Study on the Grinding Kinetics and Magnetic Separation of Low-Grade Vanadiferous Titanomagnetite Concentrate
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Experimental Methods
3. Results and Discussion
3.1. Grinding Process and Grinding Kinetics
3.2. Particle Morphology
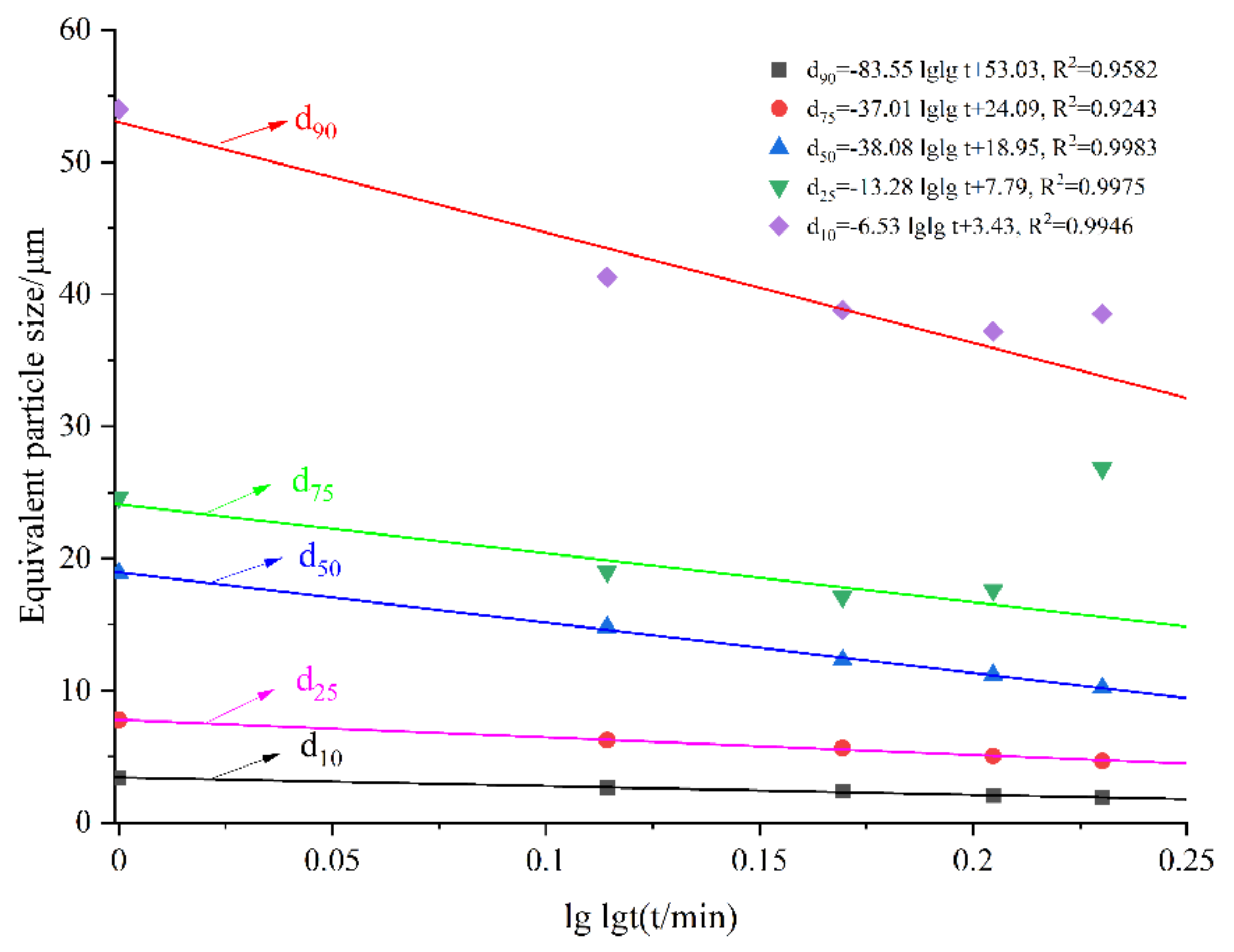
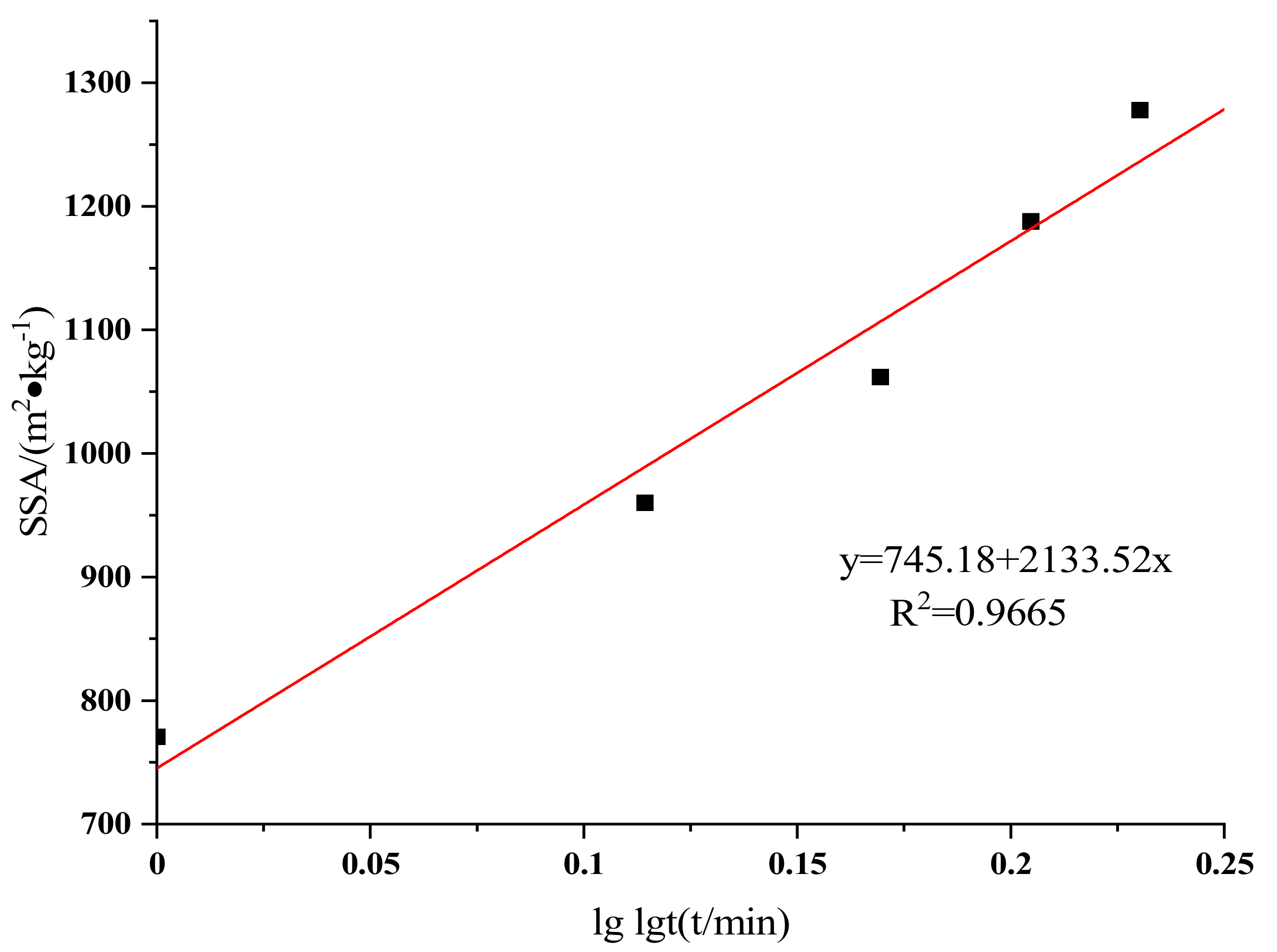
3.3. Equivalent Particle Size and Specific Surface Area
3.4. Particle Size Distribution Features
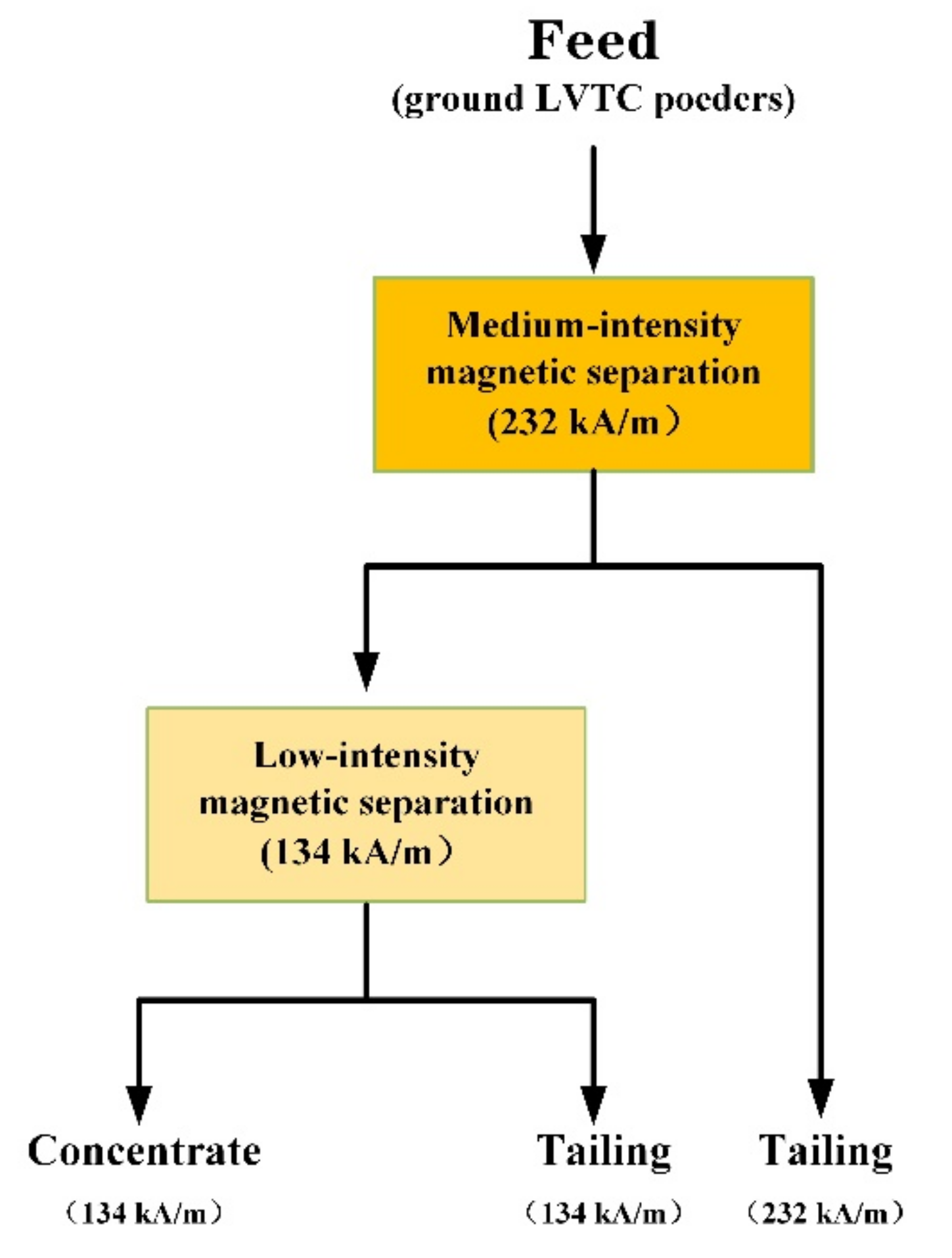
3.5. Magnetic Separation
3.5.1. Effect of Grinding Time on the Grade of Magnetic Separation
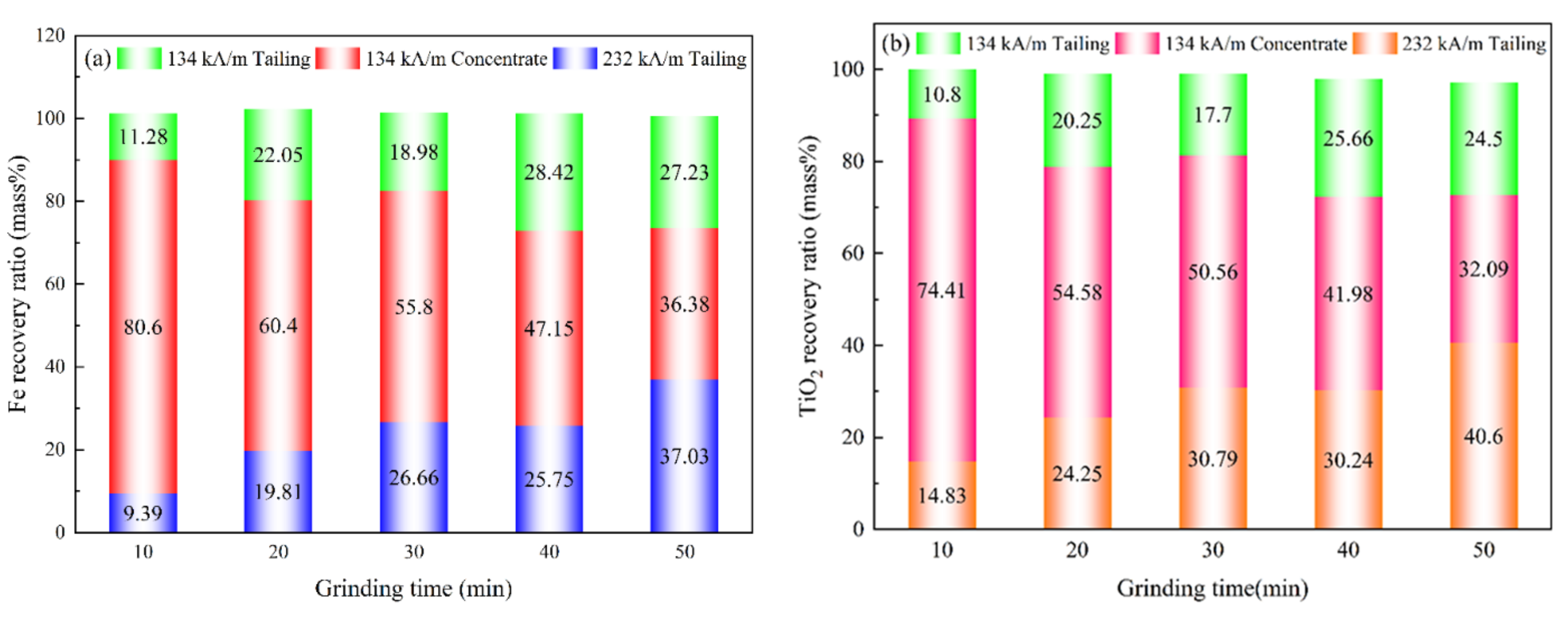
3.5.2. Effect of Grinding Time on the Recovery Ratio of Magnetic Separation
3.5.3. XRD Pattern Analysis of Magnetic Separation Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, J.Y.; Tang, Y.; Yang, K.; Rouff, A.A.; Elzinga, E.J.; Huang, J.H. Leaching characteristics of vanadium in mine tailings and soils near a vanadium titanomagnetite mining site. J. Hazard. Mater. 2014, 264, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Yang, S.T.; Zhou, M.; Jiang, T.; Wang, Y.J.; Xue, X.X. Effect of basicity on sintering behavior of low-titanium vanadium–titanium magnetite. Trans. Nonferrous Met. Soc. China 2015, 25, 2087–2094. [Google Scholar] [CrossRef]
- Zheng, F.Q.; Chen, F.; Guo, Y.F.; Jiang, T.; Travyanov, A.Y.; Qiu, G.Z. Kinetics of hydrochloric acid leaching of titanium from titanium-bearing electric furnace slag. JOM 2016, 68, 1476–1484. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Reduction kinetics of oxidized vanadium titano-magnetite pellets using carbon monoxide and hydrogen. J. Alloy. Compd. 2017, 706, 546–553. [Google Scholar] [CrossRef]
