Characterization of a Medium Mn-Ni Steel Q&P Treated by a High Partitioning Temperature Cycle
Abstract
:1. Introduction
- The shift of the martensitic transformation temperature to a lower value, allowing the selection of near room temperature as the quenching stop temperature (QT). In this approach, the key is to obtain the optimum austenite volume fraction and stability with room temperature quenching [18,19,20]. The benefit is clear in terms of process control, as the control of the quenching temperature during Q&P to higher temperatures requires sophisticated cooling equipment.
- The stabilization of austenite not only by carbon, but also by Mn enrichment of austenite. Early assumptions considered that the Q&P process implies the partitioning of only carbon from martensite to austenite by diffusion through an immobile martensite/austenite interface. However, a later study considered the possible interface migration during partitioning, highlighting the importance of interface migration to adjust the retained austenite fraction [21]. Afterward, many publications experimentally analyzed and modeled the martensite/austenite interface migration and the formation of austenite and provided evidence for the partitioning of both interstitial carbon and substitutional Mn during the Q&P processing [22,23,24,25]. In these publications, the Mn content was equal to or higher than 3 wt.% and the partitioning temperature (PT) was limited to 450 °C. It was observed that the partitioning of Mn only occurred a few nanometers away from the interface. In [21], it was presumed that at elevated partitioning temperatures, interface migration was likely to be of the greatest importance due to the higher mobility of iron and substitutional alloying elements.
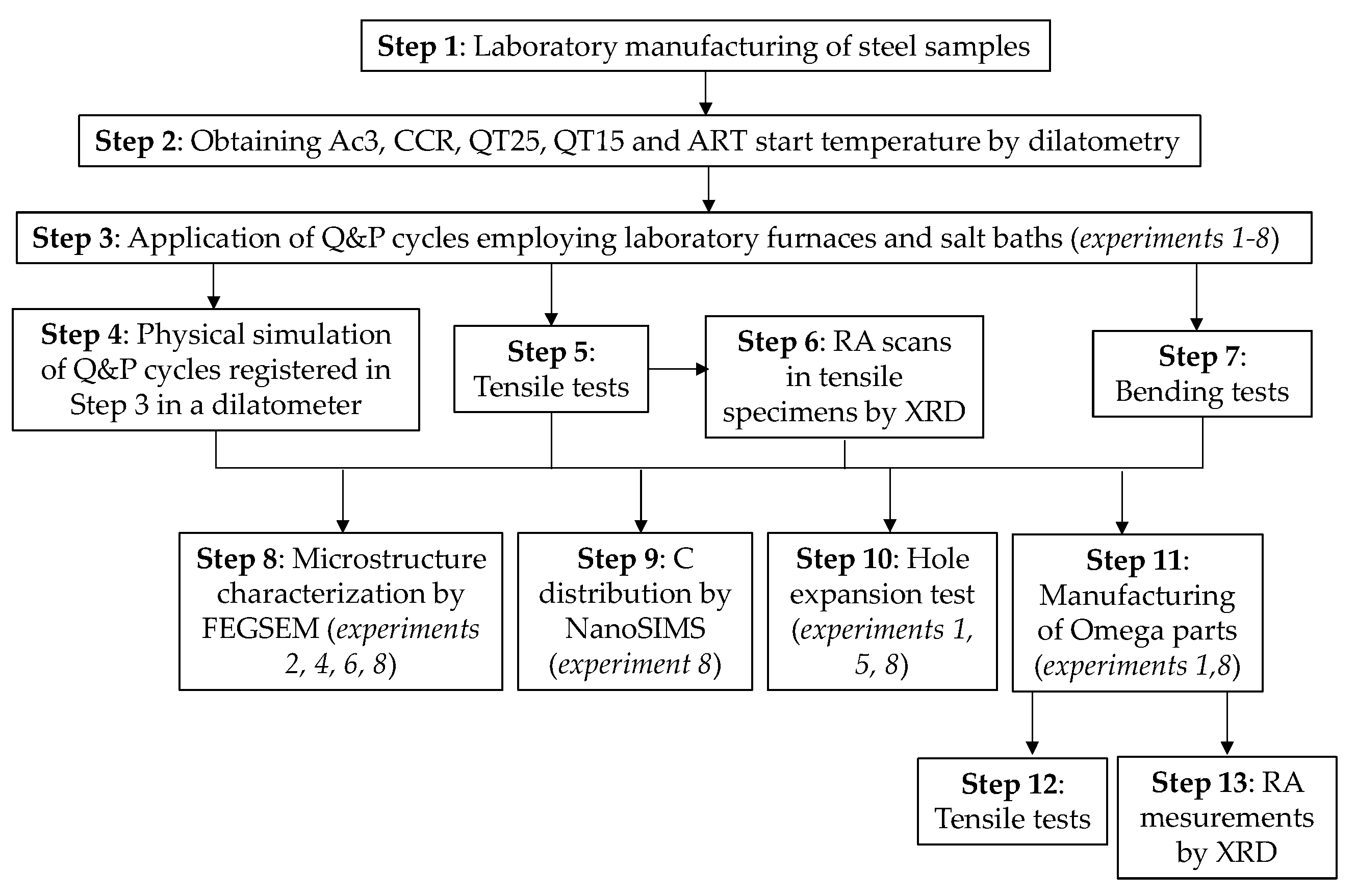
2. Materials and Methods
3. Results
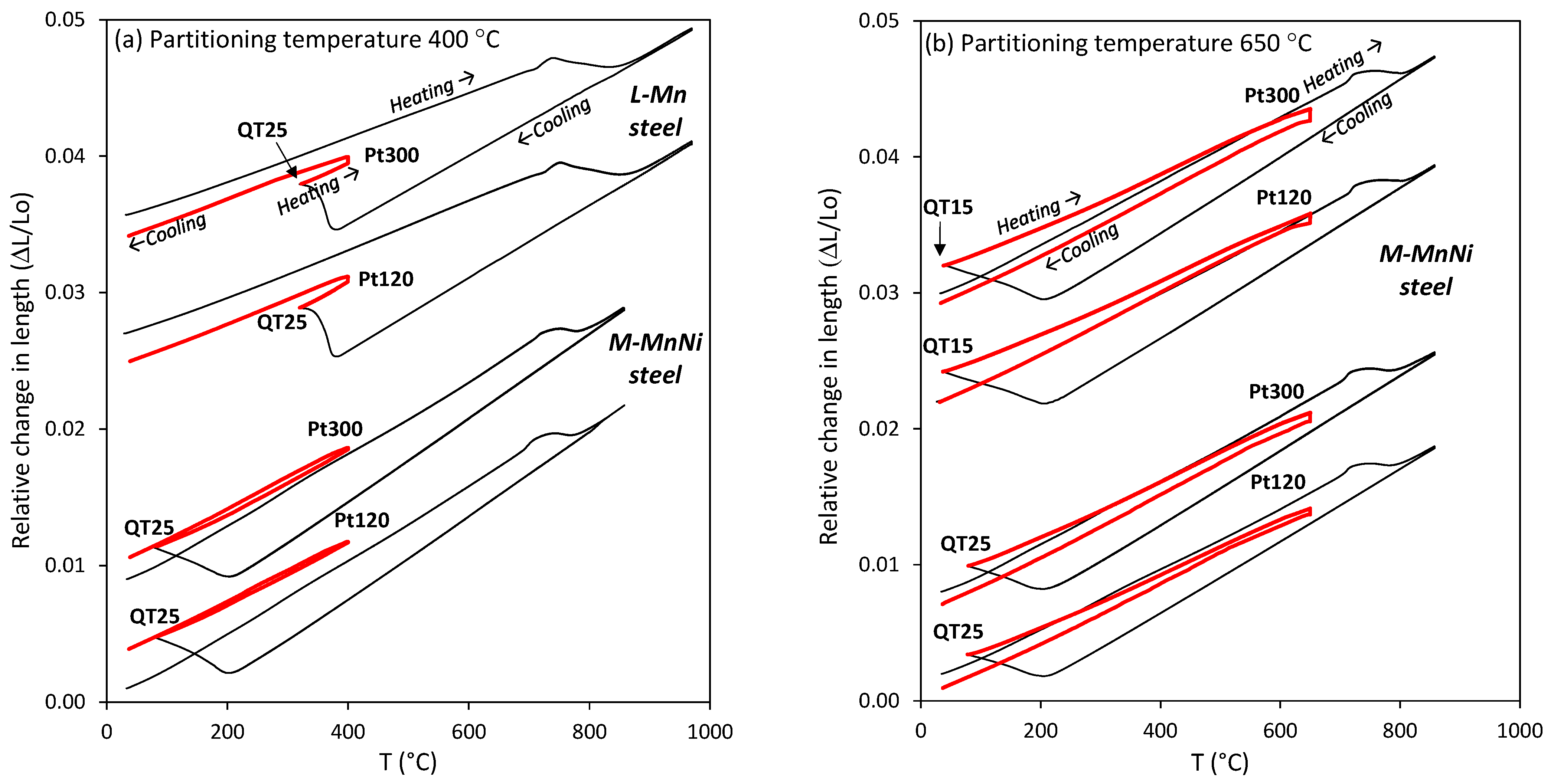
3.1. Dilatometry Curves of the Q&P Heat Treatments
3.2. Microstructure after Q&P Heat Treatments
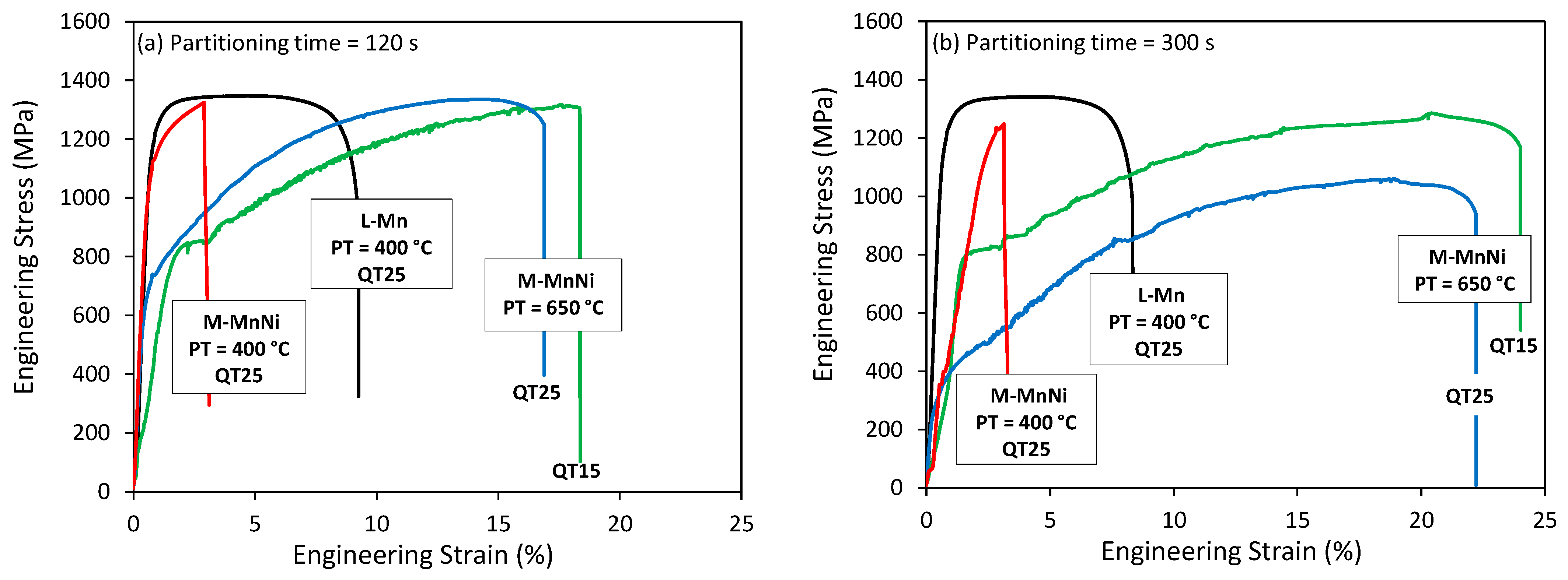
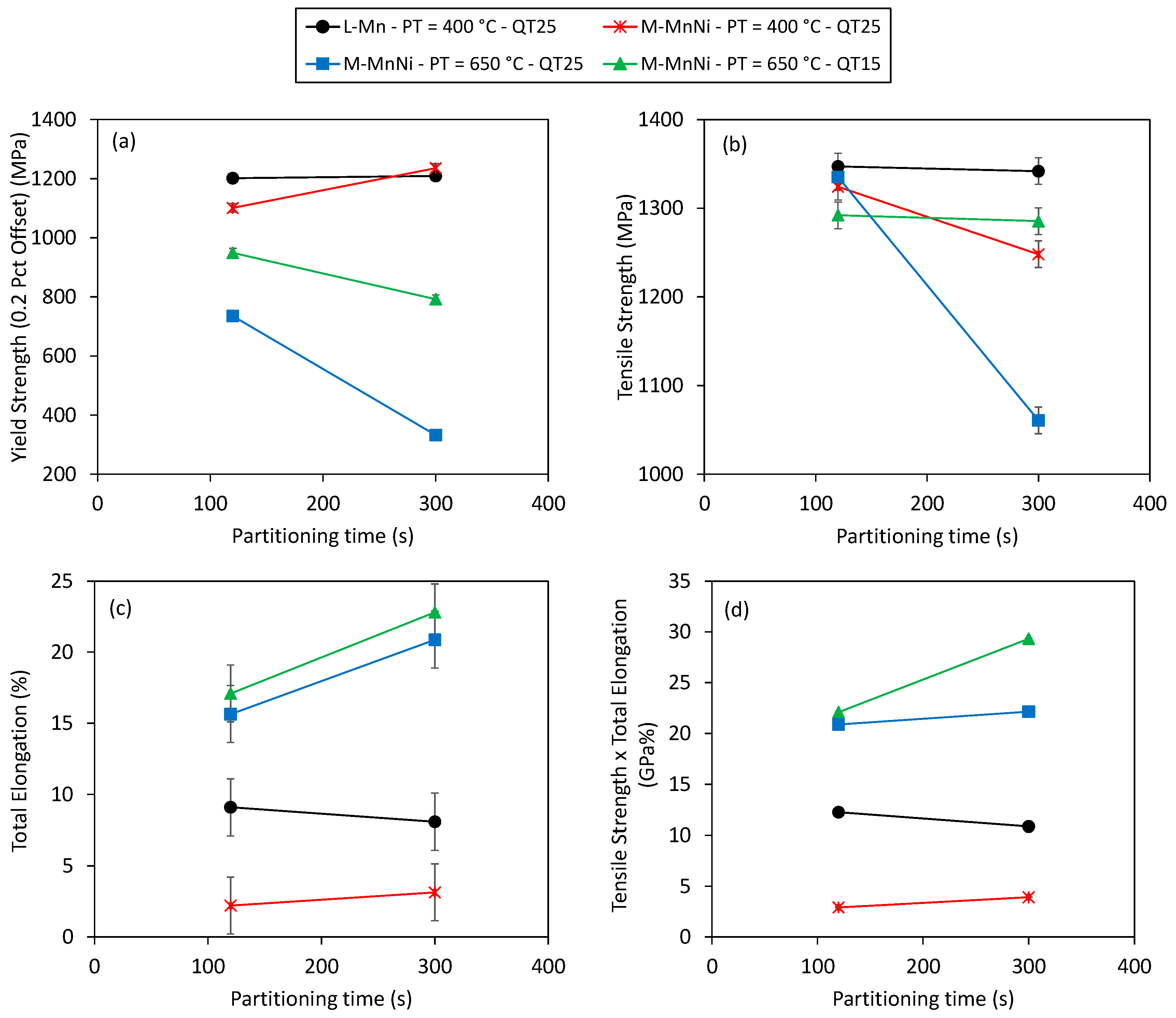
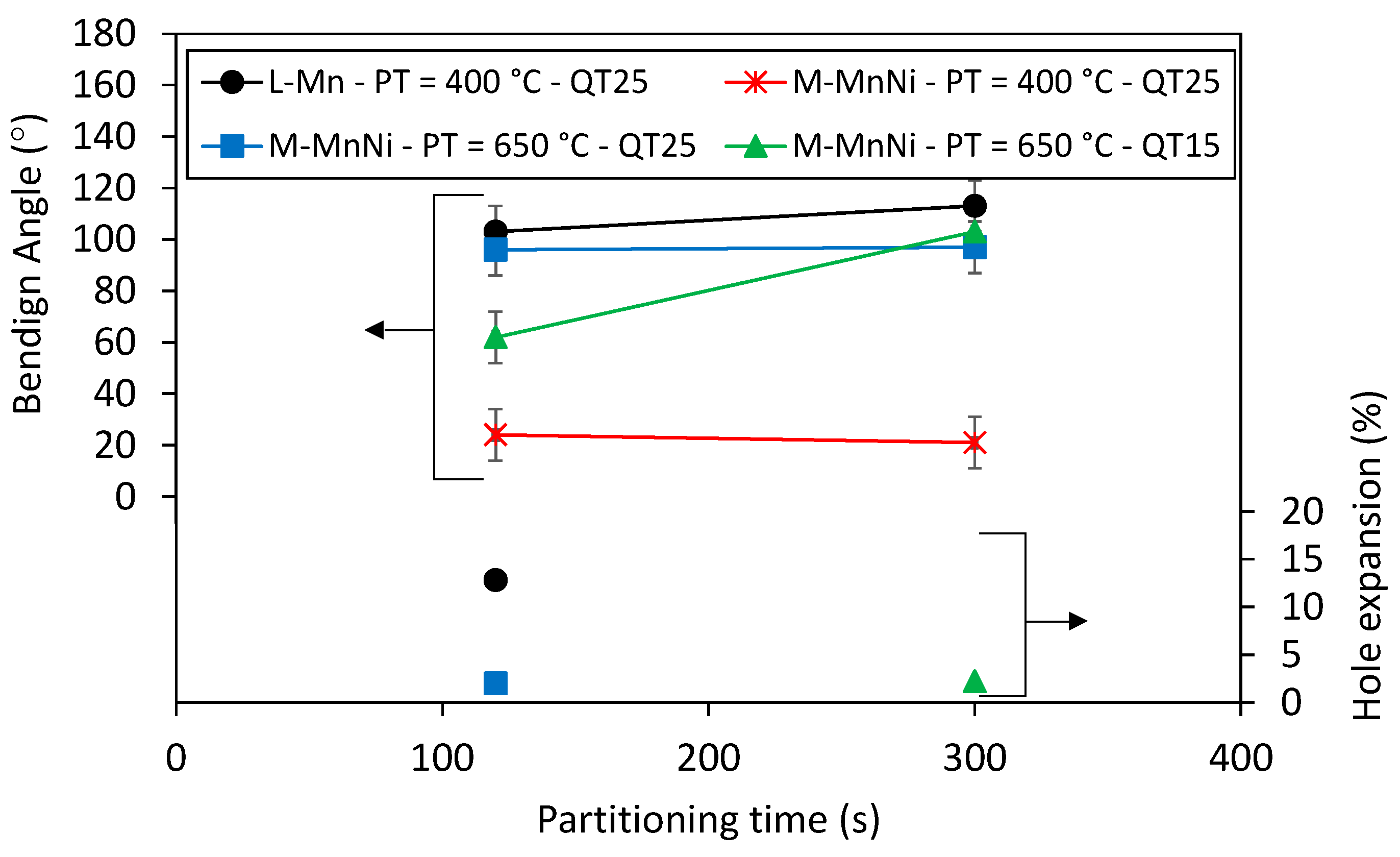
3.3. Mechanical Properties after Q&P Thermal Treatments
- Tensile curves indicated good global formability, which denotes the ability of the material to undergo plastic deformation without formation of a localized neck or distributing strain uniformly.
