Effect of Ge Addition on Magnetic Properties and Crystallization Mechanism of FeSiBPNbCu Nanocrystalline Alloy with High Fe Content
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
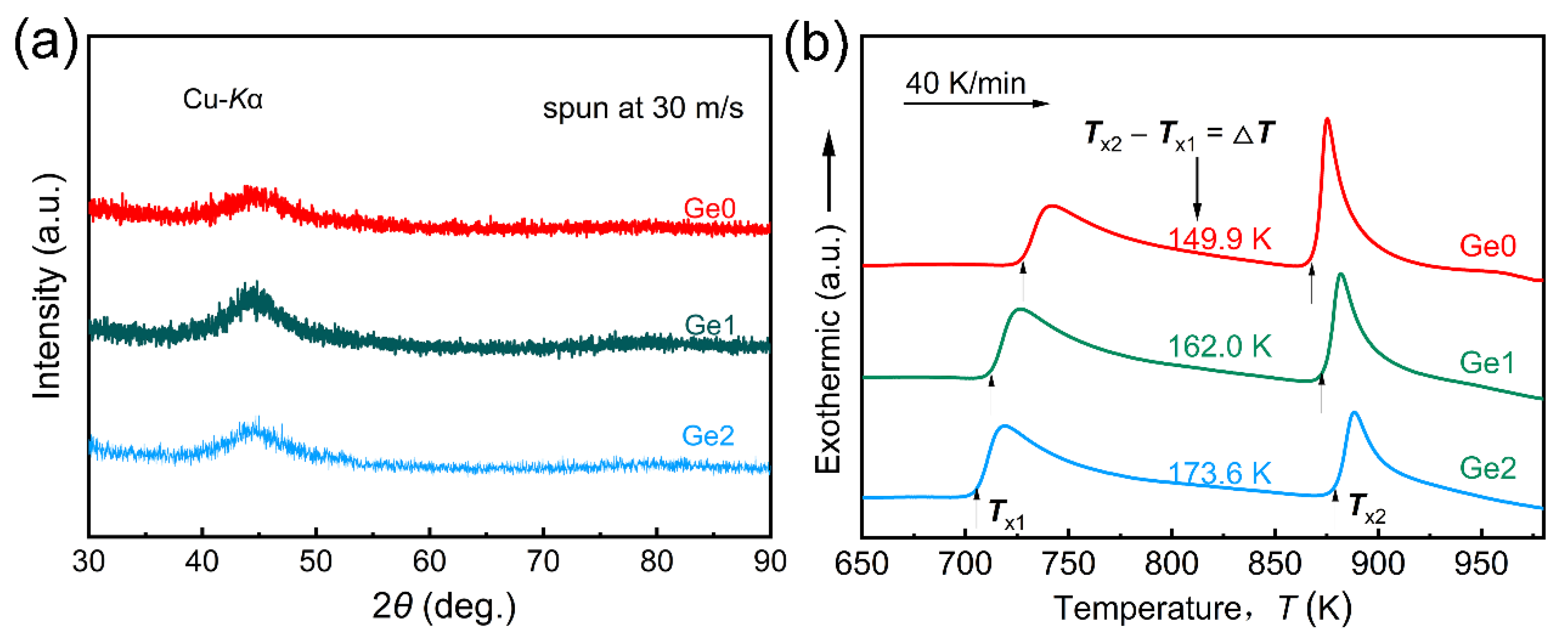
- As the Ge content increases, the onset crystallization temperature of the first crystallization reaction (Tx1) and that of the second crystallization temperature (Tx2) of the as-spun Fe80.2Si3B12-xP2Nb2Cu0.8Gex (x = 0, 1, 2 at.%) amorphous alloy ribbons shift to the low- and high-temperature directions, respectively, resulting in an increase in the heat treatment window ΔT (= Tx2 − Tx1) from 149.9 K for x = 0 to 173.6 K for x = 2 in the Ge2. A larger ΔT is better for promoting the precipitation of α-Fe and inhibiting the precipitation of borides and phosphides, which is more conducive to the preparation of dense and uniform nanocrystals.
