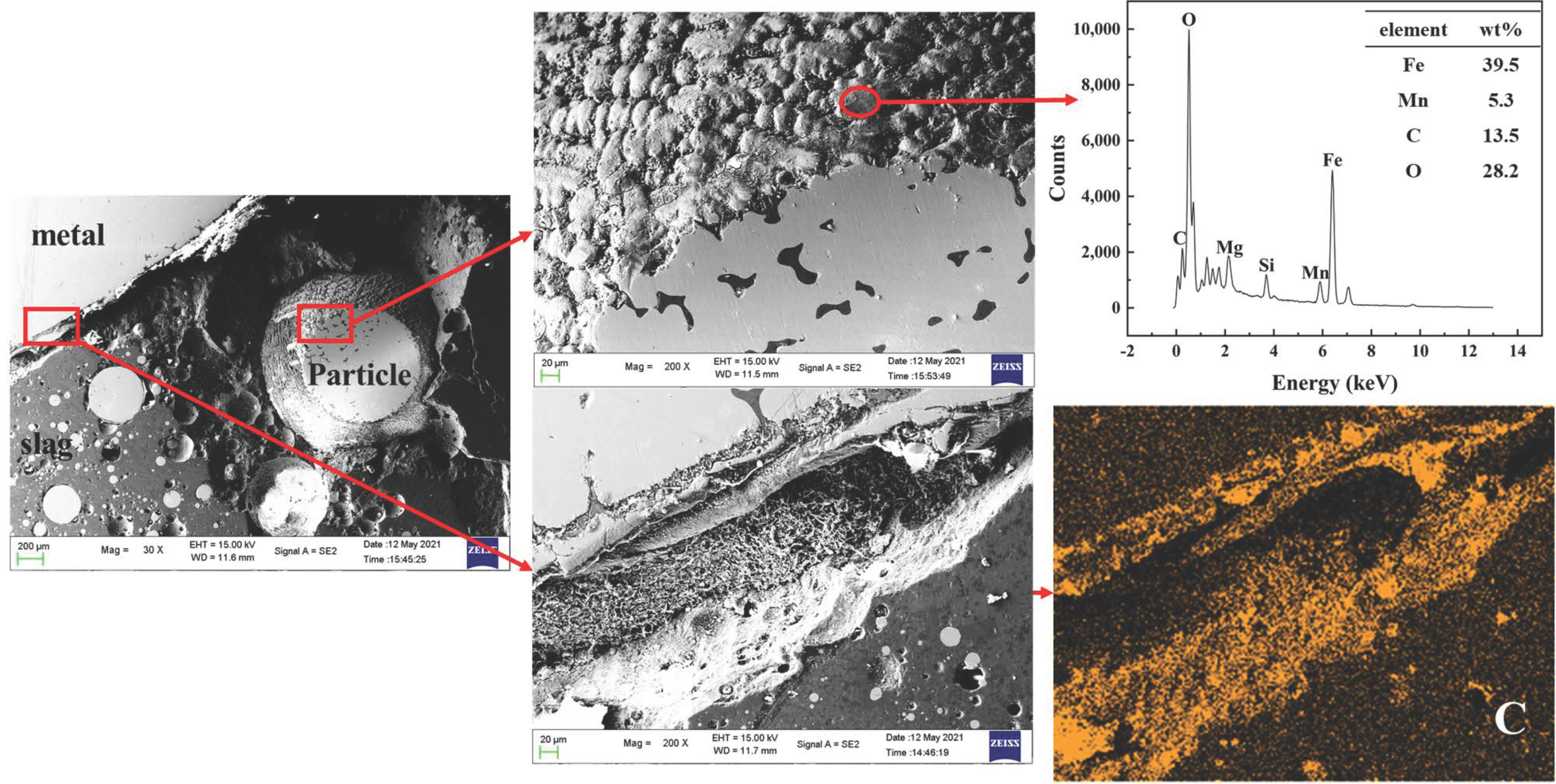
3.1. Evolution of Morphology
3.1.1. Fe-5 Mass % Al Alloy and High SiO2 Flux
3.1.2. Fe-5Al Alloy and Low SiO2 Flux
3.1.3. Fe-13Mn-5Al Alloy and Low SiO2 Flux
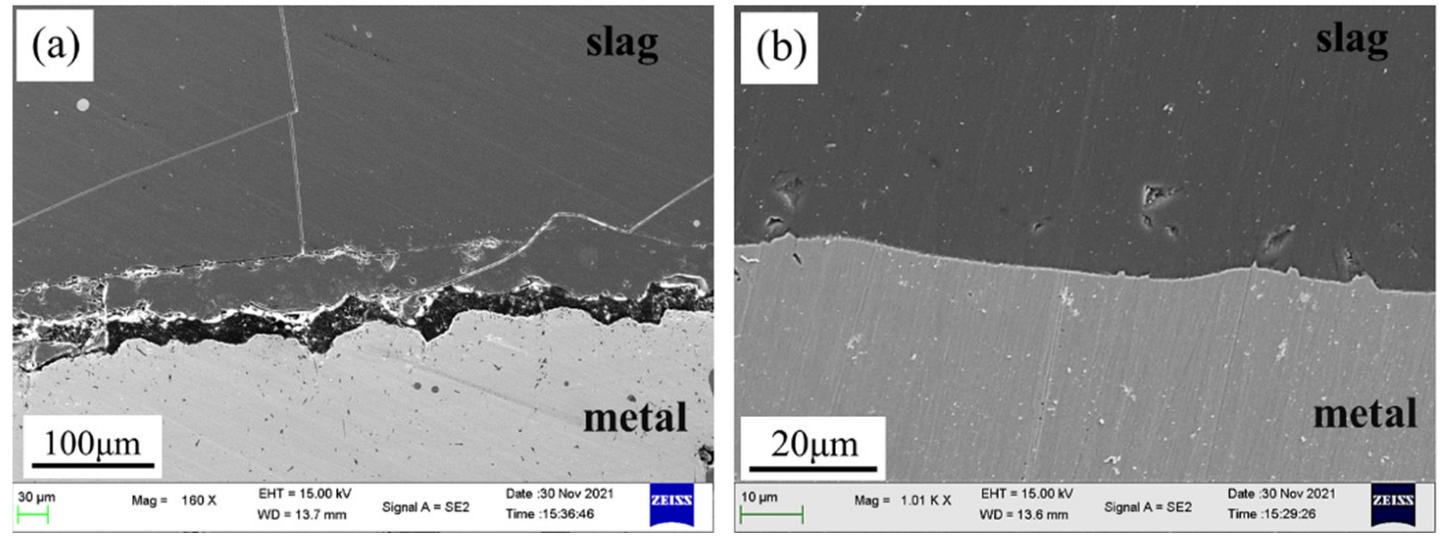
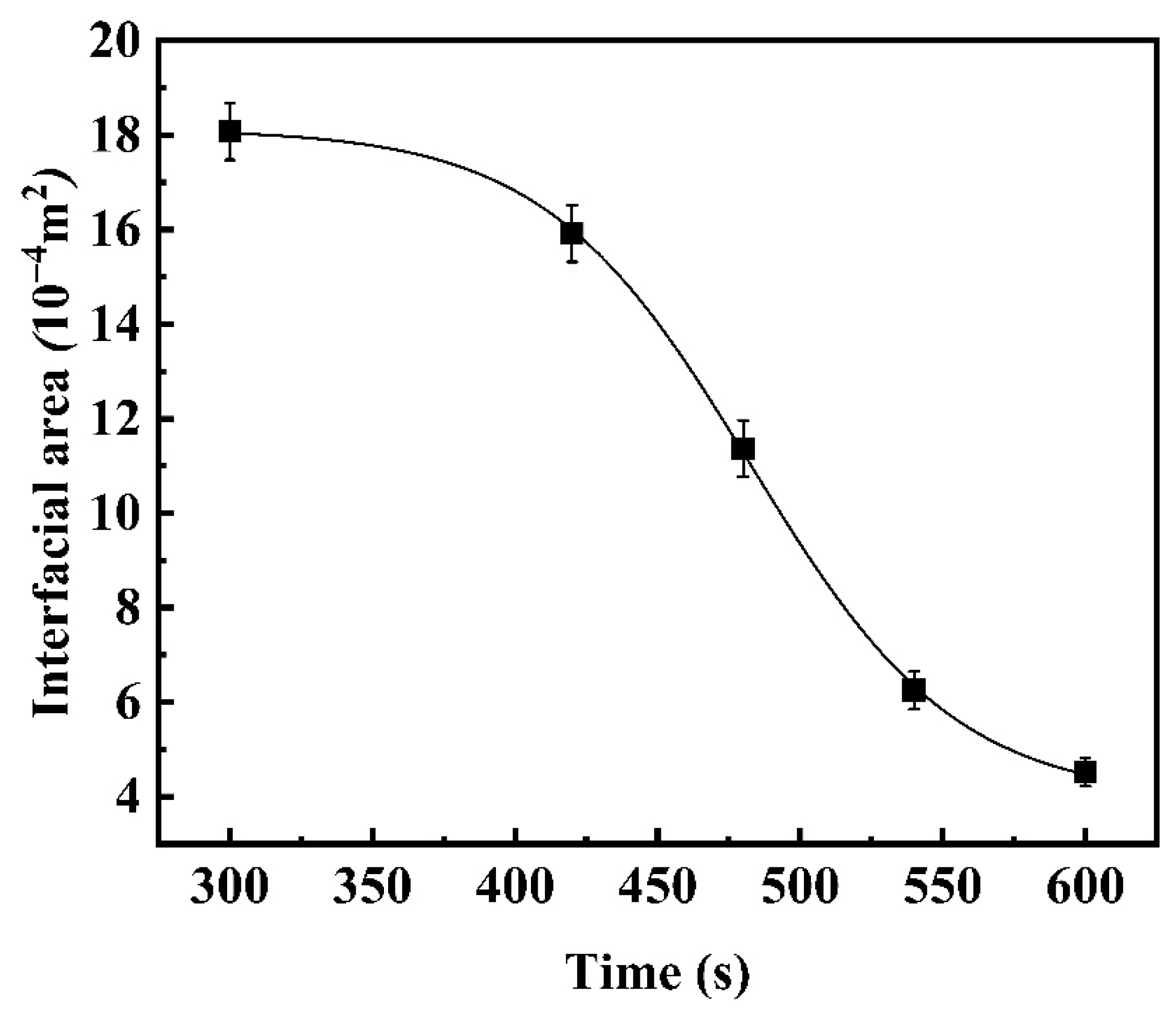
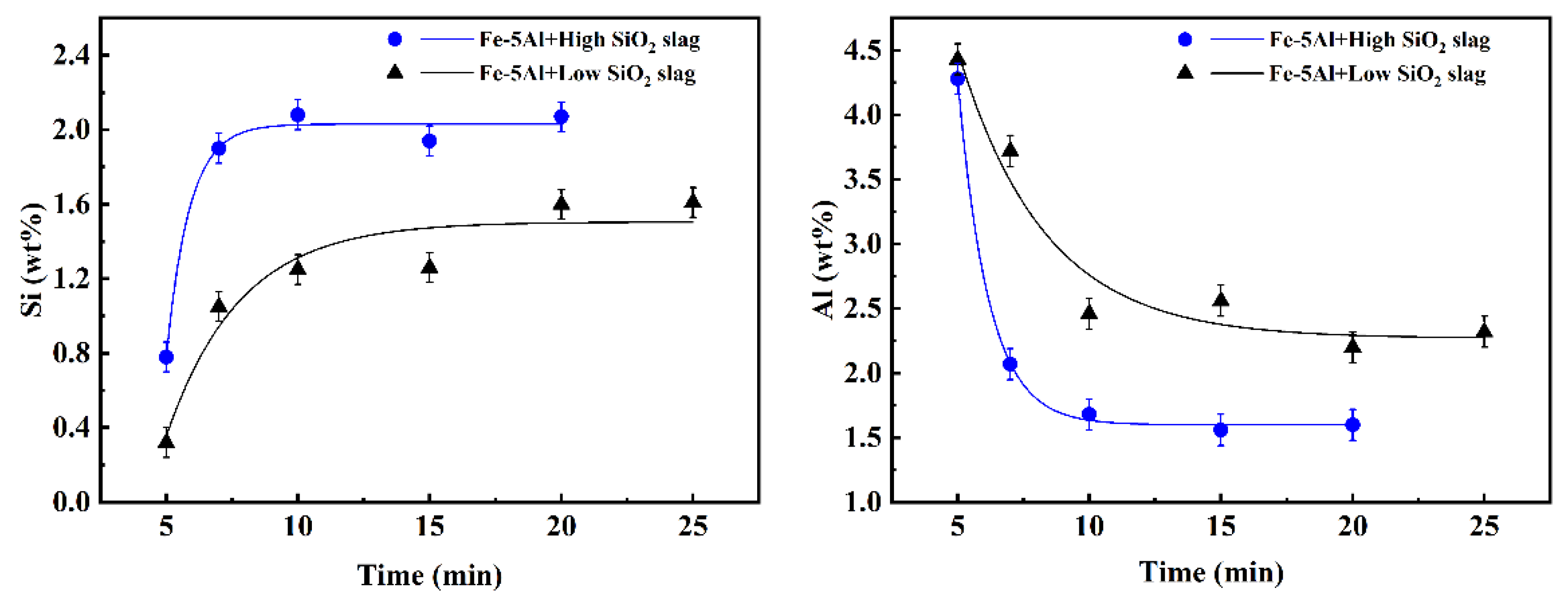
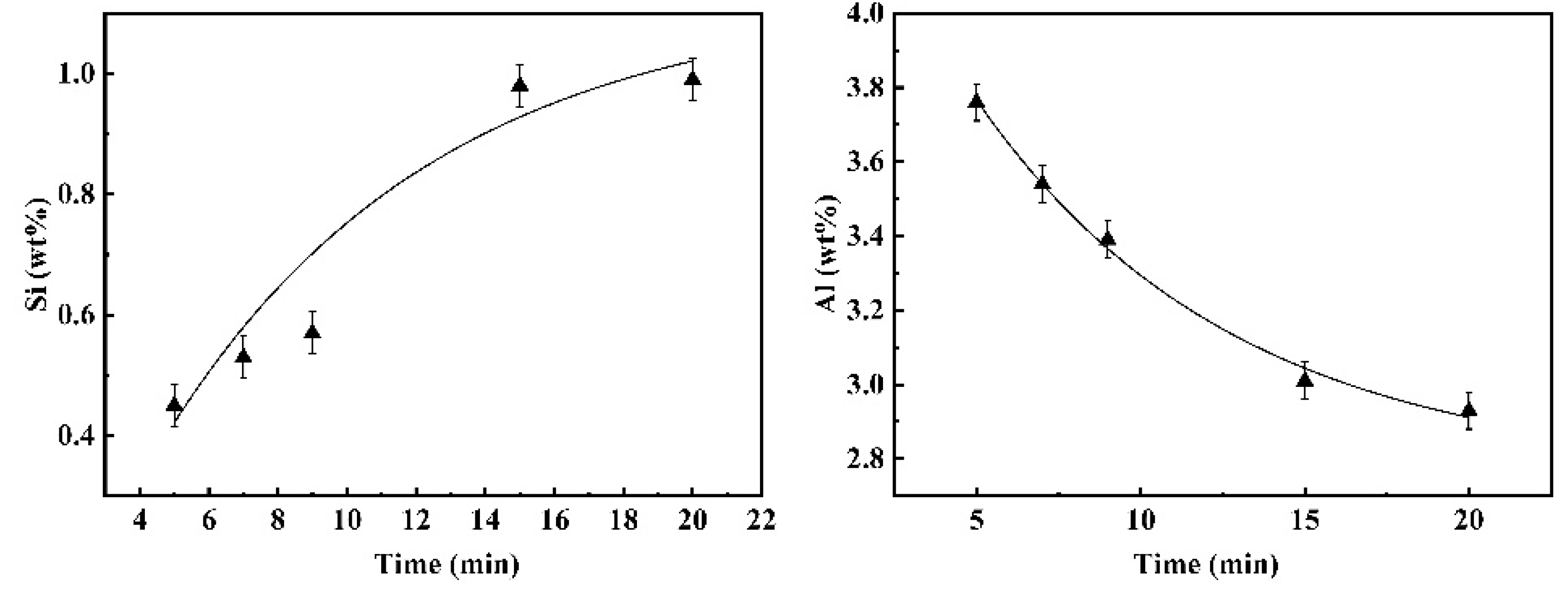
