Effect of Sealing Treatments on Erosion–Corrosion of a Fe-Based Amorphous Metallic Coating in 3.5 wt.% NaCl Solution with 2 wt.% Sand
Abstract
:1. Introduction
2. Experimental
2.1. Materials and the Preparation of Three Sealed Coatings
- (1)
- Preparation of stearic acid–sealed AMC (SAS coating):
- (2)
- Preparation of aluminum phosphate–sealed AMC (APS coating):
- (3)
- Preparation of cerium salt–sealed AMC (CSS coating):
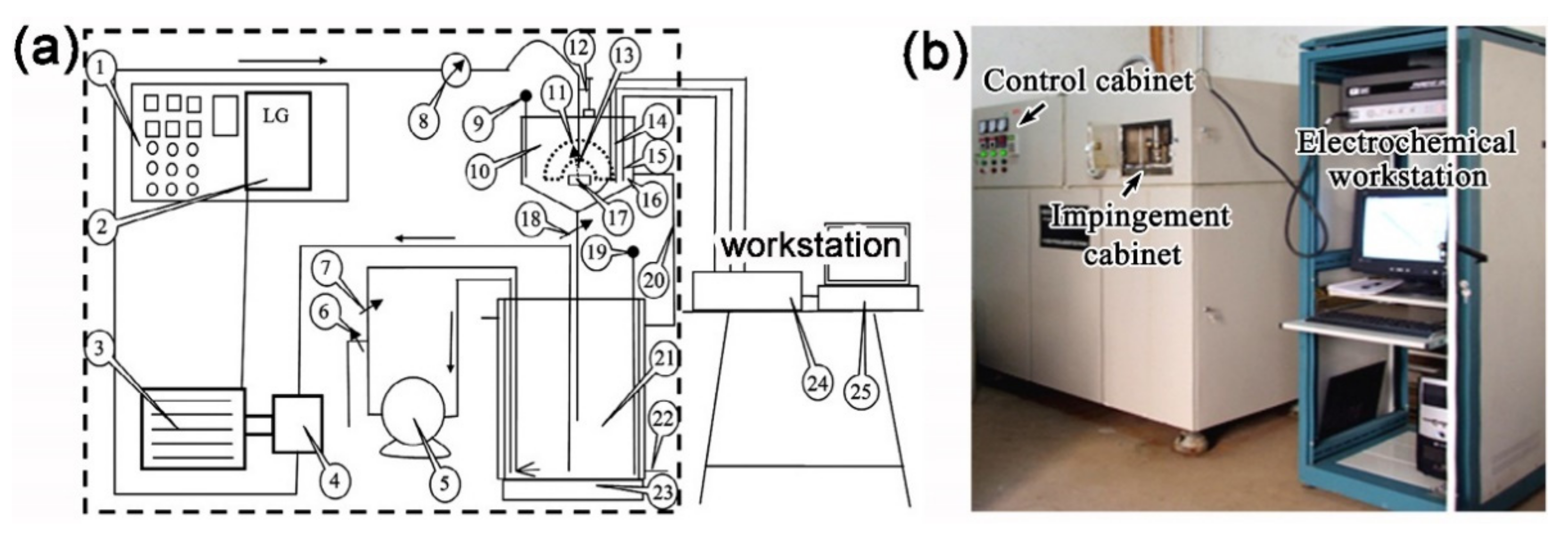
2.2. Evaluation of Erosion–Corrosion Resistance
2.3. Surface Characterization
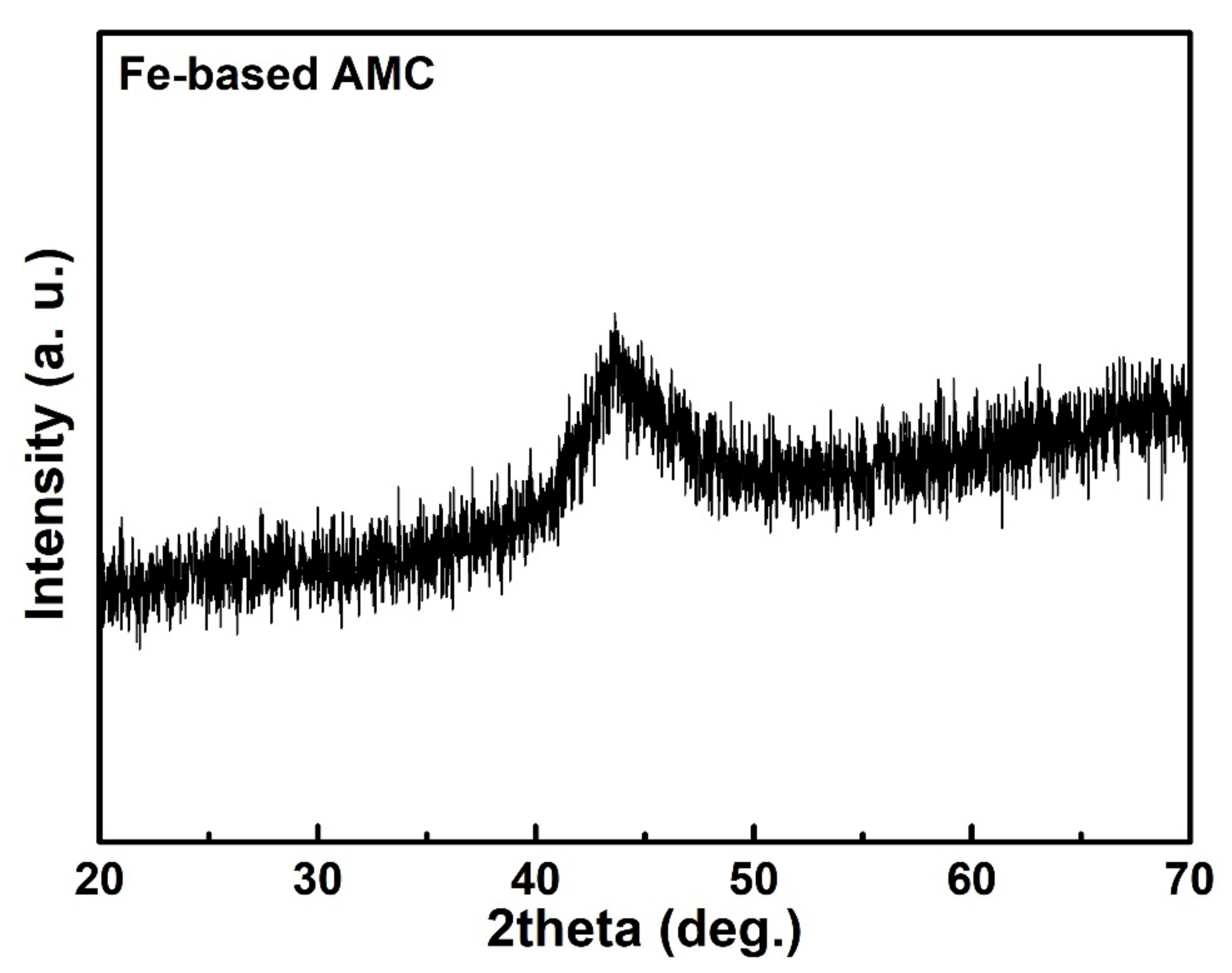
2.4. Coating Characterization
3. Results and Discussion
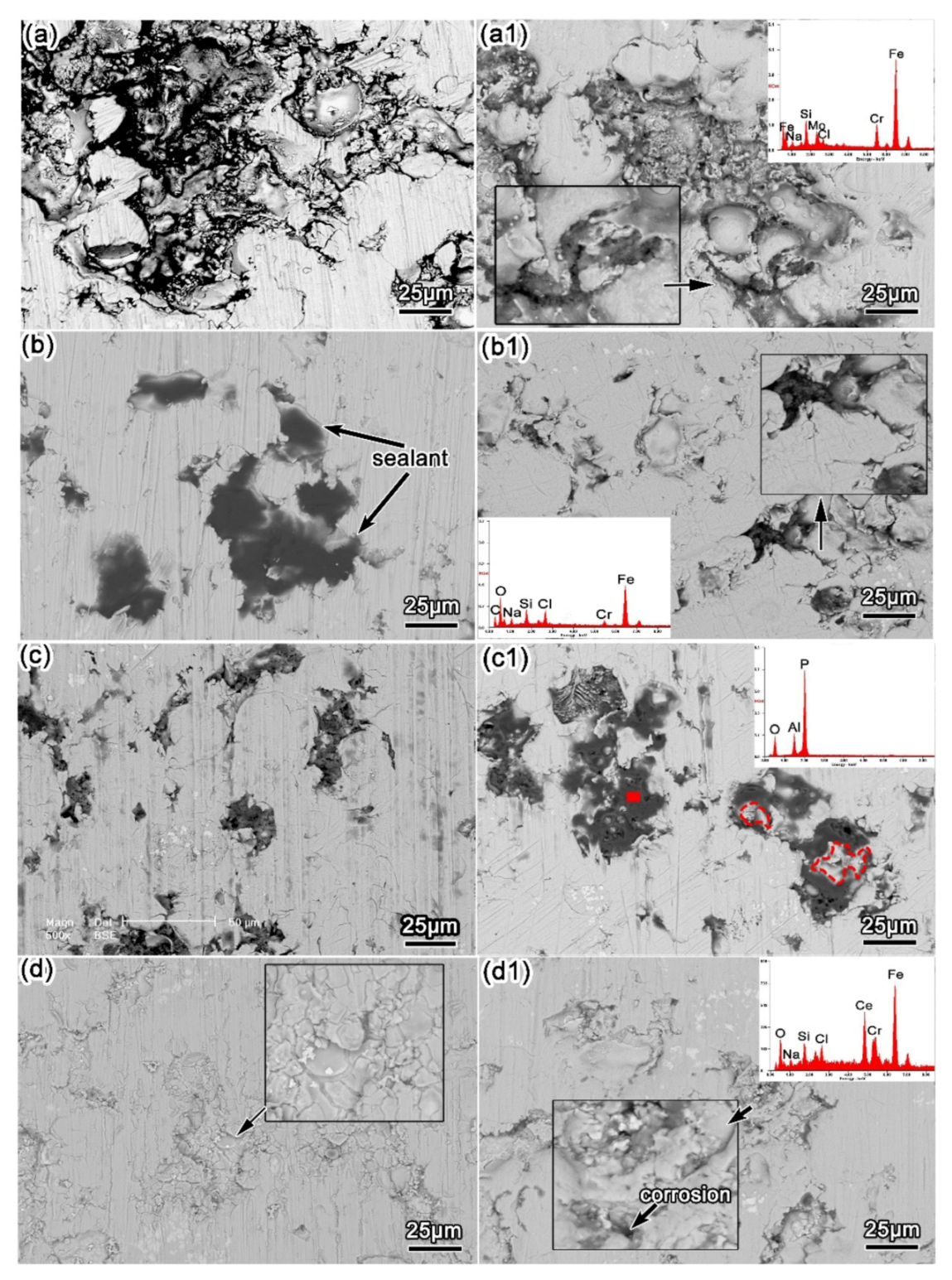
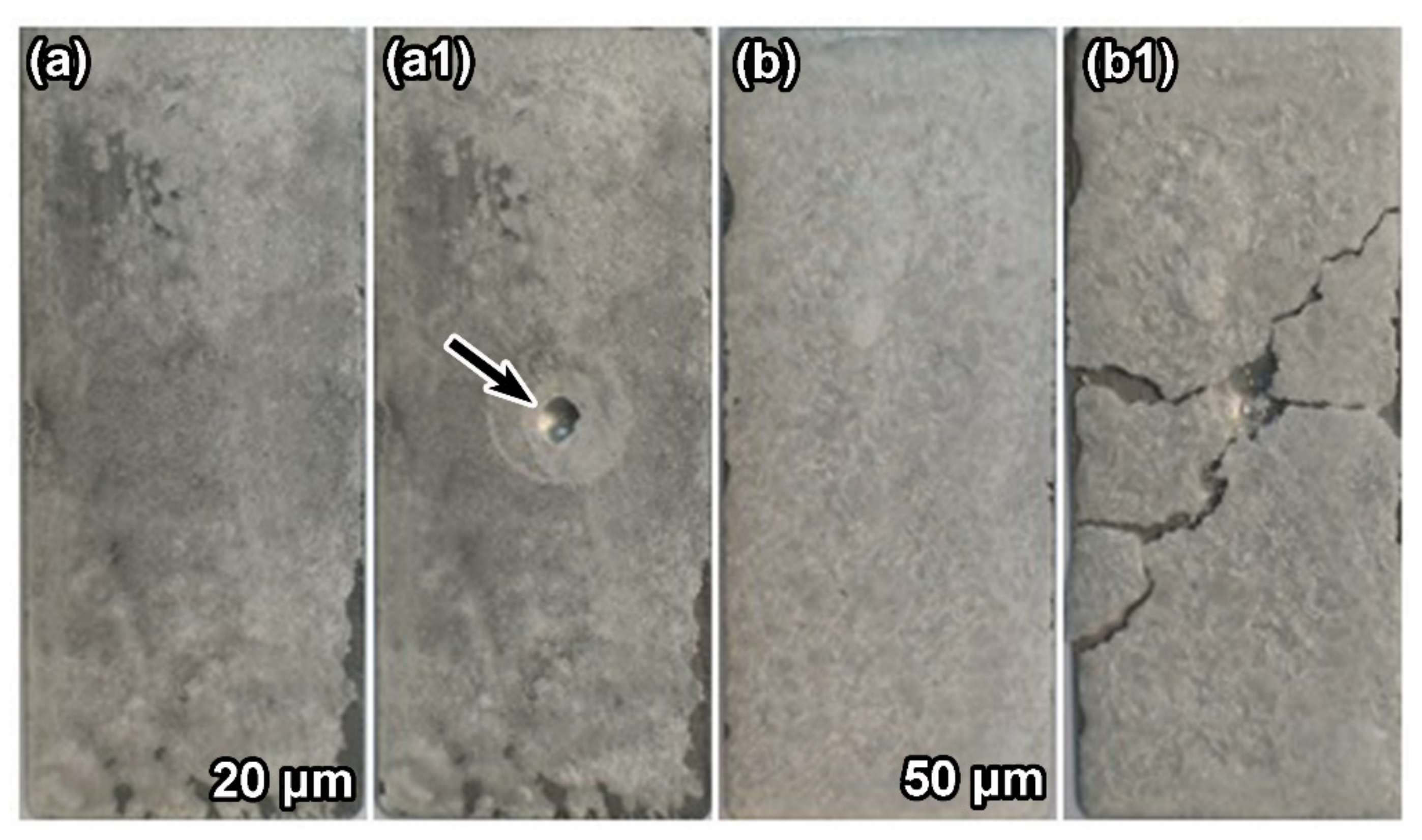
3.1. Surface Morphology
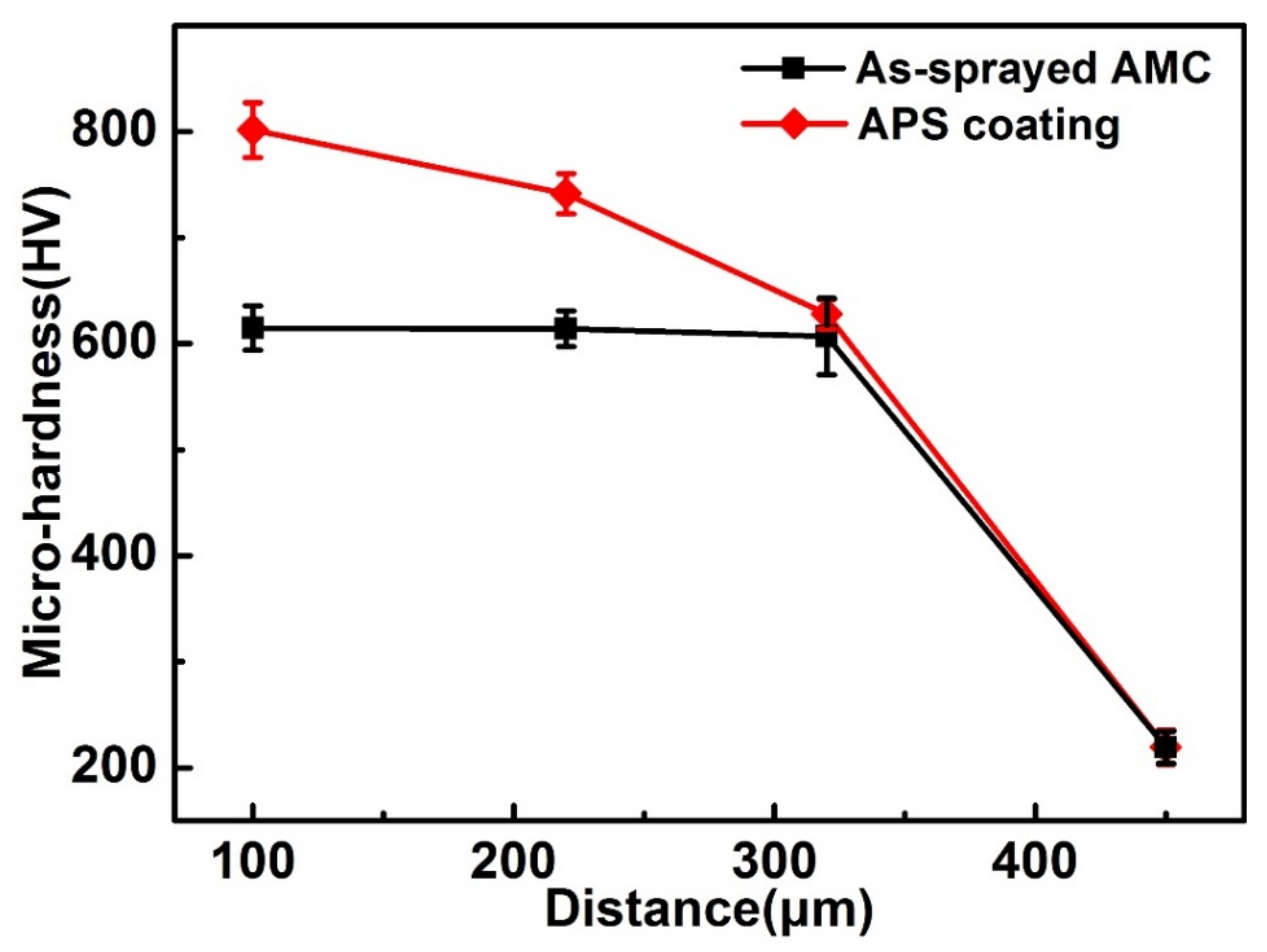
3.2. Three-Dimensional Roughness
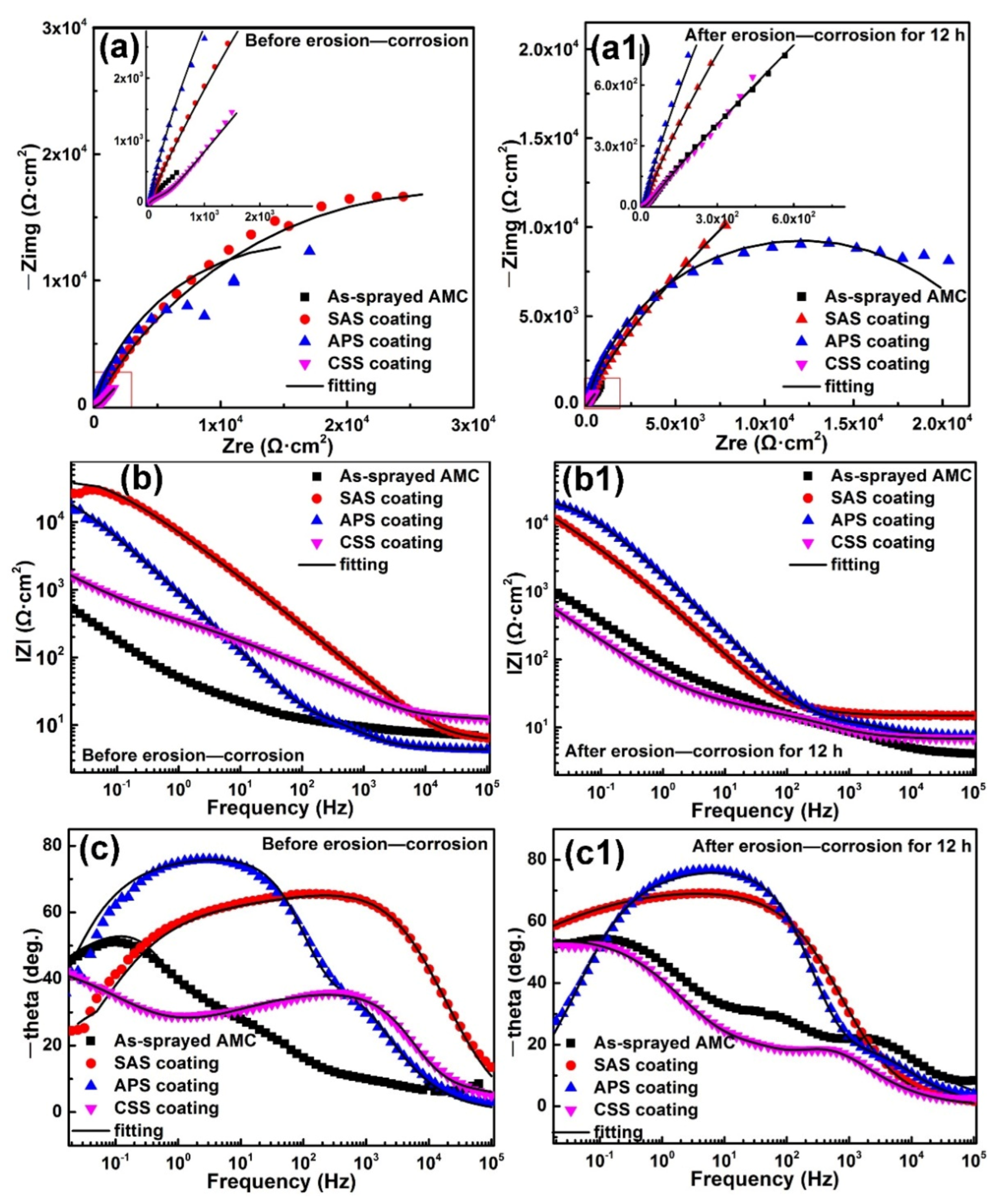
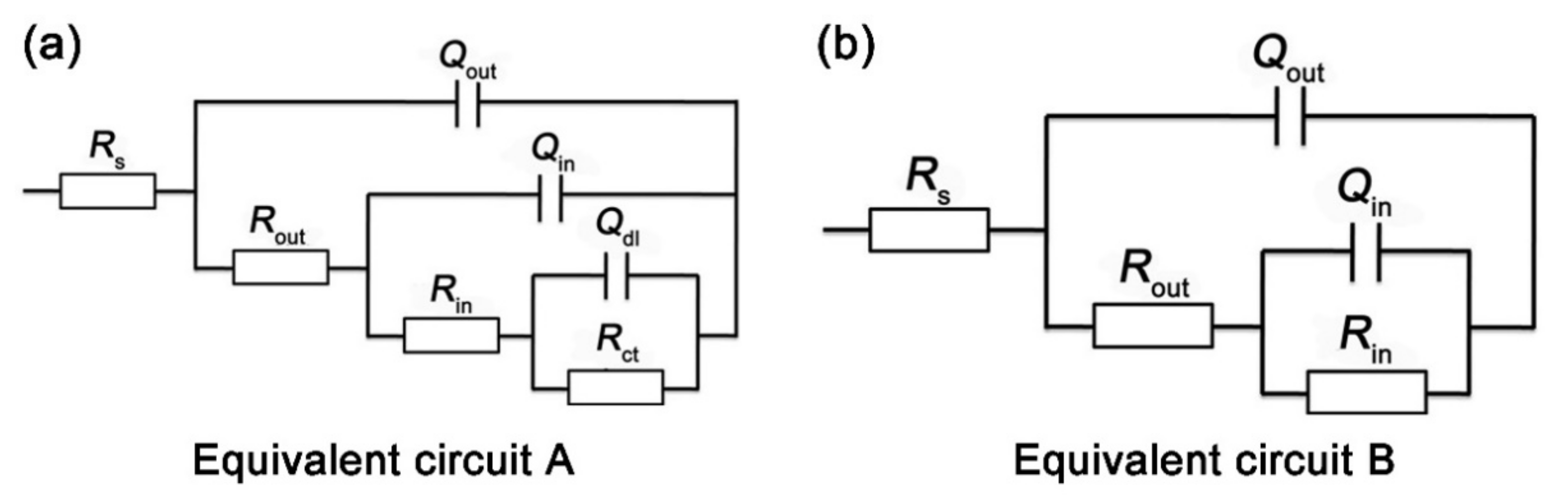
3.3. EIS Tests
3.4. Critical Flow Velocity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.; Zheng, Y. Critical flow velocity phenomenon in erosion-corrosion of pipelines: Determination methods, mechanisms and applications. J. Pipeline Sci. Eng. 2021, 1, 63–73. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Hou, W.L.; Chang, X.C.; Wang, J.Q. Slurry erosion–corrosion behaviour of high-velocity oxy-fuel (HVOF) sprayed Fe-based amorphous metallic coatings for marine pump in sand-containing NaCl solutions. Corros. Sci. 2011, 53, 3177–3185. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y.; Zhou, X.; He, S.; Sun, W.; Wang, J. Determination of the critical flow velocities for erosion–corrosion of passive materials under impingement by NaCl solution containing sand. Corros. Sci. 2014, 88, 187–196. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.L.