- Yu, J.W.; Hu, N.; Xiao, H.X.; Gao, P.; Sun, Y.S. Reduction behaviors of vanadium-titanium magnetite with H2 via a fluidized bed. Powder Technol. 2021, 385, 83–91. [Google Scholar] [CrossRef]
- Xu, C.B.; Zhang, Y.M.; Liu, T.; Huang, J. Characterization and pre-concentration of low-grade vanadium-titanium magnetite ore. Minerals 2017, 7, 137. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.D.; Lv, X.W.; Jiang, Y.Y.; Ling, J.W.; Yan, Z.M. Blast furnace ironmaking process with super-high TiO2 in the slag: Viscosity and melting properties of the slag. Metall. Mater. Trans. B 2019, 51, 722–731. [Google Scholar] [CrossRef]
- Chen, S.Y.; Fu, X.J.; Chu, M.S.; Liu, Z.G.; Tang, J. Life cycle assessment of the comprehensive utilisation of vanadium titano-magnetite. J. Clean. Prod. 2015, 101, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Wang, Y.; You, Z.X.; Wen, L.Y.; Lv, X.W. Carbonization and nitridation of vanadium-bearing titanomagnetite during carbothermal reduction with coal. J. Mater. Res. Technol. 2020, 9, 4272–4282. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Xie, X.L.; Wang, S.; Zheng, F.Q. Gas-based reduction of vanadium titano-magnetite concentrate: Behavior and mechanisms. Int. J. Miner. Metall. Mater. 2017, 24, 10–17. [Google Scholar] [CrossRef]
- Yang, H.F.; Jing, L.L.; Zhang, B.G. Recovery of iron from vanadium tailings with coal-based direct reduction followed by magnetic separation. J. Hazard. Mater. 2011, 185, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Guo, Y.F.; Qiu, G.Z.; Jiang, T.; Chen, F. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium-titanium magnetite concentrate. Trans. Nonferrous Met. Soc. China 2014, 24, 3372–3377. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.; Go, B.; Lee, S.; Park, S.; Jeon, H.S. Optimized magnetic separation for efficient recovery of V and Ti enriched concentrates from vanadium-titanium magnetite ore: Effect of grinding and magnetic intensity. Powder Technol. 2021, 391, 282–291. [Google Scholar] [CrossRef]
- Lund, C.; Lamberg, P.; Lindberg, T. Practical way to quantify minerals from chemical assays at Malmberget iron ore operations—An important tool for the geometallurgical program. Miner. Eng. 2013, 49, 7–16. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Separation and recovery of iron and titanium from oxidized vanadium titano-magnetite by gas-based reduction roasting and magnetic separation. J. Mater. Res. Technol. 2019, 8, 3036–3043. [Google Scholar] [CrossRef]
- Matijašić, G.; Kurajica, S. Grinding kinetics of amorphous powder obtained by sol–gel process. Powder Technol. 2010, 197, 165–169. [Google Scholar] [CrossRef]
- Zhao, R.C.; Han, Y.X.; He, M.Z.; Li, Y.J. Grinding kinetics of quartz and chlorite in wet ball milling. Powder Technol. 2017, 305, 418–425. [Google Scholar] [CrossRef]
- Long, H.M.; Chun, T.J.; Wang, P.; Meng, Q.M.; Di, Z.X.; Li, J.X. Grinding kinetics of vanadium-titanium magnetite concentrate in a damp mill and its properties. Metall. Mater. Trans. B 2016, 47, 1765–1772. [Google Scholar] [CrossRef]
- Choi, W.S.; Chung, H.Y.; Yoon, B.R.; Kim, S.S. Applications of grinding kinetics analysis to fine grinding characteristics of some inorganic materials using a composite grinding media by planetary ball mill. Powder Technol. 2001, 115, 209–214. [Google Scholar] [CrossRef]
