Electrochemical Short-Time Testing Method for Simulating the Degradation Behavior of Magnesium-Based Biomaterials
Abstract
:1. Introduction
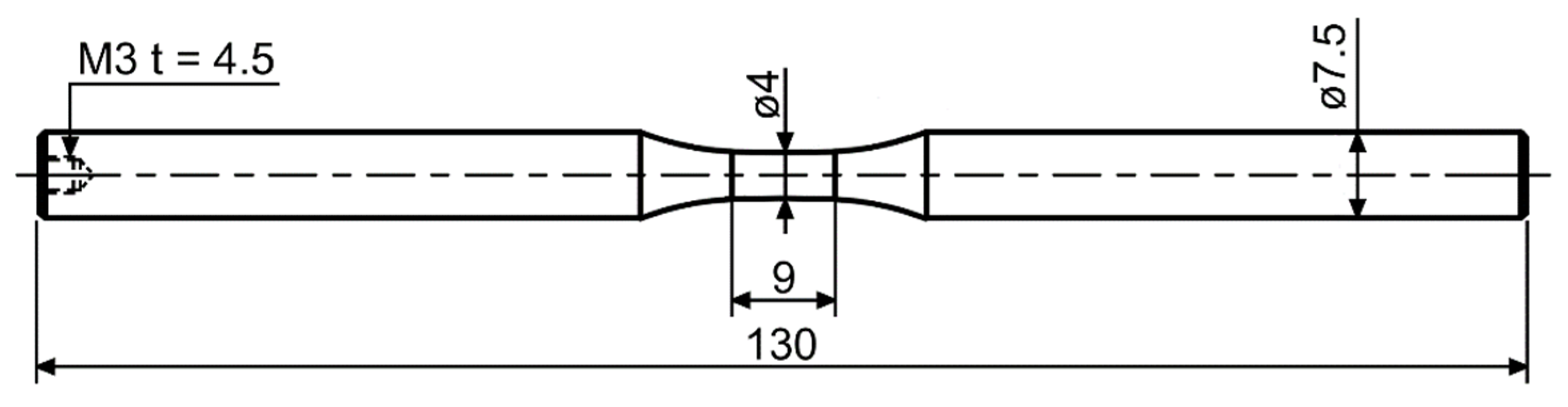
2. Materials and Methods
2.1. Corrosion Investigations
2.1.1. Potentiodynamic Polarization
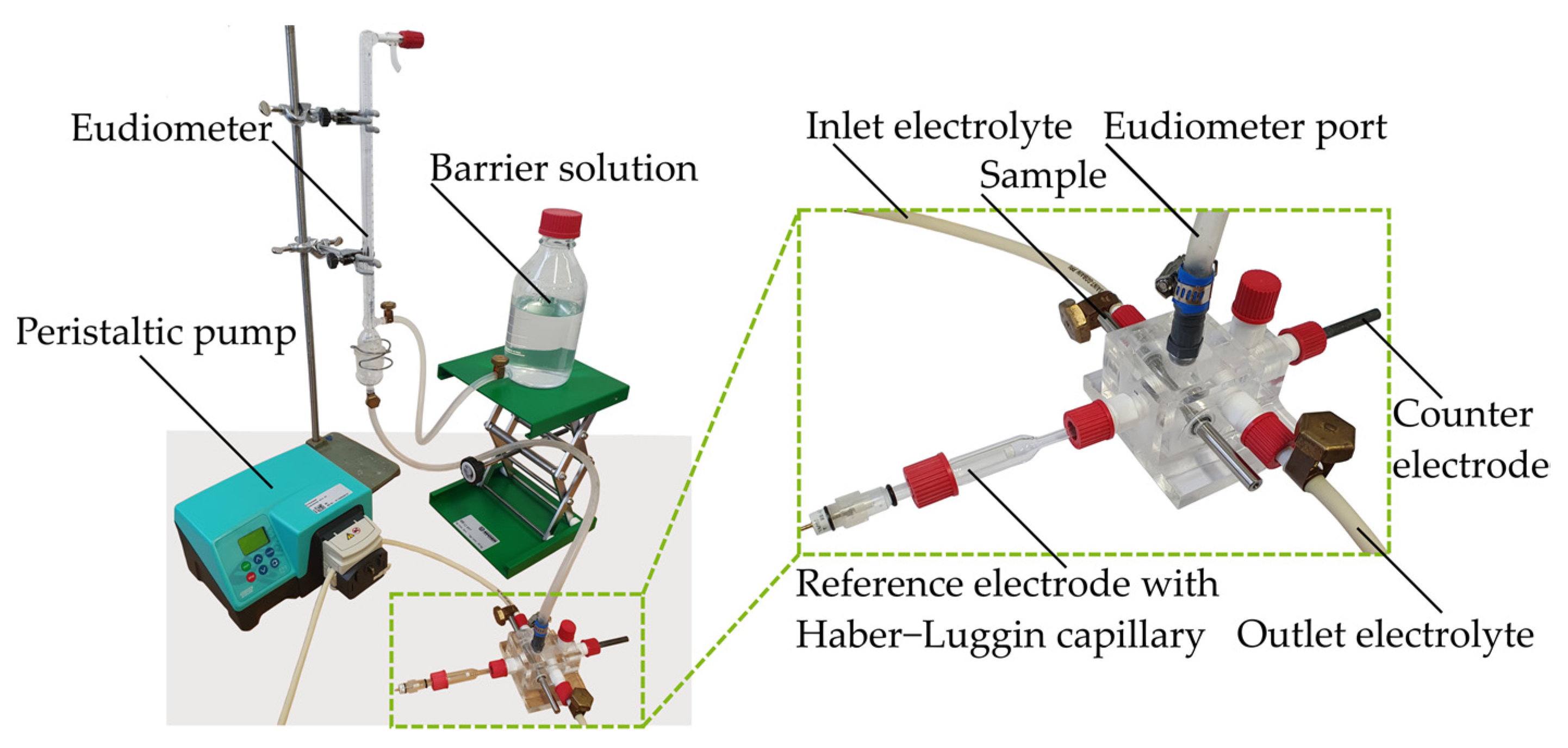
2.1.2. Immersion Tests with Galvanostatic Polarization
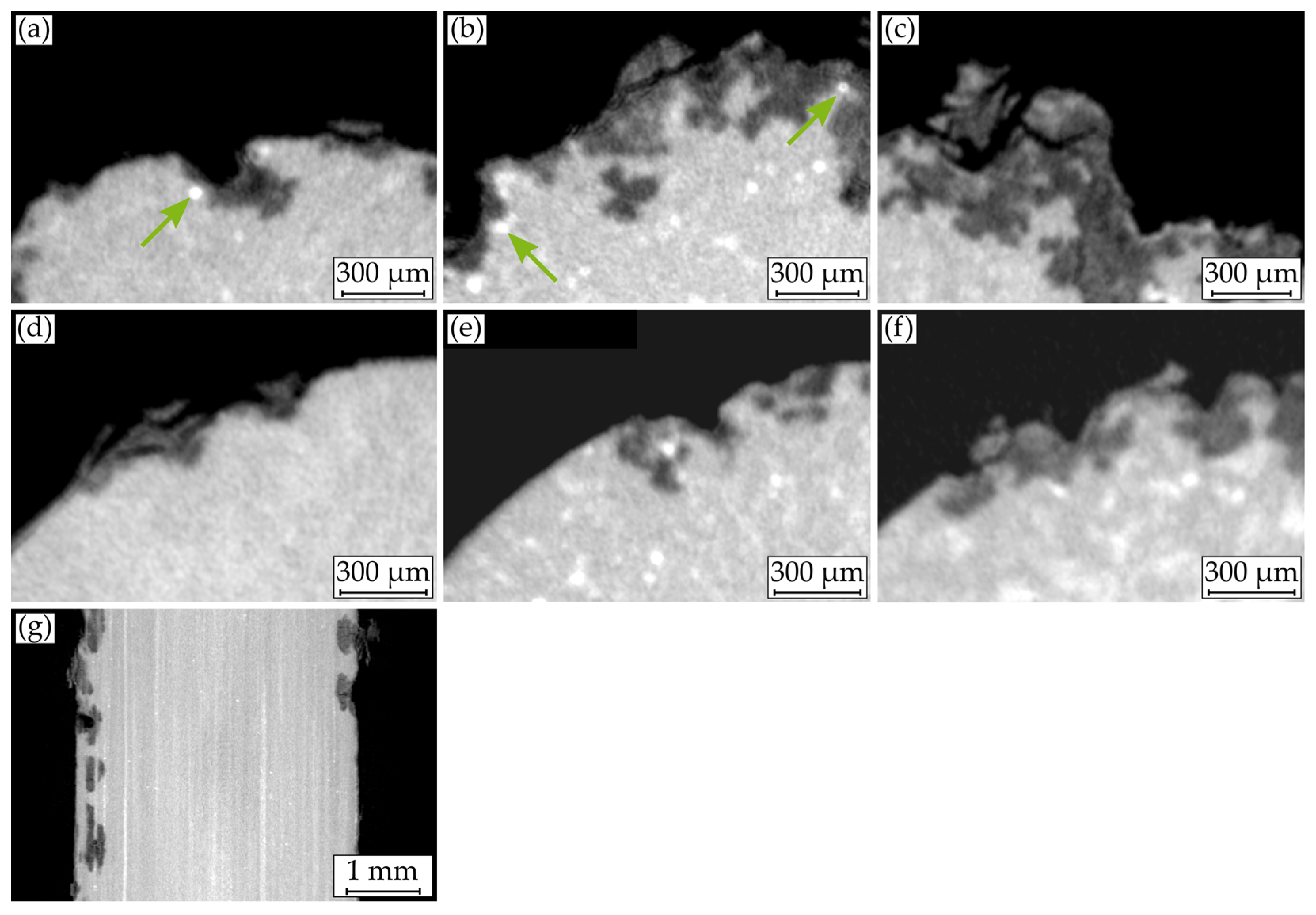
2.1.3. µ-Computed Tomography (µCT)
3. Results
3.1. Potentiodynamic Polarization
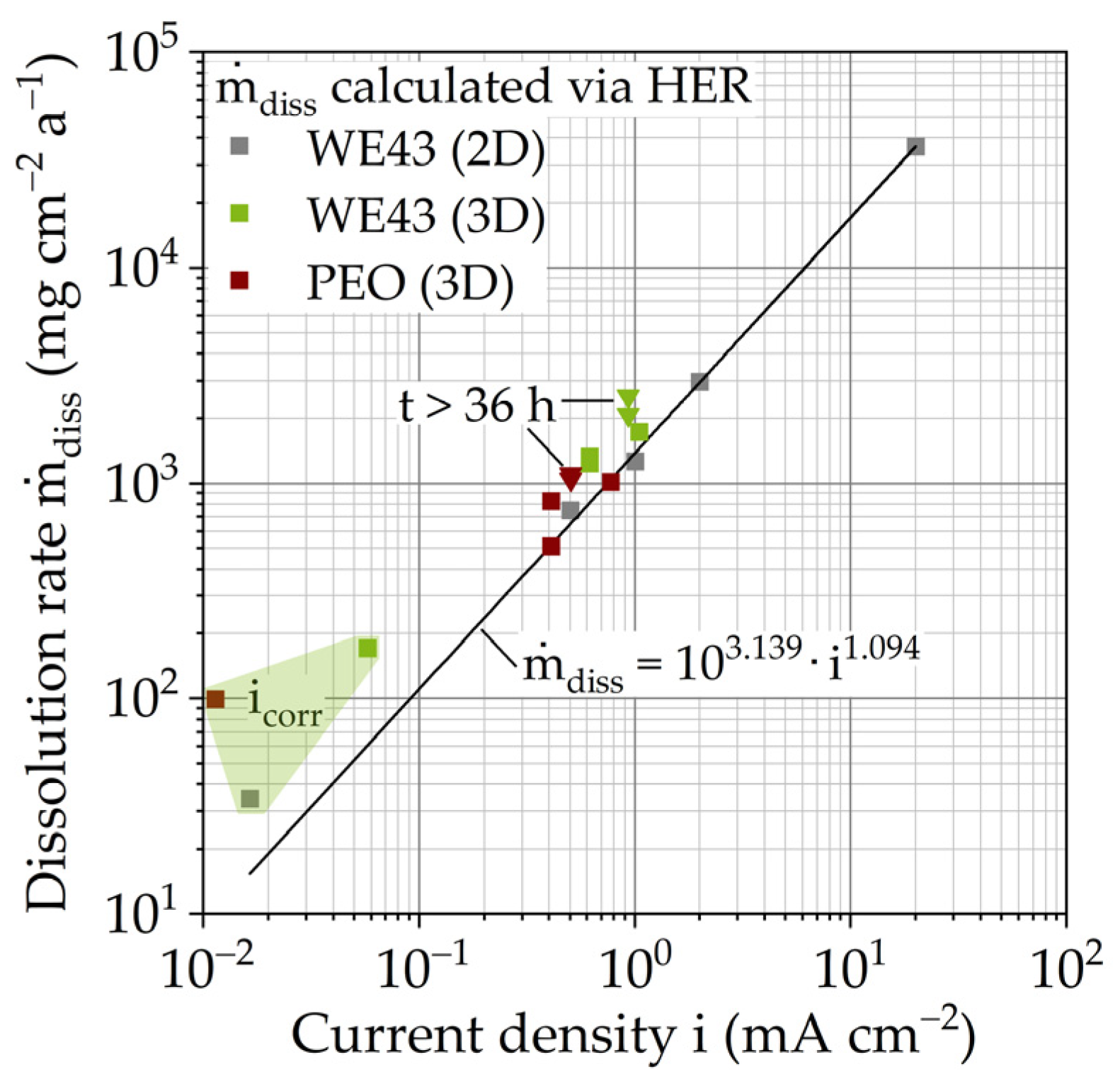
3.2. Immersion Tests with Galvanostatic Polarization
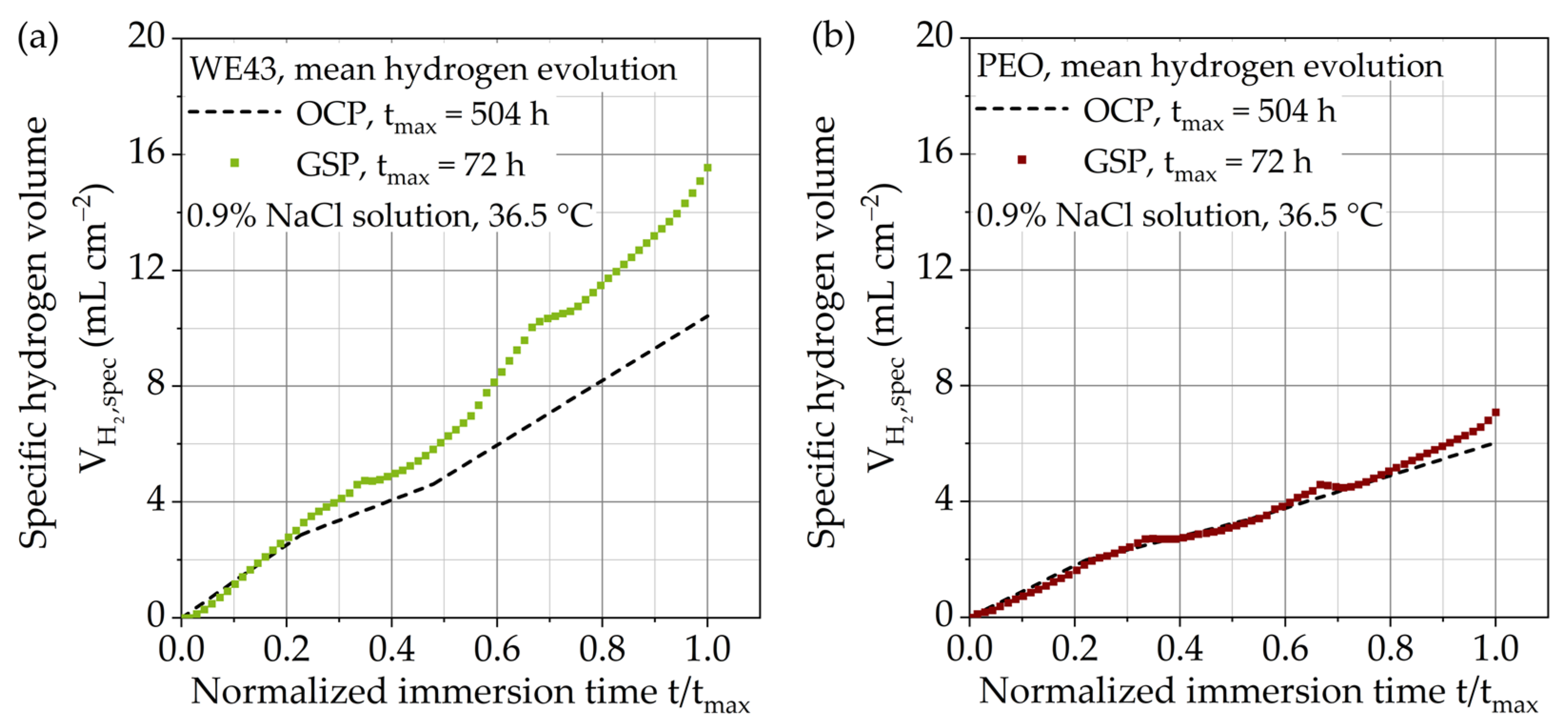
3.2.1. Specific Hydrogen Volume
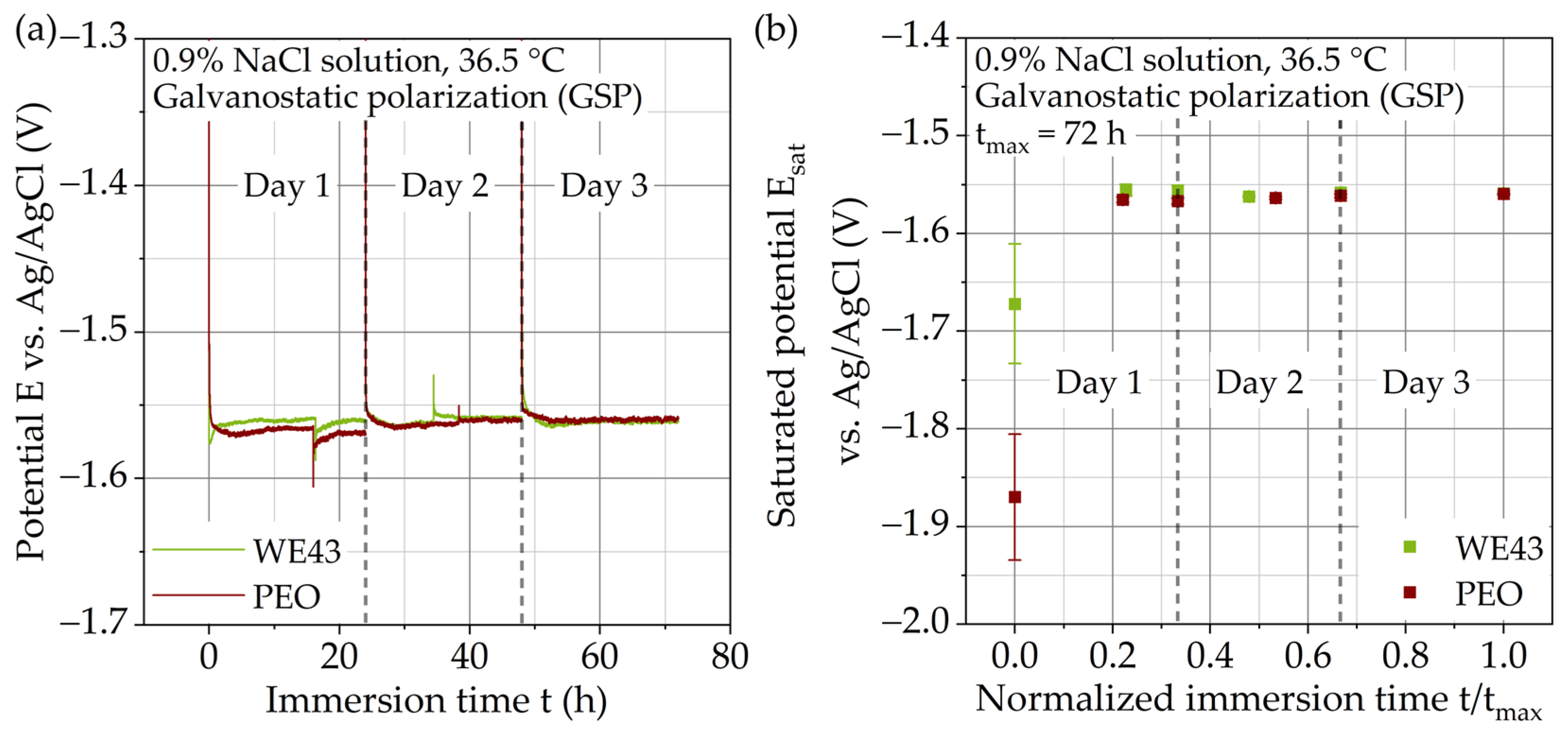
3.2.2. Potential–Time Curves
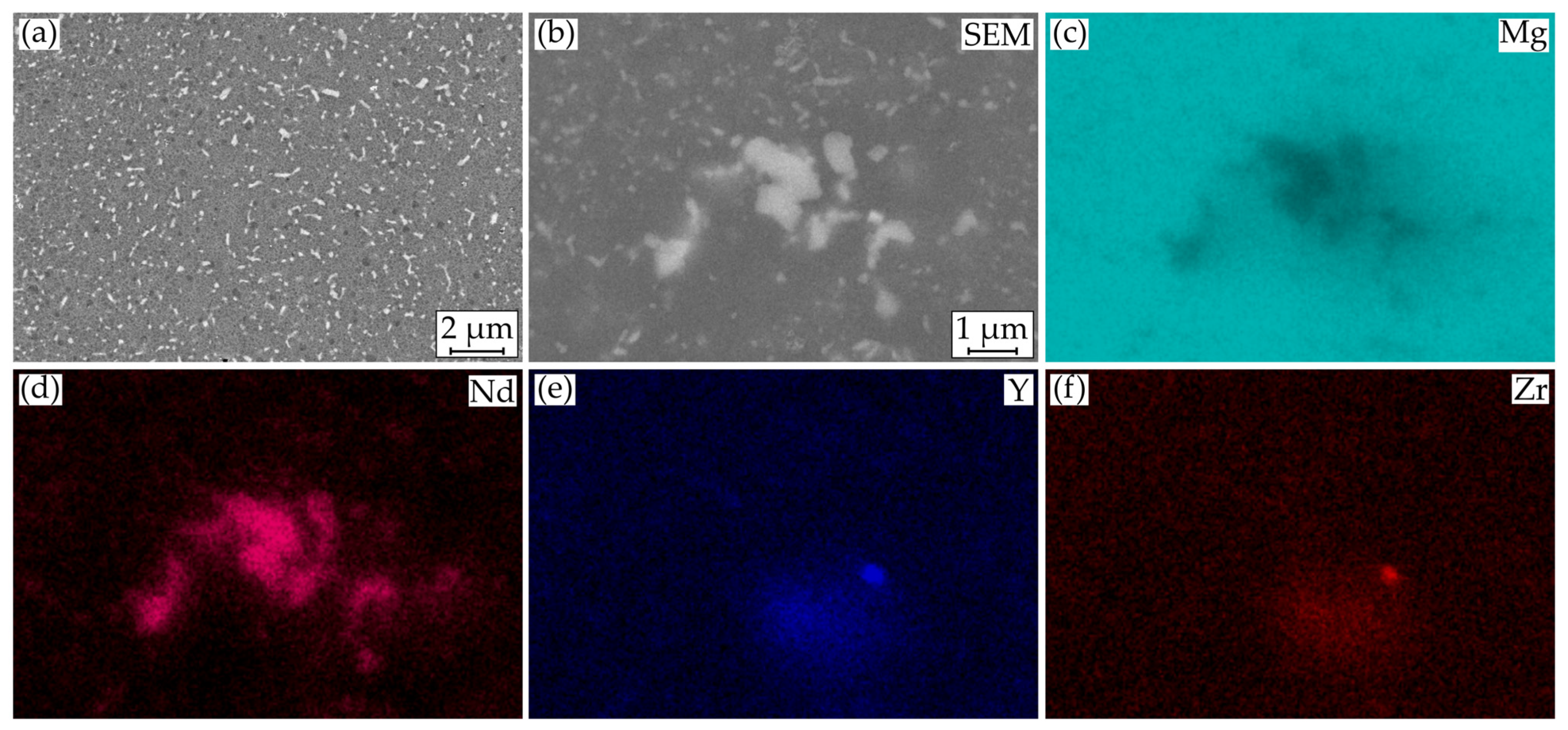
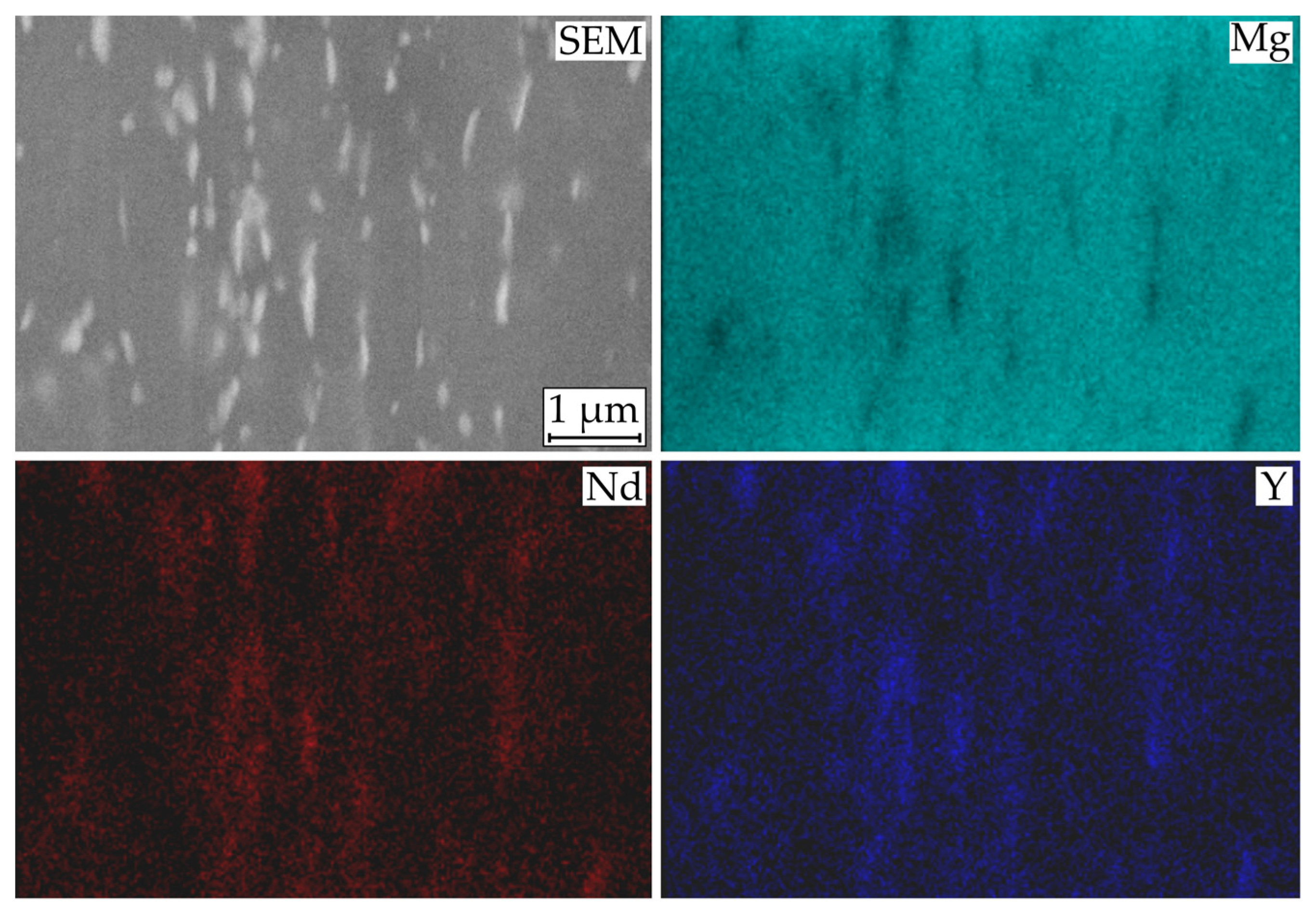
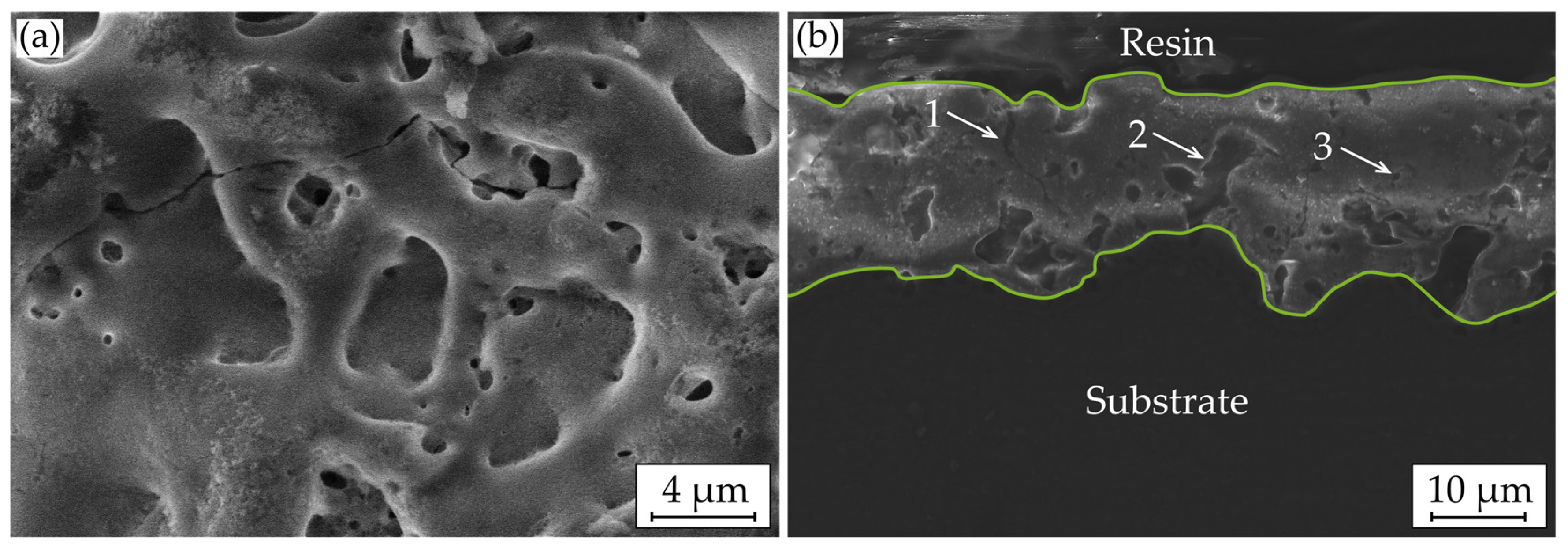
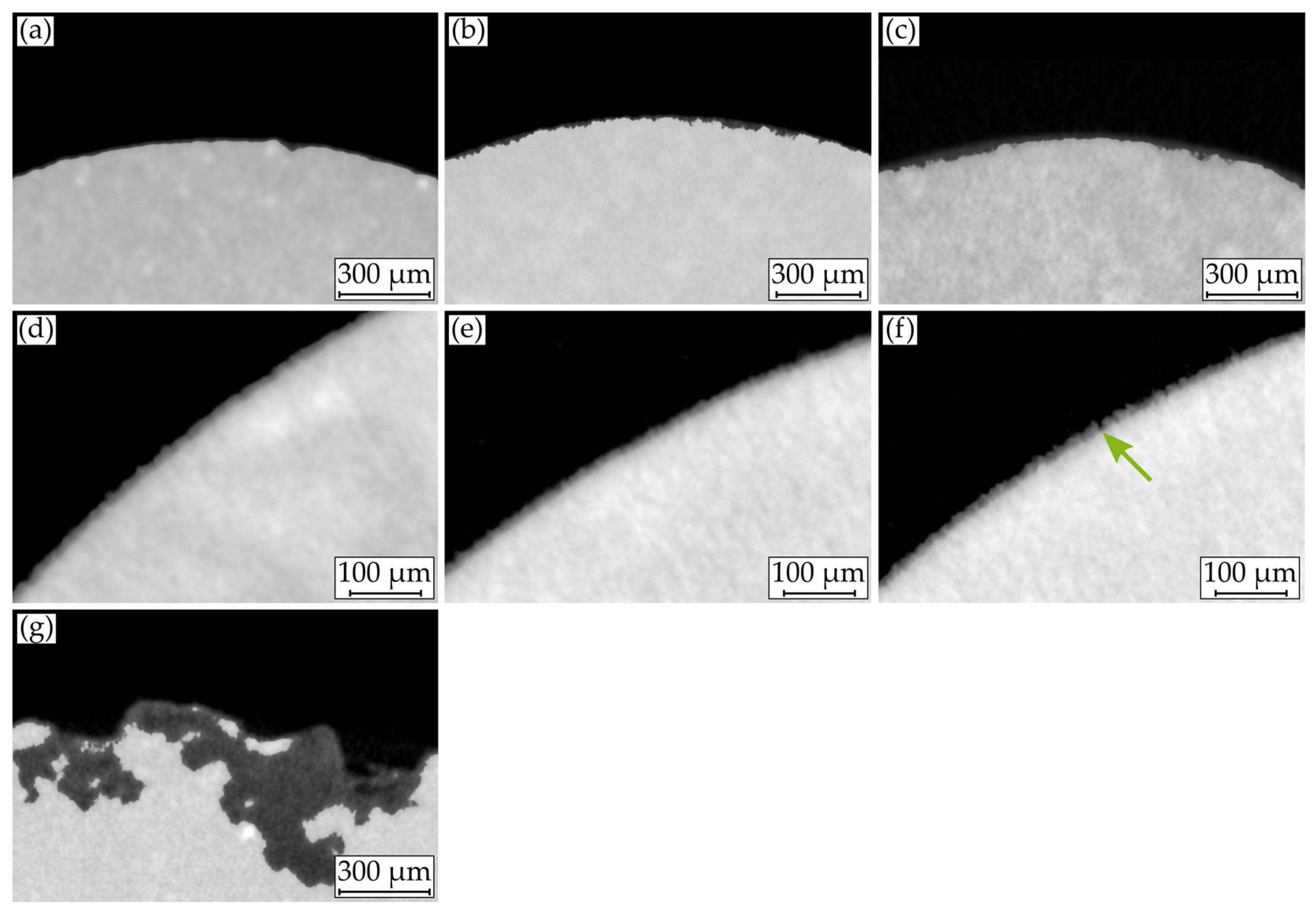
3.3. Macroscopic Corrosion Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Aspects Med. 2003, 24, 3–9. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [Green Version]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An updated on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Farraro, K.F.; Kim, K.E.; Woo, S.L.; Flowers, J.R.; McCullough, M.B. Revolutionizing orthopaedic biomaterials: The potential of biodegradable and bioresorbable magnesium-based materials for functional tissue engineering. J. Biomech. 