Exploration on the Effect of Pretreatment Conditions on Hydrogen-Induced Defects in Pure Titanium by Positron Annihilation Spectroscopy
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Sample Preparation and Process
2.2. Electrolytic Hydrogen Charging Experiment
2.3. Sample Characterization and Research Methods
3. Results and Discussion
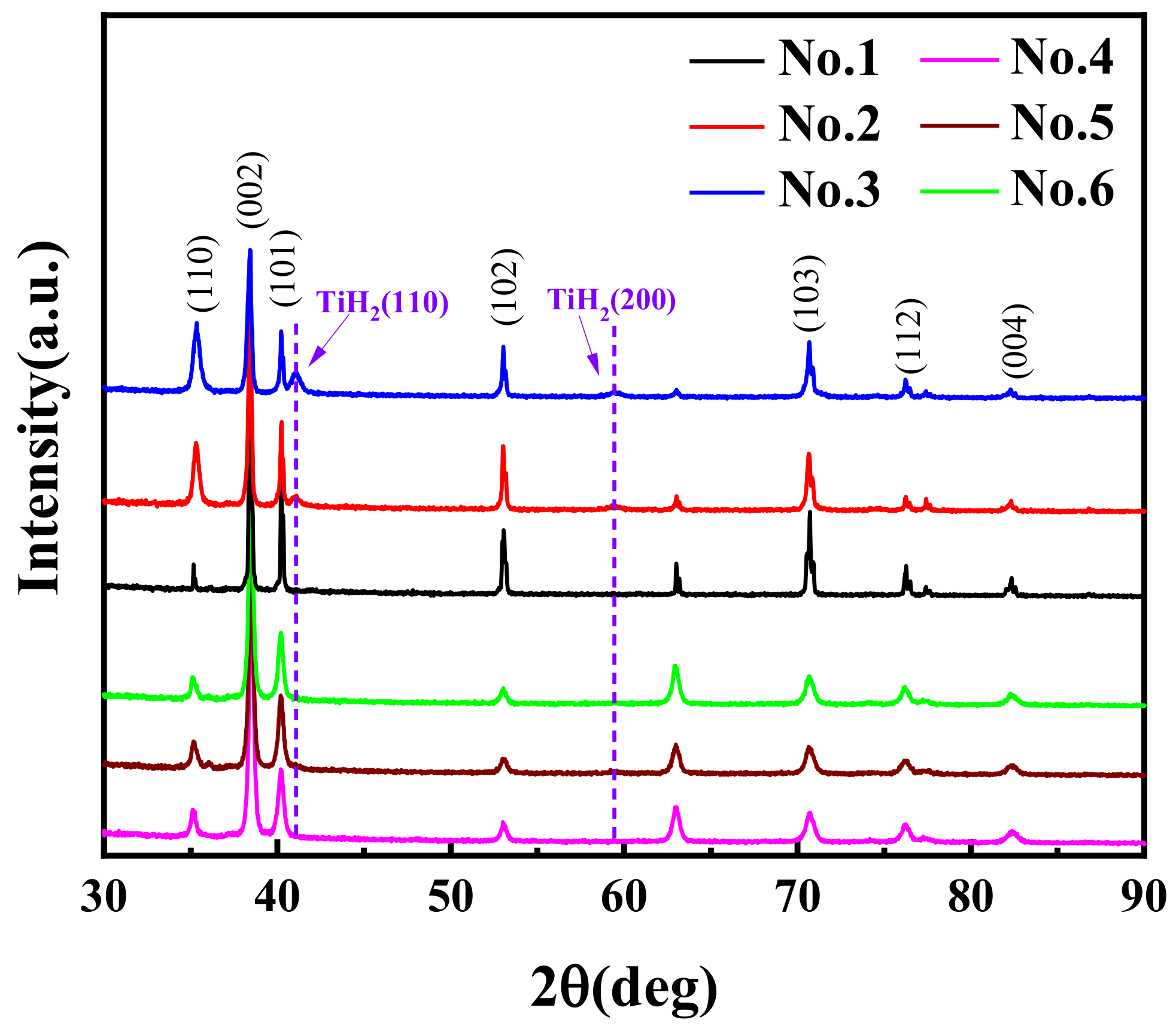
3.1. Analysis of Phase Composition in Hydrogenated Samples
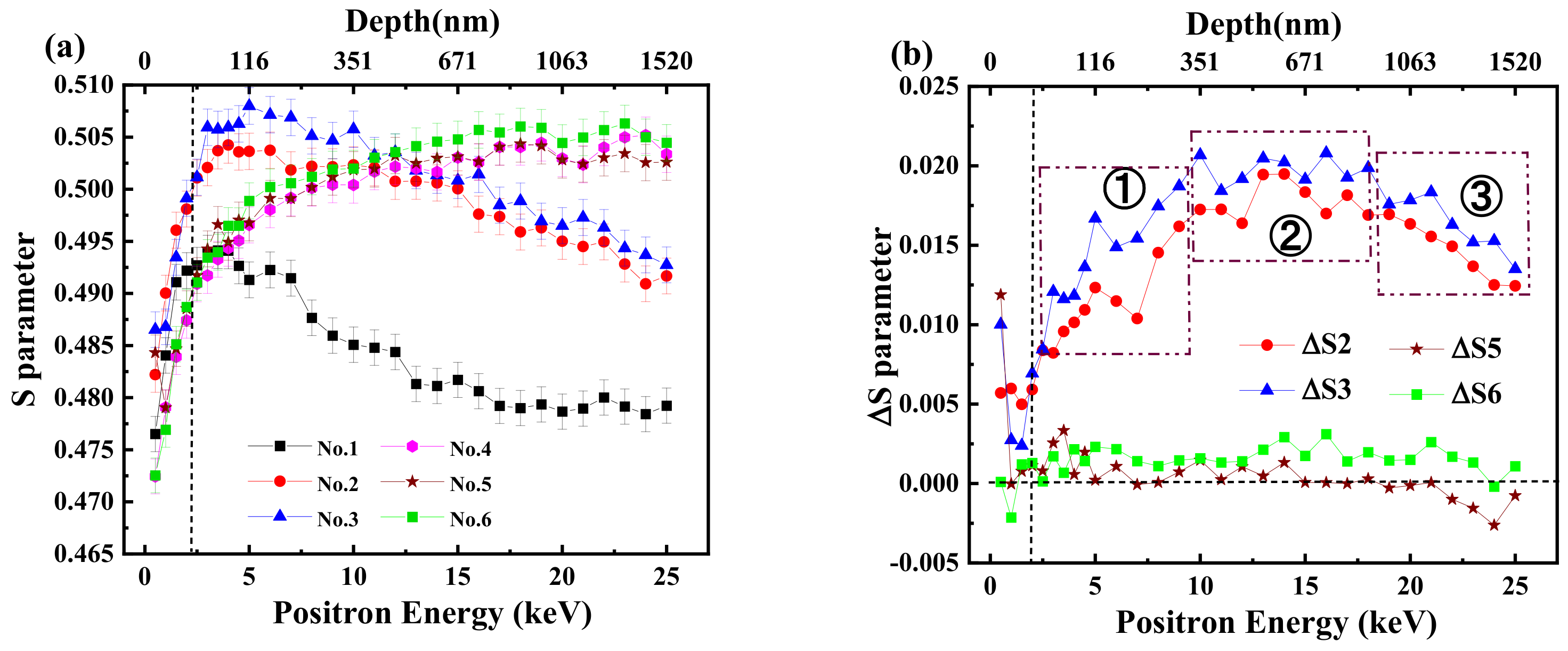
3.2. Formation of Vacancy-Type Defects before and after Electrolytic Hydrogen Charging
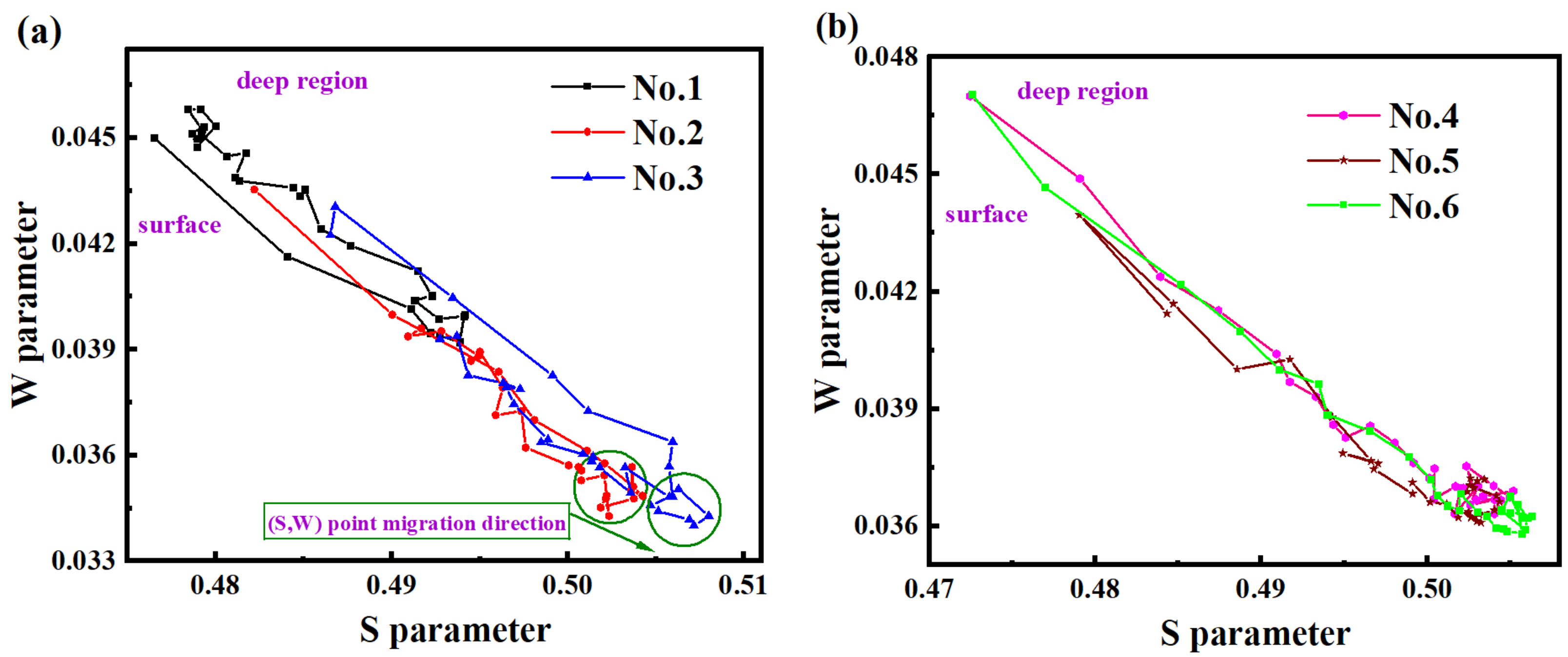
3.3. Analysis of Defect Structure in Hydrogen-Charged Samples
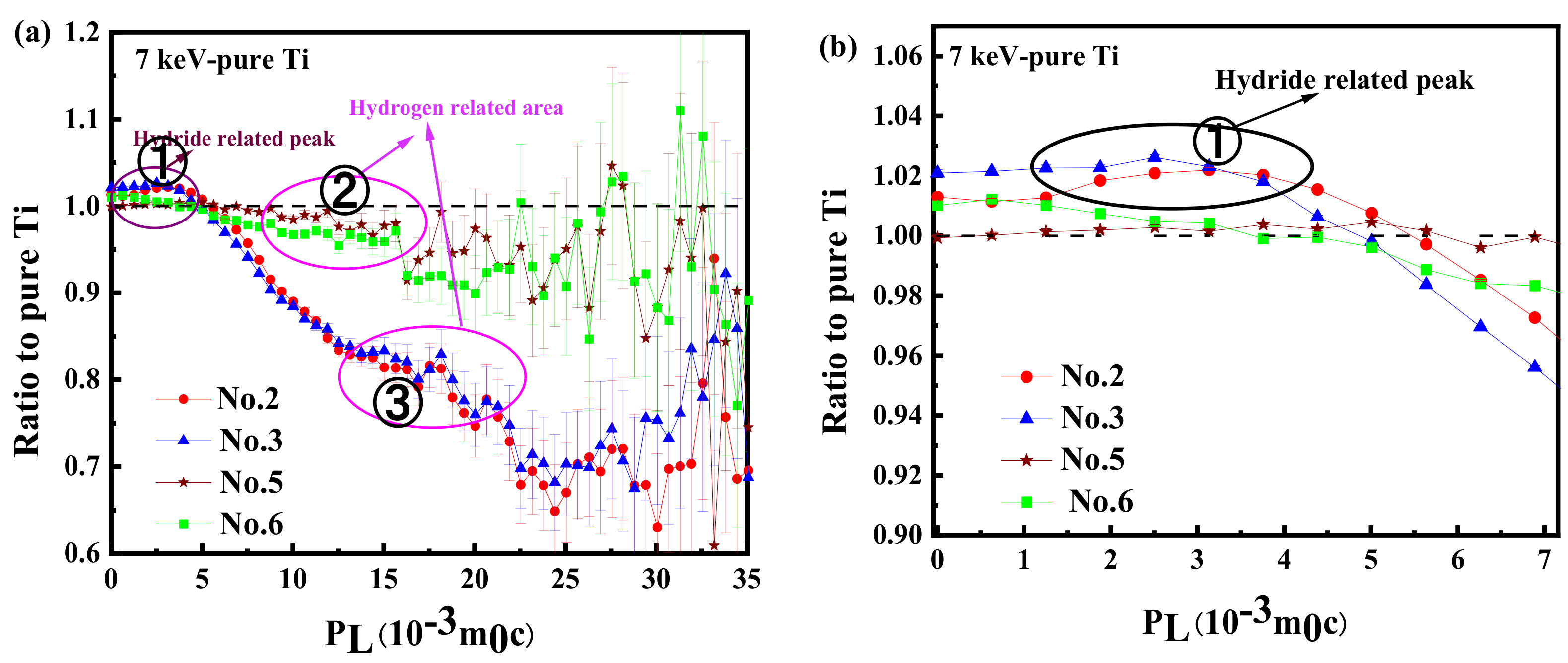
3.4. Analysis of Elemental Information of Electrolytic Hydrogen Charging Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Young, K.; Nei, J. The Current Status of Hydrogen Storage Alloy Development for Electrochemical Applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Zhao, D.; Wang, Y.; Xue, R.; Shen, Z.; Li, X. The mechanism of hydrogen storage in carbon materials. Int. J. Hydrog. Energy 2007, 32, 2513–2517. [Google Scholar]
- Momida, H.; Asari, Y.; Nakamura, Y.; Tateyama, Y. Hydrogen-enhanced vacancy embrittlement of grain boundaries in iron. Phys. Rev. B 2013, 88, 144107–144113. [Google Scholar] [CrossRef]
- Nagumo, M.; Nakamura, M.; Takai, K. Hydrogen thermal desorption relevant to delayed-fracture susceptibility of high-strength steels. Metall. Trans. A 2001, 32, 339–347. [Google Scholar] [CrossRef]
- Machida, A.; Saitoh, H.; Hattori, T.; Sano-Furukawa, A.; Aoki, K. Hexagonal Close-Packed Iron Hydride behind the Conventional Phase Diagram. Sci. Rep. 2019, 9, 12290. [Google Scholar] [CrossRef] [Green Version]