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Chang, X.C.; Hou, W.L.; Wang, J.Q. Effect of processing parameters on the microstructures and corrosion behaviour of high-velocity oxy-fuel (HVOF) sprayed Fe-based amorphous metallic coatings. Mater. Corros. 2013, 64, 801–810. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Wang, S.; Gu, X.; Wang, J. Characterisation of three-dimensional porosity in an Fe-based amorphous coating and its correlation with corrosion behaviour. Corros. Sci. 2015, 93, 211–221. [Google Scholar] [CrossRef]
- Shin, D.; Hamed, A. Influence of micro–structure on erosion resistance of plasma sprayed 7YSZ thermal barrier coating under gas turbine operating conditions. Wear 2018, 396–397, 34–47. [Google Scholar] [CrossRef]
- Farmer, J.; Wong, F.; Haslam, J.; Estill, J.; Branagan, D.; Yang, N.; Blue, C. Development, processing and testing of high-performance corrosion-resistant HVOF coatings. In Proceedings of the Global 2003 Topical Meeting at the American Nuclear Society Conference, New Orleans, LA, USA, 16–20 November 2003; pp. 1–6. [Google Scholar]
- Liu, M.; Hu, H.; Zheng, Y.; Wang, J.; Gan, Z.; Qiu, S. Effect of sol-gel sealing treatment loaded with different cerium salts on the corrosion resistance of Fe-based amorphous coating. Surf. Coat. Technol. 2019, 367, 311–326. [Google Scholar] [CrossRef]
- Liu, M.; Hu, H.; Zheng, Y. Effects of three sealing methods of aluminum phosphate sealant on corrosion resistance of the Fe-based amorphous coating. Surf. Coat. Technol. 2017, 309, 579–589. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Zheng, Y.; Ke, W.; Sun, W.; Wang, J. Effect of porosity sealing treatments on the corrosion resistance of high-velocity oxy-fuel (HVOF)-sprayed Fe-based amorphous metallic coatings. Surf. Coat. Technol. 