Cooling Rate Controlled Aging of a Co-Free Fe-Ni-Cr-Mo-Ti-Al Maraging Steel
Abstract
:1. Introduction
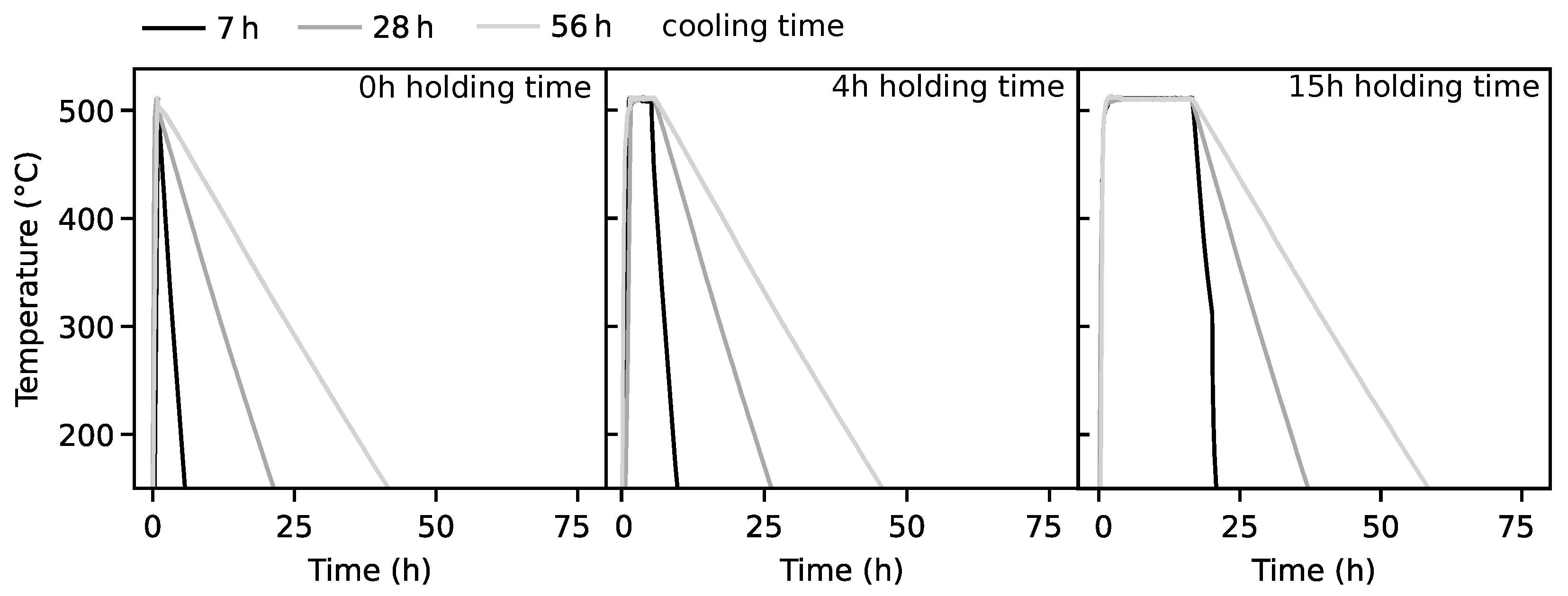
2. Materials and Methods
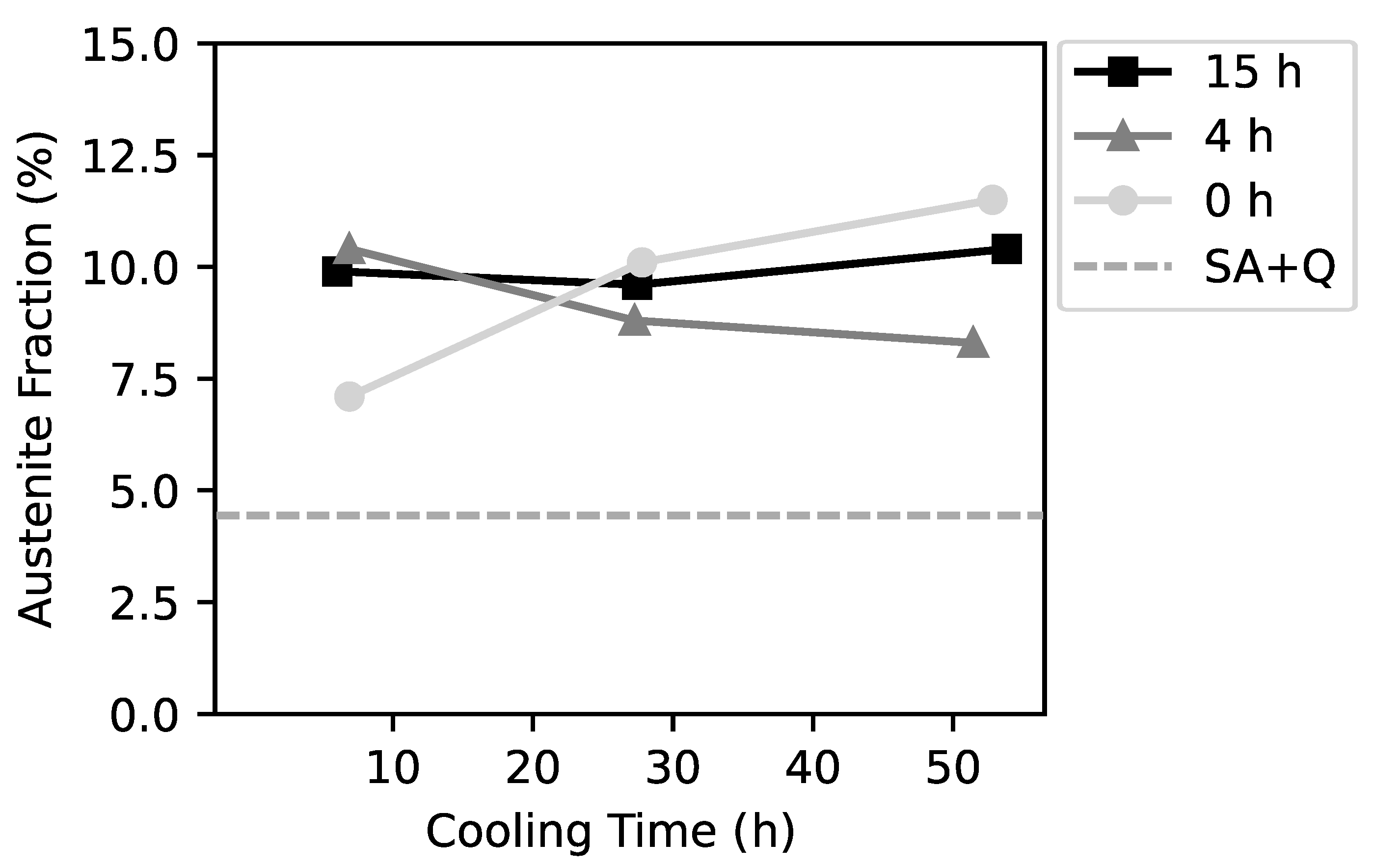
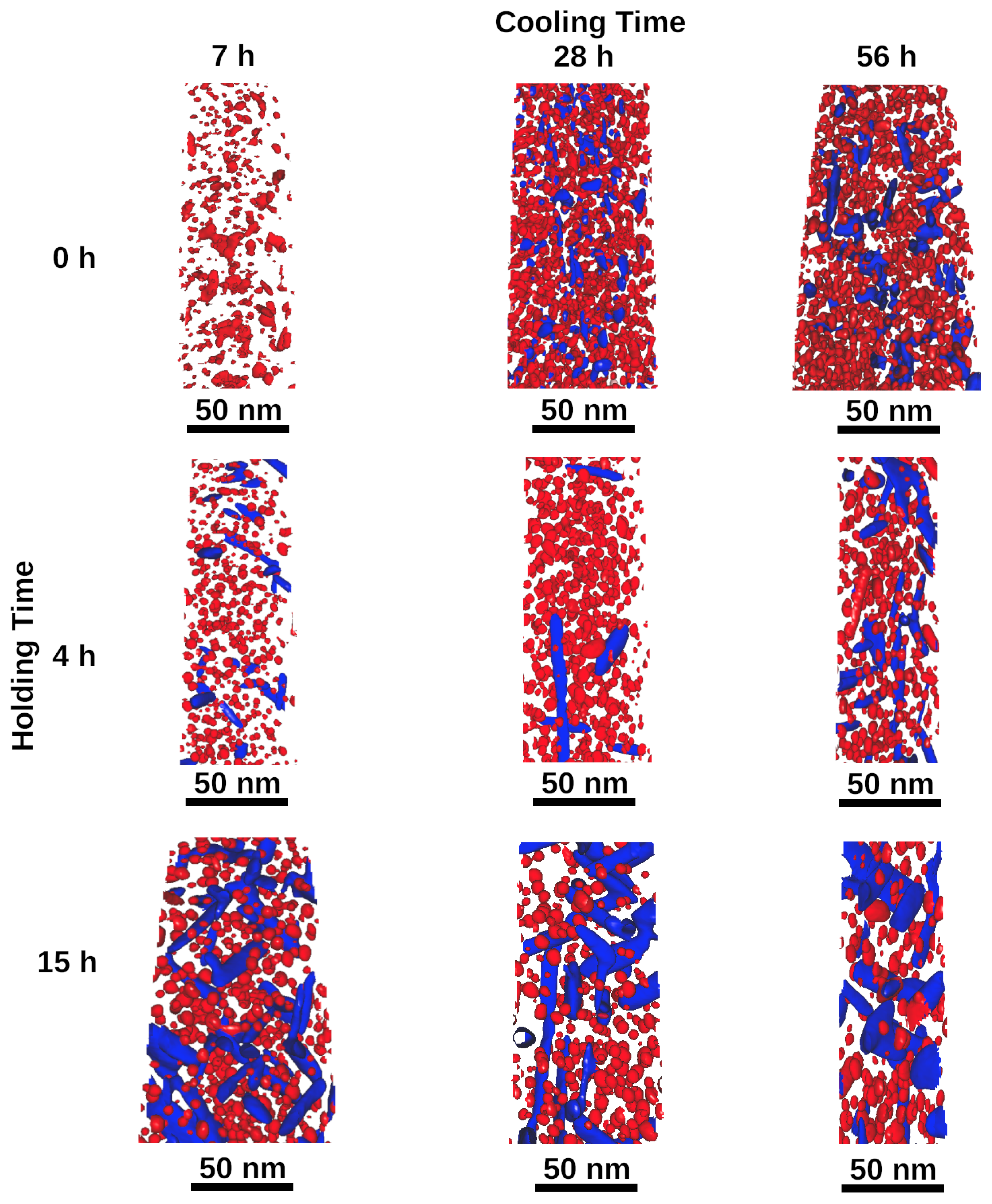
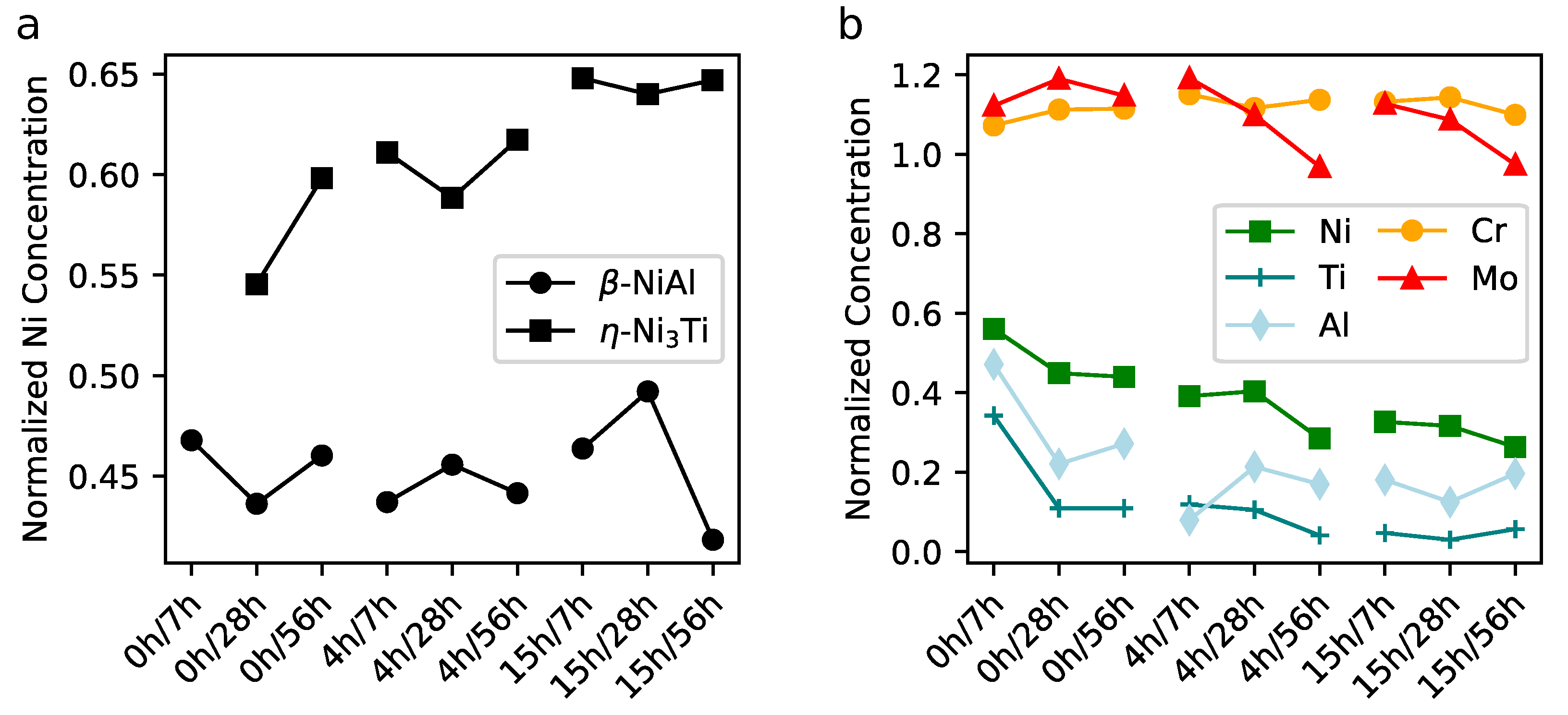
3. Results
4. Discussion
5. Conclusions
- A slow cooling rate can be treated as an extension of the aging heat treatment, that uses lower temperatures. Longer cooling times can result in a higher volume fraction of precipitates and a higher hardness level.
- Solid solution hardening can play an important role for the total strength in under-peak aged precipitation hardened materials and peak hardness can be achieved with early stages of the precipitates.
- The highest hardness of this alloy can be achieved with a long cooling time and without significant loss of impact toughness. There is no clear correlation between the secondary aging effects (austenite reversion and change of impact toughness) and the cooling time.
- Aging heat treatments with a short aging time and long cooling times can be successfully used as an alternative to conventional aging heat treatments. Furthermore, no detrimental effect of long cooling times was found, when longer aging times were used. This knowledge can be useful for large work pieces, where air cooling can result in different cooling rates between the surface and the core of the work piece.