TiO2–SnO2 Nanocomposites for Photocatalytic Environmental Remediation under UV-Light
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of SnO2, TiO2 Nanoparticles (NPs) and TiO2–SnO2 Nanocomposite
2.3. Structural Characterization
2.4. Photocatalytic Degradation of Methyl Orange (MO) Dye
3. Results and Discussion
3.1. XRD Analysis
3.2. Morphological and Structure Analysis of TiO2–SnO2 Nanocomposite
3.3. Optical Properties
3.4. PL Spectra
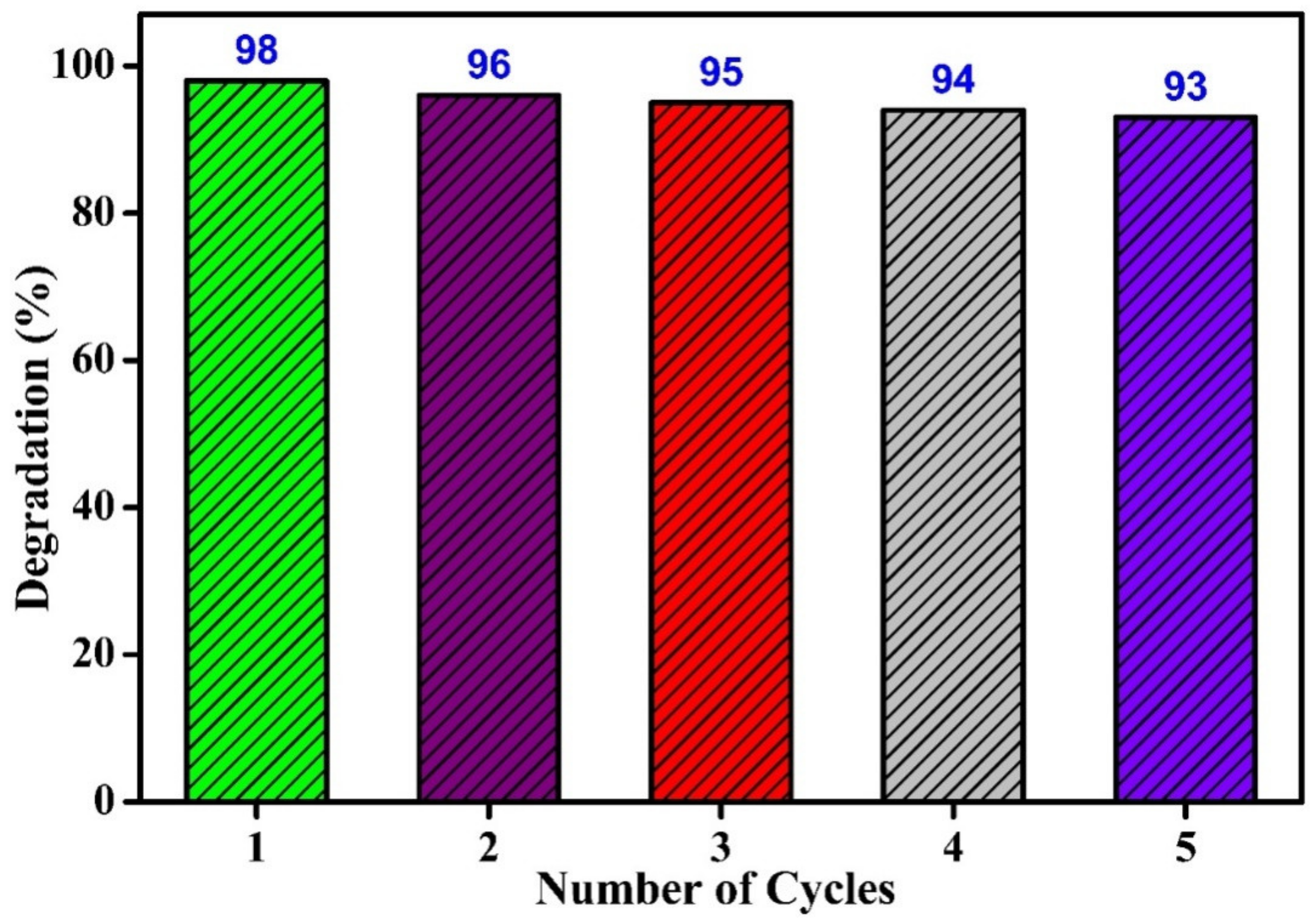
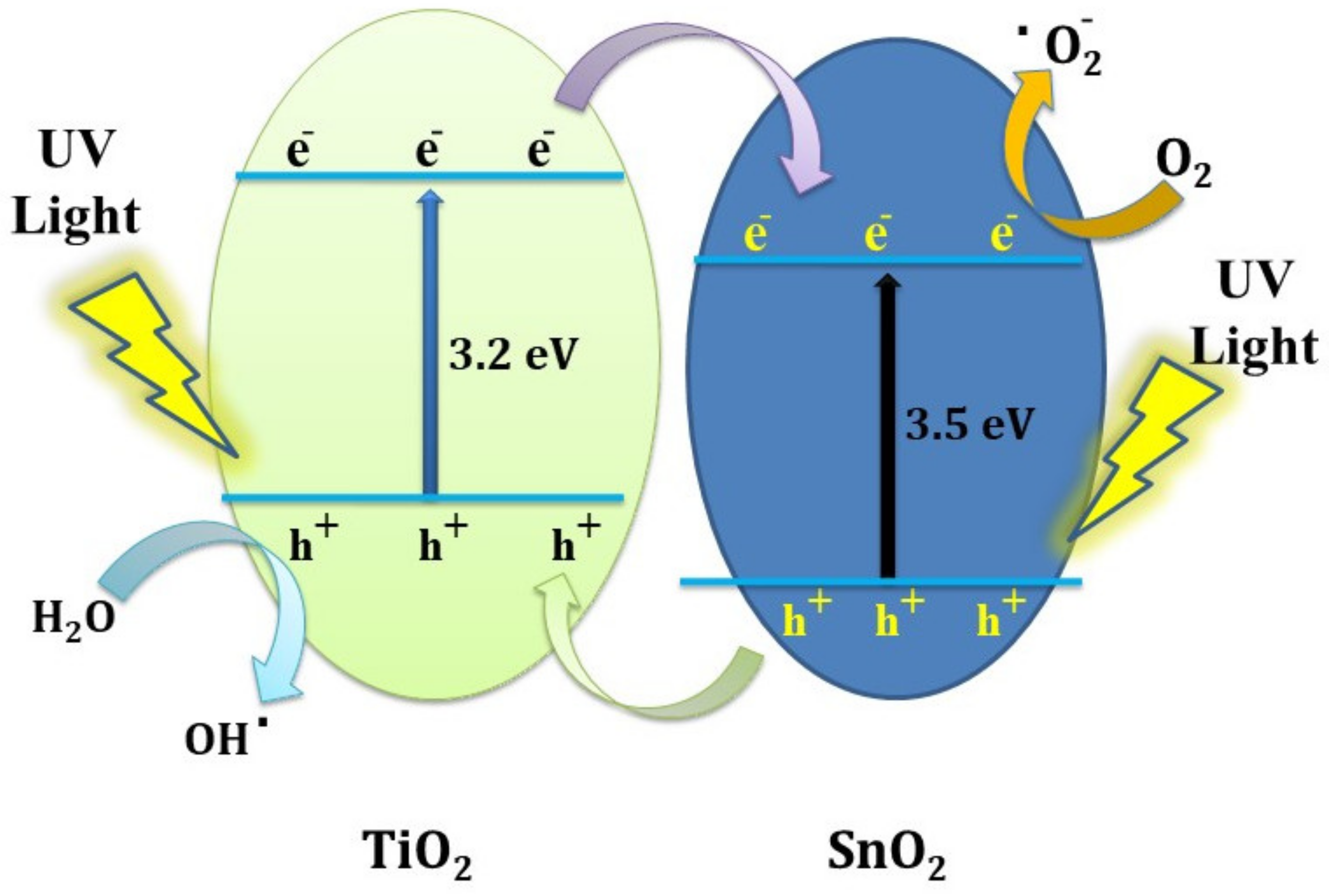
3.5. Photocatalytic Degradation of Methyl Orange Applying UV Light
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aspland, J.R. Textile Dyeing and Coloration; AATCC: Research Triangle Park, NC, USA, 1997. [Google Scholar]
- Mahmood, A.; Khan, S.U.-D.; Rana, U.A. Theoretical designing of novel heterocyclic azo dyes for dye sensitized solar cells. J. Comput. Electron. 2014, 13, 1033–1041. [Google Scholar] [CrossRef]
- Sha, Y.; Mathew, I.; Cui, Q.; Clay, M.; Gao, F.; Zhang, X.J.; Gu, Z. Rapid degradation of azo dye methyl orange using hollow cobalt nanoparticles. Chemosphere 2016, 144, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, R.; Hadjel, M.; Benderdouche, N. Heterogenous Photocatalysis Treatement of Azo Dye Methyl Orange by Nano Composite TiO2/Diatomite. Orient. J. Chem. 2015, 31, 1611–1620. [Google Scholar] [CrossRef]