2014, 47, 1979–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, A.; Atrens, A. Understanding Magnesium corrosion−A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Singh Raman, R.K.; Jafari, S.; Harandi, S.E. Corrosion fatigue fracture of magnesium alloys in bioimplant applications: A review. Eng. Fract. Mech. 2015, 137, 97–108. [Google Scholar] [CrossRef]
- Byun, S.-H.; Lim, H.-K.; Cheon, K.-H.; Lee, S.-M.; Kim, H.E.; Lee, J.-H. Biodegradable magnesium alloy (WE43) in bone-fixation plate and screw. J. Biomed. Mater. Res. 2020, 108, 2505–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger, D.; Galli, S.; Zeller-Plumhoff, B.; Wieland, F.; Peruzzi, N.; Wiese, B.; Heuser, P.; Moosmann, J.; Wennerberg, A.; Willumeit-Römer, R. High-resolution ex vivo analysis of the degradation and osseointegration of Mg-xGd implant screws in 3D. Bioact. Mater. 2022, 13, 37–52. [Google Scholar] [CrossRef]
- Rendenbach, C.; Fischer, H.; Kopp, A.; Schmidt-Bleek, K.; Kreiker, H.; Stumpp, S.; Thiele, M.; Duda, G.; Hanken, H.; Beck-Broichsitter, B.; et al. Improved in vivo osseointegration and degradation behavior of PEO surface-modified WE43 magnesium plates and screws after 6 and 12 months. Mater. Sci. Eng. C 2021, 129, 112380. [Google Scholar] [CrossRef] [PubMed]