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Decker, R.F.; Novak, C.J.; Landig, T.W. Developments and projected trends in maraging steels. JOM 1967, 19, 60–66. [Google Scholar] [CrossRef]
- Sha, W.; Guo, Z. Maraging Steels: Modelling of Microstructure, Properties and Applications, 1st ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2009. [Google Scholar]
- Wilm, A. Physikalisch-metallurgische Untersuchungen über magnesiumhaltige Aluminiumlegierungen. Metallurgie 1911, 8, 225. [Google Scholar]
- Ardell, A.J. Precipitation hardening. Metall. Trans. A 1985, 16, 2131–2165. [Google Scholar] [CrossRef]
- Ahmadi, M.R.; Povoden-Karadeniz, E.; Öksüz, K.I.; Falahati, A.; Kozeschnik, E. A model for precipitation strengthening in multi-particle systems. Comput. Mater. Sci. 2014, 91, 173–186. [Google Scholar] [CrossRef]
- Schober, M.; Schnitzer, R.; Leitner, H. Precipitation evolution in a Ti-free and Ti-containing stainless maraging steel. Ultramicroscopy 2009, 109, 553–562. [Google Scholar] [CrossRef]
- Li, K.; Yu, B.; Misra, R.D.; Han, G.; Liu, S.; Shang, C.J. Strengthening of cobalt-free 19Ni3Mo1.5Ti maraging steel through high-density and low lattice misfit nanoscale precipitates. Mater. Sci. Eng. A 2018, 715, 174–185. [Google Scholar] [CrossRef]