- Bordulev, Y.S.; Laptev, R.S.; Kudiiarov, V.N.; Lider, A.M. Investigation of Commercially Pure Titanium Structure during Accumulation and Release of Hydrogen by Means of Positron Lifetime and Electrical Resistivity Measurements. Adv. Mater. Res. 2014, 880, 93–100. [Google Scholar] [CrossRef]
- Kumar, J.; Saxena, S. Electrochemical investigation of hydrogen absorption in titanium-based intermetallics. Int. J. Hydrog. Energy 1989, 14, 331–337. [Google Scholar] [CrossRef]
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. Atomic mechanism and prediction of hydrogen embrittlement in iron. Nat. Mater. 2013, 12, 145–151. [Google Scholar] [CrossRef]
- Laptev, R.S.; Kudiiarov, V.N.; Bordulev, Y.S.; Mikhaylov, A.A.; Lider, A.M. Gas-phase hydrogenation influence on defect behavior in titanium-based hydrogen-storage material. Prog. Nat. Sci. 2017, 27, 105–111. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, H.; Shi, S. Mechanical properties of titanium hydride. J. Alloys Compd. 2007, 436, 82–85. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Dan, E. The hydrogen embrittlement of titanium-based alloys. JOM 2005, 57, 46–49. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrog. Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W. Hydrogen Embrittlement Understood. Metall. Trans. A 2015, 46, 1085–1103. [Google Scholar] [CrossRef]
- Thomas, S.; Ott, N.; Schaller, R.F.; Yuwono, J.A.; Volovitch, P.; Sundararajan, G. The effect of absorbed hydrogen on the dissolution of steel. Heliyon 2016, 2, e00209. [Google Scholar] [CrossRef]
