Investigation into the Corrosion Wear Resistance of CoCrFeNiAlx Laser-Clad Coatings Mixed with the Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Coatings
2.2. Microstructure Characterization
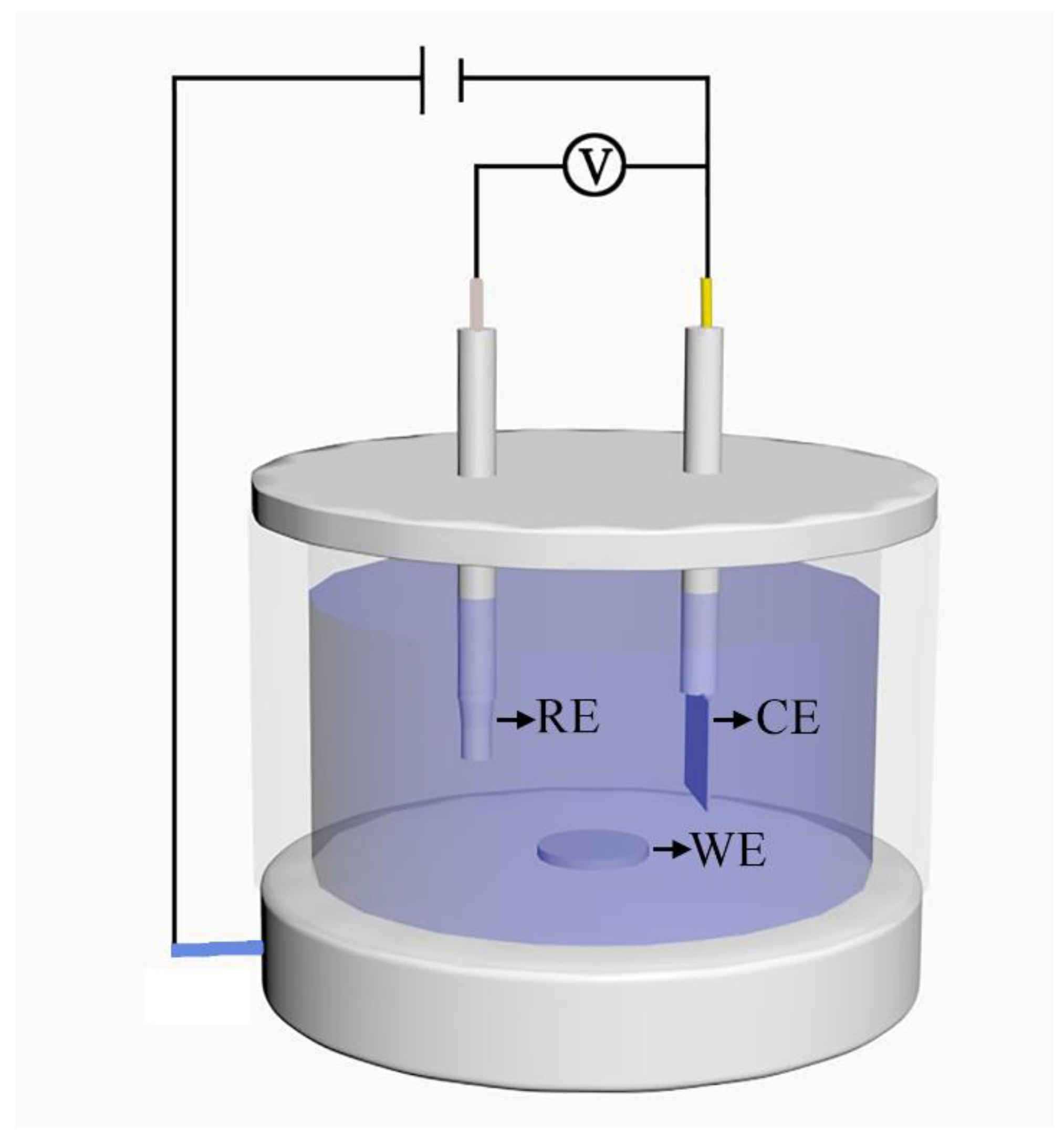
2.3. Electrochemical Performance
2.4. Mechanical Performance