- Devi, L.G.; Reddy, K.M. Enhanced photocatalytic activity of silver metallized TiO2 particles in the degradation of an azo dye methyl orange: Characterization and activity at different pH values. Appl. Surf. Sci. 2010, 256, 3116–3121. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Lou, X.W.; Xie, Y. Two-dimensional nanosheets for photoelectrochemical water splitting: Possibilities and opportunities. Sci. Direct 2013, 8, 598–618. [Google Scholar] [CrossRef]
- Zulfiqar, M.; Sufian, S.; Bahadar, A.; Lashari, N.; Rabat, N.E.; Mansor, N. Surface-fluorination of TiO2 photocatalysts for remediation of water pollution: A review. J. Clean. Prod. 2021, 317, 128354. [Google Scholar] [CrossRef]
- Kumar, S.S.; Muruganandham, T.; Jaabir, M.S.M. Photocatalytic Degradation of Methyl Orange using TiO2/SnO2 Binary Nano Composite. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 514–522. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Al-Salim, N.I.; Bagshaw, S.A.; Bittar, A.; Kemmitt, T.; McQuillan, A.J.; Mills, A.M.; Ryan, M.J. Characterisation and activity of sol–gel-prepared TiO2 photocatalysts modified with Ca, Sr or Ba ion additives. J. Mater. Chem. 2000, 10, 2358–2363. [Google Scholar] [CrossRef]
- Konovalova, T.A.; Kispert, L.D.; Konovalov, V.V. Surface Modification of TiO2 Nanoparticles with Carotenoids. EPR Study. J. Phys. Chem. B 1999, 103, 4672–4677. [Google Scholar] [CrossRef]