- Dutta, A.; Gupta, P.; Mukherjee, N. Proton irradiation studies on pure Ti and Ti-6Al-4V. Nucl. Instrum. Methods Phys. Res. 2016, 387, 63–72. [Google Scholar] [CrossRef]
- Wang, T.; Grambole, D.; Grötzschel, R.; Herrmann, F.; Kreißig, U.; Eichhorn, F. Mobility and retention of implanted hydrogen in Ti225 titanium alloy. Surf. Coat. Technol. 2002, 158, 139–145. [Google Scholar] [CrossRef]
- Ang, C.; Silva, C.; Shih, C.; Koyanagi, T.; Katoh, Y.; Zinkle, S.J. Anisotropic swelling and microcracking of neutron irradiated Ti3AlC2–Ti5Al2C3 materials. Scr. Mater. 2016, 114, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Jean, Y.C. Hydrogen-induced defects of AISI 316 stainless steel studied by variable energy Doppler broadening energy spectra. Phys. Status Solidi 2004, 201, 917–922. [Google Scholar] [CrossRef]
- Číže, J. Characterization of lattice defects in metallic materials by positron annihilation spectroscopy: A review. J. Mater. Sci. Technol. 2018, 34, 577–598. [Google Scholar] [CrossRef]
- Chen, H.; Hung, W.; Lo, C.H.; Huang, S.; Cheng, M.; Liu, G. Free-Volume Depth Profile of Polymeric Membranes Studied by Positron Annihilation Spectroscopy: Layer Structure from Interfacial Polymerization. Macromolecules 2007, 40, 7542–7557. [Google Scholar] [CrossRef]
- Selim, F.A. Positron annihilation spectroscopy of defects in nuclear and irradiated materials—A review. Mater. Charact. 2021, 174, 110952. [Google Scholar] [CrossRef]
- Chejara, N.; Gupta, S.K.; Palsania, H.S.; Meena, D. Study of defect production in neutron irradiated Cu, Ni and Pb using positron annihilation spectroscopy (PAS). Mater Today Proc. 2021, 42, 1651–1656. [Google Scholar] [CrossRef]