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Analyses
3.2. Microstructural Characterization
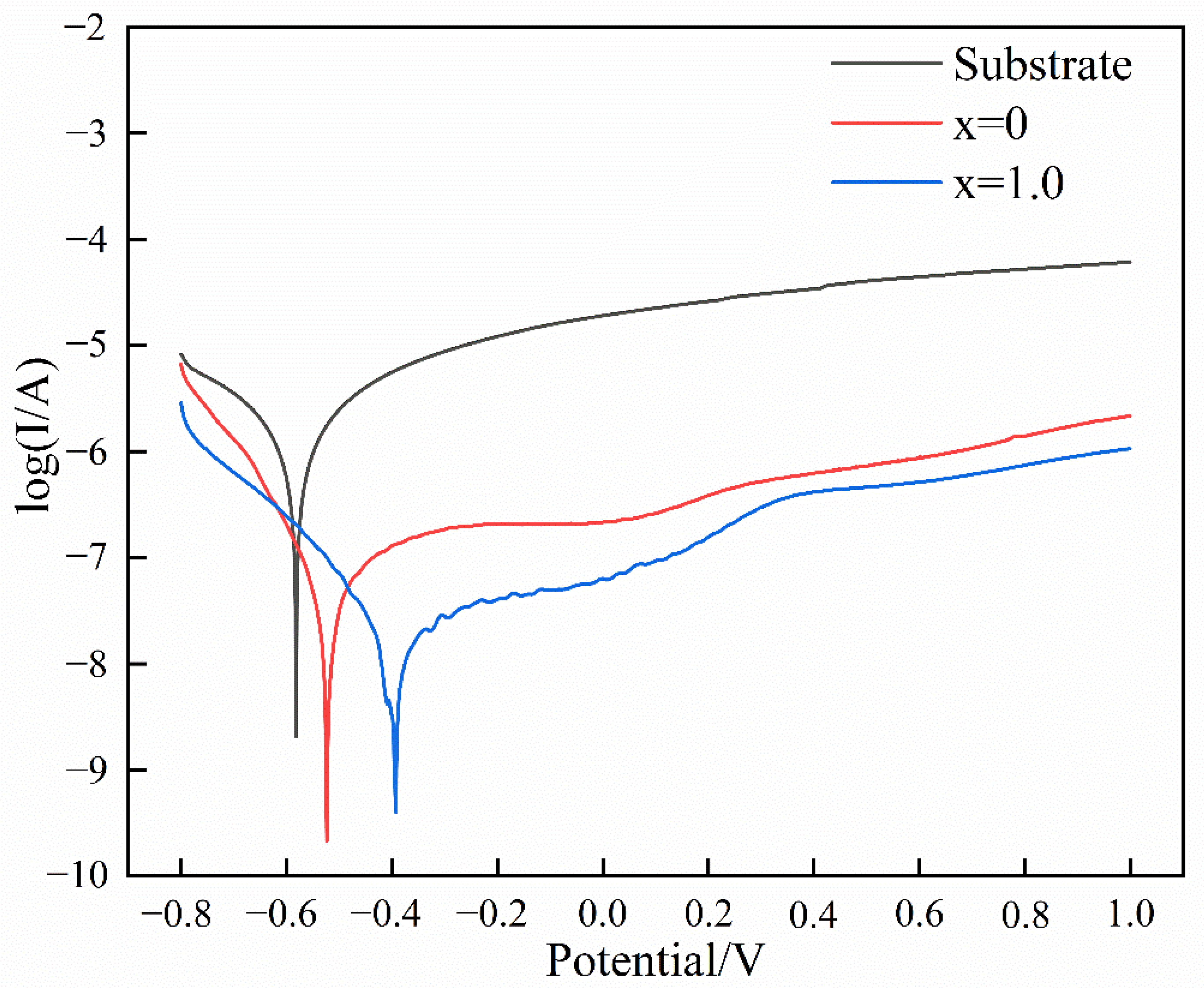
3.3. Corrosion Resistance
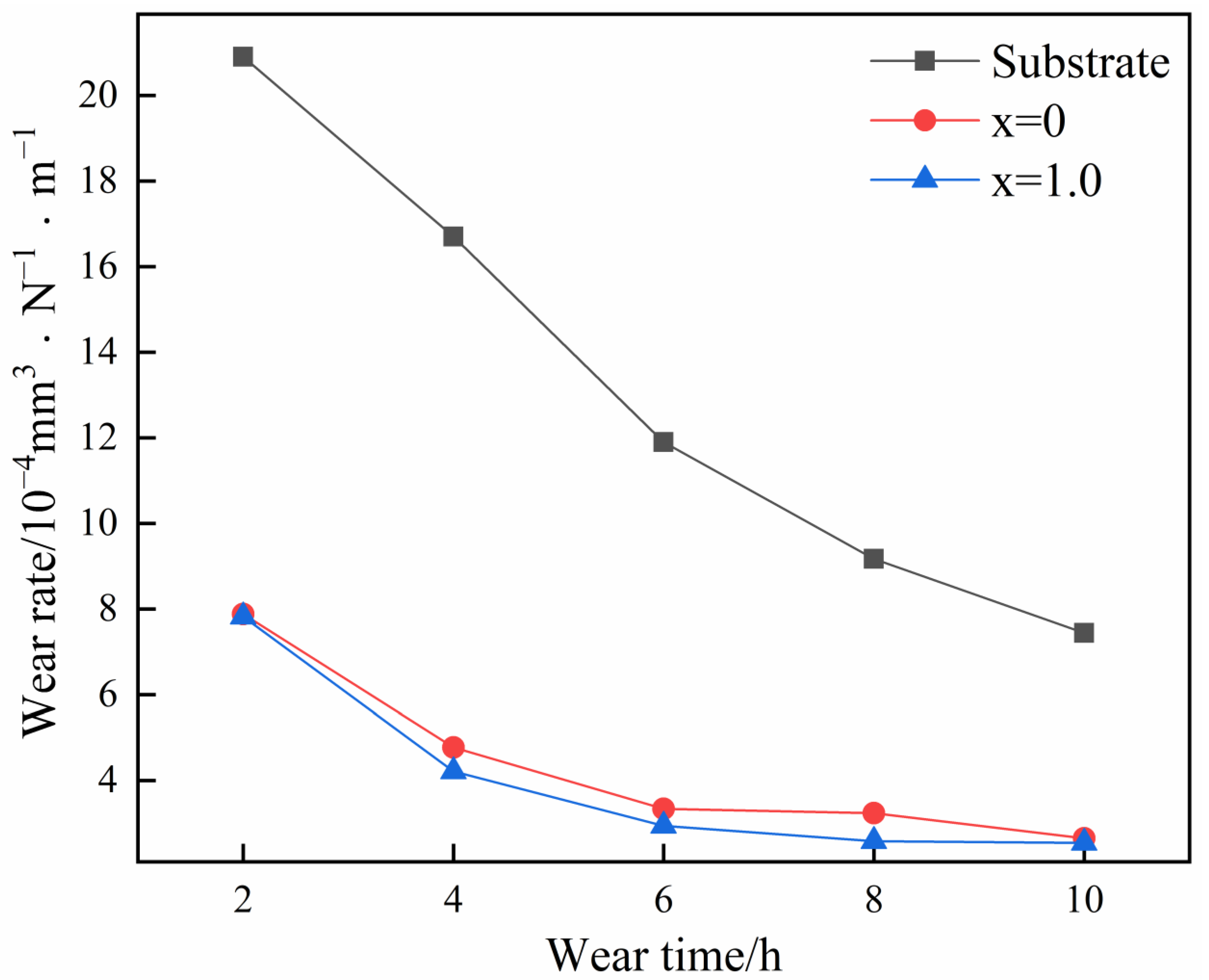
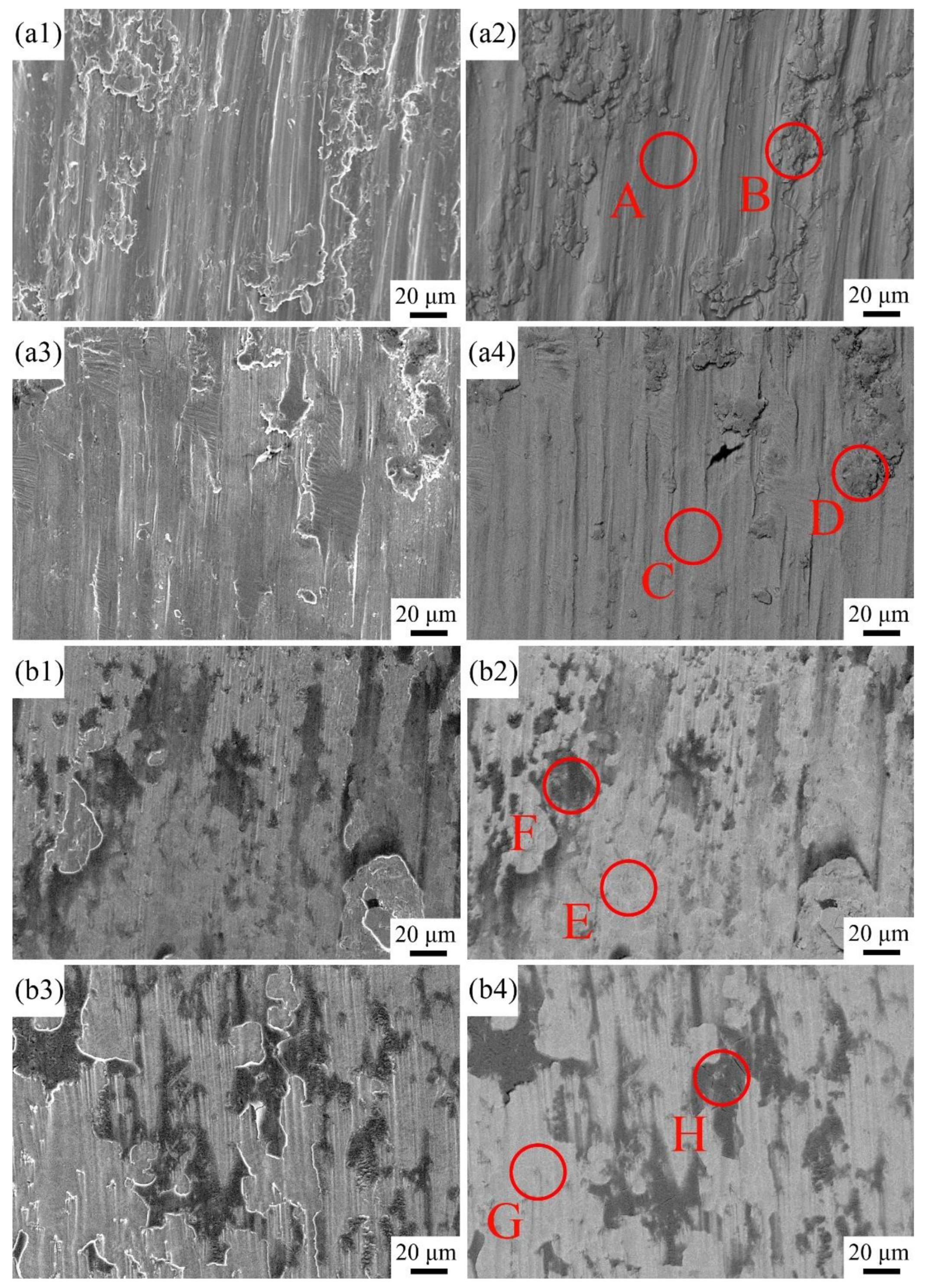
3.4. Corrosion Wear Resistance
4. Conclusions
- (1)
- CoCrFeNiAlx HEA coatings (x = 0 and 1.0) were fabricated on Ti6Al4V via laser cladding. Primary α(Ti), α(Ti) + Ti2Ni eutectics and TiC dendrites were synthesized. The introduction of Al improved microstructural uniformity by reducing the area fraction of the eutectics and refining TiC dendrites.
- (2)
- The coatings exhibited more outstanding corrosion resistance than the substrate in the 3.5 wt.% NaCl solution due to their lower corrosion potential, corrosion current density, and current density in the stable stage. The introduction of Al accelerated the formation of the oxidation film and reduced its cracking susceptibility, further improving corrosion resistance.