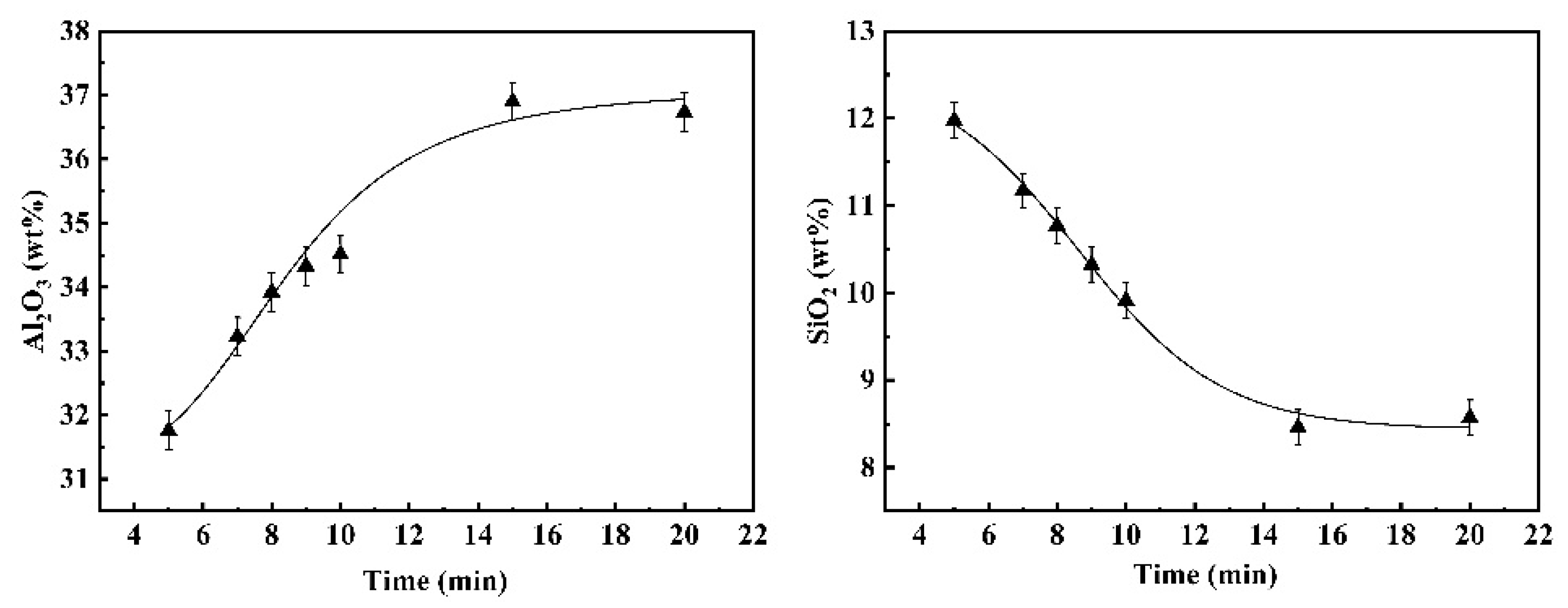
3.2. Kinetic Analysis of the Global Slag/Metal Reaction
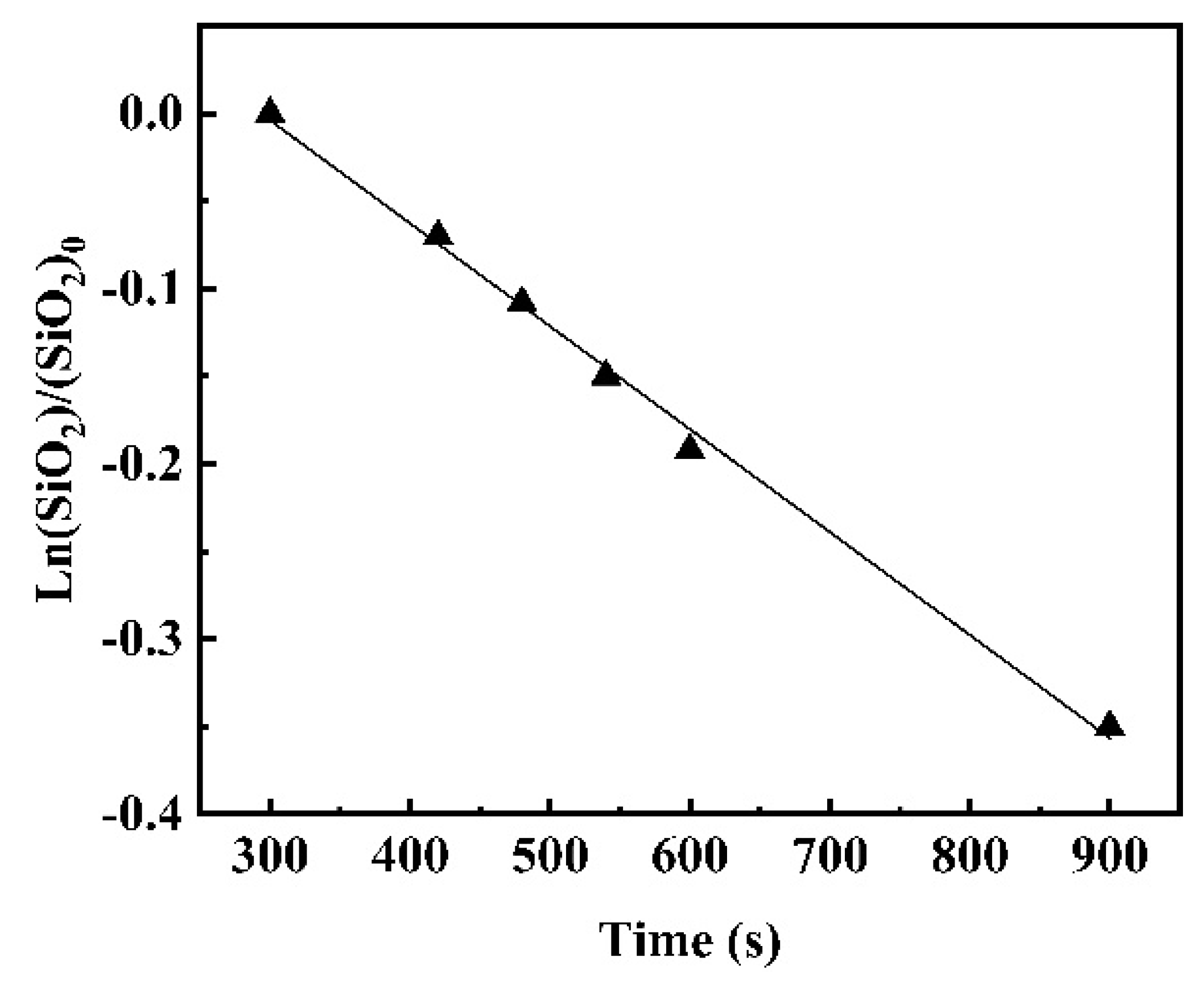
3.2.1. Reaction Kinetics between Fe-5Al Metal and Flux
3.2.2. Reaction Kinetics between Fe-13Mn-5Al Metal and Flux
4. Conclusions
- (1)
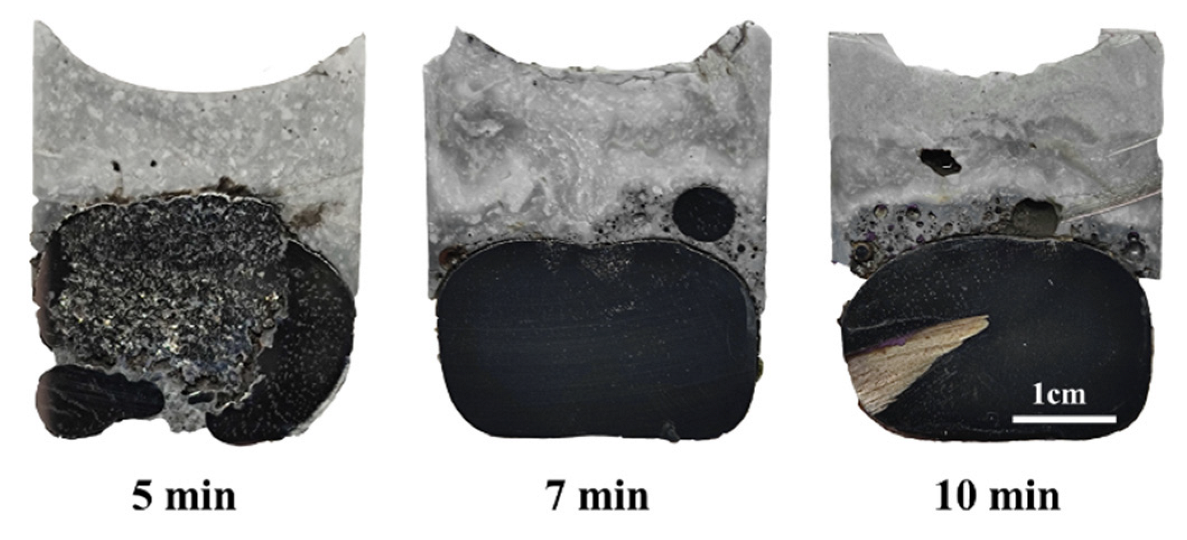
- For the reaction either between high Al steel and high SiO2 