- An, X.; Zhang, H.; Zhu, T.; Wang, Q.; Zhang, P.; Song, Y.; Wan, M.; Yang, T.; Cao, X. Exploration of vacancy defect formation and evolution in low-energy ion implanted pure titanium. Int. J. Hydrog. Energy 2022, 47, 8467–8479. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, P.; Lu, E.; Guo, L.; Wang, B.; Cao, X. Correlation between Cu precipitates and irradiation defects in Fe–Cu model alloys investigated by positron annihilation spectroscopy. Acta Mater. 2016, 103, 658–664. [Google Scholar] [CrossRef]
- Wang, B.; Ma, Y.; Zhang, Z.; Yu, R.; Wang, P. Performance of the Beijing pulsed variable-energy positron beam. Appl. Surf. Sci. 2007, 255, 119–121. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Z.; Gao, X.; Cui, M.; Li, B.; Sun, J. Positron annihilation Doppler broadening spectroscopy study on Fe-ion irradiated NHS steel. Nucl. Instrum. Methods Phys. Res. Sect. B 2015, 344, 5–10. [Google Scholar] [CrossRef]
- Qiu, J.; Xin, Y.; Ju, X.; Guo, L.; Wang, B.; Zhong, Y. Investigation by slow positron beam of defects in CLAM steel induced by helium and hydrogen implantation. Nucl. Instrum. Methods Phys. Res. Sect. B 2009, 267, 3162–3165. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Zhang, P.; Wang, S.; Wang, B. The influence of rhenium addition on the distribution of vacancy-type defects in tungsten. J. Nucl. Mater. 2021, 553, 153045. [Google Scholar] [CrossRef]
- Schultz, P.J.; Lynn, K.G. Interaction of positron beams with surfaces, thin films, and interfaces. Rev. Mod. Phys. 1988, 60, 701–779. [Google Scholar] [CrossRef]