- (3)
- The coatings demonstrated a lower wear rate than the substrate at different wear times in the 3.5 wt.% NaCl solution, transitioning more rapidly into the stable wear stage. The introduction of Al reduced the wear rate in stable wear to a certain extent (2.07 × 10−4 mm3·N−1·m−1 for the coating with Al and 2.68 × 10−4 mm3·N−1·m−1 for the coating without Al).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lv, Y.H.; Li, J.; Tao, Y.F.; Hu, L.F. Oxidation behaviors of the TiNi/Ti2Ni matrix composite coatings with different contents of TaC addition fabricated on Ti6Al4V by laser cladding. J. Alloys Compd. 2016, 679, 202–212. [Google Scholar] [CrossRef]
- Obadele, B.A.; Olubambi, P.A.; Andrews, A.; Pityana, S.; Mathew, M.T. Electrochemical behaviour of laser-clad Ti6Al4V with CP Ti in 0.1 M oxalic acid solution. J. Alloys Compd. 2015, 646, 753–759. [Google Scholar] [CrossRef]
- Zakerin, N.; Morshed-Behbahani, K. Perspective on the passivity of Ti6Al4V alloy in H2SO4 and NaOH solutions. J. Mol. Liq. 2021, 333, 115947. [Google Scholar] [CrossRef]
- He, Y.; Xiao, H.; Gan, W.; Yu, Q.; Yin, F. The Electrochemical Dissolution Behavior Research of Titanium Alloy under Different Electrolyte. Procedia CIRP 2018, 68, 751–756. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Li, Q.-A.; Fu, S.-L.; Wang, J.-Z. Corrosion and tribocorrosion behaviors of AISI 316 stainless steel and Ti6Al4V alloys in artificial seawater. Trans. Nonferrous Met. Soc. China 2014, 24, 1022–1031. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, F.; Shao, Y.; Lu, B.; Lu, T.; Guo, Z. Electrochemical Corrosion Behavior of Powder Metallurgy Ti6Al4V Alloy. J. Mater. Eng. Perform. 2020, 30, 556–564. [Google Scholar] [CrossRef]
- Hu, S.; Li, T.; Su, Z.; Liu, D. Research on suitable strength, elastic modulus and abrasion resistance of Ti–Zr–Nb medium entropy alloys (MEAs) for implant adaptation. Intermetallics 2022, 140, 107401. [Google Scholar] [CrossRef]
- Jin, G.; Cai, Z.; Guan, Y.; Cui, X.; Liu, Z.; Li, Y.; Dong, M.; Zhang, D. High temperature wear performance of laser-cladded FeNiCoAlCu high-entropy alloy coating. Appl. Surf. Sci. 2018, 445, 113–122. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Z.; Zhang, W.; Huang, B.; Zhao, H.; Ren, L. Superior antiwear biomimetic artificial joint based on high-entropy alloy coating on porous Ti6Al4V. Tribol. Int. 2021, 158, 106937. [Google Scholar] [CrossRef]
- Motallebzadeh, A.; Peighambardoust, N.S.; Sheikh, S.; Murakami, H.; Guo, S.; Canadinc, D. Microstructural, mechanical and electrochemical characterization of TiZrTaHfNb and Ti1.5ZrTa0.5Hf0.5Nb0.5 refractory high-entropy alloys for biomedical applications. Intermetallics 2019, 113, 106572. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Dubey, A.K. Recent trends in laser cladding and surface alloying. Opt. Laser Technol. 2021, 134, 106619. [Google Scholar] [CrossRef]
- Seo, J.-W.; Kim, J.C.; Kwon, S.-J.; Jun, H.-K. Effects of Laser Cladding for Repairing and Improving Wear of Rails. Int. J. Precis. Eng. Manuf. 2019, 20, 1207–1217. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, J.; Zhang, L.; Tsutsumi, S.; Feng, J.; Ma, N. Impacts of laser cladding residual stress and material properties of functionally graded layers on titanium alloy sheet. Addit. Manuf. 2020, 35, 101303. [Google Scholar] [CrossRef]
- Kolodziejczak, P.; Golanski, D.; Chmielewski, T.; Chmielewski, M. Microstructure of Rhenium Doped Ni-Cr Deposits Produced by Laser Cladding. Materials 2021, 14, 2745. [Google Scholar] [CrossRef]