- Tensile strength values were not significantly affected, while yield strength showed a noteworthy reduction. The decrease in yield strength was particularly strong for the QT25 and 300 s partitioning time condition. The lower yield strength can be detrimental for certain automotive applications that require anti-intrusion capacity. The low yield to tensile strength ratio can also be related to poor hole expansion capacity.
- Total elongation values after high partitioning temperature treatments were notably higher, ranging from 16% to 23%. In this case, the values were higher for the QT15 condition and increased with the partitioning time. The product of tensile strength and total elongation revealed outstanding properties after high PT treatments, particularly in the QT15 and 300 s partitioning time condition, which resulted in 29 GPa%. In comparison, the QT25 condition resulted in a lower product of tensile strength and total elongation close to 22 GPa%, which is a more common value for the standard TRIP grades [40].
- Continuous yielding was observed in the QT25 condition, whereas discontinuous yielding appeared in the QT15 condition. Among all Advanced High-Strength Steels, intercritically annealed medium Mn steels most frequently present discontinuous yielding [41,42]. In addition to discontinuous yielding, the Portevin–Le Chatelier (PLC) effect was another plastic instability observed in M-MnNi steel after high partitioning temperature treatment. This effect is characterized by serrated plastic flow in the tensile curves and normally is proposed to be the consequence of dynamic strain aging (DSA) [43].
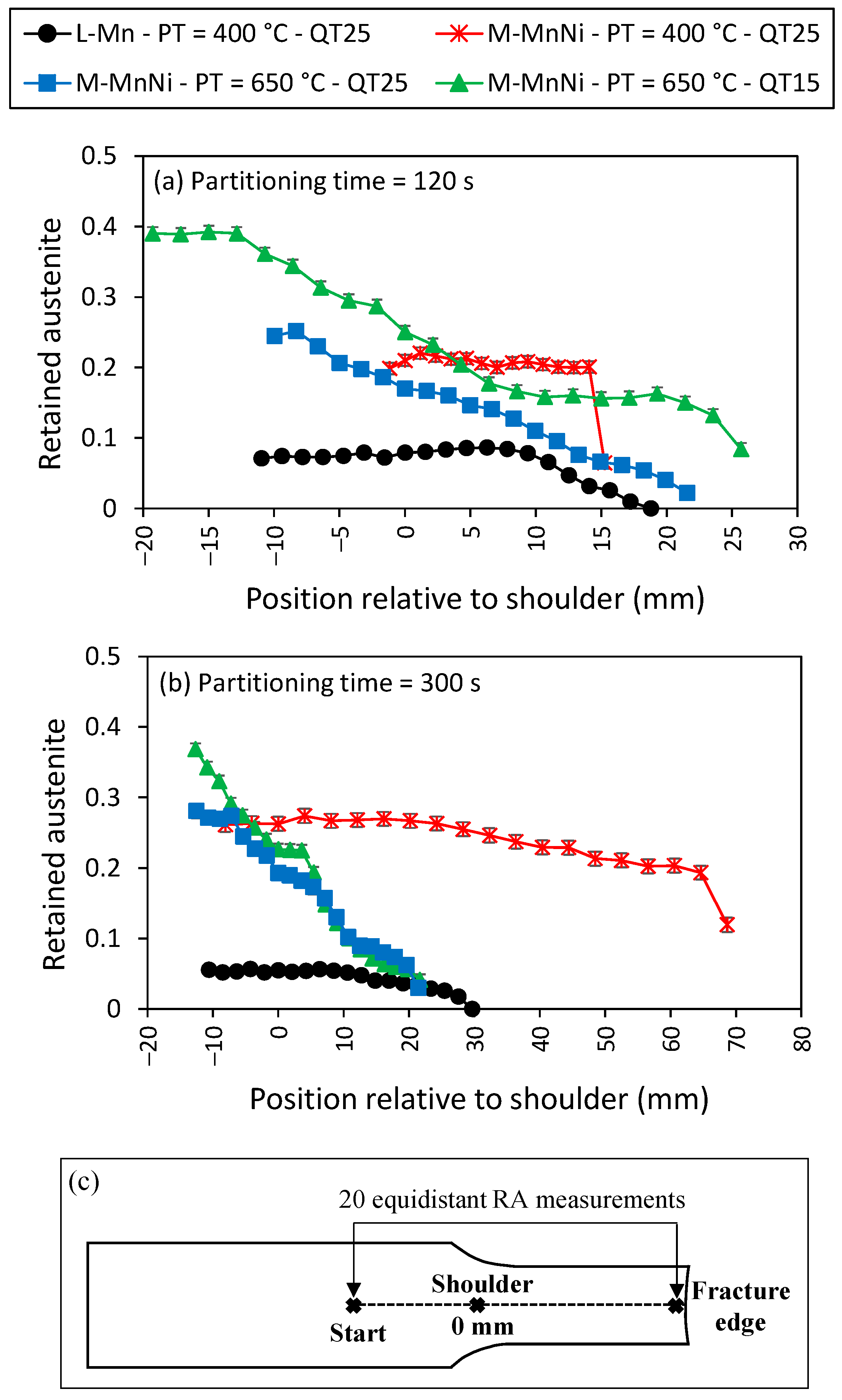
3.4. Retained Austenite Volume Fraction along Tensile Specimen Length
- The line scan for L-Mn steel shows a very flat retained austenite profile on the specimen head and radius, and then a continuous decrease in austenite content to 0% at the fracture tip. The reduction of austenite only in the plastically deformed zone of the samples suggests that the austenite transformation was induced solely by plastic strain.
- In contrast, the M-MnNi steel with the high temperature partitioning cycle shows a progressive decrease in austenite content from the head of the specimen, through the shoulder and on the plastically deformed gauge length. This behavior is common for the two quenching temperature conditions considered. Given that the shoulder and head of the tensile specimen only experienced elastic stresses during the tensile tests, the transition in austenite content on the head and radius was interpreted as being the result of stress-induced transformation, with the austenite reduction to the fracture tip being related to strain-induced transformation.
- The third distinct case was exemplified by M-MnNi steel given a low-temperature partitioning cycle, which showed an almost completely constant austenite content from head to within 1 mm of the fracture tip, when there is a sharp drop in the austenite fraction from 0.20 to below 0.07 and from 0.19 to 0.11, in 120 s and 300 s partitioning time conditions, respectively. As shown before, M-MnNi steel exhibited, in the low PT condition, low ductility and high yield strength (Figure 9), so it is likely that the only transformation mechanism that could operate is stress-induced transformation.