- Leitner, H.; Schober, M.; Schnitzer, R.; Zinner, S. Strengthening behavior of Fe-Cr-Ni-Al-(Ti) maraging steels. Mater. Sci. Eng. A 2011, 528, 5264–5270. [Google Scholar] [CrossRef]
- Schnitzer, R.; Schober, M.; Zinner, S.; Leitner, H. Effect of Cu on the evolution of precipitation in an Fe-Cr-Ni-Al-Ti maraging steel. Acta Mater. 2010, 58, 3733–3741. [Google Scholar] [CrossRef]
- Kürnsteiner, P.; Wilms, M.B.; Weisheit, A.; Barriobero-Vila, P.; Jägle, E.A.; Raabe, D. Massive nanoprecipitation in an Fe-19Ni-xAl maraging steel triggered by the intrinsic heat treatment during laser metal deposition. Acta Mater. 2017, 129, 52–60. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, H.; Wu, Y.; Liu, X.; Chen, H.; Yao, M.; Gault, B.; Ponge, D.; Raabe, D.; Hirata, A.; et al. Ultrastrong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhang, S.L.; Ding, Y.L.; Ge, Q.L.; Su, J. Effect of aging temperature on the precipitation behavior and mechanical properties of Fe–Cr–Ni maraging stainless steel. Mater. Sci. Eng. A 2021, 806, 140763. [Google Scholar] [CrossRef]
- Nakagawa, H.; Yokota, H.; Miyazaki, T. Effects of aging temperature on the microstructures and mechanical properties of a precipitation hardening martensitic stainless steel containing retained austenite. J. Mater. Sci. 2000, 35, 2245–2253. [Google Scholar] [CrossRef]