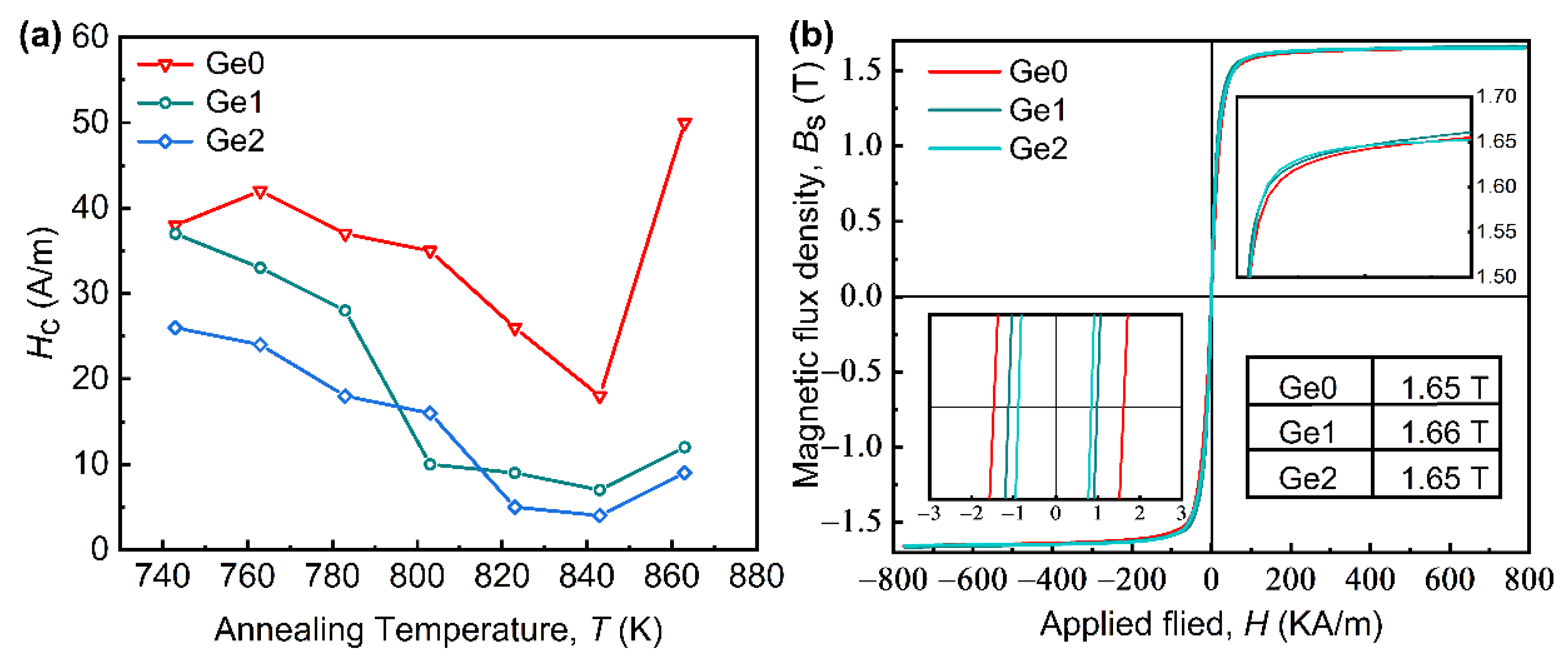
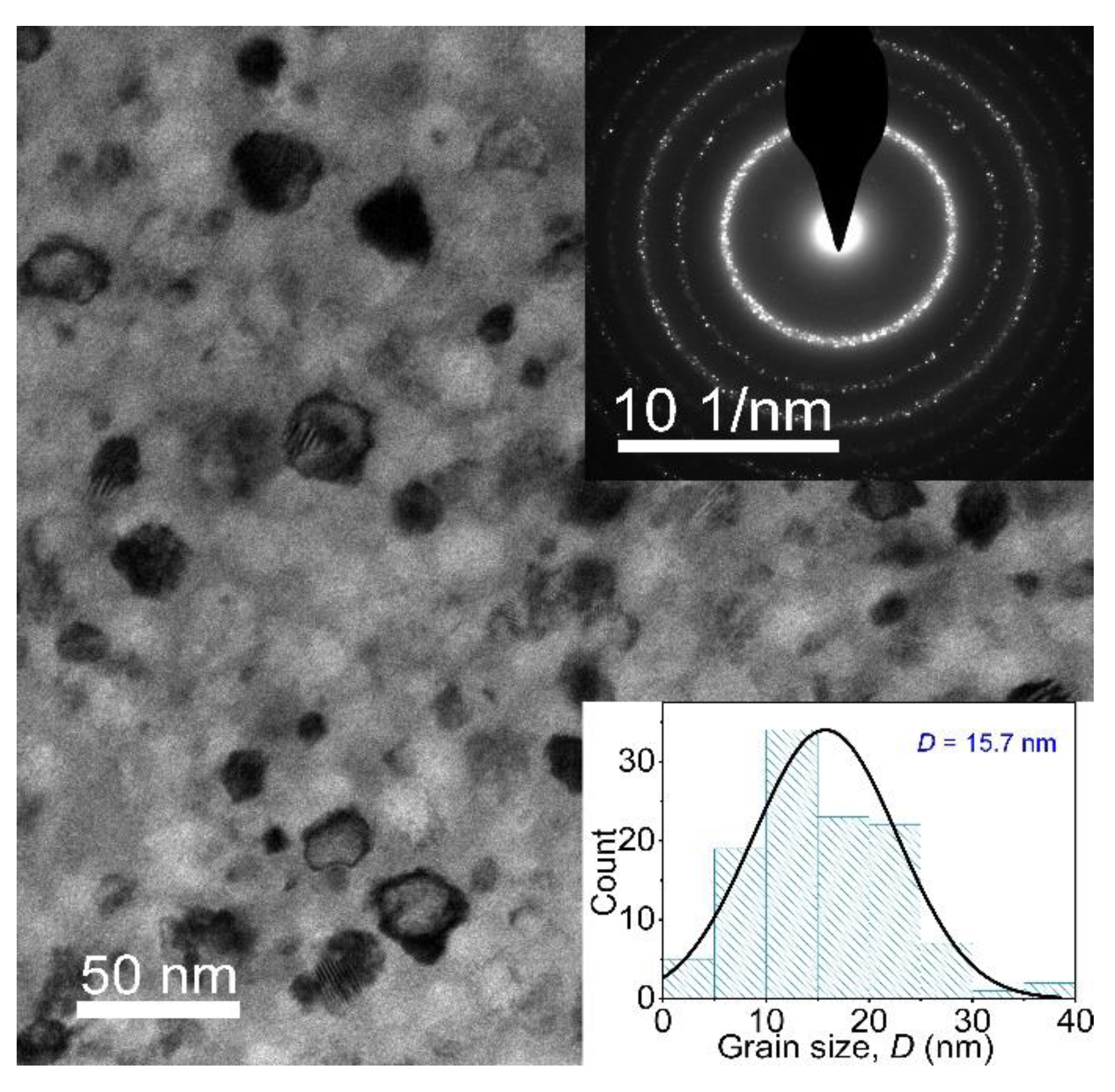
- The Fe80.2Si3B10P2Nb2Cu0.8Ge2 nanocrystalline alloys with a small grain size of 15.7 nm were obtained by annealing the corresponding amorphous alloy ribbons at a temperature of 843 K for 10 min. The Fe80.2Si3B10P2Nb2Cu0.8Ge2 nanocrystalline alloy exhibits excellent magnetic properties with a high Bs of 1.65 T, small Hc of 3 A/m, which are considered to be derived from uniform and dense nanocrystalline structures.
- Both the nucleation crystallization activation energy (Ex1) and the growth crystallization activation energy (Ep1) for the primary crystallization reaction of the as-spun Fe-based alloy ribbons decrease with increasing the Ge content.