- Shadanbaz, S.; Walker, J.; Staiger, M.P.; Dias, G.J.; Pietak, A. Growth of calcium phosphates on magnesium substrates for corrosion control in biomedical applications via immersion techniques. J. Biomed. Mater. Res. B. Appl. Biomater. 2013, 101, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.F.; Kim, Y.C.; Han, H.S.; Lee, G.C.; Seok, H.K.; Lee, J.C. In vitro dynamic degradation behavior of new magnesium alloy for orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 807–815. [Google Scholar] [CrossRef]
- Nidadavolu, E.P.S.; Feyerabend, F.; Ebel, T.; Willumeit-Romer, R.; Dahms, M. On the determination of magnesium degradation rates under physiological conditions. Materials 2016, 9, 627. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, A.H.M.; Luthringer, J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, Y.; Chen, X.; Luthringer-Feyerabend, B.; Xue, J.; Zander, D.; Willumeit-Römer, R.; Kainer, K.U.; Hort, N. Revisiting the tolerance limit of Fe impurity in biodegradable magnesium. Scripta Mater. 2022, 212, 114509. [Google Scholar] [CrossRef]
- Öcal, E.B.; Esen, Z.; Aydinol, K.; Dericioglu, A.F. Comparison of the short and long-term degradation behaviors of as-cast pure Mg, AZ91 and WE43 alloys. Mater. Chem. Phys. 2020, 241, 122350. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Cheng, S.-C.; Liang, L.-X.; Zhang, J.-C.; Li, S.-Q.; Wang, Z.-L.; Zeng, R.