- Iwasaki, M.; Hara, M.; Kawada, H.; Tada, H.; Ito, S. Cobalt Ion-Doped TiO2 Photocatalyst Response to Visible Light. J. Colloid Interface Sci. 2000, 224, 202–204. [Google Scholar] [CrossRef]
- Ito, S.; Inoue, S.; Kawada, H.; Hara, M.; Iwasaki, M.; Tada, H. Low-Temperature Synthesis of Nanometer-Sized Crystalline TiO2 Particles and Their Photoinduced Decomposition of Formic Acid. J. Colloid Interface Sci. 1999, 216, 59–64. [Google Scholar] [CrossRef]
- Ding, X.-Z.; Liu, X.-H. Synthesis and microstructure control of nanocrystalline titania powders via a sol–gel process. Mater. Sci. Eng. A 1997, 224, 210–215. [Google Scholar] [CrossRef]
- Moon, J.; Takagi, H.; Fujishiro, Y.; Awano, M. Preparation and characterization of the Sb-doped TiO2 photocatalysts. J. Mater. Sci. 2001, 36, 949–955. [Google Scholar] [CrossRef]
- Ovenstone, J. Preparation of novel titania photocatalysts with high activity. J. Mater. Sci. 2001, 36, 1325–1329. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Chen, J.; Tian, L.; Huang, L.; Tu, M.; Tan, S. Structure design and photocatalytic properties of one-dimensional SnO2-TiO2 composites. Nanoscale Res. Lett. 2015, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Chai, S.; Zhao, G.; Li, P.; Lei, Y.; Zhang, Y.-N.; Li, D. Novel Sieve-Like SnO2/TiO2 Nanotubes with Integrated Photoelectrocatalysis: Fabrication and Application for Efficient Toxicity Elimination of Nitrophenol Wastewater. J. Phys. Chem. C 2011, 115, 18261–18269. [Google Scholar] [CrossRef]