- The non-isothermal crystallization kinetics study shows that the value of the local Avrami exponent, n, for the crystallization is less than 1 in the whole crystallization process, reflecting the crystallization mechanism of the direct growth of pre-existing nuclei.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herzer, G. Modern soft magnets: Amorphous and nanocrystalline materials. Acta Mater. 2013, 61, 718–734. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Oguma, S.; Yamauchi, K. New Fe-based soft magnetic alloys composed of ultrafine grain structure. J. Appl. Phys. 1988, 64, 6044–6046. [Google Scholar] [CrossRef]
- Suzuki, K.; Parsons, R.; Zang, B.; Onodera, K.; Kishimoto, H.; Kato, A. Copper-free nanocrystalline soft magnetic materials with high saturation magnetization comparable to that of Si steel. Appl. Phys. Lett. 2017, 110, 012407. [Google Scholar] [CrossRef]
- Urata, A.; Matsumoto, H.; Yoshida, S.; Makino, A. Fe–B–P–Cu nanocrystalline soft magnetic alloys with high Bs. J. Alloys Compd. 2011, 509, S431–S433. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, W.; Hu, H.; Liu, P.; Wu, J.; Zhang, B. Roles of Co element in Fe-based bulk metallic glasses utilizing industrial FeB alloy as raw material. Prog. Nat. Sci. Mater. Int. 2017, 27, 503–506. [Google Scholar] [CrossRef]
- Wang, F.; Inoue, A.; Han, Y.; Kong, F.L.; Zhu, S.L.; Shalaan, E.; Al-Marzouki, F.; Obaid, A. Excellent soft magnetic Fe-Co-B-based amorphous alloys with extremely high saturation magnetization above 1.85 T and low coercivity below 3 A/m. J. Alloys Compd. 2017, 711, 132–142. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, H.; Wang, M.; Zhang, Y.; Ma, Z.; Zhao, Y.; Yang, W. Effects of Ni substitution for Fe on magnetic properties of Fe80−xNixP13C7 (x= 0–30) glassy ribbons. J. Non-Cryst. Solids 2017, 463, 68–71. [Google Scholar] [CrossRef]
- Liu, T.; He, A.; Kong, F.; Wang, A.; Dong, Y.; Zhang, H.; Wang, X.; Ni, H.; Yang, Y. Heterostructured crystallization mechanism and its effect on enlarging the processing window of Fe-based nanocrystalline alloys. J. Mater. Sci. Technol. 2021, 68, 53–60. [Google Scholar] [CrossRef]
- Li, H.; Wang, A.; Liu, T.; Chen, P.; He, A.; Li, Q.; Luan, J.; Liu, C. Design of Fe-based nanocrystalline alloys with superior magnetization and manufacturability. Mater. Today 2021, 42, 49–56. [Google Scholar] [CrossRef]
- Bai, F.; Dong, Y.; Xie, L.; Li, Q.; He, A.; Jia, X.; Li, J.; Wang, X. Effect of pre-existing nuclei on microstructure and magnetic properties of high Bs FINEMET-like nanocrystalline alloys. J. Mater. Sci. 2021, 56, 9254–9262. [Google Scholar] [CrossRef]
- Liu, T.; Kong, F.; Xie, L.; Wang, A.; Chang, C.; Wang, X.; Liu, C. Fe (Co) SiBPCCu nanocrystalline alloys with high Bs above 1.83 T. J. Magn. Magn. Mater. 2017, 441, 174–179. [Google Scholar] [CrossRef]
- Li, Y.; Jia, X.; Zhang, W.; Zhang, Y.; Xie, G.; Qiu, Z.; Luan, J.; Jiao, Z. Formation and crystallization behavior of Fe-based amorphous precursors with pre-existing α-Fe nanoparticles—Structure and magnetic properties of high-Cu-content Fe-Si-B-Cu-Nb nanocrystalline alloys. J. Mater. Sci. Technol. 2021, 65, 171–181. [Google Scholar] [CrossRef]