-C. In vitro corrosion resistance of layer-by-layer assembled polyacrylic acid multilayers induced Ca-P coating on magnesium alloy AZ31. Bioact. Mater. 2020, 5, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Victoria-Hernandez, J.; Jiang, P.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.; Yi, S.; Letzig, D.; Feyerabend, F. In vitro evaluation of the ZX11 magnesium alloy as potential bone plate: Degradability and mechanical integrity. Acta Biomater. 2019, 97, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Van Gaalen, K.; Gremse, F.; Benn, F.; McHugh, P.E.; Kopp, A.; Vaughan, T.J. Automated ex-situ detection of pitting corrosion and its effect on the mechanical integrity of rare earth magnesium alloy—WE43. Bioact. Mater. 2022, 8, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Rassat, S.D.; Helderandt, D.J.; Caldwell, D.D.; Aardahl, C.L.; Autrey, T.; Linehan, J.C.; Rappe, K.G. Automated gas burette system for evolved hydrogen measurements. Rev. Sci. Instrum. 2008, 79, 084103. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corr. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
- Esmaily, M.; Zeng, Z.; Mortazavi, A.N.; Gullino, A.; Choudhary, S.; Derra, T.; Benn, F.; D’Elia, F.; Müther, M.; Thomas, S.; et al. A detailed microstructural and corrosion analysis of magnesium alloy WE43 manufactured by selective laser melting. Addit. Manuf. 2020, 35, 101312. [Google Scholar] [CrossRef]