- Sabelová, V.; Kršjak, V.; Kuriplach, J.; Petriska, M.; Slugeň, V.; Šimeg Veterníková, J. Characterization of helium implanted Fe–Cr alloys by means of positron annihilation methods. J. Nucl. Mater. 2014, 450, 54–58. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, H.; Cao, X.; Jin, S.; Zhang, P.; Xiao, A. Formation and recovery of Cu precipitates in Fe–Cu model alloys under varying heat treatment. Phys. Status Solidi A 2017, 214, 1600785. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, T.; Jin, S.; Kuang, P.; Zhang, P.; Lu, E. Detection of helium in irradiated Fe9Cr alloys by coincidence Doppler broadening of slow positron annihilation. Appl. Phys. A Mater. Sci. Process. 2017, 123, 177. [Google Scholar] [CrossRef]
- Wang, Q.; An, X.; Zhu, T.; Wan, M.; Cao, X. Effect of electrochemical hydrogen charging on defect structure in titanium. J. Alloys Compd. 2021, 885, 160909. [Google Scholar] [CrossRef]
- Connétable, D.; Huez, J.; Andrieu, E.; Mijoule, C. First-principles study of diffusion and interactions of vacancies and hydrogen in hcp-titanium. J. Phys. Condens. Matter. 2011, 23, 405401. [Google Scholar] [CrossRef]
- Souza, G.; Foerster, C.E.; Lepienski, C.M.; Kuromoto, N.K.; Silva, S.; Schreiner, W.H. Structural, chemical and tribo-mechanical surface features of Ti and nitrided Ti submitted to hydrogen low energy implantation. Mater. Chem. Phys. 2010, 124, 443–452. [Google Scholar] [CrossRef]
- Yong, X.; Xin, J.; Jie, Q.; Guo, L.; Chen, J.; Zheng, Y. Vacancy-type defects and hardness of helium implanted CLAM steel studied by positron-annihilation spectroscopy and nano-indentation technique. Fusion Eng. Des. 2012, 87, 432–436. [Google Scholar]