- Tada, H.; Hattori, A.; Tokihisa, Y.; Imai, K.; Tohge, N.; Ito, S. A Patterned-TiO2/SnO2 Bilayer Type Photocatalyst. J. Phys. Chem. B 2000, 104, 4585–4587. [Google Scholar] [CrossRef]
- Spanhel, L.; Weller, H.; Henglein, A. Photochemistry of semiconductor colloids. 22. Electron ejection from illuminated cadmium sulfide into attached titanium and zinc oxide particles. J. Am. Chem. Soc. 1987, 109, 6632–6635. [Google Scholar] [CrossRef]
- Gopidas, K.R.; Bohorquez, M.; Kamat, P.V. Photophysical and photochemical aspects of coupled semiconductors: Charge-transfer processes in colloidal cadmium sulfide-titania and cadmium sulfide-silver(I) iodide systems. J. Phys. Chem. 1990, 94, 6435–6440. [Google Scholar] [CrossRef]
- Serpone, N.; Borgarello, E.; Grätzel, M. Visible light induced generation of hydrogen from H2S in mixed semiconductor dispersions; Improved efficiency through inter-particle electron transfer. J. Chem. Soc. Chem. Commun. 1984, 342–344. [Google Scholar] [CrossRef]
- Hotchandani, S.; Kamat, P.V. Charge-transfer processes in coupled semiconductor systems. Photochemistry and photoelectrochemistry of the colloidal cadmium sulfide-zinc oxide system. J. Phys. Chem. 1992, 96, 6834–6839. [Google Scholar] [CrossRef]