flux or between high Al steel and low SiO2 flux, the spontaneous emulsification of metal droplets in slag and slag fractions in metal, as well fluctuation of interface, occur abruptly at the beginning of the reaction. Although the intensity for the case of low SiO2 flux is lower relatively, it still can be observed apparently.
- (2)
- The duration of these dynamic interfacial phenomena is affected distinctively by the reaction system. The dynamic interfacial phenomena occurred in the system composed of Fe-5Al alloy and flux can disappear rapidly with the progressing of reaction, whereas for the case of reaction between Fe-13Mn-5Al alloy and flux, the emulsified metal droplets and slag fractions can maintain and stay stably.
- (3)
- For different reaction systems, the dynamic interfacial phenomena have significant and specific effect on the global reaction kinetics and reaction mechanism. As for the reaction between Fe-5Al alloy and flux, the dynamic interfacial phenomena result in a considerable increment of interfacial area, which consequently causes an extremely rapid global reaction controlled by the mass transfer of [Al] in molten steel with a mass transfer coefficient of . However, the reaction between Fe-13Mn-5Al alloy and flux is comparatively very slow due to the trivial effect or contribution from the dynamic interfacial phenomena, and its rate-controlling step may be the transfer of (SiO2) in molten slag with a mass transfer coefficient of .
