[(AlxTi1−x)-(FeCoNi)12](AlxTi1−x)0.5Cr2.5 High-Entropy Alloy Coating by Laser Cladding
Abstract
:1. Introduction
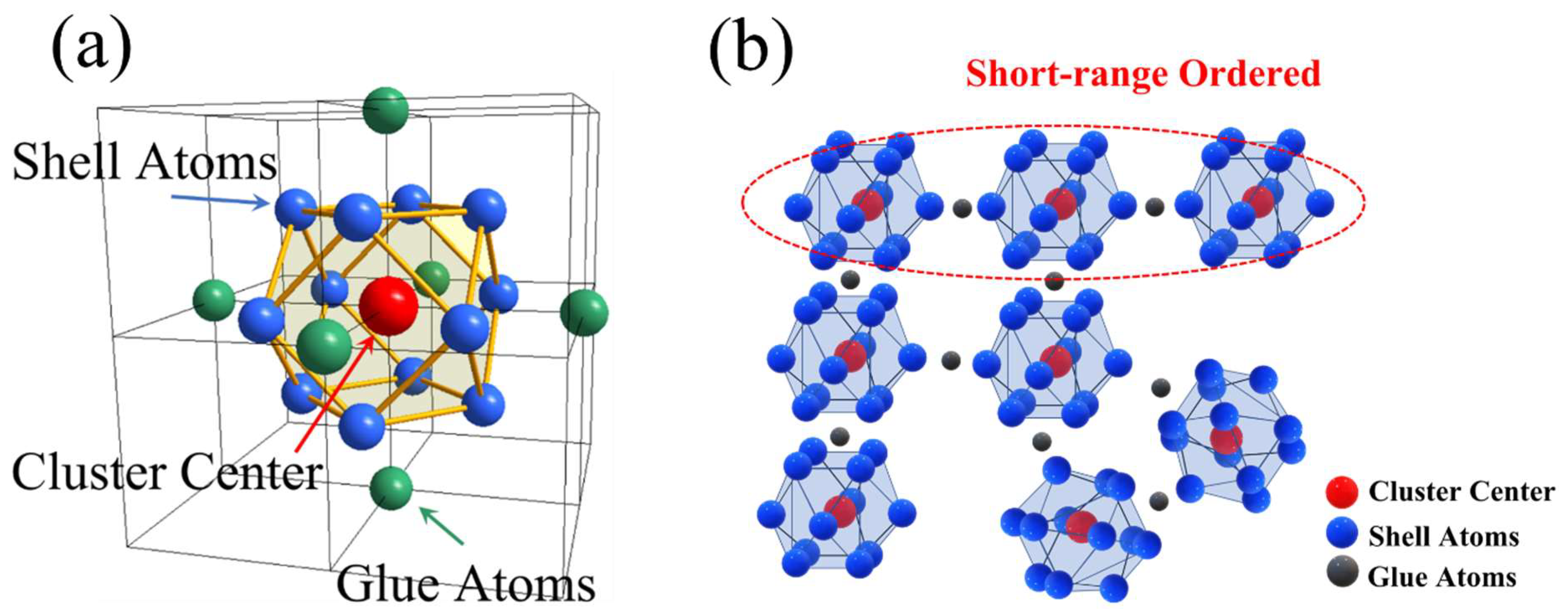
2. Compositional Design Based on Cluster-Plus-Glue-Atom Model
3. Material and Methods
4. Results
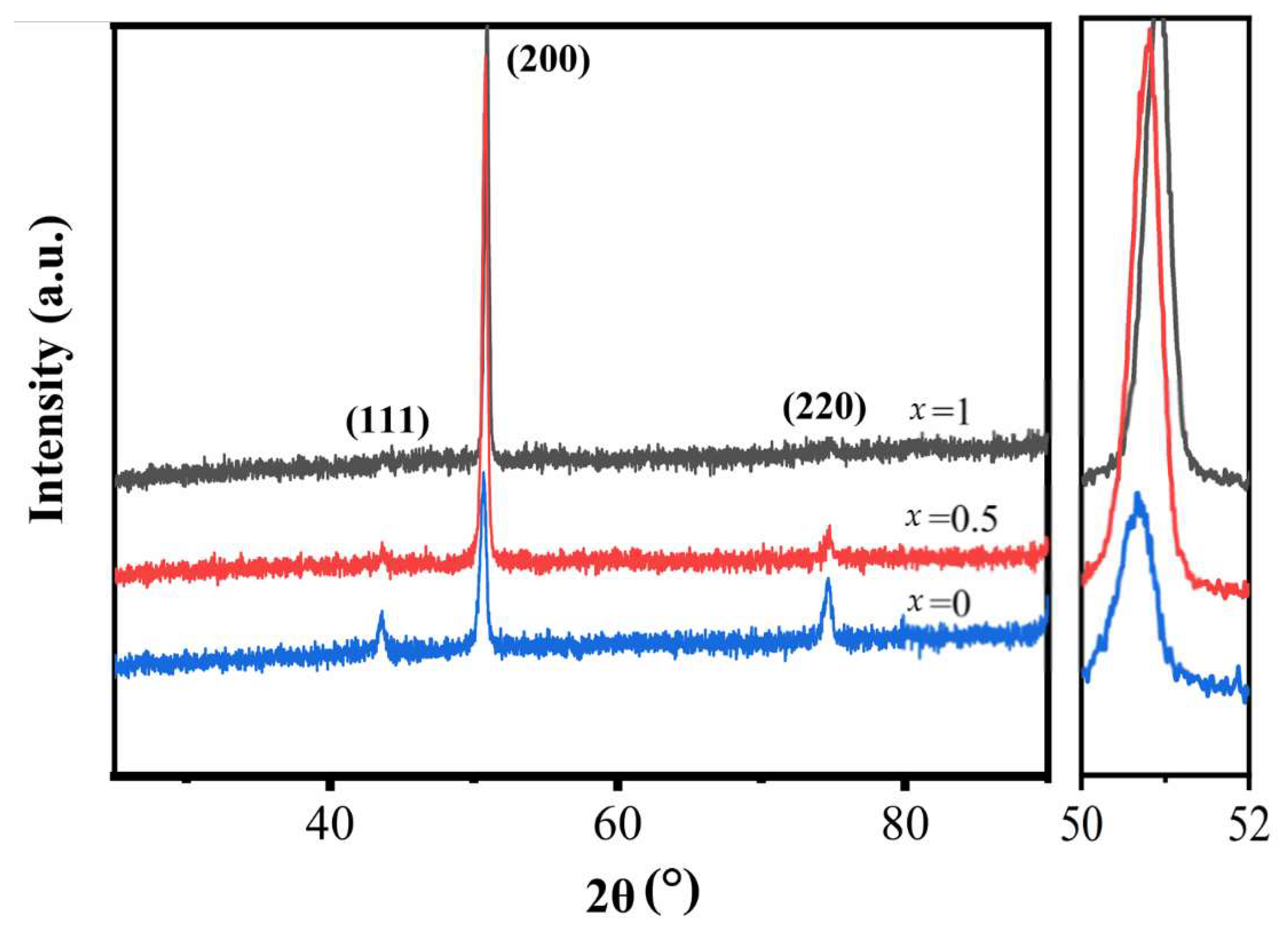
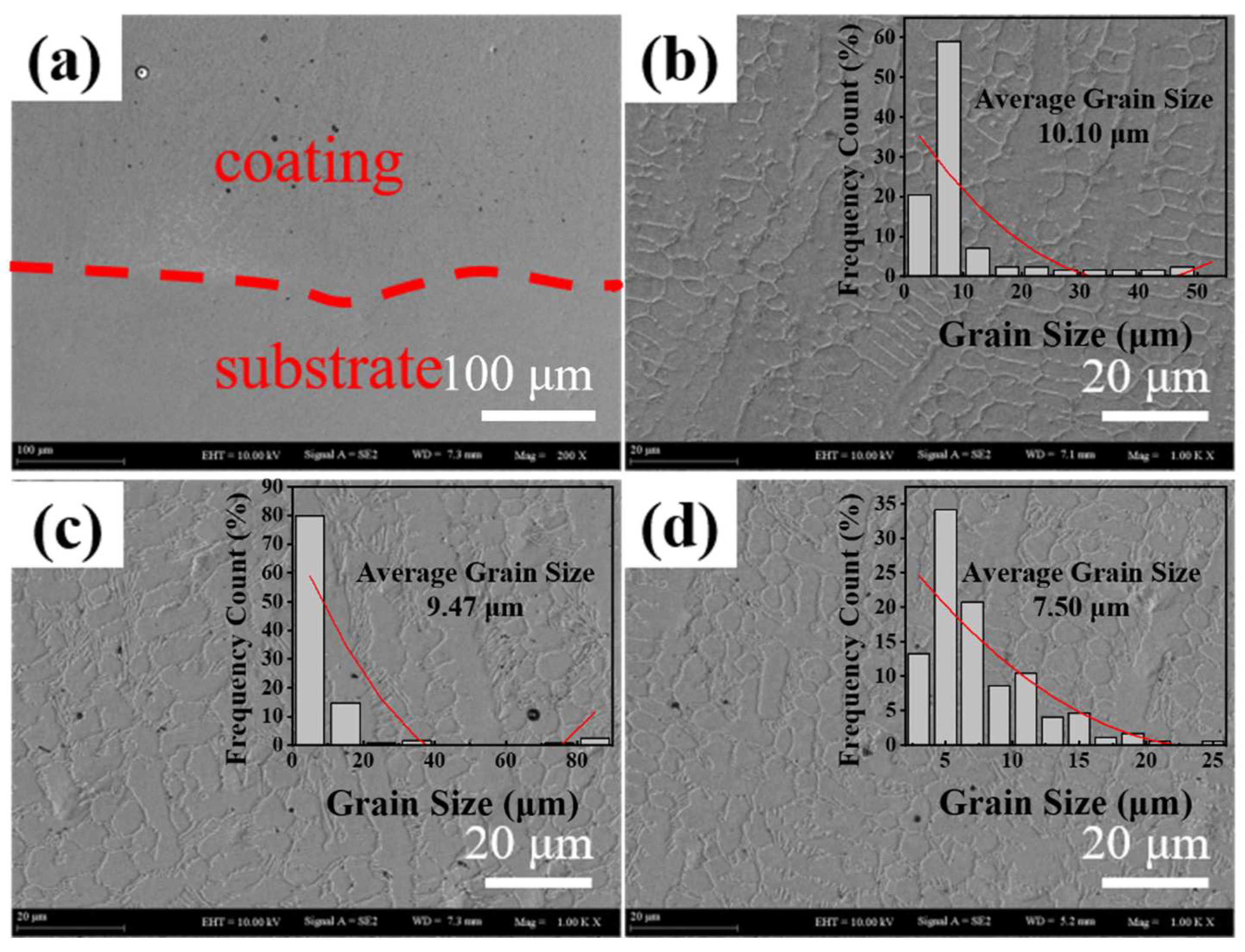
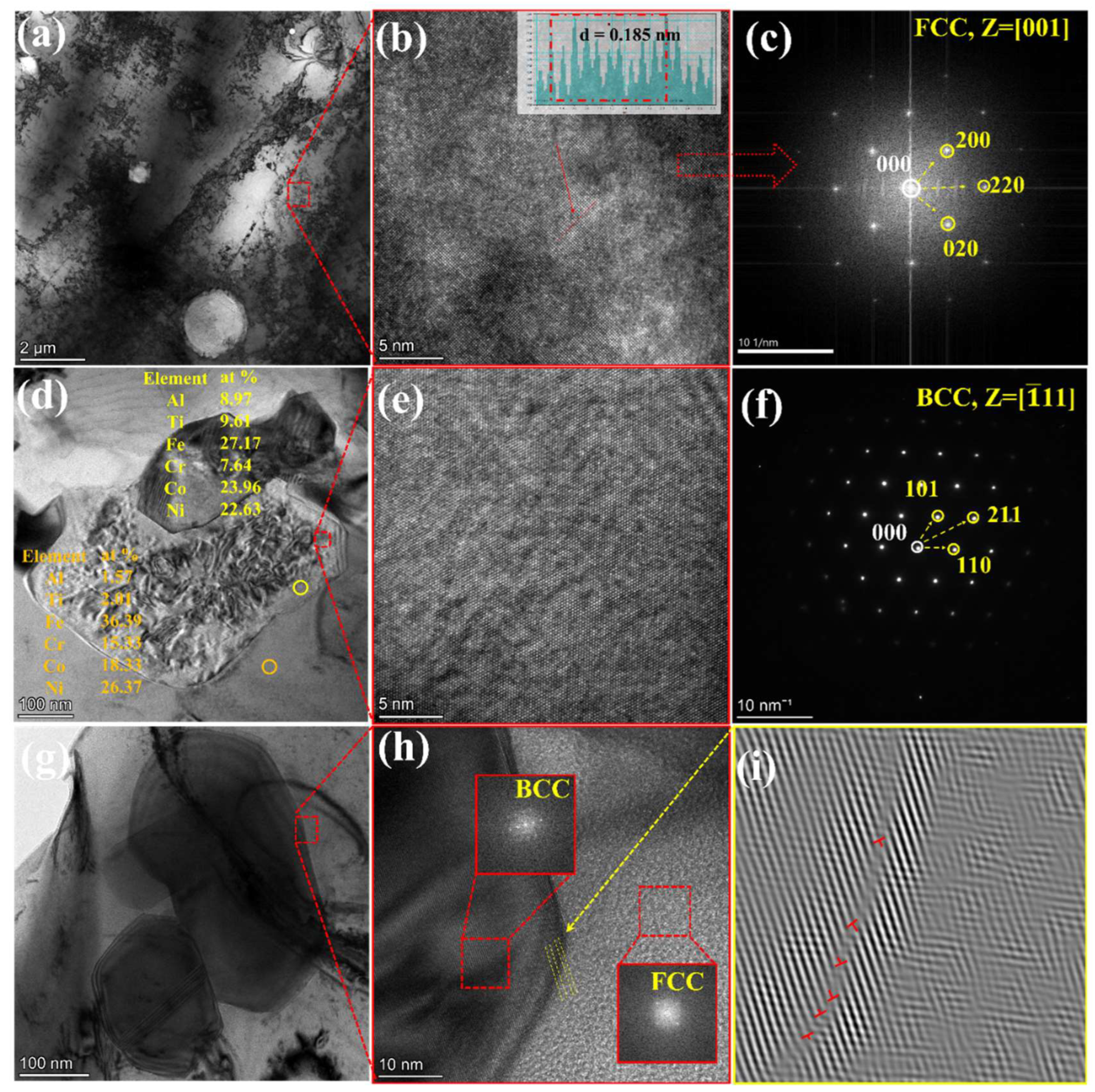
4.1. Microstructural Analysis
| Element | ΔHmix (kJ/mol) | |||||
|---|---|---|---|---|---|---|
| Fe | Co | Ni | Cr | Al | Ti | |
| Fe | - | −1 | −2 | −1 | −11 | −17 |
| Co | - | 0 | −4 | −19 | −28 | |
| Ni | - | −7 | −22 | −35 | ||
| Cr | - | −10 | −7 | |||
| Al | - | −30 | ||||
| Element | Atomic Radius (Å) | Crystal | Tm (K) | VEC |
|---|---|---|---|---|
| Fe | 1.24 | BCC | 1811 | 8 |
| Co | 1.25 | HCP | 1768 | 9 |
| Ni | 1.25 | FCC | 1728 | 10 |
| Cr | 1.25 | BCC | 2180 | 6 |
| Al | 1.43 | FCC | 933 | 3 |
| Ti | 1.46 | HCP | 1941 | 4 |
| Alloy Composition | Tm(K) | ΔSmix(J/K·mol) | ΔHmix(kJ/mol) | Ω | δ | VEC |