- Sato, T.; Masaki, K.; Sato, K.-I.; Fujishiro, Y.; Okuwaki, A. Photocatalytic properties of layered hydrous titanium oxide/CdS–ZnS nanocomposites incorporating CdS–ZnS into the interlayer. J. Chem. Technol. Biotechnol. 1996, 67, 339–344. [Google Scholar] [CrossRef]
- Machida, M.; Ma, X.W.; Taniguchi, H.; Yabunaka, J.-I.; Kijima, T. Pillaring and photocatalytic property of partially substituted layered titanates, Na2Ti3-x Mx O7 and K2Ti4-x Mx O9 (M = Mn, Fe, Co, Ni, Cu). J. Mol. Catal. A Chem. 2000, 155, 131–142. [Google Scholar] [CrossRef]
- Bedja, I.; Kamat, P.V. Capped Semiconductor Colloids. Synthesis and Photoelectrochemical Behavior of TiO2-Capped SnO2Nanocrystallites. J. Phys. Chem. 1995, 99, 9182–9188. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Kamat, P.V. Enhanced Rates of Photocatalytic Degradation of an Azo Dye Using SnO2/TiO2 Coupled Semiconductor Thin Films. Environ. Sci. Technol. 1995, 29, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, D.; Wang, X.; Yang, X.; Lu, L. Rapid Synthesis of Nanocrystalline TiO2/SnO2 Binary Oxides and Their Photoinduced Decomposition of Methyl Orange. J. Solid State Chem. 2002, 165, 193–198. [Google Scholar] [CrossRef]