- Kano, J.; Miyazaki, M.; Saito, F. Ball mill simulation and powder characteristics of ground talc in various types of mill. Adv. Powder Technol. 2000, 11, 333–342. [Google Scholar] [CrossRef]
- Djamarani, K.M.; Clark, I.M. Characterization of particle size based on fine and coarse fractions. Powder Technol. 1997, 93, 101–108. [Google Scholar] [CrossRef]
- Rosin, P.O.; Rammler, E.J. Laws governing the fineness of powdered coal. J. Inst. Fuel 1933, 7, 29–36. [Google Scholar]
- Reid, K.J. A solution to the batch grinding equation. Chem. Eng. Sci. 1965, 20, 953–963. [Google Scholar] [CrossRef]
- Ozao, R.; Ochiai, M. Thermal analysis and self-similarity law in particle size distribution of powder samples. Part 3. Thermochim. Acta 1992, 208, 161–167. [Google Scholar] [CrossRef]
- Menéndez-Aguado, J.M.; Peña-Carpio, E.; Sierra, C. Particle size distribution fitting of surface detrital sediment using the Swrebec function. J. Soils Sediments 2015, 15, 2004–2011. [Google Scholar] [CrossRef]
- Osorio, A.M.; Menéndez-Aguado, J.M.; Bustamante, O.; Restrepo, G.M. Fine grinding size distribution analysis using the Swrebec function. Powder Technol. 2014, 258, 206–208. [Google Scholar] [CrossRef]
- González-Tello, P.; Camacho, F.; Vicaria, J.M.; González, P.A. A modified Nukiyama–Tanasawa distribution function and a Rosin–Rammler model for the particle-size-distribution analysis. Powder Technol. 2008, 186, 278–281. [Google Scholar] [CrossRef]