2011, 206, 1307–1318. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.; Sun, W.; Gao, Y.; Wang, J. Enhanced corrosion resistance in Fe-based amorphous coatings through eliminating Cr-depleted zones. Corros. Sci. 2018, 136, 161–173. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S.; Sun, W.; Wang, J. Influence of oxidation related structural defects on localized corrosion in HVAF-sprayed Fe-based metallic coatings. Surf. Coat. Technol. 2018, 335, 205–218. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y. Effects of surface treatments on the corrosion and erosion-corrosion of 304 stainless steel in 3.5% NaCl solution. Corros. Sci. 2016, 112, 657–668. [Google Scholar] [CrossRef]
- Arun, S.; Sooraj, P.; Hariprasad, S.; Arunnellaiappan, T.; Rameshbabu, N. Fabrication of superhydrophobic coating on PEO treated zirconium samples and its corrosion resistance. Mater. Today Proc. 2020, 27, 2056–2060. [Google Scholar] [CrossRef]
- Cui, X.J.; Lin, X.Z.; Liu, C.H.; Yang, R.S.; Zheng, X.W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhao, P.H.; Zhao, J.M. The influences of sealing methods on corrosion behavior of anodized aluminum alloys in NaCl solutions. Surf. Coat. Technol. 2003, 166, 237–242. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Ma, A.; Hu, H.; Zheng, Y.; Yang, B.; Wang, J. Influence of sealing treatment on the corrosion behavior of HVAF sprayed Al-based amorphous/nanocrystalline coating. Surf. Coat. Technol. 2018, 353, 263–273. [Google Scholar] [CrossRef]
- Shao, F.; Yang, K.; Zhao, H.; Liu, C.; Wang, L.; Tao, S. Effects of inorganic sealant and brief heat treatments on corrosion behavior of plasma sprayed Cr2O3–Al2O3 composite ceramic coatings. Surf. Coat. Technol. 2015, 276, 8–15. [Google Scholar] [CrossRef]