- Shi, Z.; Jia, J.X.; Atrens, A. Galvanostatic anodic polarization of WE43. J. Magnes. Alloy. 2014, 2, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Jia, J.X.; Atrens, A. Galvanostatic polarization curves and galvanic corrosion of high purity Mg in 3.5% NaCl saturated with Mg(OH)2. Corr. Sci. 2012, 60, 296–308. [Google Scholar] [CrossRef]
- Huang, J.; Song, G.-L.; Zhu, Y.; Zheng, D.; Wang, Z. The anodically polarized Mg surface products and accelerated hydrogen evolution. J. Magnes. Alloy. 2022; in press. [Google Scholar] [CrossRef]
- Thomas, S.; Medhekar, N.V.; Frankel, G.S.; Birbilis, N. Corrosion mechanism and hydrogen evolution on Mg. Curr. Opin. Solid State Mater. Sci. 2015, 19, 85–94. [Google Scholar] [CrossRef]
- Klein, M.; Frieling, G.; Walther, F. Corrosion fatigue assessment of creep-resistant magnesium alloys DieMag422 and AE42. Eng. Fract. Mech. 2017, 185, 33–45. [Google Scholar] [CrossRef]
- Jung, O.; Porchetta, D.; Schroeder, M.-L.; Klein, M.; Wegner, N.; Walther, F.; Feyerabend, F.; Barbeck, M.; Kopp, A. In vivo simulation of magnesium degradability using a new fluid dynamic bench testing approach. Int. J. Mol. Sci. 2019, 20, 4859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartjen, P.; Wegner, N.; Ahmadi, P.; Matthies, L.; Nada, O.; Fuest, S.; Yan, M.; Knipfer, C.; Gosau, M.; Walther, F.; et al. Toward tailoring the degradation rate of magnesium-based biomaterials for various medical applications: Assessing corrosion, cytocompatibility and immunological effects. Int. J. Mol. Sci. 2021, 22, 971. [Google Scholar] [CrossRef]