- Ouchterlony, F. The Swebrec© function: Linking fragmentation by blasting and crushing. Min. Technol. 2005, 114, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.W.; Qin, Y.H.; Gao, P.; Han, Y.X.; Li, Y.J. An innovative approach for determining the grinding media system of ball mill based on grinding kinetics and linear superposition principle. Powder Technol. 2021, 378, 172–181. [Google Scholar] [CrossRef]
- Rodríguez, B.Á.; García, G.G.; Coello-Velázquez, A.L.; Menéndez-Aguado, J.M. Product size distribution function influence on interpolation calculations in the Bond ball mill grindability test. Int. J. Miner. Process. 2016, 157, 16–20. [Google Scholar] [CrossRef]
- Liu, S.H.; Li, Q.L.; Xie, G.S.; Li, L.H.; Xiao, H.L. Effect of grinding time on the particle characteristics of glass powder. Powder Technol. 2016, 295, 133–141. [Google Scholar] [CrossRef]
- Ahmadi, R.; Hashemzadehfini, M.; Parian, M.A. Rapid determination of Bond rod-mill work index by modeling the grinding kinetics. Adv. Powder Technol. 2013, 24, 441–445. [Google Scholar] [CrossRef]
- Liu, S.X.; Shen, L.L.; Wang, Q.P.; Niu, F.S. Investigation on crushing kinetic equation of ball milling of quartz powder. Adv. Mater. Res. 2010, 156, 812–816. [Google Scholar] [CrossRef]
- Tavares, L.M.; Carvalho, R.M. Impact work index prediction from continuum damage model of particle fracture. Miner. Eng. 2007, 20, 1368–1375. [Google Scholar] [CrossRef]
- Kotake, N.; Daibo, K.; Yamamoto, T.; Kanda, Y. Experimental investigation on a grinding rate constant of solid materials by a ball mill—effect of ball diameter and feed size. Powder Technol. 2004, 143, 196–203. [Google Scholar] [CrossRef]
- Frances, C. On modelling of submicronic wet milling processes in bead mills. Powder Technol. 2004, 143, 253–263. [Google Scholar] [CrossRef]