- Conde, F.F.; Escobar, J.D.; Oliveira, J.P.; Béreš, M.; Jardini, A.L.; Bose, W.W.; Avila, J.A. Effect of thermal cycling and aging stages on the microstructure and bending strength of a selective laser melted 300-grade maraging steel. Mater. Sci. Eng. A 2019, 758, 192–201. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, X.; Ma, D.; Tong, M.; Wang, T.; Xu, B.; Sun, M.; Li, D. Effect of aging temperature on the austenite reversion and mechanical properties of a Fe-10Cr-10Ni cryogenic maraging steel. J. Mater. Res. Technol. 2021, 11, 98–111. [Google Scholar] [CrossRef]
- Niu, M.C.; Yang, K.; Luan, J.H.; Wang, W.; Jiao, Z.B. Cu-assisted austenite reversion and enhanced TRIP effect in maraging stainless steels. J. Mater. Sci. Technol. 2022, 104, 52–58. [Google Scholar] [CrossRef]
- Wang, M.; Tasan, C.C.; Ponge, D.; Raabe, D. Spectral TRIP enables ductile 1.1 GPa martensite. Acta Mater. 2016, 111, 262–272. [Google Scholar] [CrossRef]
- Gault, B.; Moody, M.; Cairney, J.M.; Ringer, S.P. Atom Probe Microscopy; Springer: New York, NY, USA, 2012. [Google Scholar]