- Wegner, N.; Walther, F. Assessment of galvanostatic anodic polarization to accelerate the corrosion of the bioresorbable magnesium alloy WE43. Appl. Sci. 2021, 11, 2128. [Google Scholar] [CrossRef]
- Zeng, Z.; Stanford, N.; Davies, C.H.J.; Nie, J.-F.; Birbilis, N. Magnesium extrusion alloys: A review of developments and prospects. Int. Mater. Review 2019, 64, 27–62. [Google Scholar] [CrossRef]
- Imandoust, A.; Barrett, C.D.; Al-Samman, T.; Inal, K.A.; El Kadiri, H. A review on the effect of rare-earth elements on texture evolution during processing of magnesium alloys. J. Mater. Sci. 2017, 52, 1–29. [Google Scholar] [CrossRef]
- Xiang, C.; Gupta, N.; Coelho, P.; Cho, K. Effect of microstructure on tensile and compressive behavior of WE43 alloy in as cast and heat treated conditions. Mater. Sci. Eng. A 2018, 710, 74–85. [Google Scholar] [CrossRef]
- Mraied, H.; Wang, W.; Cai, W. Influence of chemical heterogeneity and microstructure on the corrosion resistance of biodegradable WE43 magnesium alloys. J. Mater. Chem. B 2019, 7, 6399–6411. [Google Scholar] [CrossRef] [PubMed]