- Delagrammatikas, G.; Delagrammatikas, M.; Tsimas, S. Particle size distributions a new approach. Powder Technol. 2007, 176, 57–65. [Google Scholar] [CrossRef]
- Kotake, N.; Suzuki, K.; Asahi, S.; Kanda, Y. Experimental study on the grinding rate constant of solid materials in a ball mill. Powder Technol. 2002, 122, 101–108. [Google Scholar] [CrossRef]
- Liu, S.H.; Li, Q.L.; Song, J.W. Study on the grinding kinetics of copper tailing powder. Powder Technol. 2018, 330, 105–113. [Google Scholar] [CrossRef]
- Wang, F.W.; Dai, H.X.; Lv, Y.C.; Zhang, L. Dynamic capture behavior of ferromagnetic particles based on fully coupled multiphysics model of particle-fluid interactions. Miner. Eng. 2019, 138, 238–245. [Google Scholar] [CrossRef]
- Silin, I.; Hahn, K.; Gürsel, D.; Kremer, D.; Gronen, L.; Stopić, S.; Friedrich, B.; Wotruba, H. Mineral processing and metallurgical treatment of lead vanadate ores. Minerals 2020, 10, 197. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.S.; Meng, Q.Y.; Yuan, Z.T.; Xu, Y.K.; Li, L.X. New cognitions into three new classified titanaugite in mineralogical characteristics and fundamental performances. Miner. Eng. 2021, 169, 106962. [Google Scholar] [CrossRef]
- Yuan, Z.T.; Zhao, X.; Meng, Q.Y.; Xu, Y.K.; Li, L.X. Effect of selective coating of magnetite on improving magnetic separation of ilmenite from titanaugite. Miner. Eng. 2020, 149, 106267. [Google Scholar] [CrossRef]
- Wang, F.W.; Tang, D.D.; Gao, L.K.; Dai, H.X.; Wang, P.R.; Gong, Z.H. Magnetic entrainment mechanism of multi-type intergrowth particles for Low-Intensity magnetic separation based on a multiphysics model. Miner. Eng. 2020, 149, 106264. [Google Scholar] [CrossRef]