- Knuuttila, J.; Ahmaniemi, S.; Mäntylä, T. Wet abrasion and slurry erosion resistance of thermally sprayed oxide coatings. Wear 1999, 232, 207–212. [Google Scholar] [CrossRef]
- Leivo, E.M.; Vippola, M.S.; Sorsa, P.; Vuoristo, P.; Mntyl, T.A. Wear and corrosion properties of plasma sprayed Al2O3 and Cr2O3 coatings sealed by aluminum phosphates. J. Therm. Spray. Technol. 1997, 6, 205–210. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Llamazares, Y.; Matykina, E.; Yerokhin, A.; Pardo, A. Influence of sealing post-treatments on the corrosion resistance of PEO coated AZ91 magnesium alloy. Appl. Surf. Sci. 2018, 433, 653–667. [Google Scholar] [CrossRef]
- Van Phuong, N.; Fazal, B.R.; Moon, S. Cerium- and phosphate-based sealing treatments of PEO coated AZ31 Mg alloy. Surf. Coat. Technol. 2017, 309, 86–95. [Google Scholar] [CrossRef]
- Dong, Q.; Ba, Z.; Jia, Y.; Chen, Y.; Lv, X.; Zhang, X.; Wang, Z. Effect of solution concentration on sealing treatment of Mg-Al hydrotalcite film on AZ91D Mg alloy. J. Magnes. Alloy. 2017, 5, 320–325. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; He, X.; Sun, F. Effect of cerium additive on aluminum-based chemical conversion coating on AZ91D magnesium alloy. Appl. Surf. Sci. 2013, 280, 467–473. [Google Scholar] [CrossRef]
- Yu, X.W.; Yan, C.W.; Cao, C.A. Study on the rare earth sealing procedure of the porous film of anodized 2024 aluminum alloy. J. Mater. Sci. Technol. 2003, 19, 51–53. [Google Scholar]
- Toor, I.U.; Alashwan, Z.; Badr, H.M.; Ben-Mansour, R.; Shirazi, S.A. Effect of Jet Impingement Velocity and Angle on CO2 Erosion–Corrosion with and without Sand for API 5L-X65 Carbon Steel. Materials 2020, 13, 2198. [Google Scholar] [CrossRef]
- Yi, J.; Hu, H.; Wang, Z.; Zheng, Y. On the critical flow velocity for erosion-corrosion of Ni-based alloys in a saline-sand solution. Wear 2020, 458–459, 203417. [Google Scholar] [CrossRef]