- Darband, B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Krüger, D.; Zeller-Plumhoff, B.; Wiese, B.; Yi, S.; Zuber, M.; Wieland, F.; Moosmann, J.; Willumeit-Römer, R. Assessing the microstructure and in vitro degradation behavior of Mg-xGd srew implants using µCT. J. Magnes. Alloy. 2021, 9, 2207–2222. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Corrosion resistance of WE43 and AZ91D magnesium alloys with phosphate PEO coating. Corr. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Bahmani, A.; Arthanari, S.; Shin, K.S. Formulation of corrosion rate of magnesium alloys using microstructural parameters. J. Magnes. Alloy. 2020, 8, 134–149. [Google Scholar] [CrossRef]
- Yang, Y.; Scenini, F.; Curioni, M. A study on magnesium corrosion by real-time imaging and electrochemical methods: Relationship between local processes and hydrogen evolution. Electrochim. Acta 2016, 198, 174–184. [Google Scholar] [CrossRef]
- Coy, A.E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Susceptibility of rare-earth-magnesium alloys to micro-galvanic corrosion. Corr. Sci. 2010, 52, 3896–3906. [Google Scholar] [CrossRef]
- Jafari, S.; Singh Raman, R.K.; Davies, C.H.J. Corrosion fatigue of magnesium alloy in modified simulated body fluid. Eng. Fract. Mech. 2015, 137, 2–11. [Google Scholar] [CrossRef]


| Material | Corrosion Rate (103 mg cm−2 a−1) [30] | Time Interval (h) | Current Density i (mA cm−2) | |
|---|---|---|---|---|
| t0 | ta | |||