- Guo, X.F.; Dai, S.J.; Wang, Q.Q. Influence of different comminution flowsheets on the separation of vanadium titano-magnetite. Miner. Eng. 2020, 149, 106268. [Google Scholar] [CrossRef]
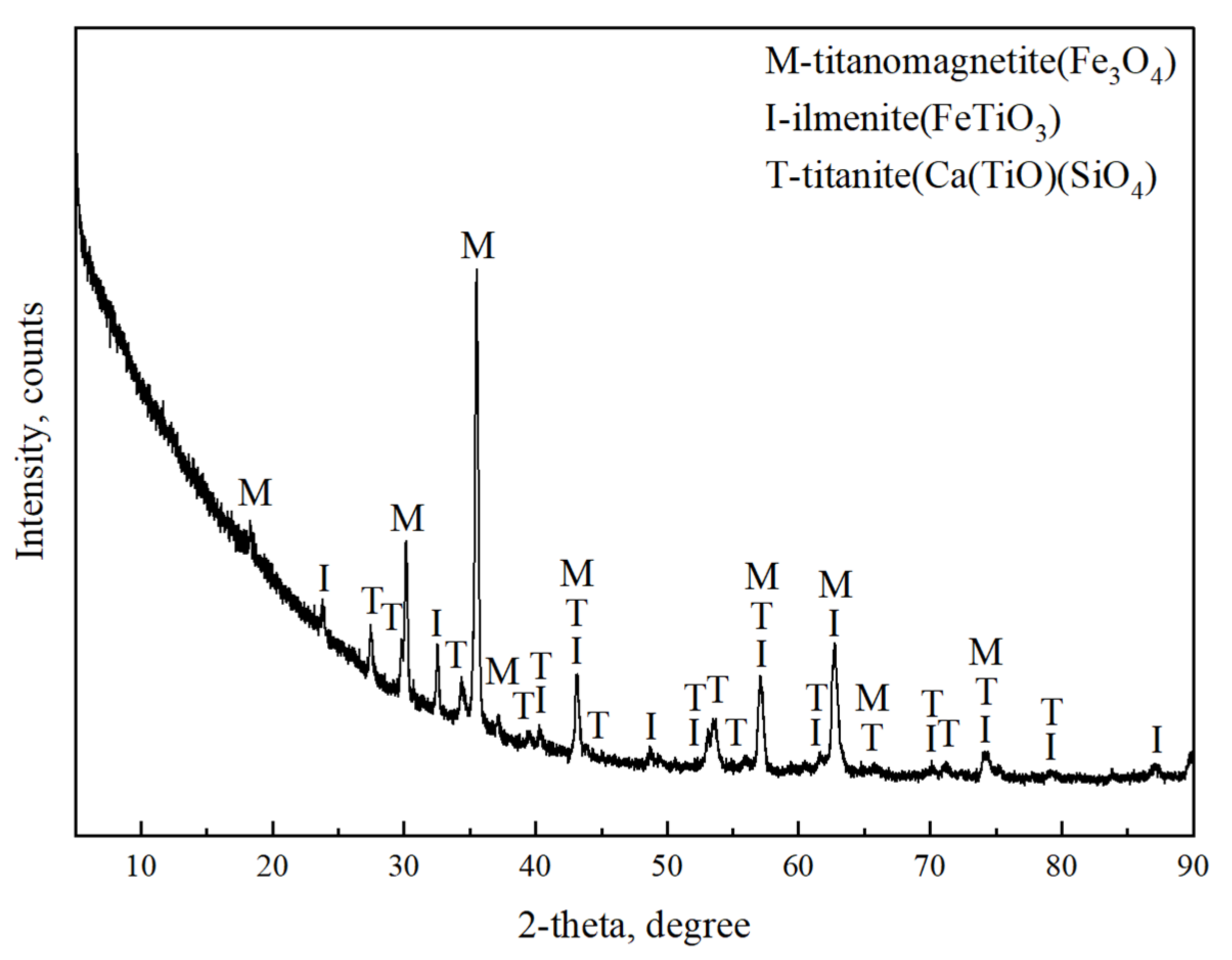



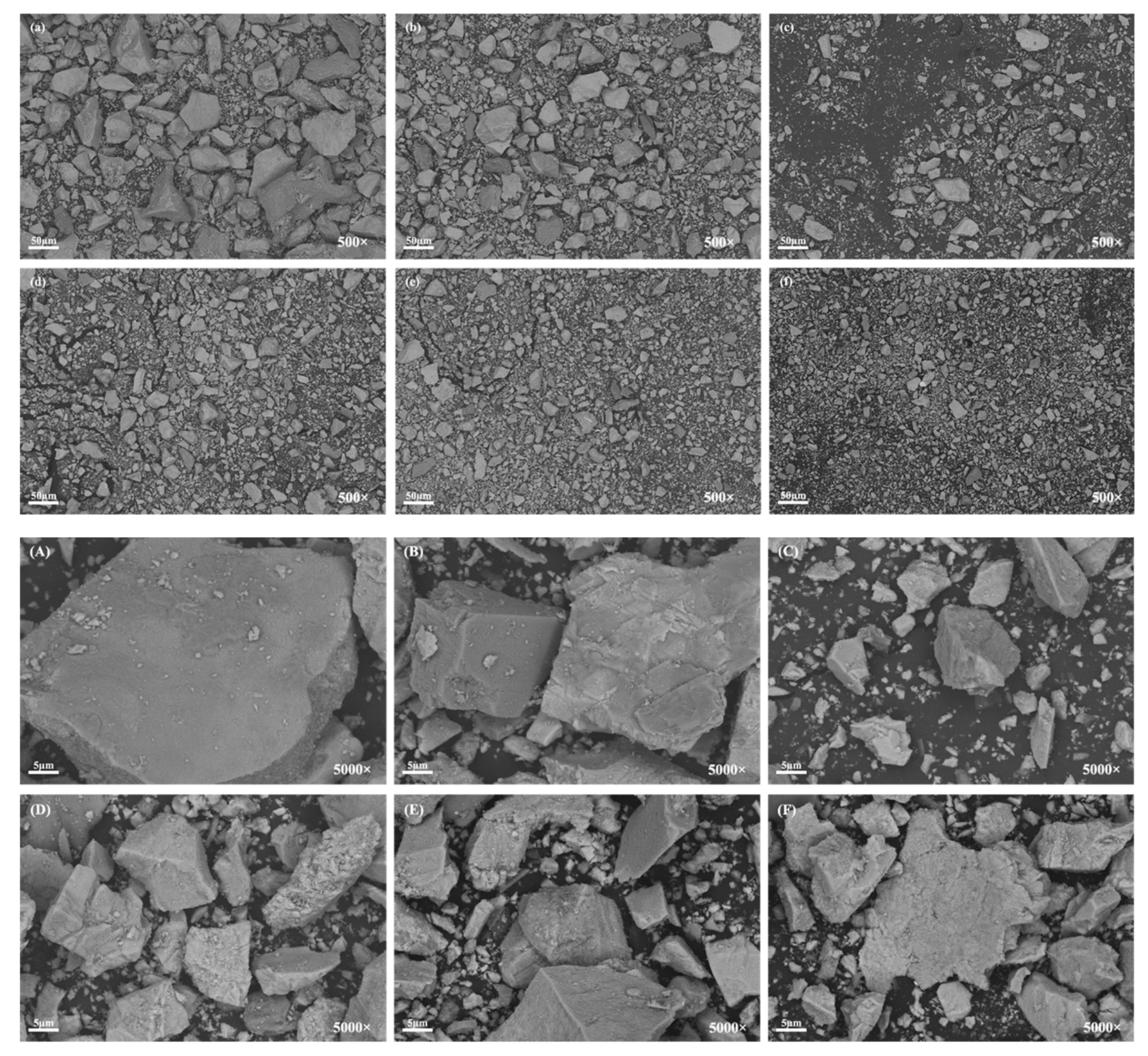




| TFe | FeO | TiO2 | SiO2 | CaO | Al2O3 | V2O5 | MgO | MnO | ZnO | SrO | K2O | Na2O | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 43.75 | 18.4 | 17.33 | 10.98 | 5.06 | 2.61 | 2.34 | 0.87 | 0.66 | 0.08 | 0.07 | 0.05 | 0.04 | 0.04 |
| Mineral Phase | Fe Mass Fraction | Fe Distribution | Ti Mass Fraction | Ti Distribution |
|---|---|---|---|---|
| Titanomagnetite | 35.83 | 83.09 | 5.26 | 50.79 |
| Ilmenite | 5.53 | 5.53 | 3.90 | 37.68 |
| Titanite | Minor | Minor | 1.18 | 11.37 |
| Gangue | 1.76 | 4.08 | 0.02 | 0.16 |
| Total | 43.12 | 99.99 | 10.35 | 100.00 |
| Grinding Time/Min | 0.99 μm | 1.88 μm | 5.92 μm | 9.86 μm | 18.7 μm | 45.6 μm |
|---|---|---|---|---|---|---|
| 10 | 97.93 | 93.77 | 78.25 | 66.32 | 43.48 | 12.26 |
| 20 | 97.63 | 93.25 | 74.95 | 60.94 | 40.7 | 4.89 |
| 30 | 97.34 | 92.71 | 71.74 | 56.27 | 32.58 | 5.09 |
| 40 | 96.7 | 91.24 | 70.74 | 54.89 | 29.95 | 5.44 |
| 50 | 96.32 | 90.23 | 67.59 | 51.17 | 27.83 | 6.16 |
| Parameter | 0.99 μm | 1.88 μm | 5.92 μm | 9.86 μm | 18.7 μm | 45.6 μm |
|---|---|---|---|---|---|---|
| Kt | 3.81821 | 4.44516 | 4.55423 | 4.56275 | 4.59818 | 4.60931 |
| M | 0.60708 | 0.27755 | 0.15307 | 0.08534 | 0.02266 | 0.01004 |
| R2 | 0.95623 | 0.97434 | 0.96573 | 0.98743 | 0.97658 | 0.96057 |