3.5. Characterization of Cold-Formed Q&P Omega Parts
4. Discussion
4.1. Microstructure Evolution during Q&P Cycles
4.1.1. Microstructure Evolution during Low Partitioning Temperature Cycles (PT = 400 °C)
4.1.2. Microstructure Evolution during High Partitioning Temperature Cycles (PT = 650 °C)
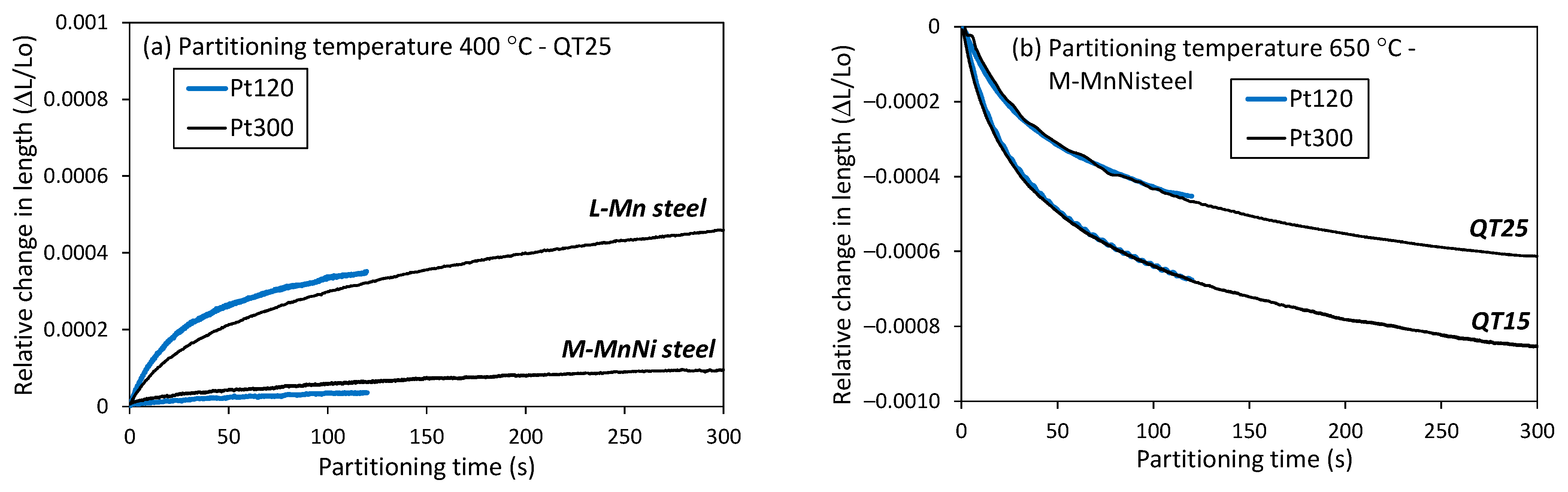
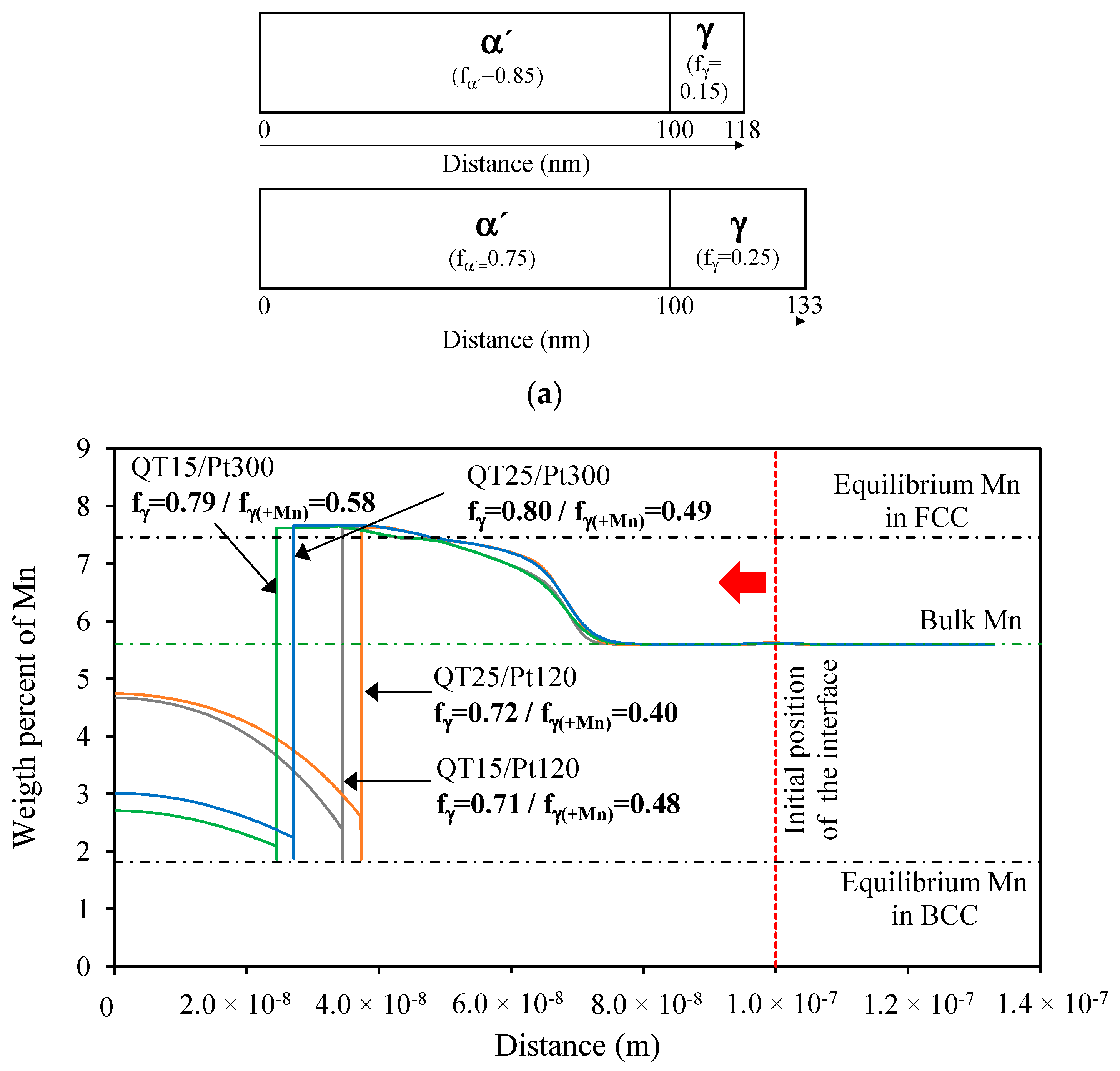
- The comparison among the dilatometry traces obtained with the two QT conditions (Figure 5b) reveals that the lower initial austenite volume fraction (QT15 condition) resulted in a more pronounced contraction of the dilatometry sample. This means that the amount of newly formed austenite was higher in QT15. Ding et al. [29] also found that the contraction in partitioning increased with a decreasing amount of pre-existing austenite. In any case, the curves revealed that equilibrium was not reached in any of the conditions applied, as contraction continuously decreased without showing a plateau.