|---|---|---|---|---|---|---|
| 0 | 1849.28 | 12.90 | −11.90 | 2.00 | 4.91% | 8.06 |
| 0.5 | 1801.91 | 12.25 | −10 | 2.21 | 4.59% | 8.02 |
| 1 | 1849.28 | 12.90 | −11.90 | 2.00 | 4.91% | 8.06 |
4.2. Corrosion Resistance
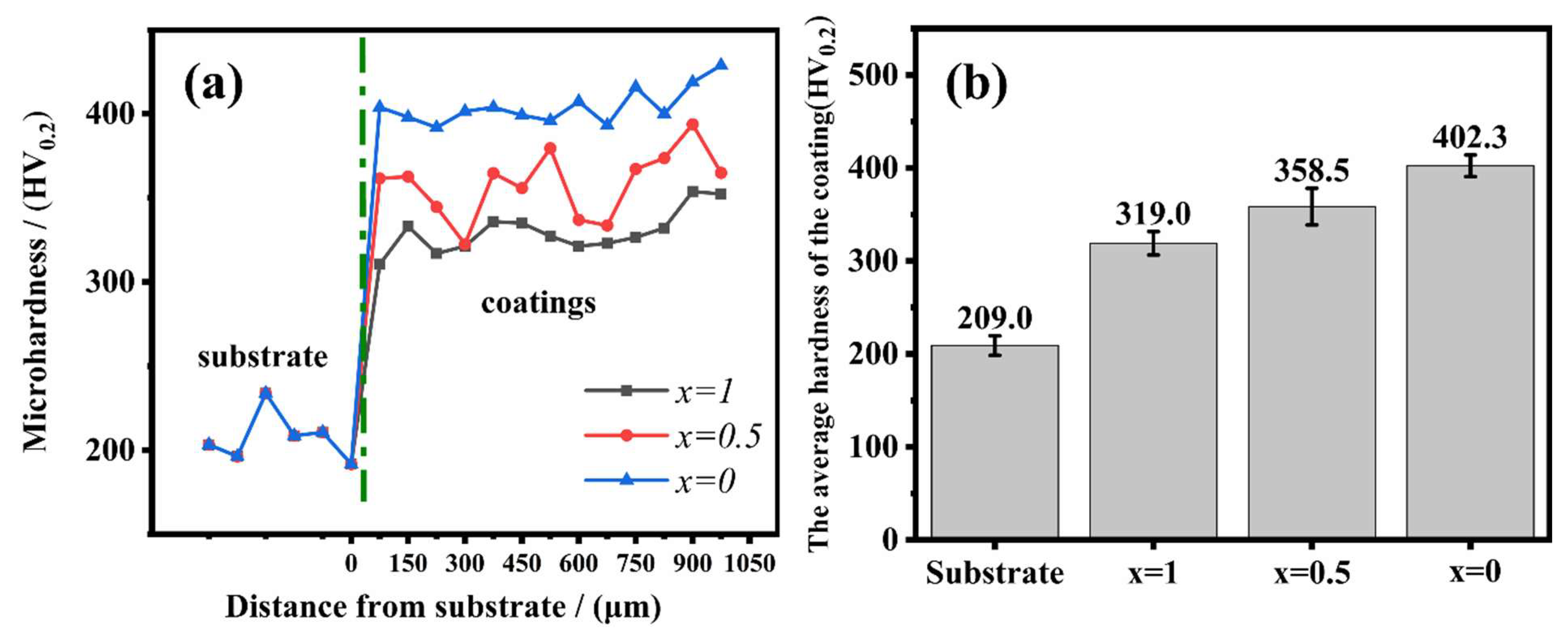
4.3. Microhardness
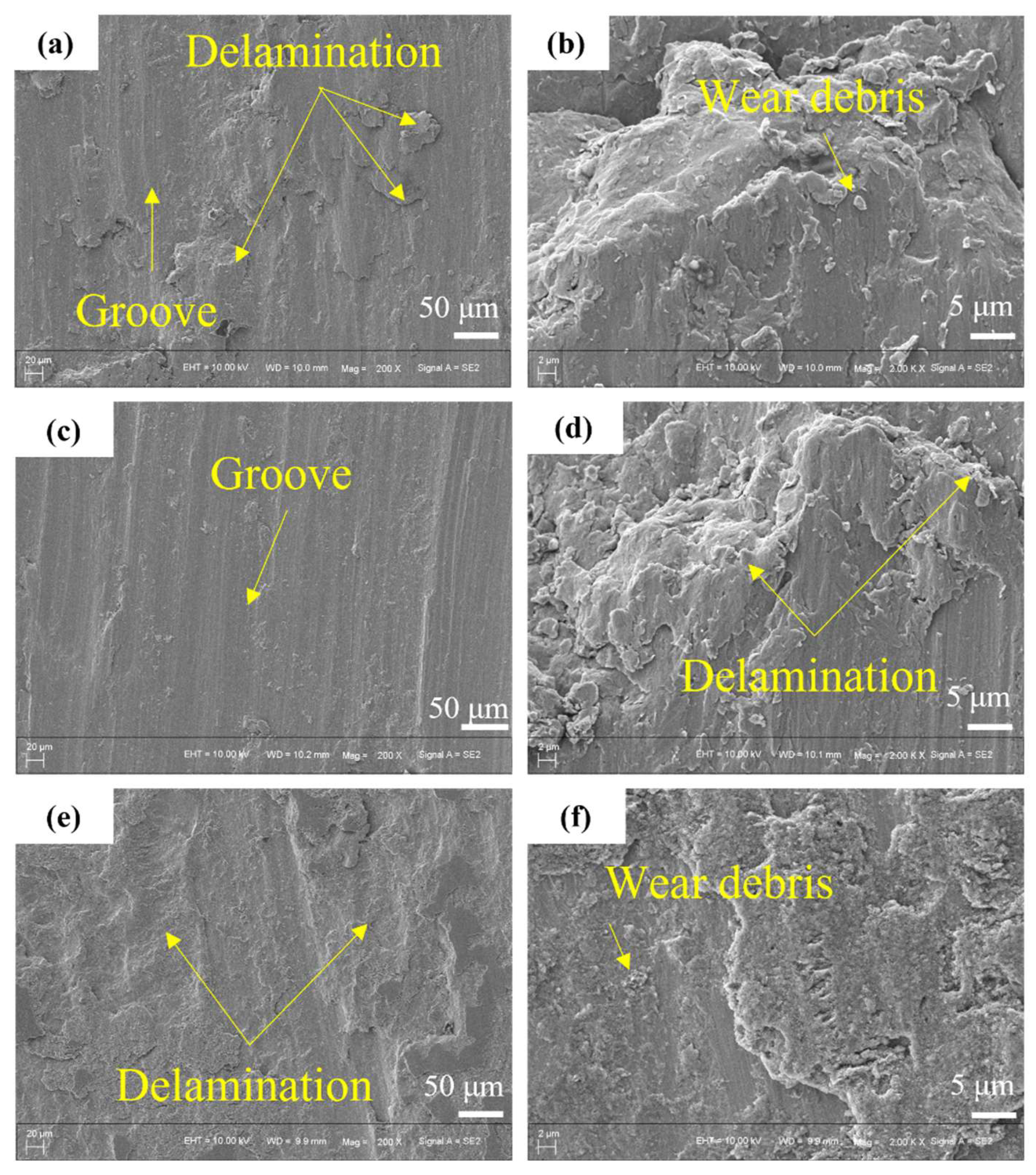
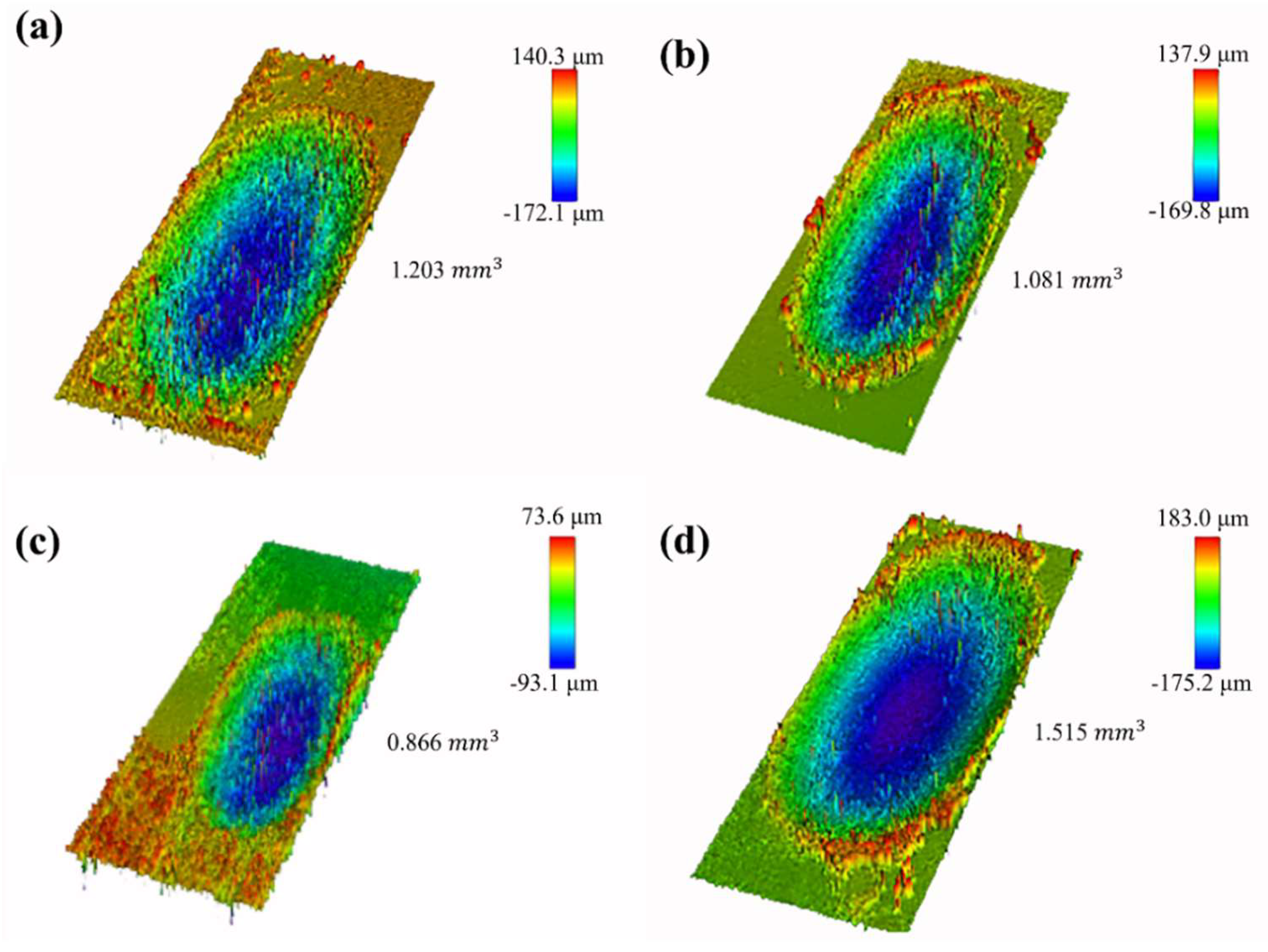
4.4. Wear Resistance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, F.Y.; Yu, J.K.; Xiao, J.J.; Qiao, Q.; Yang, H.B.; Guo, Y.Q. Preparation of FeCoNiCr High Entropy Alloy Coatings and Optimization of Process Parameters. Rare Met. Mater. Eng. 2021, 50, 2337–2342. [Google Scholar]
- Qi, Y.; Cao, T.; Zong, H.; Wu, Y.; He, L.; Ding, X.; Jiang, F.; Jin, S.; Sha, G.; Sun, J. Enhancement of strength-ductility balance of heavy Ti and Al alloyed FeCoNiCr high-entropy alloys via boron doping. J. Mater. Sci. Technol. 2021, 75, 154–163. [Google Scholar] [CrossRef]