- Zeisl, S.; Lassnig, A.; Hohenwarter, A.; Mendez-Martin, F. Precipitation behaviour of a Co-free Fe-Ni-Cr-Mo-Ti-Al maraging steel after severe plastic deformation. Mater. Sci. Eng. A 2021, 833, 142416. [Google Scholar] [CrossRef]
- Jägle, E.A.; Choi, P.P.; Raabe, D. The maximum separation cluster analysis algorithm for atom-probe tomography: Parameter determination and accuracy. Microsc. Microanal. 2014, 20, 1662–1671. [Google Scholar] [CrossRef]
- Galindo-Nava, E.I.; Rivera-Díaz-Del-Castillo, P.E. A model for the microstructure behaviour and strength evolution in lath martensite. Acta Mater. 2015, 98, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Warlimont, H.; Martienssen, W. Springer Handbooks of Materials Data, 2nd ed.; Springer Nature: Cham, Switzerland, 2018. [Google Scholar]
- Schnitzer, R.; Radis, R.; Nöhrer, M.; Schober, M.; Hochfellner, R.; Zinner, S.; Povoden-Karadeniz, E.; Kozeschnik, E.; Leitner, H. Reverted austenite in PH 13-8 Mo maraging steels. Mater. Chem. Phys. 2010, 122, 138–145. [Google Scholar] [CrossRef]
- Galindo-Nava, E.I.; Rainforth, W.M.; Rivera-Díaz-del Castillo, P.E. Predicting microstructure and strength of maraging steels: Elemental optimisation. Acta Mater. 2016, 117, 270–285. [Google Scholar] [CrossRef]