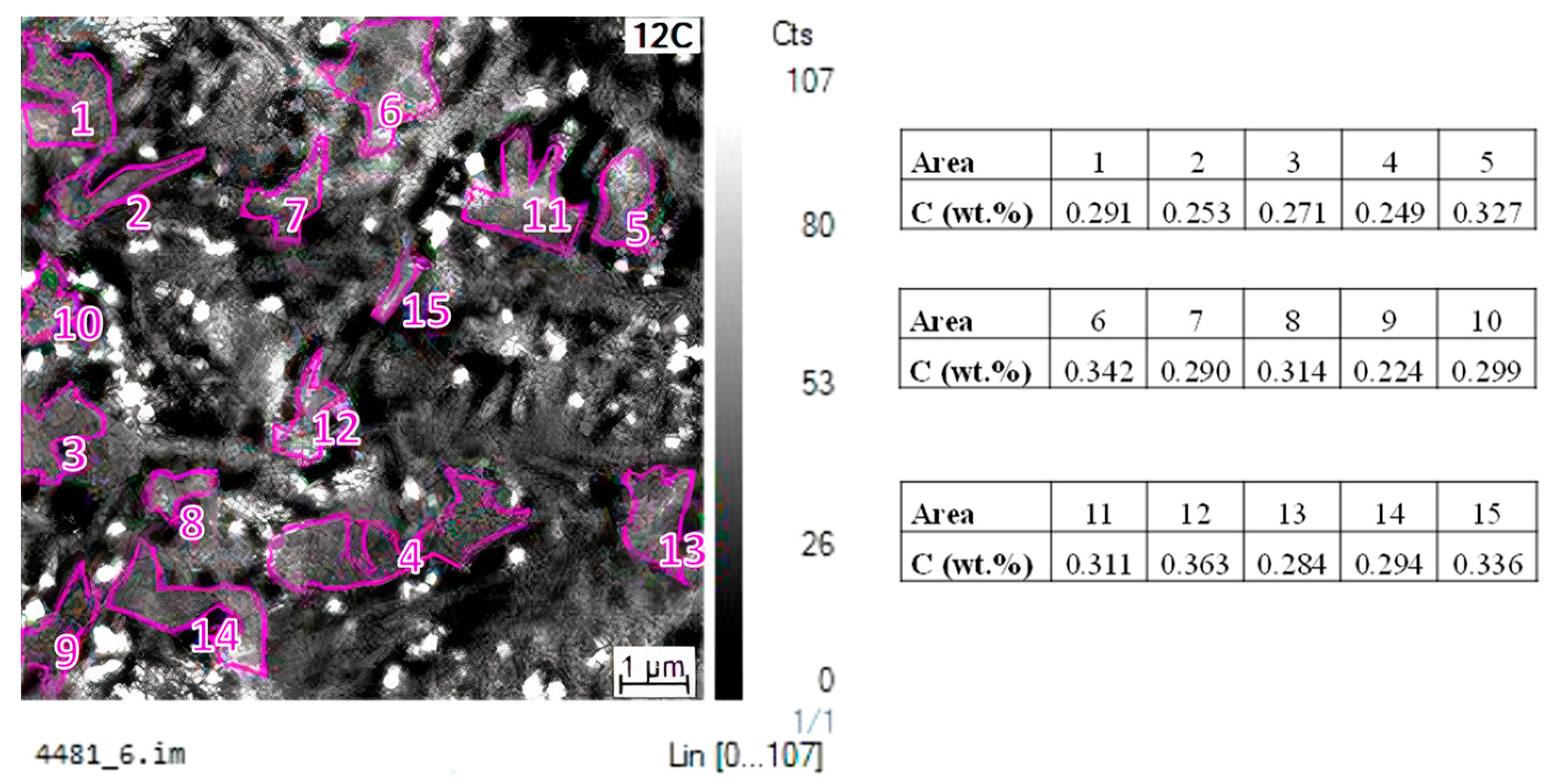
- After partitioning, secondary martensite transformation was not observed, regardless of the QT condition. However, FEGSEM images revealed the formation of secondary martensite, particularly for the QT25 condition. This means that the fraction was low and/or that the transformation occurred below the temperature where the dilatometry ended, which was around 35 °C. Moreover, the QT15 condition was expected to result in less secondary martensite formed in the final cooling. This can be explained based on the influence of the initial austenite size on Mn partitioning, as follows. The measured carbon content seemed to be rather low to stabilize such an amount of austenite at room temperature. This means that there are other factors that allow such stabilization. In many publications on medium Mn steels, the austenite stability is explained by Mn partitioning between ferrite and austenite during long-term soaking in the intercritical temperature range [4,8,47]. However, some investigations demonstrate that Mn partitioning occurs also in short annealing periods. De Moor et al. [48] pointed out that the high density of dislocations and the grain boundaries should facilitate Mn diffusion. In [13], the concentration of Mn in austenite reached 11.6 wt.% after annealing for a short time of 180 s in 6 wt.% Mn-containing steel. Therefore, in this work, a certain Mn partitioning could be expected, contributing to austenite stabilization. Additionally, the relatively strong partitioning of Mn is also facilitated by the small size of austenite grains (short diffusion paths). Based on this, the finer austenite grains in the QT15 condition could be favorable for Mn partitioning and result in more effective austenite stabilization. Finally, the finer structure at QT15 is expected to result in a finer austenite after partitioning, and hence in a more stable austenite [49].
4.2. Analysis of Mn and Ni Partitioning Using DICTRA
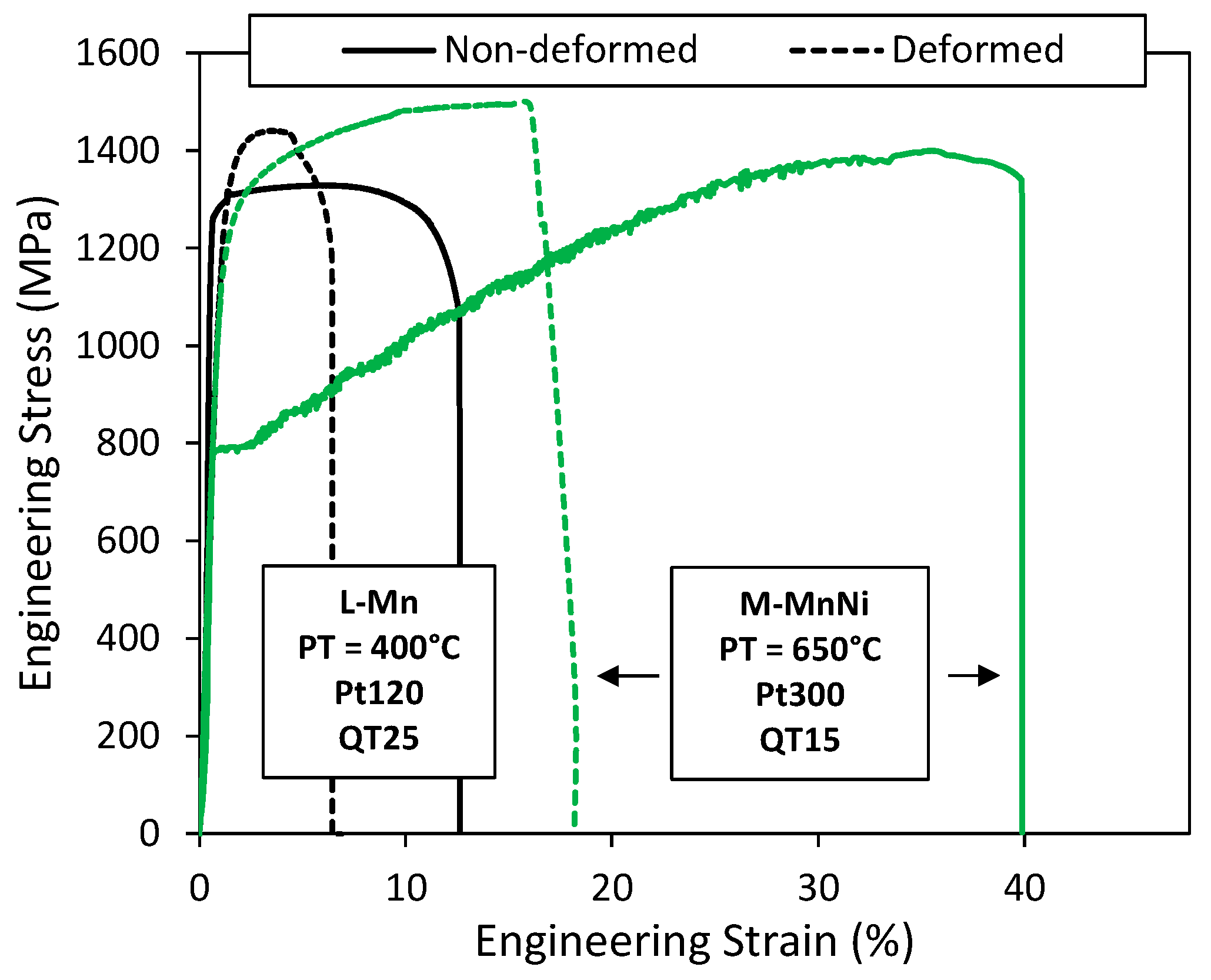
4.3. Mechanical Stability of Austenite
- In L-Mn steel, the austenite fraction was only 0.08 before deformation; therefore, very little stress-assisted transformation was estimated to have occurred, and almost all the austenite appeared to be consumed by strain-induced transformation above the yield stress. This is probably close to ideal behavior, in that the austenite that is present appears to be progressively consumed during plastic straining, without transformation at lower stress levels.
- In M-MnNi steel at high PT, retained austenite content was rather high before deformation. Under mechanical loading, significant stress-induced transformation occurred below the yield stress. Thus, stress-assisted austenite transformation contributed to the initial yielding behavior, resulting in a low yield strength. The austenite, which was stable at stresses above the yield stress, provided significant TRIP-assisted ductility. However, probably, the overall austenite stability was lower than in the case of low-temperature partitioning cycles.
- The transformation stability of austenite is affected by the local carbon content in austenite, the grain size, morphology, the constraining effect of the phases surrounding the austenite and the crystallographic orientation of the austenite with respect to the loading direction [60,61,62]. In this case, the carbon content was significantly lower than in L-Mn steel, which likely strongly determined the mechanical stability. Additionally, Hidalgo et al. [63] demonstrated that austenite surrounded by a stronger martensite matrix was mechanically more stable than austenite surrounded by a weaker martensite. Thereby, in the present case, the weaker martensite at higher temperatures would likely also contribute to decreasing austenite stability. The grain size effect on transformation driving force and austenite stability is poorly understood for small austenite grain sizes and non-polygonal austenite, both of which are present in these types of Q&P/austenite reversion annealing microstructures. As a result, more theoretical work is required before a robust material design framework can be made available to allow the optimization of austenite’s mechanical stability.