- Ramasamy, P.; Stoica, M.; Bera, S.; Calin, M.; Eckert, J. Effect of replacing Nb with (Mo and Zr) on glass forming ability, magnetic and mechanical properties of FeCoBSiNb bulk metallic glass. J. Alloys Compd. 2017, 707, 78–81. [Google Scholar] [CrossRef]
- Li, Y.; Jia, X.; Xu, Y.; Chang, C.; Xie, G.; Zhang, W. Soft magnetic Fe-Si-B-Cu nanocrystalline alloys with high Cu concentrations. J. Alloys Compd. 2017, 722, 859–863. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Zhang, X.; Wu, J.; He, A.; Dong, Y. Effect of P microalloying on magnetic properties and structure of FeSiBNbCu nanocrystalline alloy. J. Mater. Sci. Mater. Electron. 2021, 32, 4177–4184. [Google Scholar] [CrossRef]
- Zhang, C.; Chi, Q.; Zhang, J.; Dong, Y.; He, A.; Zhang, X.; Geng, P.; Li, J.; Xiao, H.; Song, J.; et al. Correlation among the amorphous forming ability, viscosity, free-energy difference and interfacial tension in Fe–Si–B–P soft magnetic alloys. J. Alloys Compd. 2020, 831, 154784. [Google Scholar] [CrossRef]
- Murugaiyan, P.; Mitra, A.; Patro, A.K.; Roy, R.K.; Churyukanova, M.; Kaloshkin, S.; Shuvaeva, E.; Panda, A.K. Role of P on amorphization, microstructure, thermo-physical and soft magnetic properties of Fe-rich FeB(P)SiNbCu melt-spun alloys. J. Magn. Magn. Mater. 2019, 492, 165723. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; He, A.; Xie, L.; Li, F.; Chang, L.; Xiao, H.; Li, H.; Wang, T. Improvement of SMPs in Fe-Si-B-P-C-Cu-Nb alloys via harmonizing P and B. J. Magn. Magn. Mater. 2020, 506, 166757. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, H.; Sun, C.; Chen, C.; Hao, J. Effects of the substitution of Si with P on the glass forming ability, crystallization behavior, and magnetic properties of FeCuNbSiBP atomized powder. J. Magn. Magn. Mater. 2022, 550, 169087. [Google Scholar] [CrossRef]
- Cremaschi, V.; Sánchez, G.; Sirkin, H. Magnetic properties and structural evolution of FINEMET alloys with Ge addition. Physica B 2004, 354, 213–216. [Google Scholar] [CrossRef]
- Chang, C.; Xiang, Z.; Zhao, C.; Wang, A.; Song, R.; Pan, D. Enlarging heat-treatment processing windows of Fe-Si-B-P-Cu-M nanocrystalline alloys by doping transition metal elements. J. Alloys Compd. 2021, 860, 158420. [Google Scholar] [CrossRef]
- Wang, F.; Inoue, A.; Han, Y.; Zhu, S.L.; Kong, F.L.; Zanaeva, E.; Liu, G.D.; Shalaan, E.; Al-Marzouki, F.; Obaid, A. Soft magnetic Fe-Co-based amorphous alloys with extremely high saturation magnetization exceeding 1.9 T and low coercivity of 2 A/m. J. Alloys Compd. 2017, 723, 376–384. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Z.; Han, Y. Effect of Ge addition on structure and soft magnetic properties of Si-rich Fe-based nanocrystalline alloys. J. Alloys Compd. 2016, 672, 332–335. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, K.; Li, Q. Comparative study of non-isothermal crystallization kinetics between Fe80P13C7 bulk metallic glass and melt-spun glassy ribbon. J. Alloys Compd. 2012, 540, 6–15. [Google Scholar] [CrossRef]