- Senkov, O.; Miller, J.; Miracle, D.; Woodward, C. Accelerated exploration of multi-principal element alloys with solid solution phases. Nat. Commun. 2015, 6, 6529. [Google Scholar] [CrossRef]
- Bagdasaryan, A.; Pshyk, A.; Coy, L.; Kempiński, M.; Pogrebnjak, A.; Beresnev, V.; Jurga, S. Structural and mechanical characterization of (TiZrNbHfTa)N/WN multilayered nitride coatings. Mater. Lett. 2018, 229, 364–367. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, L.; Xu, L.; Prashanth, K.; Zhang, N.; Ma, X.; Jia, Y.; Xu, Y.; Jia, Y.; Wang, G. Frictional Wear and Corrosion Behavior of AlCoCrFeNi High-Entropy Alloy Coatings Synthesized by Atmospheric Plasma Spraying. Entropy 2020, 22, 740. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. High-temperature wear behaviour of AlCoCrFeNiTi0.5 coatings produced by HVOF. Surf. Coat. Technol. 2020, 403, 126379. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.; Zhang, C.; Guan, M.; Tan, J. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Shang, C.; Axinte, E.; Sun, J.; Li, X.; Li, P.; Du, J.; Qiao, P.; Wang, Y. CoCrFeNi(W1−xMox) high-entropy alloy coatings with excellent mechanical properties and corrosion resistance prepared by mechanical alloying and hot pressing sintering. Mater. Des. 2017, 117, 193–202. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Yang, B.; Liaw, P.K. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Chen, P.; Hao, J. Microstructural characterization and corrosion behaviour of AlCoCrFeNiTix high-entropy alloy coatings fabricated by laser cladding. Surf. Coat. Technol. 2019, 361, 63–74. [Google Scholar] [CrossRef]
- Xiang, C.; Zhang, Z.; Fu, H.; Han, E.-H.; Zhang, H.; Wang, J. Microstructure and corrosion behavior of AlCoCrFeNiSi0.1 high-entropy alloy. Intermetallics 2019, 114, 106599. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Yan, S.; Yu, G.; Chen, J.; He, J.; Yin, F. Microstructure evolution and mechanical properties of atmosphere plasma sprayed AlCoCrFeNi high-entropy alloy coatings under post-annealing. J. Alloys Compd. 