- Xiong, G.; Wei, G.; Yang, X.; Lu, L.; Wang, X. Characterization and size-dependent magnetic properties of Ba3Co2Fe24O41 nanocrystals synthesized through a sol-gel method. J. Mater. Sci. 2000, 35, 931–936. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Lu, L.; Wang, X. Synthesis of substituted M- and W-type barium ferrite nanostructured powders by stearic acid gel method. J. Alloy. Compd. 1996, 237, 45–48. [Google Scholar] [CrossRef]
- Deshmukh, S.M.; Tamboli, M.S.; Shaikh, H.; Babar, S.B.; Hiwarale, D.P.; Thate, A.G.; Shaikh, A.F.; Alam, M.A.; Khetre, S.M.; Bamane, S.R. A Facile Urea-Assisted Thermal Decomposition Process of TiO2 Nanoparticles and Their Photocatalytic Activity. Coatings 2021, 11, 165. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Zou, B.; Zhang, H.; Wang, J.; Schubert, U.; Rui, Y. Black SnO2–TiO2 Nanocomposites with High Dispersion for Photocatalytic and Photovoltalic Applications. ACS Appl. Nano Mater. 2020, 3, 4265–4273. [Google Scholar] [CrossRef]
- Jilani, A.; Ansari, M.O.; Rehman, G.U.; Shakoor, M.B.; Hussain, S.Z.; Othman, M.H.D.; Ahmad, S.R.; Dustgeer, M.R.; Alshahrie, A. Phenol removal and hydrogen production from water: Silver nanoparticles decorated on polyaniline wrapped zinc oxide nanorods. J. Ind. Eng. Chem. 2022, in press. [Google Scholar] [CrossRef]