- Ismail, A.; Irshad, H.M.; Zeino, A.; Toor, I.H. Electrochemical Corrosion Performance of Aromatic Functionalized Imidazole Inhibitor under Hydrodynamic Conditions on API X65 Carbon Steel in 1 M HCl Solution. Arab. J. Sci. Eng. 2019, 44, 5877–5888. [Google Scholar] [CrossRef]
- Hu, H.X.; Guo, X.M.; Zheng, Y.G. Comparison of the cavitation erosion and slurry erosion behavior of cobalt-based and nickel-based coatings. Wear 2019, 428–429, 246–257. [Google Scholar] [CrossRef]
- Toor, I.U.; Irshad, H.M.; Badr, H.M.; Samad, M.A. The Effect of Impingement Velocity and Angle Variation on the Erosion Corrosion Performance of API 5L-X65 Carbon Steel in a Flow Loop. Metals 2018, 8, 402. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Hu, H.; Wang, Z.; Zheng, Y. Comparison of critical flow velocity for erosion-corrosion of six stainless steels in 3.5 wt% NaCl solution containing 2 wt% silica sand particles. Wear 2018, 416–417, 62–71. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y.; Sun, W.; Wang, J. Erosion–corrosion of HVOF-sprayed Fe-based amorphous metallic coating under impingement by a sand-containing NaCl solution. Corros. Sci. 2013, 76, 337–347. [Google Scholar] [CrossRef]
- Hu, H.; Zheng, Y.; Qin, C. Comparison of Inconel 625 and Inconel 600 in resistance to cavitation erosion and jet impingement erosion. Nucl. Eng. Des. 2010, 240, 2721–2730. [Google Scholar] [CrossRef]
- Vippola, M.; Ahmaniemi, S.; Keränen, J.; Vuoristo, P.; Lepistö, T.; Mäntylä, T.; Olsson, E. Aluminum phosphate sealed alumina coating: Characterization of microstructure. Mater. Sci. Eng. A 2002, 323, 1–8. [Google Scholar] [CrossRef]