- An, X.; Zhu, T.; Wan, M.; Li, Y.; Cao, X. Investigation of the interaction between hydrogen and irradiation defects in titanium by using positron annihilation spectroscopy. Int. J. Hydrog. Energy 2021, 46, 13162–13170. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, B.; Song, L.; Liu, X.; Song, Y.; Liu, Y.; Zhang, P.; Cao, X.; Xu, Q. Sensitivity of positrons at hydrogen storage sites in FeCr alloy containing vacancy and helium atom. Int. J. Hydrog. Energy 2020, 45, 15571–15577. [Google Scholar] [CrossRef]
- An, X.; Zhu, T.; Wang, Q.; Song, Y.; Liu, J.; Zhang, P. Interaction Mechanism of Dislocation and Hydrogen in Austenitic 316 Stainless Steel. Acta Metall. Sin. 2021, 57, 913–920. [Google Scholar]
- Zhu, T.; Jin, S.; Gong, Y.; Lu, E.; Song, L.; Xu, Q. The influence of dislocation and hydrogen on thermal helium desorption behavior in Fe9Cr alloys. J. Nucl. Mater. 2017, 495, 244–248. [Google Scholar] [CrossRef]
- Zhang, S.; Cizek, J.; Yao, Z.; Oleksandr, M.; Kong, X.; Liu, C.; van Dijk, N.; van der Zwaag, S. Self healing of radiation-induced damage in Fe–Au and Fe–Cu alloys: Combining positron annihilation spectroscopy with TEM and ab initio calculations. J. Alloys Compd. 2020, 817, 15765. [Google Scholar] [CrossRef]
- Song, Y.; Guo, Z.; Yang, R. Influence of interstitial elements on the bulk modulus and theoretical strength of a-titanium: A first-principles study. Philos. Mag. A 2002, 82, 1345–1359. [Google Scholar]
- Brusa, R.S.; Deng, W.; Karwasz, P.; Zecca, A. Doppler-broadening measurements of positron annihilation with high-momentum electrons in pure elements. Nucl. Instrum. Methods Phys. Res. 2002, 194, 519–531. [Google Scholar] [CrossRef] [Green Version]