- Rezaei-Shahreza, P.; Seifoddini, A.; Hasani, S. Non-isothermal kinetic analysis of nano-crystallization process in (Fe41Co7Cr15Mo14Y2C15)94B6 amorphous alloy. Thermochim. Acta 2017, 652, 119–125. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Pratap, A.; Lad, K.N.; Rao, T.L.S.; Majmudar, P.; Saxena, N.S. Kinetics of crystallization of amorphous Cu50Ti50 alloy. J. Non-Cryst. Solids 2004, 345–346, 178–181. [Google Scholar] [CrossRef]
- Blazquez, J.; Conde, C.; Conde, A. Non-isothermal approach to isokinetic crystallization processes: Application to the nanocrystallization of HITPERM alloys. Acta Mater. 2005, 53, 2305–2311. [Google Scholar] [CrossRef]
- Malek, J. The applicability of Johnson-Mehl-Avrami model in the thermal analysis of the crystallization kinetics of glasses. Thermochim. Acta 1995, 267, 61–73. [Google Scholar] [CrossRef]
- Ramasamy, P.; Stoica, M.; Taghvaei, A.H.; Prashanth, K.G.; Ravi, K.; Eckert, J. Kinetic analysis of the non-isothermal crystallization process, magnetic and mechanical properties of FeCoBSiNb and FeCoBSiNbCu bulk metallic glasses. J. Appl. Phys. 2016, 119, 073908. [Google Scholar] [CrossRef]
- Nakamura, K.; Katayama, K.; Amano, T. Some Aspects of Nonisothermal Crystallization of Polymers. II. Consideration of Isokinetic Condition. J. Appl. Polym. Sci. 1973, 17, 1031–1041. [Google Scholar] [CrossRef]
- Paul, T.; Loganathan, A.; Agarwal, A.; Harimkar, S.P. Kinetics of isochronal crystallization in a Fe-based amorphous alloy. J. Alloys Compd. 2018, 753, 679–687. [Google Scholar] [CrossRef]
- Peng, C.; Chen, Z.H.; Zhao, X.Y.; Zhang, A.L.; Zhang, L.K.; Chen, D. Crystallization kinetics of Zr60Cu25Fe5Al10 bulk metallic glass. J. Non-Cryst. Solids 2014, 405, 7–11. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wang, L.; Chen, H.; Cheng, X.; Zhong, X.; Feng, Y. The formation and crystallization of amorphous Al65Fe20Zr15. J. Non-Cryst. Solids 2008, 354, 5555–5558. [Google Scholar] [CrossRef]




| Sample | Tc (K) | Tx1 (K) | Tp1 (K) | Ex1 (kJ/mol) | Ep1 (kJ/mol) |
|---|---|---|---|---|---|
| Ge0 | 553 | 725.9 | 740.5 | 268.6 | 491.3 |
| Ge1 | 557 | 710.3 | 725.5 | 252.2 | 459.7 |
| Ge2 | 562 | 702.3 | 718.1 | 250.9 | 446.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Bai, F.; Dong, Y.; Xie, L.; Li, Q.; He, A.; Li, J. Effect of Ge Addition on Magnetic Properties and Crystallization Mechanism of FeSiBPNbCu Nanocrystalline Alloy with High Fe Content. Metals 2022, 12, 640. https://doi.org/10.3390/met12040640
Zhang H, Bai F, Dong Y, Xie L, Li Q, He A, Li J. Effect of Ge Addition on Magnetic Properties and Crystallization Mechanism of FeSiBPNbCu Nanocrystalline Alloy with High Fe Content. Metals. 2022; 12(4):640. https://doi.org/10.3390/met12040640
Chicago/Turabian StyleZhang, Haijie, Fushan Bai, Yaqiang Dong, Lei Xie, Qiang Li, Aina He, and Jiawei Li. 2022. "Effect of Ge Addition on Magnetic Properties and Crystallization Mechanism of FeSiBPNbCu Nanocrystalline Alloy with High Fe Content" Metals 12, no. 4: 640. https://doi.org/10.3390/met12040640
APA StyleZhang, H., Bai, F., Dong, Y., Xie, L., Li, Q., He, A., & Li, J. (2022). Effect of Ge Addition on Magnetic Properties and Crystallization Mechanism of FeSiBPNbCu Nanocrystalline Alloy with High Fe Content. Metals, 12(4), 640. https://doi.org/10.3390/met12040640