- AlAbduljabbar, F.A.; Haider, S.; Ali, F.A.A.; Alghyamah, A.A.; Almasry, W.A.; Patel, R.; Mujtaba, I.M. Efficient Photocatalytic Degradation of Organic Pollutant in Wastewater by Electrospun Functionally Modified Polyacrylonitrile Nanofibers Membrane Anchoring TiO2 Nanostructured. Membranes 2021, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Jilani, A.; Rehman, G.U.; Ansari, M.O.; Othman, M.H.D.; Hussain, S.Z.; Dustgeer, M.R.; Darwesh, R. Sulfonated polyaniline-encapsulated graphene@graphitic carbon nitride nanocomposites for significantly enhanced photocatalytic degradation of phenol: A mechanistic study. New J. Chem. 2020, 44, 19570–19580. [Google Scholar] [CrossRef]
- Wang, C.; Shao, C.; Zhang, X.; Liu, Y. SnO2 Nanostructures-TiO2 Nanofibers Heterostructures: Controlled Fabrication and High Photocatalytic Properties. Inorg. Chem. 2009, 48, 7261–7268. [Google Scholar] [CrossRef] [PubMed]
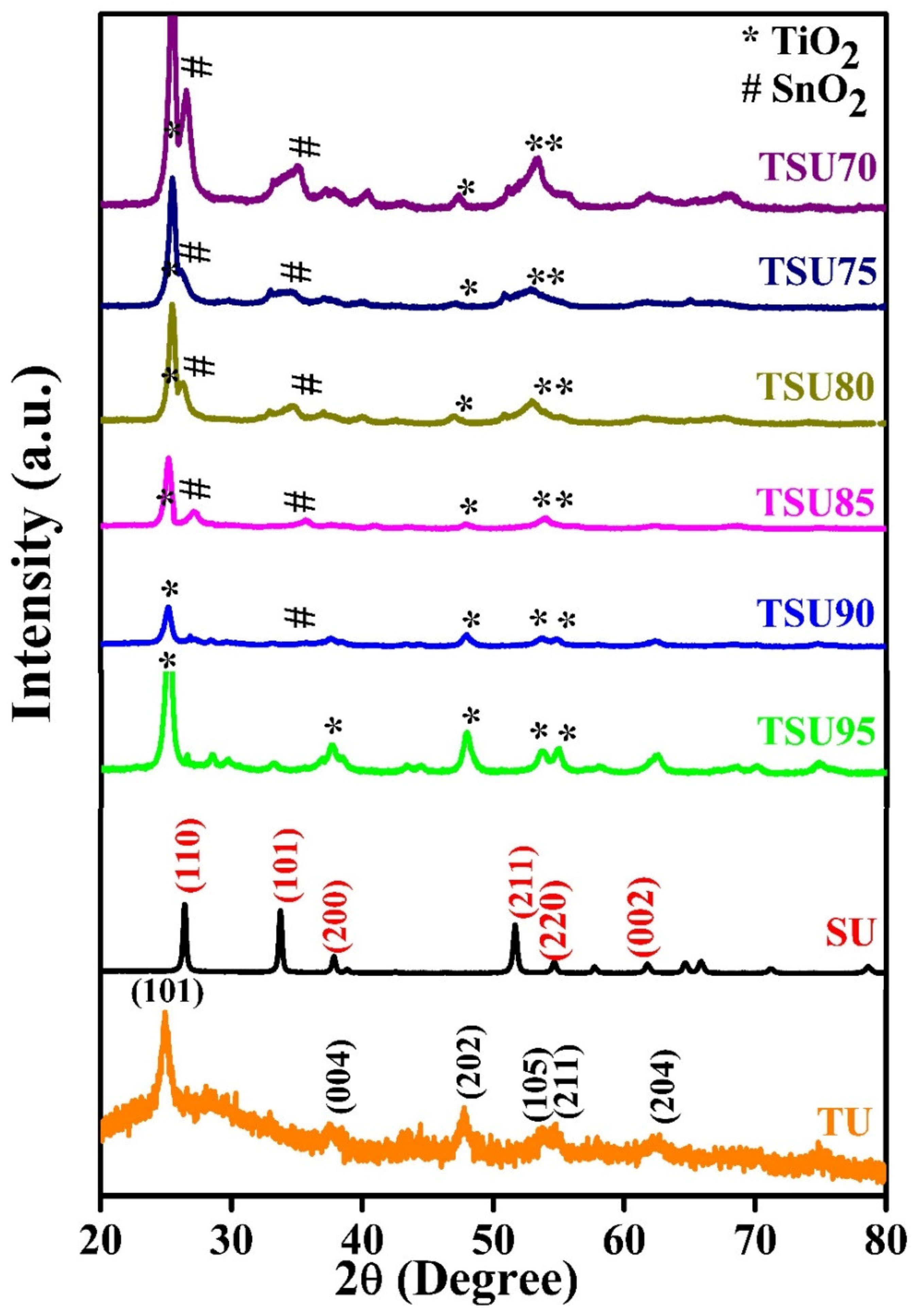

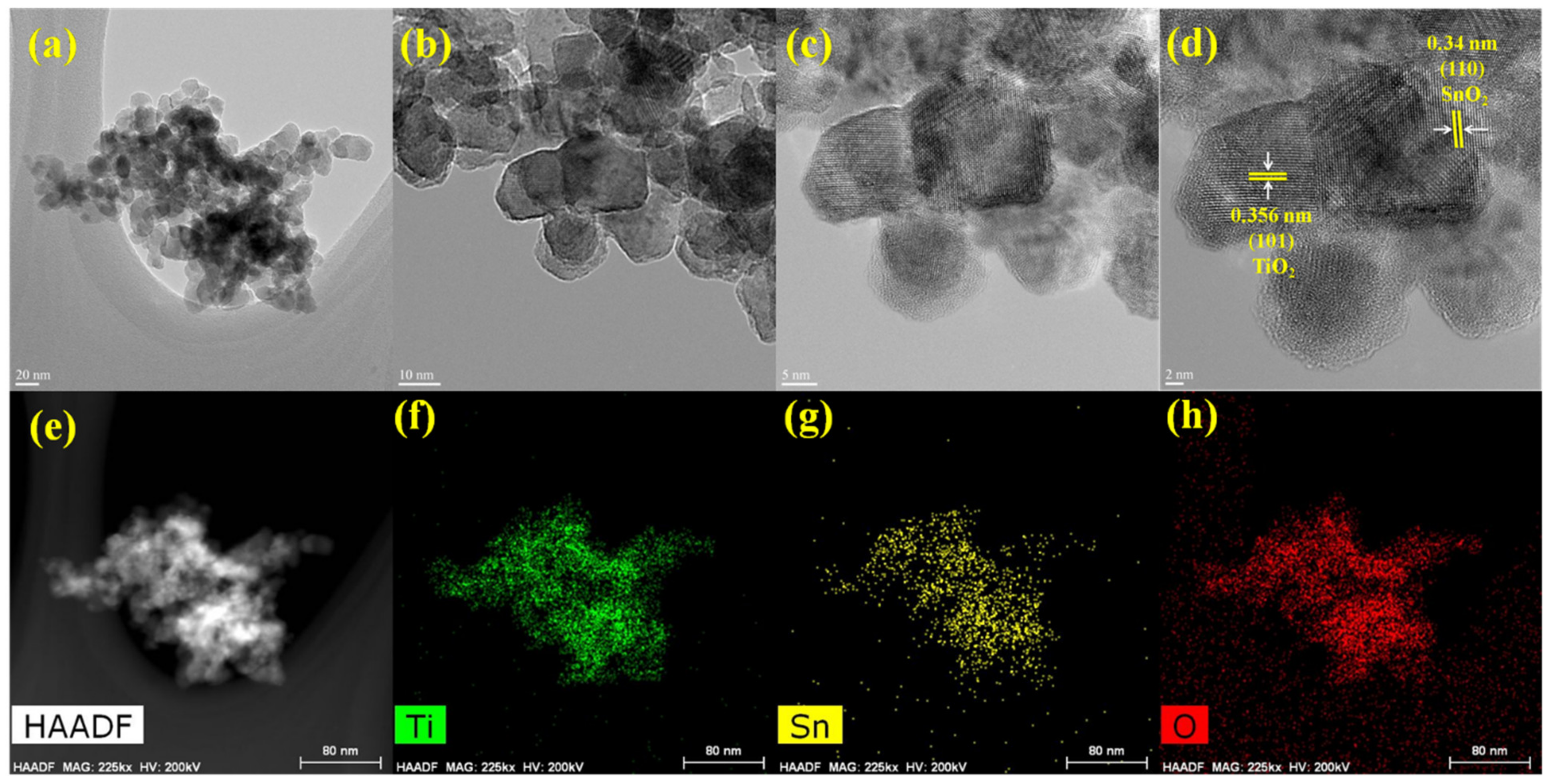

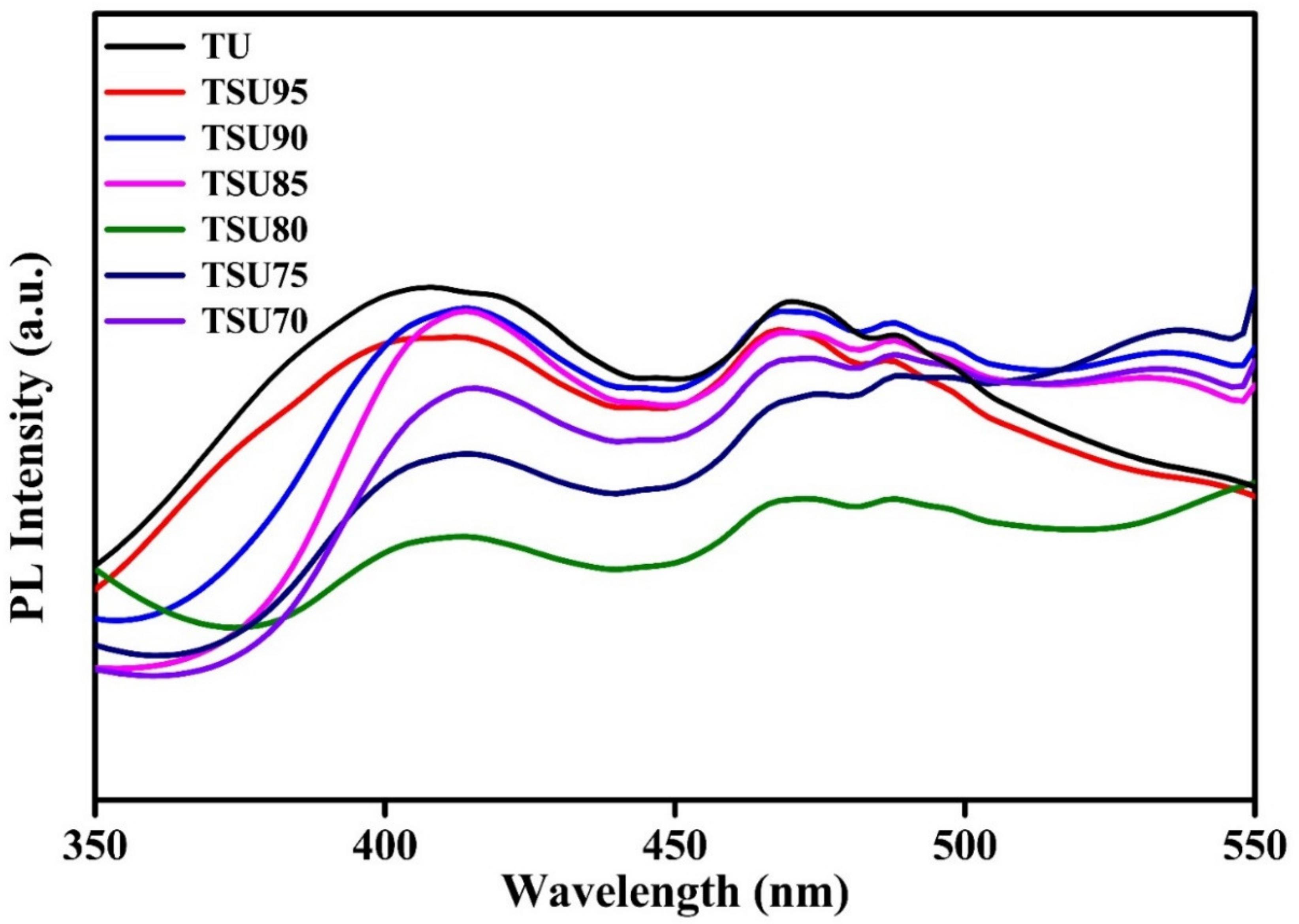
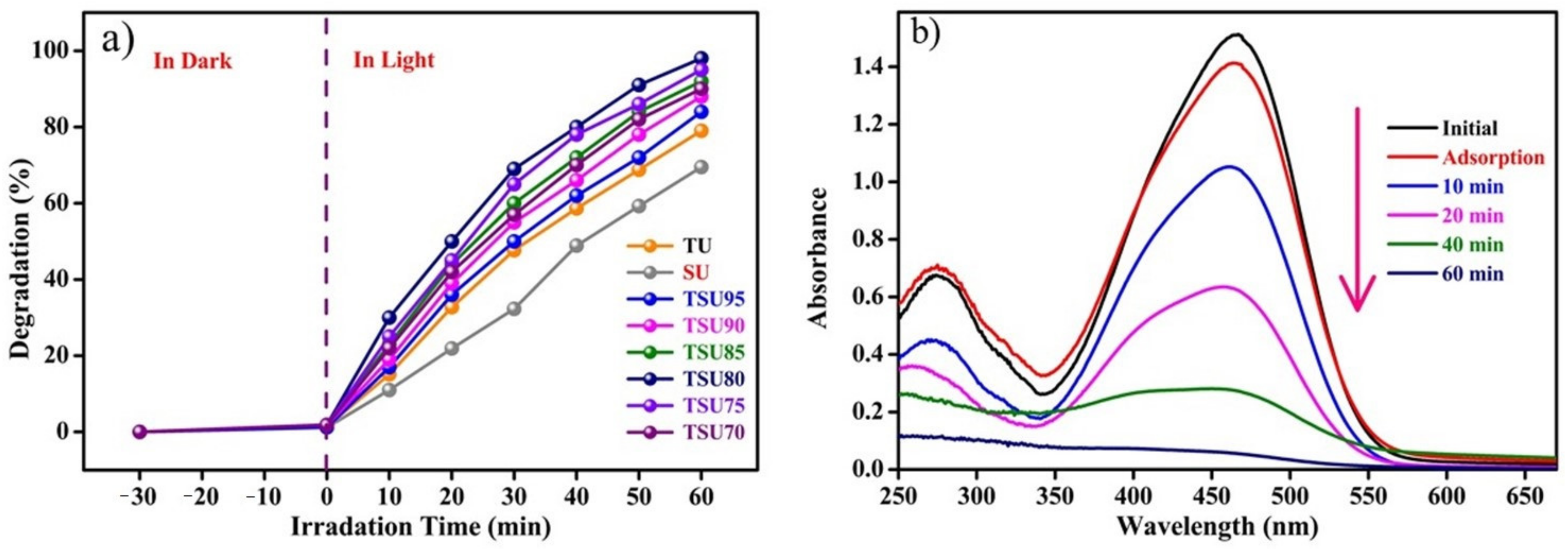
| Sr. No | Sample | Weight Ratio of Precursors (mg) (TiOSO4·H2O:SnCl4·H2O) | Crystallite Size (nm) | Band Gap (eV) |
|---|---|---|---|---|
| 1 | Pure TiO2 (TU) | 1000 | 14 | 3.22 |
| 2 | Pure SnO2 (SU) | 1000 | 25.96 | 3.5 |
| 3 | TSU95 | 950:50 | 13.43 | 3.25 |
| 4 | TSU90 | 900:100 | 14.05 | 3.23 |
| 5 | TSU85 | 850:150 | 13.5 | 3.25 |
| 6 | TSU80 | 800:200 | 13.75 | 3.03 |
| 7 | TSU75 | 750:250 | 13.78 | 3 |