| Samples | Grinding Time /min | SSA (m2/kg) | D10 /μm | D25 /μm | D50 /μm | D75 /μm | D90 /μm |
| Raw LVTC | - | 393.2 | 7.25 | 15.3 | 42 | 52 | 112 |
| LVTC powder | 10 | 770.8 | 3.43 | 7.78 | 18.9 | 24.6 | 54 |
| 20 | 960.2 | 2.65 | 6.25 | 14.8 | 19 | 41.3 | |
| 30 | 1062 | 2.4 | 5.65 | 12.3 | 17.1 | 38.8 | |
| 40 | 1188 | 2.07 | 5.05 | 11.2 | 17.6 | 37.2 | |
| 50 | 1278 | 1.91 | 4.69 | 10.2 | 26.8 | 38.5 |
| Model | Parameters | Grinding Time | ||||
|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | ||
| RRB | d* (measured) | 21.2 | 18.7 | 14.5 | 13.6 | 12.7 |
| d* (fitting) | 22.9 | 18.3 | 15.6 | 14.5 | 13.4 | |
| n | 0.90 | 1.02 | 0.93 | 1.00 | 0.94 | |
| R2 | 0.9983 | 0.9983 | 0.9984 | 0.9987 | 0.9980 | |
| Swebrec | X50 | 18.7 | 14.5 | 11.2 | 10.8 | 9.86 |
| Xmax | 144 | 127 | 98.1 | 163 | 186 | |
| b | 4.69 | 5.15 | 4.82 | 4.60 | 4.66 | |
| R2 | 0.9965 | 0.9966 | 0.9990 | 0.9967 | 0.9969 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xing, Z.; Cheng, G.; Xue, X.; Ding, X. Study on the Grinding Kinetics and Magnetic Separation of Low-Grade Vanadiferous Titanomagnetite Concentrate. Metals 2022, 12, 575. https://doi.org/10.3390/met12040575
Liu J, Xing Z, Cheng G, Xue X, Ding X. Study on the Grinding Kinetics and Magnetic Separation of Low-Grade Vanadiferous Titanomagnetite Concentrate. Metals. 2022; 12(4):575. https://doi.org/10.3390/met12040575
Chicago/Turabian StyleLiu, Jinsheng, Zhenxing Xing, Gongjin Cheng, Xiangxin Xue, and Xueyong Ding. 2022. "Study on the Grinding Kinetics and Magnetic Separation of Low-Grade Vanadiferous Titanomagnetite Concentrate" Metals 12, no. 4: 575. https://doi.org/10.3390/met12040575