- (4)
- Through the kinetic analysis, we have a better understanding of the reaction mechanism between CaO- SiO2 flux in molten steel with high Al content. It is helpful to predict the changing trend of flux composition in the process of continuous casting of high Mn-high Al steel and improve the production efficiency. Additionally, it is meaningful to analyze which type of mold powder is more suitable for continuous casting operation. However, to predict well the change of mold powder composition in the continuous casting process, more continuous casting process reaction models need to be established based on multi-component reaction model, and the accuracy of the continuous casting model should be verified by actual production.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, K.K.H.A.; Jin, K.G.; Han, S.H.; Hwang, S.W.; Choi, K.; Lee, C.S. Stacking fault energy and plastic deformation of fully austenitic high manganese steels: Effect of Al addition. Mater. Sci. Eng. A 2010, 527, 3651–3661. [Google Scholar] [CrossRef]
- Frommeyer, G.; Brüx, U.; Neumann, P. Supra-Ductile and High-Strength Manganese-TRIP/TWIP Steels for High Energy Absorption Purposes. ISIJ Int. 2003, 43, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Suikkanen, P.P.; Lang, V.T.E.; Somani, M.C. Effect of Silicon and Aluminium on Austenite Static Recrystallization Kinetics in High-strength TRIP-aided Steels. ISIJ Int. 2012, 52, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.G.A.; Kang, C.Y.A.; Shin, S.Y.B.; Hong, S.B.; Lee, S.B.; Kim, H.S.B.; Kim, K.H.C.; Kim, N.J.D. Effects of Al addition on deformation and fracture mechanisms in two high manganese TWIP steels. Mater. Sci. Eng. A 2011, 528, 2922–2928. [Google Scholar] [CrossRef]
- Lee, Y.K.; Jin, J.E. Effects of Al on microstructure and tensile properties of C-bearing high Mn TWIP steel. Acta Mater. 2012, 60, 1680–1688. [Google Scholar]
- CHO, J.; BLAZEK, K.; FRAZEE, M. Assessment of CaO–Al2O3 Based Mold Flux System for High Aluminum TRIP Casting. ISIJ Int. 2013, 53, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cui, H. Simulation for Mass Transfer Kinetics at Slag-Steel Interface during High Al Steel Continuous Casting. ISIJ Int. 2019, 59, 2256–2263. [Google Scholar]
- Zhang, Z.; Wen, G.; Tang, P.; Sridhar, S. The Influence of Al2O3/SiO2 Ratio on the Viscosity of Mold Fluxes. ISIJ Int. 2008, 48, 739–746. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, J.J.Q.Z.; Ostrovski, O.; Sasaki, Y.; Zhang, C.; Cai, D. Dynamic Wetting of High-Al Steel by CaO-SiO2- and CaO-Al2O3-Based Mold Fluxes. Metall. Mater. Trans. B 2019, 50, 2175–2185. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y.; Sridhar, S.; Wang, X.; Zhang, Z. Effect of Al2O3/SiO2 Ratio on the Viscosity and Structure of Slags. ISIJ Int. 2012, 52, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Hanao, M.; Kawamoto, M.; Yamanaka, A. Influence of Mold Flux on Initial Solidification of Hypo-Peritectic Steel in a Continuous Casting Mold. ISIJ Int. 2012, 52, 1310–1319. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, Q.; Zhang, J. Effect of Al2O3/(B2O3+Na2O) Ratio on CaO-Al2O3-Based Mold Fluxes: Melting Property, Viscosity, Heat Transfer, and Structure. Metall. Mater. Trans. B 2019, 50, 2794–2803. [Google Scholar] [CrossRef]
- Wang, Z.; Sohn, I. Review on the High-Temperature Thermophysical Properties of Continuous Casting Mold Fluxes for Highly Alloyed Steels. ISIJ Int. 2019, 60, 2705–2716. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Sohn, I. Interfacial Kinetics of High-Al-Containing Ultra-Lightweight Steels with Calcium Silicate-Based Molten Oxides at High Temperature. Metall. Mater. Trans. B 2016, 47, 1773–1784. [Google Scholar] [CrossRef]