- Escobar, J.D.; Faria, G.A.; Wu, L.; Oliveira, J.P.; Mei, P.R.; Ramirez, A.J. Austenite reversion kinetics and stability during tempering of a Ti-stabilized supermartensitic stainless steel: Correlative in situ synchrotron X-ray diffraction and dilatometry. Acta Mater. 2017, 138, 92–99. [Google Scholar] [CrossRef]
- Kooiker, H.; Perdahcıoğlu, E.S.; van den Boogaard, A.H. Combined athermal and isothermal martensite to austenite reversion kinetics, experiment and modelling. Mater. Des. 2020, 196, 109124. [Google Scholar] [CrossRef]
- Schnitzer, R.; Zickler, G.A.; Lach, E.; Clemens, H.; Zinner, S.; Lippmann, T.; Leitner, H. Influence of reverted austenite on static and dynamic mechanical properties of a PH 13-8 Mo maraging steel. Mater. Sci. Eng. A 2010, 527, 2065–2070. [Google Scholar] [CrossRef]




| Fe | Ni | Cr | Mo | Ti | Al | C | |
|---|---|---|---|---|---|---|---|
| wt.% | bal. | 12.3 | 10.2 | 2.0 | 1.2 | 1.5 | <0.03 |
| at.% | bal. | 11.6 | 10.8 | 1.2 | 1.4 | 3.1 | <0.14 |
| Condition | -NiAl | -Ni3Ti | |||||
|---|---|---|---|---|---|---|---|
| Central Element | dMax (nm) | NMin | Central Element | dMax (nm) | NMin | ||
| 0-7 | Ti | 0.95 | 75 | ||||
| 0-28 | Al | 0.75 | 130 | Ti | 0.95 | 400 | |
| 0-56 | Al | 0.80 | 150 | Ti | 0.96 | 450 | |
| 4-7 | Al | 0.78 | 150 | Ti | 0.93 | 800 | |
| 4-28 | Al | 0.80 | 150 | Ti | 0.98 | 800 | |
| 4-56 | Al | 0.75 | 130 | Ti | 0.98 | 800 | |
| 15-7 | Al | 0.70 | 80 | Ti | 0.94 | 700 | |