- Ahmaniemi, S.; Vippola, M.; Vuoristo, P.; Mäntylä, T.; Buchmann, M.; Gadow, R. Residual stresses in aluminium phosphate sealed plasma sprayed oxide coatings and their effect on abrasive wear. Wear 2002, 252, 614–623. [Google Scholar] [CrossRef]
- Morris, J.H.; Perkins, P.G.; Rose, A.E.A.; Smith, W.E. The chemistry and binding properties of aluminium phosphates. Chem. Soc. Rev. 1977, 6, 173–194. [Google Scholar] [CrossRef]
- Dean, S.D. Velocity-accelerated corrosion testing and predictions. Mater. Perform. 1990, 29, 61–67. [Google Scholar]
- Li, L.; Wang, Z.; Zheng, Y. Interaction between pitting corrosion and critical flow velocity for erosion-corrosion of 304 stainless steel under jet slurry impingement. Corros. Sci. 2019, 158, 108084. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Zhang, Q.; Kang, P. Characterization of Ce conversion coating on Gr-f/6061Al composite surface for corrosion protection. J. Alloys Compd. 2008, 461, 389–394. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.; Arrabal, R.; Merino, S.; Viejo, F.; Carboneras, M. Effect of Ce surface treatments on corrosion resistance of A3xx.x/SiCp composites in salt fog. Surf. Coat. Technol. 2006, 200, 2938–2947. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Qi, W.; Wang, J. Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel. Corros. Sci. 2016, 110, 57–70. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, X.; Yang, L.; Wang, X.; Chen, J.; Wang, Z.; Zhou, H.; Zou, J.; Wang, F. Effect of aging treatment on microstructure and corrosion behavior of a Fe-18Cr-15Mn-0.66N stainless steel. J. Mater. Sci. Technol. 2021, 107, 197–206. [Google Scholar] [CrossRef]
- Tang, Y.B.; Shen, X.W.; Liu, Z.H.; Qiao, Y.X.; Yang, L.L.; Lu, D.H.; Zou, J.S.; Xu, J. Corrosion behaviors of selective laser melted inconel 718 alloy in NaOH solution. Acta Metall. Sin. 2022, 58, 324–333. [Google Scholar]