- Lin, Y.; Yao, J.; Lei, Y.; Fu, H.; Wang, L. Microstructure and properties of TiB2 –TiB reinforced titanium matrix composite coating by laser cladding. Opt. Lasers Eng. 2016, 86, 216–227. [Google Scholar] [CrossRef]
- Wang, W.F.; Jin, L.S.; Yang, J.G.; Sun, F.J. Directional growth whisker reinforced Ti-base composites fabricated by laser cladding. Surf. Coat. Technol. 2013, 236, 45–51. [Google Scholar] [CrossRef]
- Mahamood, R.M.; Akinlabi, E.T. Effect of the Scanning Speed of Treatment on the Microstructure, Microhardness, Wear, and Corrosion Behavior of Laser Metal-Deposited Ti–6AL–4V/TiC Composite. Mater. Sci. 2017, 53, 76–85. [Google Scholar] [CrossRef]
- Zhang, T.G.; Zhuang, H.F.; Zhang, Q.; Yao, B.; Yang, F. Influence of Y2O3 on the microstructure and tribological properties of Ti-based wear-resistant laser-clad layers on TC4 alloy. Ceram. Int. 2020, 46, 13711–13723. [Google Scholar] [CrossRef]
- Yanan, L.; Ronglu, S.; Wei, N.; Tiangang, Z.; Yiwen, L. Effects of CeO2 on microstructure and properties of TiC/Ti2Ni reinforced Ti-based laser cladding composite coatings. Opt. Lasers Eng. 2019, 120, 84–94. [Google Scholar] [CrossRef]
- Li, N.; Xiong, Y.; Xiong, H.; Shi, G.; Blackburn, J.; Liu, W.; Qin, R. Microstructure, formation mechanism and property characterization of Ti + SiC laser cladded coatings on Ti6Al4V alloy. Mater. Charact. 2019, 148, 43–51. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Chen, C.; Liu, J.; Zhao, L.; Dai, J. Fabrication of Co-Based Coatings on Titanium Alloy by Laser Cladding with CeO2 Addition. Mater. Manuf. Processes 2016, 31, 1461–1467. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Chen, C.; Liu, J.; Zhao, L. Microstructures and properties of TiN reinforced Co-based composite coatings modified with Y2O3 by laser cladding on Ti–6Al–4V alloy. J. Alloys Compd. 2015, 650, 178–184. [Google Scholar] [CrossRef]
- Li, W.; Xu, P.; Wang, Y.; Zou, Y.; Gong, H.; Lu, F. Laser synthesis and microstructure of micro- and nano-structured WC reinforced Co-based cladding layers on titanium alloy. J. Alloys Compd. 2018, 749, 10–22. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Liu, J.; Chen, C.; Dai, J.; Zhao, Z. Microstructure and wear property of the Ti5Si3/TiC reinforced Co-based coatings fabricated by laser cladding on Ti-6Al-4V. Opt. Laser Technol. 2017, 92, 156–162. [Google Scholar] [CrossRef]
- Wu, F.; Chen, T.; Wang, H.; Liu, D. Effect of Mo on Microstructures and Wear Properties of In Situ Synthesized Ti(C,N)/Ni-Based Composite Coatings by Laser Cladding. Materials 2017, 10, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.L.; Li, J.; Chen, J.L.; Song, R.; Shao, J.Z.; Qu, C.C. Effect of the heat treatment time on microstructure and fracture toughness of laser-clad TiB2-TiC pseudoeutectic reinforced TiNi/Ti2Ni intermetallic matrix composite coating. Metall. Res. Technol. 2016, 113, 304. [Google Scholar] [CrossRef]
- Liu, K.; Yan, H.; Zhang, P.; Zhao, J.; Yu, Z.; Lu, Q. Wear Behaviors of TiN/WS2 + hBN/NiCrBSi Self-Lubricating Composite Coatings on TC4 Alloy by Laser Cladding. Coatings 2020, 10, 747. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A.; Das, A.K.; Dixit, A.R. Parametric study and characterization of AlN-Ni-Ti6Al4V composite cladding on titanium alloy. Surf. Coat. Technol. 2018, 349, 37–49. [Google Scholar] [CrossRef]
- Li, W.G.; Li, J.; Xu, Y.S. Optimization of Corrosion Wear Resistance of the NiCrBSi Laser-Clad Coatings Fabricated on Ti6Al4V. Coatings 2021, 11, 960. [Google Scholar] [CrossRef]