- Brooks, G.A.; Rhamdhani, M.A.; Coley, K.S.; Subagyo; Pan, Y. Transient Kinetics of Slag Metal Reactions. Metall. Mater. Trans. B 2009, 40, 353–362. [Google Scholar] [CrossRef]
- Wang, Q.W.Q.; Qiu, S.Q.S.; Zhao, P.Z.P. Kinetic analysis of alumina change in mold slag for high aluminum steel during continuous casting. Metall. Mater. Trans. B 2012, 43, 424–430. [Google Scholar] [CrossRef]
- Yu, X.Y.X.; Wen, G.W.G.; Tang, P.T.P.; Ma, F.M.F.; Wang, H.W.H. Behavior of Mold Slag Used for 20Mn23Al Nonmagnetic Steel During Casting. J. Iron Steel Res. Int. 2011, 18, 20–25. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.; Cho, J.; Park, M.; Lee, H.; Kang, Y. A Reaction between High Mn-High Al Steel and CaO-SiO2-Type Molten Mold Flux: Part I. Composition Evolution in Molten Mold Flux. Metall. Mater. Trans. B 2013, 44, 299–308. [Google Scholar] [CrossRef]
- Kang, Y.B.; Kim, M.S.; Lee, S.W.; Cho, J.W.; Park, M.S.; Lee, H.G. A Reaction between High Mn-High Al Steel and CaO-SiO2-Type Molten Mold Flux: Part II. Reaction Mechanism, Interface Morphology, and Al2O3 Accumulation in Molten Mold Flux. Metall. Mater. Trans. B 2013, 44, 309–316. [Google Scholar] [CrossRef]
- Kim, M.; Park, M.; Kang, S.; Park, J.; Kang, Y. A Reaction between High Mn–High Al Steel and CaO–SiO2-Type Molten Mold Flux: Reaction Mechanism Change by High Al Content ([pct Al]0 = 5.2) in the Steel and Accumulation of Reaction Product at the Reaction Interface. ISIJ Int. 2018, 58, 686–695. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Sridhar, S.; Fruehan, R.J. Kinetics of Reduction of SiO2 in SiO2-Al2O3-CaO Slags by Al in Fe-Al(-Si) Melts. Metall. Mater. Trans. B 2014, 45, 1380–1388. [Google Scholar] [CrossRef]
- Chung, Y.; Cramb, A.W. Dynamic and equilibrium interfacial phenomena in liquid steel-slag systems. Metall. Mater. Trans. B 2000, 31, 957–971. [Google Scholar] [CrossRef]
- Rhamdhani, M.A.A.B.; Brooks, G.A.D.; Coley, K.S.E. Analysis of interfacial area changes during spontaneous emulsification of metal droplets in slag. Metall. Mater. Trans. B 2006, 37, 1087–1091. [Google Scholar] [CrossRef]
- Assis, A.N.A.; Warnett, J.B.; Spooner, S.B.; Fruehan, R.J.A.; Williams, M.A.B.; Sridhar, S.B. Spontaneous Emulsification of a Metal Drop Immersed in Slag Due to Dephosphorization: Surface Area Quantification. Metall. Mater. Trans. B 2015, 46, 568–576. [Google Scholar] [CrossRef]
- Rhamdhani, M.A.A.B.; Coley, K.S.C.; Brooks, G.A.D.E. Analysis of the source of dynamic interfacial phenomena during reaction between metal droplets and slag. Metall. Mater. Trans. B 2005, 36, 591–604. [Google Scholar] [CrossRef]
- Rhamdhani, M.A.A.; Brooks, G.A.B.C.; Coley, K.S.A. Kinetics of metal/slag reactions during spontaneous emulsification. Metall. Mater. Trans. B 2005, 36, 219–227. [Google Scholar] [CrossRef]
- Lee, J.; Le, T.H.; Shin, M. Density and Surface Tension of Liquid Fe-Mn Alloys. Metall. Mater. Trans. B 2011, 42, 546–549. [Google Scholar] [CrossRef]







| Slag | CaO | SiO2 | Al2O3 | MgO | CaF2 | C |
|---|---|---|---|---|---|---|
| High SiO2 Flux | 41.1 | 36.7 | 14.7 | 4.9 | - | 0.04 |
| Low SiO2 Flux | 37.8 | 13.0 | 31.4 | 10.3 | 5.8 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Zhang, J.; Yan, B. Interfacial Phenomena and Reaction Kinetics between High Al Molten Steel and CaO-SiO2-Type Flux. Metals 2022, 12, 391. https://doi.org/10.3390/met12030391
Zhao B, Zhang J, Yan B. Interfacial Phenomena and Reaction Kinetics between High Al Molten Steel and CaO-SiO2-Type Flux. Metals. 2022; 12(3):391. https://doi.org/10.3390/met12030391
Chicago/Turabian StyleZhao, Bingbing, Jie Zhang, and Baijun Yan. 2022. "Interfacial Phenomena and Reaction Kinetics between High Al Molten Steel and CaO-SiO2-Type Flux" Metals 12, no. 3: 391. https://doi.org/10.3390/met12030391