- Wang, Z.; Hu, H.; Zheng, Y.; Ke, W.; Qiao, Y. Comparison of the corrosion behavior of pure titanium and its alloys in fluoride-containing sulfuric acid. Corros. Sci. 2016, 103, 50–65. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y.; Sun, W.; Wang, J. Effect of applied potential on passivation and erosion–corrosion of a Fe-based amorphous metallic coating under slurry impingement. Corros. Sci. 2014, 82, 115–124. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y.; Sun, W.; Wang, J. Effect of heat treatment on the structure, cavitation erosion and erosion–corrosion behavior of Fe-based amorphous coatings. Tribol. Int. 2015, 90, 393–403. [Google Scholar] [CrossRef]
- Lu, B.; Luo, J. Correlation between surface-hardness degradation and erosion resistance of carbon steel—Effects of slurry chemistry. Tribol. Int. 2015, 83, 146–155. [Google Scholar] [CrossRef]
- Grewal, H.; Agrawal, A.; Singh, H. Slurry erosion performance of Ni–Al2O3 based composite coatings. Tribol. Int. 2013, 66, 296–306. [Google Scholar] [CrossRef]
- Divakar, M.; Agarwal, V.; Singh, S. Effect of the material surface hardness on the erosion of AISI316. Wear 2005, 259, 110–117. [Google Scholar] [CrossRef]
- Hong, S.; Wei, Z.; Wang, K.; Gao, W.; Wu, Y.; Lin, J. The optimization of microbial influenced corrosion resistance of HVOF sprayed nanostructured WC-10Co-4Cr coatings by ultrasound-assisted sealing. Ultrason. Sonochem. 2021, 72, 105438. [Google Scholar] [CrossRef]
- Si, L.Q.; Wang, Z.H.; Zhou, Z.H.; Jiang, S.Q.; Cheng, J.B.; Wang, G. Influence of Sealing Treatments with Ultrasonic Excitation on Corrosion Resistance of Plasma-sprayed Al2O3-13wt% TiO2 Coatings. Rare Met. Mat. Eng. 2012, 41, 223–227. [Google Scholar]