- Qu, C.C.; Li, J.; Bai, L.L.; Shao, J.Z.; Song, R.; Chen, J.L. Effects of the thickness of the pre-placed layer on microstructural evolution and mechanical properties of the laser-clad coatings. J. Alloys Compd. 2015, 644, 450–463. [Google Scholar] [CrossRef]
- Li, M.; Huang, J.; Zhu, Y.Y.; Li, Z.G. Effect of heat input on the microstructure of in-situ synthesized TiN–TiB/Ti based composite coating by laser cladding. Surf. Coat. Technol. 2012, 206, 4021–4026. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Chew, Y.; Bi, G.J.; Zhu, Z.G.; Ng, F.L.; Weng, F.; Liu, S.B.; Nai, S.M.L.; Lee, B.Y. Microstructure and enhanced strength of laser aided additive manufactured CoCrFeNiMn high entropy alloy. Mater. Sci. Eng. A 2019, 744, 137–144. [Google Scholar] [CrossRef]
- Lee, C.; Song, G.; Gao, M.C.; Feng, R.; Chen, P.; Brechtl, J.; Chen, Y.; An, K.; Guo, W.; Poplawsky, J.D.; et al. Lattice distortion in a strong and ductile refractory high-entropy alloy. Acta Mater. 2018, 160, 158–172. [Google Scholar] [CrossRef]
- Li, R.; Niu, P.; Yuan, T.; Cao, P.; Chen, C.; Zhou, K. Selective laser melting of an equiatomic CoCrFeMnNi high-entropy alloy: Processability, non-equilibrium microstructure and mechanical property. J. Alloys Compd. 2018, 746, 125–134. [Google Scholar] [CrossRef]
- Zhao, P.; Li, J.; Zhang, Y.; Li, X.; Xia, M.M.; Yuan, B.G. Wear and high-temperature oxidation resistances of AlNbTaZrx high-entropy alloys coatings fabricated on Ti6Al4V by laser cladding. J. Alloys Compd. 2021, 862, 158405. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Vilar, R.; Shen, J. Dry sliding wear behavior of laser clad TiVCrAlSi high entropy alloy coatings on Ti–6Al–4V substrate. Mater. Des. 2012, 41, 338–343. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Z.; Wu, M.; Weng, F.; Liu, T.; Zhan, X. Influence of Specific Energy on Microstructure and Properties of Laser Cladded FeCoCrNi High Entropy Alloy. Metals 2020, 10, 1464. [Google Scholar] [CrossRef]
- Juan, Y.; Li, J.; Jiang, Y.; Jia, W.; Lu, Z. Wear-resistant single-phase high-entropy alloy coatings prepared on Ti6Al4V with laser cladding. Mater. Tehnol. 2019, 53, 751–758. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Jiang, Y.; Kang, D.; Juan, Y.; Lu, Z. Investigation into corrosion and wear behaviors of laser-clad coatings on Ti6Al4V. Mater. Res. Express 2020, 7, 016587. [Google Scholar] [CrossRef]
- Park, B.J.; Chang, H.J.; Kim, D.H.; Kim, W.T. In situ formation of two amorphous phases by liquid phase separation in Y–Ti–Al–Co alloy. Appl. Phys. Lett. 2004, 85, 6353–6355. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Physics 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Akira, T.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Gharahshiran, M.R.K.; Khoshakhlagh, A.; Khalaj, G.; Bakhtiari, H.; Banihashemi, A.R. Effect of postweld heat treatment on interface microstructure and metallurgical properties of explosively welded bronze—carbon steel. J. Cent. South Univ. 2018, 25, 1849–1861. [Google Scholar] [CrossRef]
- Shim, S.H.; Pouraliakbar, H.; Hong, S.I. Hierarchical structured as-cast CrFeNiMn0.5Cu0.5 high entropy alloy with excellent tensile strength/ductility properties. Scr. Mater. 2022, 210, 114473. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Ríos, C.T.; Antunes, R.A. Electronic properties of the passive films formed on CoCrFeNi and CoCrFeNiAl high entropy alloys in sodium chloride solution. J. Mater. Res. Technol. 2020, 9, 13879–13892. [Google Scholar] [CrossRef]