- In M-MnNi steel at low PT, given the observed extremely sharp transition in austenite to the fracture tip, it was hypothesized that local high elastic stresses around microcracks or other damage features produced local massive stress-induced transformations and an early fracture.
4.4. Relationship between Mechanical Properties and Microstructure
5. Conclusions
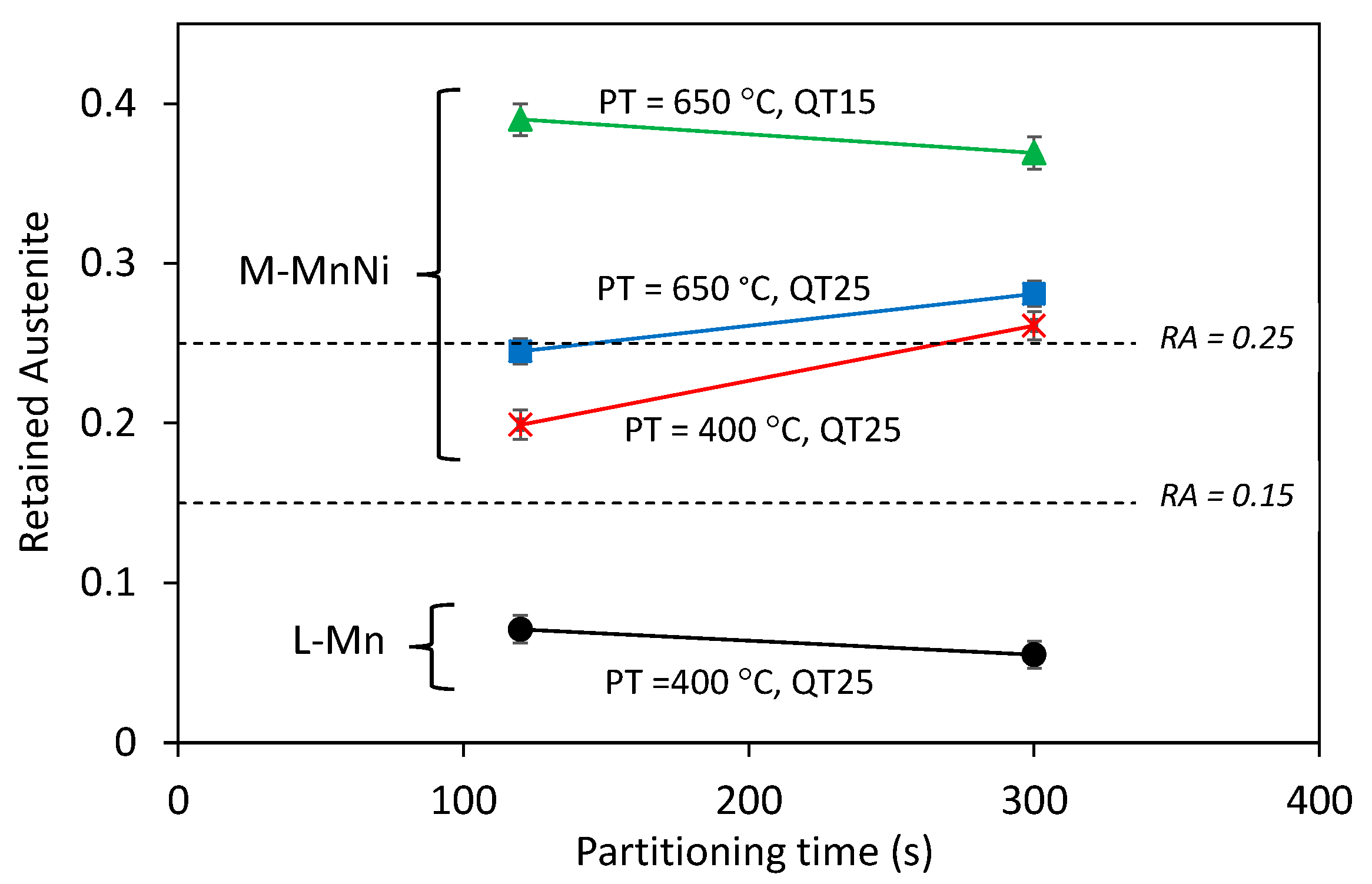
- High volume fractions of austenite, in the range of 0.25–0.40, were obtained after short partitioning times, which was explained by the fact that new austenite could form not only by nucleating new grains but also by the direct growth of initial austenite, enhancing the kinetics of austenite reversion.
- Quenching temperature strongly influenced both the final microstructure features and the austenite’s thermal stability. The lowest QT condition led to a final microstructure with more and finer retained austenite and finer MA islands.
- The medium Mn-Ni steel and associated high partitioning temperature Q&P cycles resulted in very promising tensile strength/elongation balances. The best results (29 GPa%) were obtained in the lowest QT condition. Under mechanical loading, significant stress-induced transformation was expected to have occurred below the yield stress. The austenite that was stable at stresses above the yield stress provided significant TRIP-assisted ductility. However, the overall austenite stability was considered to be relatively low due to the low carbon content of austenite and the constraining effect of the weaker surrounding martensite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matlock, D.K.; Speer, J.G.; De Moor, E.; Gibss, P. Recent developments in advanced high strength sheet steels for automotive applications: An overview. Jestech 2012, 15, 1–12. [Google Scholar]
- Radhakanta, R. Special issue on medium manganese steels. Mater. Sci. Technol. 2019, 35, 2039–2044. [Google Scholar] [CrossRef]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Miller, R.L. Ultrafine-Grained Microstructures and Mechanical Properties of Alloy Steels. Metall. Trans. 1972, 3, 905–912. [Google Scholar] [CrossRef]
- Furukawa, T. Dependence of strength—Ductility characteristics on thermal history in low carbon 5 wt.% Mn steels. Mater. Sci. Technol. 1989, 5, 465–470. [Google Scholar] [CrossRef]
- Gibbs, P.J.; De Moor, E.; Merwin, M.J.; Clausen, B.; Speer, J.G.; Matlock, D.K. Austenite stability effects on tensile behavior of manganese-enriched-austenite transformation-induced plasticity steel. Metall. Mater. Trans. A 2011, 42A, 3691–3702. [Google Scholar] [CrossRef]
- Arlazarov, A.; Gouné, M.; Bouaziz, O.; Hazotte, A.; Petitgand, G.; Barges, P. Evolution of microstructure and mechanical properties of medium Mn during double annealing. Mater. Sci. Eng. A 2012, 542, 31–39. [Google Scholar] [CrossRef]
- Kim, D.E.; Park, Y.K.; Lee, O.Y.; Jin, K.G.; Kim, S.J. Formation of retained austenite and mechanical properties of 4~8%Mn hot rolled TRIP steels. Korean J. Mater. Res. 2005, 15, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Merwin, M.J. Microstructure and properties of cold rolled and annealed low carbon manganese TRIP steels. In Proceedings of the AIST Steel Properties & Applications Conference, Detroit, MI, USA, 16–20 September 2007. [Google Scholar]
- Shi, J.; Sun, X.; Wang, M.; Hui, W.; Dong, H.; Cao, W. Enhanced work-hardening behavior and mechanical properties in ultrafine-grained steels with large-fractioned metastable austenite. Scr. Mater. 2010, 63, 815–818. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Bokinala, P.K.; Mitra, R.; Chakrabarti, D. Relative effect of C and Mn on strength-toughness of medium Mn steels. Mater. Sci. Technol. 2019, 35, 55–67. [Google Scholar] [CrossRef]
- Du, P.J.; Yang, D.P.; Bai, M.K.; Xiong, X.C.; Wu, D.; Wang, G.D.; Yi, H.L. Austenite stabilisation by two step partitioning of manganese and carbon in a Mn-TRIP steel. Mater. Sci. Technol. 2019, 35, 2084–2091. [Google Scholar] [CrossRef]
- Lee, S.; De Cooman, B.C. On the Selection of the Optimal Intercritical Annealing Temperature for Medium Mn TRIP Steel. Metall. Mater. Trans. A 2011, 44, 5018–5024. [Google Scholar] [CrossRef] [Green Version]
- Fonstein, N. Advanced High Strength Sheet Steels, Physical Metallurgy, Design, Processing and Properties; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- De Moor, E.; Matlock, D.K.; Speer, J.G.; Fojer, C.; Penning, J. Comparison of Hole Expansion Properties of Quench & Partitioned, Quenched & Tempered and Austempered Steels; SAE Technical Paper Series. In Proceedings of the SAE International 2012 World Congress & Exhibition, Detroit, MI, USA, 24–26 April 2012. [Google Scholar]
- Santofimia, M.; Zhao, L.; Petrov, R.; Kwakernaak, C.; Sloof, W.G.; Sietsma, J. Microstructural development during the quenching and partitioning process in a newly designed low carbon steel. Acta Mater. 2011, 59, 6059–6068. [Google Scholar] [CrossRef]
- De Moor, E.; Speer, J.G.; Matlock, D.K.; Kwak, J.-H.; Lee, S.-B. Effect of carbon and manganese on the quenching and partitioning response of CMnSi steels. ISIJ Int. 2011, 51, 137–144. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Liu, L.; Huang, M. Room temperature quenching and partitioning steel. Metall. Mater. Trans. A 2018, 49, 3167–3172. [Google Scholar] [CrossRef]
- Hou, Z.; Zhao, X.; Zhang, W.; Liu, H.; Yi, H. A medium manganese steel designed for water quenching and partitioning. Mater. Sci. Technol. 2018, 34, 1168–1175. [Google Scholar] [CrossRef]
- He, B.B.; Pan, S.; Huang, M.X. Extra work hardening in room-temperature quenching and partitioning medium Mn steel enabled by intercritical annealing. Mater. Sci. Eng. A 2020, 797, 140106. [Google Scholar] [CrossRef]
- Speer, J.G.; Hackenberg, R.E.; De Cooman, B.C.; Matlock, D.K. Influence of interface migration during annealing of martensite/austenite mixtures. Philos. Mag. Lett. 2007, 87, 379–382. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Povstugar, I.; Choi, P.-P.; Raabe, D.; Sietsma, J. Carbon redistribution in a quenched and partitioned steel analysed by atom probe tomography. In Proceedings of the 3rd International Symposium on Steel Science, The Iron and Steel Institute of Japan, Kyoto, Japan, 27–30 May 2012; pp. 155–158. [Google Scholar]