| 15-28 | Al | 0.71 | 80 | Ti | 0.94 | 800 | |
| 15-56 | Al | 0.70 | 140 | Ti | 0.94 | 800 | |
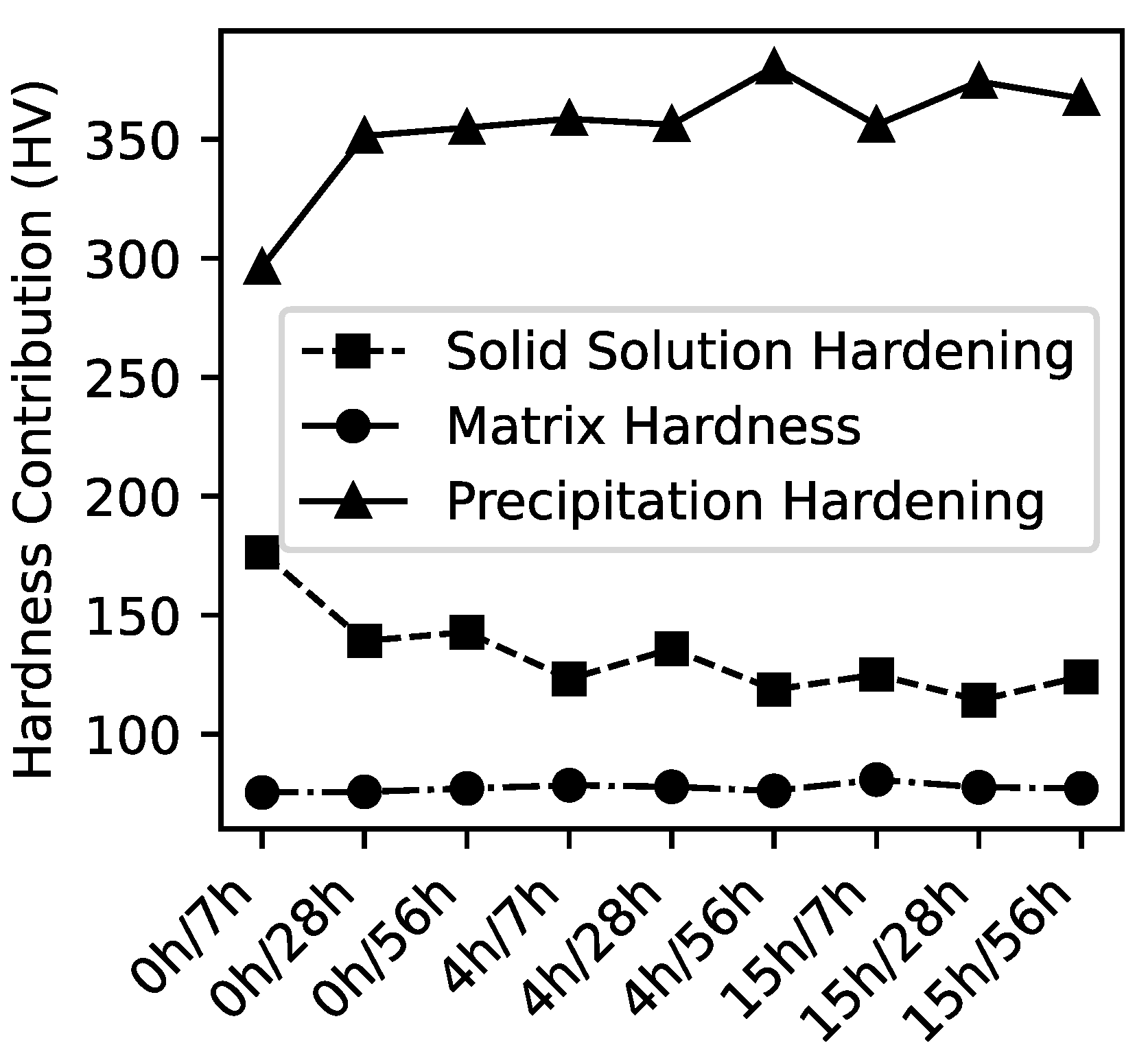
| Condition | HV | HV,0 | HV,Matrix | HV,SS | HV,P |
|---|---|---|---|---|---|
| 0-7 | 565 | 17 | 75 | 176 | 296 |
| 0-28 | 583 | 17 | 76 | 139 | 351 |
| 0-56 | 592 | 17 | 77 | 143 | 355 |
| 4-7 | 577 | 17 | 78 | 123 | 359 |
| 4-28 | 587 | 17 | 78 | 136 | 356 |
| 4-56 | 592 | 17 | 76 | 119 | 380 |
| 15-7 | 579 | 17 | 81 | 125 | 356 |
| 15-28 | 583 | 17 | 78 | 114 | 374 |
| 15-56 | 585 | 17 | 77 | 124 | 367 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeisl, S.; Schnitzer, R. Cooling Rate Controlled Aging of a Co-Free Fe-Ni-Cr-Mo-Ti-Al Maraging Steel. Metals 2022, 12, 538. https://doi.org/10.3390/met12040538
Zeisl S, Schnitzer R. Cooling Rate Controlled Aging of a Co-Free Fe-Ni-Cr-Mo-Ti-Al Maraging Steel. Metals. 2022; 12(4):538. https://doi.org/10.3390/met12040538
Chicago/Turabian StyleZeisl, Stefan, and Ronald Schnitzer. 2022. "Cooling Rate Controlled Aging of a Co-Free Fe-Ni-Cr-Mo-Ti-Al Maraging Steel" Metals 12, no. 4: 538. https://doi.org/10.3390/met12040538
APA StyleZeisl, S., & Schnitzer, R. (2022). Cooling Rate Controlled Aging of a Co-Free Fe-Ni-Cr-Mo-Ti-Al Maraging Steel. Metals, 12(4), 538. https://doi.org/10.3390/met12040538