- Lyu, P.; Gao, Q.; Peng, T.; Yuan, H.; Guan, Q.; Cai, J.; Liu, H.; Liu, X. Microstructure and properties of CoCrFeNiAlx (x = 0.1, 0.5, 1) high-entropy alloys enhanced by laser surface remelting. Mater. Charact. 2022, 185, 111717. [Google Scholar] [CrossRef]
- Rashid, R.A.R.; Abaspour, S.; Palanisamy, S.; Matthews, N.; Dargusch, M.S. Metallurgical and geometrical charac-terisation of the 316L stainless steel clad deposited on a mild steel substrate. Surf. Coat. Technol. 2017, 327, 174–184. [Google Scholar] [CrossRef]
- Gao, P.; He, D.Q.; Zheng, S.X.; Pu, J.B.; Guo, F. In situ study on friction-corrosion interaction of carbon-based and nitride-based coatings. J. Tribol. 2015, 35, 138–146. [Google Scholar] [CrossRef]








| ΔHmix | Ti | Co | Cr | Fe | Ni | Al | C |
|---|---|---|---|---|---|---|---|
| Ti | - | - | - | - | - | - | - |
| Co | −28 | - | - | - | - | - | - |
| Cr | −7 | −4 | - | - | - | - | - |
| Fe | −17 | −1 | −1 | - | - | - | - |
| Ni | −35 | 0 | −7 | −2 | - | - | - |
| Al | −30 | −19 | −10 | −11 | −22 | - | - |
| C | −109 | −42 | −61 | −50 | −39 | −39 | - |
| Coatings | Marked Zones | Elements | ||||||
|---|---|---|---|---|---|---|---|---|
| Ti | C | Co | Cr | Fe | Ni | Al | ||
| CoCrFeNi | A | 62.61 | 2.61 | 4.64 | 9.03 | 5.46 | 3.69 | 11.95 |
| B | 60.95 | 1.68 | 5.26 | 6.85 | 5.13 | 13.53 | 6.60 | |
| C | 49.93 | 1.44 | 10.51 | 3.92 | 6.87 | 18.46 | 8.87 | |
| D | 72.43 | 11.36 | 3.45 | 2.25 | 3.03 | 3.41 | 4.07 | |
| CoCrFeNiAl | E | 60.29 | 1.49 | 3.78 | 6.08 | 4.11 | 3.84 | 20.42 |
| F | 54.71 | 1.16 | 7.39 | 4.96 | 6.78 | 7.39 | 17.37 | |
| G | 76.94 | 11.62 | 1.76 | 1.04 | 1.42 | 2.18 | 5.04 | |
| Samples | Ecorr/V | Ea-s/V | Ea-s − Ecorr/V | icorr/A∙cm−2 | is/A∙cm−2 |
|---|---|---|---|---|---|
| Substrate | −0.582 | −0.070 | 0.512 | 6.620 × 10−6 | 1.436 × 10−4 |
| x = 0 | −0.524 | −0.299 | 0.225 | 2.249 × 10−7 | 1.021 × 10−6 |
| x = 1.0 | −0.393 | −0.231 | 0.161 | 1.260 × 10−7 | 2.506 × 10−7 |
| Samples | 2 h | 4 h | 6 h | 8 h | 10 h |
|---|---|---|---|---|---|
| Substrate | 2.09 × 10−3 | 1.67 × 10−3 | 1.19 × 10−3 | 9.17 × 10−4 | 7.44 × 10−4 |
| x = 0 | 7.88 × 10−4 | 4.77 × 10−4 | 3.34 × 10−4 | 3.24 × 10−4 | 2.65 × 10−4 |
| x = 1.0 | 7.82 × 10−4 | 4.21 × 10−4 | 2.94 × 10−4 | 2.58 × 10−4 | 2.54 × 10−4 |
| Samples | Times (h) | Zones | Elements | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | C | Co | Cr | Fe | Ni | Al | V | O | |||
| Substrate | 2 | A | 83.58 | 0.53 | – | – | – | – | 10.52 | 3.56 | 1.81 |
| B | 79.50 | 0.91 | – | – | – | – | 11.12 | 4.32 | 4.15 | ||
| 10 | C | 79.54 | 0.95 | – | – | – | – | 11.12 | 2.94 | 5.45 | |
| D | 73.03 | 0.91 | – | – | – | – | 8.25 | 3.48 | 14.33 | ||
| x = 1.0 | 2 | F | 57.82 | 1.26 | 4.20 | 3.44 | 3.17 | 4.38 | 3.94 | 2.19 | 19.59 |
| E | 65.16 | 0.70 | 4.87 | 4.63 | 5.85 | 5.75 | 8.05 | 3.00 | 1.99 | ||
| 10 | G | 51.11 | 1.40 | 1.52 | 5.38 | 1.26 | 1.97 | 3.88 | 1.37 | 32.12 | |
| H | 58.32 | 0.70 | 5.18 | 8.58 | 6.17 | 4.47 | 10.79 | 3.57 | 2.22 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Li, J.; Xu, Y. Investigation into the Corrosion Wear Resistance of CoCrFeNiAlx Laser-Clad Coatings Mixed with the Substrate. Metals 2022, 12, 460. https://doi.org/10.3390/met12030460
Li W, Li J, Xu Y. Investigation into the Corrosion Wear Resistance of CoCrFeNiAlx Laser-Clad Coatings Mixed with the Substrate. Metals. 2022; 12(3):460. https://doi.org/10.3390/met12030460
Chicago/Turabian StyleLi, Wanggen, Jun Li, and Yinsi Xu. 2022. "Investigation into the Corrosion Wear Resistance of CoCrFeNiAlx Laser-Clad Coatings Mixed with the Substrate" Metals 12, no. 3: 460. https://doi.org/10.3390/met12030460