- De Knijf, D.; Santofimia, M.J.; Shi, H.; Bliznuk, V.; Föjer, C.; Petrov, R.; Xu, W. In situ austenite–martensite interface mobility study during annealing. Acta Mater. 2015, 90, 161–168. [Google Scholar] [CrossRef]
- Seo, E.J.; Cho, L.; De Cooman, B.C. Kinetics of the partitioning of carbon and substitutional alloying elements during quenching and partitioning (Q&P) processing of medium Mn steel. Acta Mater. 2016, 107, 354–365. [Google Scholar] [CrossRef]
- Thomas, G.A.; Speer, J.G. Interface migration during partitioning of Q&P steel. Mater. Sci. Technol. 2014, 30, 998–1007. [Google Scholar] [CrossRef]
- Ayenampudi, S.; Celada-Casero, C.; Sietsma, J.; Santofimia, M.J. Microstructure evolution during high-temperature partitioning of a medium-Mn quenching and partitioning steel. Materialia 2019, 8, 100492. [Google Scholar] [CrossRef]
- Arribas, M.; Gutiérrez, T.; Del Molino, E.; Arlazarov, A.; De Diego-Calderón, I.; Martin, D.; De Caro, D.; Ayenampudi, S.; Santofimia, M.J. Austenite Reverse Transformation in a Q&P Route of Mn and Ni Added Steels. Metals 2020, 10, 862. [Google Scholar] [CrossRef]
- Ayenampudi, S.; Celada-Casero, C.; Arechabaleta, Z.; Arribas, M.; Arlazarov, A.; Sietsma, J.; Santofimia, M.J. Microstructural Impact of Si and Ni During High Temperature Quenching and Partitioning Process in Medium-Mn Steels. Metall. Mater. Trans. A 2021, 52, 1321–1335. [Google Scholar] [CrossRef]
- Ding, R.; Dai, Z.; Huang, M.; Yang, Z.; Zhang, C.; Chen, H. Effect of pre-existed austenite on austenite reversion and mechanical behavior of a Fe-0.2C-8Mn-2Al medium Mn steel. Acta Mater. 2018, 147, 59–69. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.-J. Effect of Ni addition on the mechanical behavior of quenching and partitioning (Q&P) steel. Mater. Sci. Eng. A 2017, 698, 183–190. [Google Scholar] [CrossRef]
- Pierce, D.T.; Coughlin, D.R.; Clarke, K.D.; De Moor, E.; Poplawsky, J.; Williamson, D.L.; Mazumder, B.; Speer, J.G.; Hood, A.; Clarke, A.J. Microstructural evolution during quenching and partitioning of 0.2C-1.5Mn-1.3Si steels with Cr or Ni additions. Acta Mater. 2018, 151, 454–469. [Google Scholar] [CrossRef]
- Valle, N.; Drillet, J.; Bouaziz, O.; Migeon, H.-N. Study of the carbon distribution in multi-phase steels using the NanoSIMS 50. Appl. Surf. Sci. 2006, 252, 7051–7053. [Google Scholar] [CrossRef]
- McDermid, J.R.; Zurob, H.S.; Bian, Y. Stability of retained austenite in high-Al, low-Si TRIP assisted steels processed via continuous galvanizing heat treatment. Metall. Mater. Trans. A 2011, 42S, 3627–3637. [Google Scholar] [CrossRef]
- Ruhl, R.; Cohen, M. Splat Quenching of Iron-Carbon Alloys. Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers. Metallurgical Society 1969, 245, 241–251. [Google Scholar]
- Dyson, D.; Holmes, B. Effect of alloying additions on the lattice parameter of austenite. J. Iron and Steel Inst. 1970, 208, 469–474. [Google Scholar]
- Clarke, A.J.; Speer, J.G.; Miller, M.K.; Hackenber, R.E.; Edmonds, D.V.; Matlock, D.K.; Rizzo, F.C.; Clarke, K.D.; De Moor, E. Carbon partitioning to austenite from martensite or bainite during the quench and partition (Q&P) process: A critical assessment. Acta Mater. 2008, 56, 16–22. [Google Scholar] [CrossRef]
- Arlazarov, A.; Ollat, M.; Masse, J.P.; Bouzat, M. Influence of partitioning on mechanical behavior of Q&P steels. Mater. Sci. Eng. A 2016, 661, 79–86. [Google Scholar] [CrossRef]
- De Knifj, D.; Petrov, R.; Föjer, C.; Kestens, L.A.I. Effect of fresh martensite on the stability of retained austenite in quenching and partitioning steel. Mater. Sci. Eng. A 2014, 615, 107–115. [Google Scholar] [CrossRef]
- Raabe, D.; Sun, B.; Kwiatkowski Da Silva, A.; Gault, B.; Yen, H.-W.; Sedighiani, K.; Sukumar, P.T.; Souza Filho, I.R.; Katnagallu, S.; Jägle, E.; et al. Current Challenges and Opportunities in Microstructure-Related Properties of Advanced High-Strength Steels. Metall. Mater. Trans. A 2020, 51, 5517–5586. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Fu, R.Y.; Krizan, D.; De Cooman, B.C. Continuous cooling transformation diagrams and properties of micro-alloyed TRIP steels. Mater. Sci. Eng. A 2006, 438–440, 296–299. [Google Scholar] [CrossRef]
- Sun, B.; Ma, Y.; Vanderesse, N.; Varanasi, R.S.; Song, W.; Bocher, P.; Ponge, D.; Raabe, D. Macroscopic to nanoscopic in situ investigation on yielding mechanisms in ultrafine grained medium Mn steels: Role of the austenite-ferrite interface. Acta Mater. 2019, 178, 10–25. [Google Scholar] [CrossRef]
- Sun, B.; Fazeli, F.; Scott, C.; Guo, B.; Aranas, C.; Chu, X.; Jahazi, M.; Yue, S. Microstructural characteristics and tensile behavior of medium manganese steels with different manganese additions. Mater. Sci. Eng. A 2018, 729, 496–507. [Google Scholar] [CrossRef]
- McCormick, P.G. A model for the Portevin-Le Chatelier effect in substitutional alloys. Acta Metall. 1972, 20, 351–354. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Sietsma, J. Volume Change Associated to Carbon Partitioning from Martensite to Austenite. Mater. Sci. Forum 2012, 706, 2290–2295. [Google Scholar] [CrossRef]
- Wei, R.; Enomoto, M.; Hadian, R.; Zurob, H.S.; Purdy, G.R. Growth of austenite from as-quenched martensite during intercritical annealing in an Fe-0.1C-3Mn-1.5Si alloy. Acta Mater. 2013, 61, 697–707. [Google Scholar] [CrossRef]
- Liu, L.; He, B.B.; Cheng, G.J.; Yen, H.W.; Huang, M.X. Optimum properties of quenching and partitioning steels achieved by balancing fraction and stability of retained austenite. Scr. Mater. 2018, 150, 1–6. [Google Scholar] [CrossRef]
- De Cooman, B.C.; Lee, S.; Kumar, S.S. Ultra-fine grained manganese TRIP steels. In Proceedings of the 2nd International Conference “Super-High Strength Steels”, Peschiera del Garda, Italy, 17–20 October 2010; pp. 1–11. [Google Scholar]
- De Moor, E.; Kang, S.; Speer, J.G.; Matlock, D.K. Manganese Diffusion in Third Generation Advanced High Strength Steels. In Proceedings of the International Conference on Mining, Material and Metallurgical Engineering, Prague, Czech Republic, 11–12 August 2014. [Google Scholar]
- Arlazarov, A.; Gouné, M.; Bouaziz, O.; Hazotte, A. Critical factors governing the thermal stability of austenite in an ultra-fined grained Medium-Mn steel. Philos. Mag. Lett. 2017, 97, 125–131. [Google Scholar] [CrossRef]
- Borgenstam, A.; Höglund, L.; Ågren, J.; Engström, A. DICTRA, a tool for simulation of diffusional transformations in alloys. J. Phase Equilibria 2000, 21, 269–280. [Google Scholar] [CrossRef]