2021, 872, 159607. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Xu, Y.; Liu, Z.; Qian, B.; Xuan, F. Selective laser melting of CoCrFeNiMn high entropy alloy powder modified with nano-TiN particles for additive manufacturing and strength enhancement: Process, particle behavior and effects. Powder Technol. 2020, 360, 509–521. [Google Scholar] [CrossRef]
- Wolff-Goodrich, S.; Marshal, A.; Pradeep, K.; Dehm, G.; Schneider, J.M.; Liebscher, C.H. Combinatorial exploration of B2/L21 precipitation strengthened AlCrFeNiTi compositionally complex alloys. J. Alloys Compd. 2021, 853, 156111. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Feng, T.; Zhang, Y.; Sha, G.; Lewandowski, J.; Liaw, P.K. High-entropy Al0.3CoCrFeNi alloy fibers with high tensile strength and ductility at ambient and cryogenic temperatures. Acta Mater. 2017, 123, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Han, B.; Li, M.; Hu, C.; Zhang, Q.; Liu, X.; Wang, Y. Investigation on microstructure and properties of laser cladded AlCoCrCuFeNi high entropy alloy coating by ultrasonic impact treatment. Intermetallics 2021, 128, 107017. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R. New Advances in High-Entropy Alloys. Entropy 2020, 22, 1158. [Google Scholar] [CrossRef]
- Yurkova, A.I.; Nakonechnyi, S.O.; Cherniavsky, V.V.; Kushnir, V.V. Nanostructured AlCoFeCrVNi and AlCoFeCrVTi high-entropy alloys resulted from mechanical alloying and sintering. Appl. Nanosci. 2021, 1–12. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of Al CoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Huang, X.; Huang, L.; Peng, H.; Liu, Y.; Liu, B.; Li, S. Enhancing strength-ductility synergy in a casting non-equiatomic NiCoCr-based high-entropy alloy by Al and Ti combination addition. Scr. Mater. 2021, 200, 113898. [Google Scholar] [CrossRef]
- Hao, C.P.; Wang, Q.; Ma, R.T.; Wang, Y.M.; Qiang, J.B.; Dong, C. Cluster-plus-glue-atom model in bcc solid solution alloys. Acta Phys. Sin. 2011, 60, 8. [Google Scholar]