| 8 | TSU70 | 700:300 | 13.77 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshmukh, S.M.; Patil, S.S.; Babar, S.B.; Alshehri, S.; Ghoneim, M.M.; Tamboli, A.M.; Lam, N.H.; Truong, N.T.N.; Kim, C.D.; Tamboli, M.S.; et al. TiO2–SnO2 Nanocomposites for Photocatalytic Environmental Remediation under UV-Light. Metals 2022, 12, 733. https://doi.org/10.3390/met12050733
Deshmukh SM, Patil SS, Babar SB, Alshehri S, Ghoneim MM, Tamboli AM, Lam NH, Truong NTN, Kim CD, Tamboli MS, et al. TiO2–SnO2 Nanocomposites for Photocatalytic Environmental Remediation under UV-Light. Metals. 2022; 12(5):733. https://doi.org/10.3390/met12050733
Chicago/Turabian StyleDeshmukh, Sandip M., Santosh S. Patil, Santosh B. Babar, Sultan Alshehri, Mohammed M. Ghoneim, Asiya M. Tamboli, Nguyen Hoang Lam, Nguyen Tam Nguyen Truong, Chang Duk Kim, Mohaseen S. Tamboli, and et al. 2022. "TiO2–SnO2 Nanocomposites for Photocatalytic Environmental Remediation under UV-Light" Metals 12, no. 5: 733. https://doi.org/10.3390/met12050733
APA StyleDeshmukh, S. M., Patil, S. S., Babar, S. B., Alshehri, S., Ghoneim, M. M., Tamboli, A. M., Lam, N. H., Truong, N. T. N., Kim, C. D., Tamboli, M. S., Khetre, S. M., & Bamane, S. R. (2022). TiO2–SnO2 Nanocomposites for Photocatalytic Environmental Remediation under UV-Light. Metals, 12(5), 733. https://doi.org/10.3390/met12050733