| Sample Number | Annealing Temperature | CD/mA·cm−2 | Time/h |
|---|---|---|---|
| No. 1 | 700 °C, 2 h | Uncharged | - |
| No. 2 | 700 °C, 2 h | 100 mA·cm−2 | 4 h |
| No. 3 | 700 °C, 2 h | 100 mA·cm−2 | 8 h |
| No. 4 | Un-annealed | Uncharged | - |
| No. 5 | Un-annealed | 100 mA·cm−2 | 4 h |
| No. 6 | Un-annealed | 100 mA·cm−2 | 8 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, S.; An, X.; Wang, Q.; Zhu, T.; Wan, M.; Zhang, P.; Ye, F.; Song, Y.; Wang, B.; Cao, X. Exploration on the Effect of Pretreatment Conditions on Hydrogen-Induced Defects in Pure Titanium by Positron Annihilation Spectroscopy. Metals 2022, 12, 595. https://doi.org/10.3390/met12040595
Jian S, An X, Wang Q, Zhu T, Wan M, Zhang P, Ye F, Song Y, Wang B, Cao X. Exploration on the Effect of Pretreatment Conditions on Hydrogen-Induced Defects in Pure Titanium by Positron Annihilation Spectroscopy. Metals. 2022; 12(4):595. https://doi.org/10.3390/met12040595
Chicago/Turabian StyleJian, Shichao, Xudong An, Qianqian Wang, Te Zhu, Mingpan Wan, Peng Zhang, Fengjiao Ye, Yamin Song, Baoyi Wang, and Xingzhong Cao. 2022. "Exploration on the Effect of Pretreatment Conditions on Hydrogen-Induced Defects in Pure Titanium by Positron Annihilation Spectroscopy" Metals 12, no. 4: 595. https://doi.org/10.3390/met12040595
APA StyleJian, S., An, X., Wang, Q., Zhu, T., Wan, M., Zhang, P., Ye, F., Song, Y., Wang, B., & Cao, X. (2022). Exploration on the Effect of Pretreatment Conditions on Hydrogen-Induced Defects in Pure Titanium by Positron Annihilation Spectroscopy. Metals, 12(4), 595. https://doi.org/10.3390/met12040595