- Apple, C.A.; Caron, R.N.; Krauss, G. Packet microstructure in Fe-0.2 pct C martensite. Metall. Trans. A 1974, 5, 593–599. [Google Scholar] [CrossRef]
- Swarr, T.; Krauss, G. The effect of structure on the deformation of as-quenched and tempered martensite in an Fe-0.2 pct C alloy. Metall. Trans. A 1976, 7A, 41–48. [Google Scholar] [CrossRef]
- Clarke, A.J. Carbon Partitioning into Austenite from Martensite in a Silicon-Containing High-Strength Sheet Steel. Ph.D. Thesis, Colorado School of Mines, 18 July 2006. [Google Scholar]
- Andrews, K.W. Empirical Formulae for the Calculation of Some Transformation Temperatures. J. Iron Steel Inst. 1965, 203, 721–727. [Google Scholar]
- Koistinen, D.P.; Marburger, R.E. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Speer, J.G.; Clarke, A.J.; Zhao, L.; Sietsma, J. Influence of interface mobility on the evolution of austenite–martensite grain assemblies during annealing. Acta Mater. 2009, 57, 4548–4557. [Google Scholar] [CrossRef] [Green Version]
- Dmitrieva, O.; Ponge, D.; Inden, G.; Millán, J.; Choi, P.; Sietsma, J.; Raabe, D. Chemical gradients across phase boundaries between martensite and austenite in steel studied by atom probe tomography and simulation. Acta Mater. 2011, 59, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Olson, G.B.; Cohen, M. Stress-assisted isothermal martensitic transformation: Application to TRIP steels. Metall. Trans. A 1982, 13, 1907–1914. [Google Scholar] [CrossRef]
- Olson, G.B.; Cohen, M. Kinetics of strain-induced martensitic nucleation. Metall. Trans. A 1975, 6, 791–795. [Google Scholar] [CrossRef]
- Van Dijk, N.H.; Butt, A.M.; Zhao, L.; Sietsma, J.; Offerman, S.E.; Wright, J.P.; Van der Zwaag, S. Thermal stability of retained austenite in TRIP steels studied by synchrotron X-ray diffraction during cooling. Acta Mater. 2005, 53, 5439–5447. [Google Scholar] [CrossRef]
- Timokhina, I.B.; Hodgson, P.D.; Pereloma, E.V. Effect of Microstructure on the Stability of Retained Austenite in Transformation-Induced-Plasticity Steels. Metall. Mater. Trans. A 2004, 35, 2331–2340. [Google Scholar] [CrossRef] [Green Version]
- De Knijf, D.; Föjer, C.; Kestens, L.A.I.; Petrov, R. Factors influencing the austenite stability during tensile testing on Quenching and Partitioning steel determined via in-situ electro backscatter diffraction. Mater. Sci. Eng. A 2015, 638, 219–227. [Google Scholar] [CrossRef]
- Hidalgo, J.; Huizenga, R.M.; Findley, K.O.; Santofimia, M.J. Interplay between metastable phases controls strength and ductility in steels. Mater. Sci. Eng. A 2019, 745, 185–194. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kawamura, K.; Urabe, T.; Hosoya, Y. Effects of microstructure on stretch-flange-formability of 980 MPa grade cold-rolled ultra high strength steel sheets. ISIJ Int. 2004, 44, 603–609. [Google Scholar] [CrossRef]
- Tsuji, N.; Ito, Y.; Saito, Y.; Minamino, Y. Strength and ductility of ultrafine grained aluminium and iron produced by ARB and annealing. Scr. Mater. 2002, 47, 893–899. [Google Scholar] [CrossRef]
- Sun, B.; Vanderesse, N.; Fazeli, F.; Scott, C.; Chen, J.; Bocher, P.; Jahazi, M.; Yue, S. Discontinuous strain-induced martensite transformation related to the Portevin-Le Chatelier effect in a medium manganese steel. Scr. Mater. 2017, 133, 9–13. [Google Scholar] [CrossRef]
- Linke, B.M.; Gerber, T.; Hatscher, A.; Salvatori, I.; Aranguren, I.; Arribas, M. Impact of Si on Microstructure and Mechanical Properties of 22MnB5 Hot stamping steel treated by Quenching & Partitioning (Q&P). Metall. Mater. Trans. A 2018, 49, 54–65. [Google Scholar] [CrossRef]




| Steel | C | Mn | Ni | Si | P | S | N |
|---|---|---|---|---|---|---|---|
| L-Mn | 0.194 | 2.01 | 0.001 | 1.49 | 0.0012 | 0.002 | 0.002 |
| M-MnNi | 0.243 | 5.60 | 1.811 | 1.44 | 0.008 | 0.002 | 0.001 |
| Experimental Conditions | Experimental Group | Variable | Control Group | ||||
|---|---|---|---|---|---|---|---|
| No | Steel | QT | PT (°C) | Pt (s) | - | - | - |
| 1 | L-Mn | QT25 | 400 | 120 | - | - | x (of group 1) |
| 2 | L-Mn | QT25 | 400 | 300 | - | - | |
| 3 | M-MnNi | QT25 | 400 | 120 | Group 1 | Steel grade | x (of group 2) |
| 4 | M-MnNi | QT25 | 400 | 300 | |||
| 5 | M-MnNi | QT25 | 650 | 120 | Group 2 | PT | x (of group 3) |
| 6 | M-MnNi | QT25 | 650 | 300 | |||
| 7 | M-MnNi | QT15 | 650 | 120 | Group 3 | QT | - |
| 8 | M-MnNi | QT15 | 650 | 300 | |||
| Steel | QT (°C) | PT (°C) | Pt (s) | YS 0.2% Offset (MPa) (Error ± 15 MPa) | TS (MPa) (Error ± 15 MPa) | YS/TS (%) | TEL in 50 mm Gauge (%) (Error ± 2%) | TS x TEL (GPa%) | Bending Angle (° (Error ± 10°)) | HER (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| L-Mn | 300 (QT25) | 400 | 120 | 1202 | 1347 | 89 | 9 | 12 | 103 | 12.80 |
| 300 | 1209 | 1342 | 90 | 8 | 11 | 113 | ||||
| M-MnNi | 75 (QT25) | 400 | 120 | 1101 | 1324 | 83 | 2 | 3 | 24 | |
| 300 | 1236 | 1248 | 99 | 3 | 4 | 21 | ||||
| Room Temperature (QT15) | 650 | 120 | 949 | 1292 | 73 | 17 | 22 | 62 | ||
| 300 | 792 | 1285 | 62 | 23 | 29 | 103 | 2.20 | |||
| 75 (QT25) | 120 | 735 | 1335 | 55 | 16 | 21 | 96 | 1.98 | ||
| 300 | 332 | 1061 | 31 | 21 | 22 | 97 |
| YS 0.2% Offset (MPa) | TS (MPa) | TEL in 30 mm Gauge (%) | RA | |
|---|---|---|---|---|
| Steel—Q&P condition | L-Mn—QT25—PT = 400 °C—Pt = 120 s | |||
| Non-deformed | 1316 | 1329 | 13 | 0.06 |
| Deformed | 1213 | 1440 | 6 | 0.02 |
| Steel—Q&P condition | M-MnNi—QT15—PT = 650 °C—Pt = 300 s | |||
| Non-deformed | 768 | 1400 | 39 | 0.24 |
| Deformed | 1117 | 1494 | 15 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arribas, M.; Del Molino, E.; Gutiérrez, T.; Arlazarov, A.; Martin, D.; De Caro, D.; Ayenampudi, S.; Santofimia, M.J. Characterization of a Medium Mn-Ni Steel Q&P Treated by a High Partitioning Temperature Cycle. Metals 2022, 12, 483. https://doi.org/10.3390/met12030483
Arribas M, Del Molino E, Gutiérrez T, Arlazarov A, Martin D, De Caro D, Ayenampudi S, Santofimia MJ. Characterization of a Medium Mn-Ni Steel Q&P Treated by a High Partitioning Temperature Cycle. Metals. 2022; 12(3):483. https://doi.org/10.3390/met12030483
Chicago/Turabian StyleArribas, Maribel, Eider Del Molino, Teresa Gutiérrez, Artem Arlazarov, David Martin, Daniele De Caro, Sudhindra Ayenampudi, and Maria J. Santofimia. 2022. "Characterization of a Medium Mn-Ni Steel Q&P Treated by a High Partitioning Temperature Cycle" Metals 12, no. 3: 483. https://doi.org/10.3390/met12030483