- Saaedi, J.; Coyle, T.W.; Arabi, H.; Mirdamadi, S.; Mostaghimi, J. Effects of HVOF Process Parameters on the Properties of Ni-Cr Coatings. J. Therm. Spray Technol. 2010, 19, 521–530. [Google Scholar] [CrossRef]
- Ice, M.; Lavernia, E. Particle Melting Behavior during High-Velocity Oxygen Fuel Thermal Spraying. J. Therm. Spray Technol. 2001, 10, 83–93. [Google Scholar] [CrossRef]
- Li, L.; Lei, J.; Yu, S.; Tian, Y.; Jiang, Q.; Pan, F. Formation and characterization of cerium conversion coatings on magnesium alloy. J. Rare Earths 2008, 26, 383–387. [Google Scholar] [CrossRef]
- Scholes, F.; Soste, C.; Hughes, A.; Hardin, S.; Curtis, P. The role of hydrogen peroxide in the deposition of cerium-based conversion coatings. Appl. Surf. Sci. 2006, 253, 1770–1780. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, Z.; Hu, H.; Zhang, L.; Zheng, Y. Effect of Sealing Treatments on Erosion–Corrosion of a Fe-Based Amorphous Metallic Coating in 3.5 wt.% NaCl Solution with 2 wt.% Sand. Metals 2022, 12, 680. https://doi.org/10.3390/met12040680
Liu M, Wang Z, Hu H, Zhang L, Zheng Y. Effect of Sealing Treatments on Erosion–Corrosion of a Fe-Based Amorphous Metallic Coating in 3.5 wt.% NaCl Solution with 2 wt.% Sand. Metals. 2022; 12(4):680. https://doi.org/10.3390/met12040680
Chicago/Turabian StyleLiu, Mingming, Zhengbin Wang, Hongxiang Hu, Lianmin Zhang, and Yugui Zheng. 2022. "Effect of Sealing Treatments on Erosion–Corrosion of a Fe-Based Amorphous Metallic Coating in 3.5 wt.% NaCl Solution with 2 wt.% Sand" Metals 12, no. 4: 680. https://doi.org/10.3390/met12040680
APA StyleLiu, M., Wang, Z., Hu, H., Zhang, L., & Zheng, Y. (2022). Effect of Sealing Treatments on Erosion–Corrosion of a Fe-Based Amorphous Metallic Coating in 3.5 wt.% NaCl Solution with 2 wt.% Sand. Metals, 12(4), 680. https://doi.org/10.3390/met12040680