| WE43 | 0.21 | 0–114.4 | 0–16.3 | 1.05 |
| 0.12 | 144.4–241.5 | 16.3–34.5 | 0.61 | |
| 0.18 | 241.5–504 | 34.5–72 | 0.94 | |
| WE43 PEO | 0.15 | 0–111.6 | 0–15.9 | 0.77 |
| 0.07 | 111.6–268.5 | 15.9–38.4 | 0.41 | |
| 0.09 | 268.5–504 | 38.4–72 | 0.51 | |
| Material | Time Interval t (h) | Current Density I (mA cm−2) | Dissolution Rate (103 mg cm−2 a−1) |
|---|---|---|---|
| WE43 | 0–16.7 | 1.05 | 1.73 |
| 16.7–24 | 0.61 | 1.33 | |
| 24–34.4 | 0.61 | 1.24 | |
| 34.4–48 | 0.94 | 2.54 | |
| 48–72 | 0.94 | 2.09 | |
| WE43 PEO | 0–16.7 | 0.77 | 1.02 |
| 16.7–24 | 0.41 | 0.83 | |
| 24–38.8 | 0.41 | 0.51 | |
| 38.8–48 | 0.51 | 1.11 | |
| 48–72 | 0.51 | 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegner, N.; Vergin, J.; Walther, F. Electrochemical Short-Time Testing Method for Simulating the Degradation Behavior of Magnesium-Based Biomaterials. Metals 2022, 12, 591. https://doi.org/10.3390/met12040591
Wegner N, Vergin J, Walther F. Electrochemical Short-Time Testing Method for Simulating the Degradation Behavior of Magnesium-Based Biomaterials. Metals. 2022; 12(4):591. https://doi.org/10.3390/met12040591
Chicago/Turabian StyleWegner, Nils, Johanna Vergin, and Frank Walther. 2022. "Electrochemical Short-Time Testing Method for Simulating the Degradation Behavior of Magnesium-Based Biomaterials" Metals 12, no. 4: 591. https://doi.org/10.3390/met12040591