- Wang, Q.; Zha, Q.; Liu, E.; Dong, C.; Wang, X.; Tan, C.; Ji, C. Composition design of high-strength martensitic precipitation hardening stainless steels based on a cluster model. Acta Met. Sin. 2012, 48, 1201. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.; Wang, Y.; Qiang, J.; Dong, C. β-Ti Alloys with Low Young’s Moduli Interpreted by Cluster-Plus-Glue-Atom Model. Met. Mater. Trans. A 2013, 44, 1872–1879. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.Y.; Cotton, J.D.; Zhang, Y. Phase Stability of Low-Density, Multiprincipal Component Alloys Containing Aluminum, Magnesium, and Lithium. JOM 2014, 66, 2009–2020. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, M.; Cui, H.; Zhao, Y.; Song, X.; Zeng, Y.; Gao, X.; Lu, F.; Wang, C.; Song, Q. Effects of Ti-to-Al ratios on the phases, microstructures, mechanical properties, and corrosion resistance of Al2-xCoCrFeNiTix high-entropy alloys. J. Alloys Compd. 2019, 805, 585–596. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Jiang, B.; Li, C.; Hao, J.; Li, X.; Dong, C.; Nieh, T. Controlled formation of coherent cuboidal nanoprecipitates in body-centered cubic high-entropy alloys based on Al2(Ni,Co,Fe,Cr)14 compositions. Acta Mater. 2018, 147, 213–225. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cui, X.; Jin, G.; Cai, Z.; Tan, N.; Lu, B.; Gao, Z. Interfacial bonding properties between cobalt-based plasma cladding layer and substrate under tensile conditions. Mater. Des. 2017, 123, 54–63. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, X.; Liu, Q. Microstructure and properties of in-situ TiN reinforced laser cladding CoCr2FeNiTi high-entropy alloy composite coatings. Surf. Coat. Technol. 2018, 344, 353–358. [Google Scholar] [CrossRef]
- Lan, H.; Liu, Q. Design of [Al-(FeCoNi)12]Al Cr3- HEAs based on cluster-plus-glue-atom model and its coating fabricated by laser cladding. Intermetallics 2020, 126, 106941. [Google Scholar] [CrossRef]
- Su, B.; Luo, L.; Wang, B.; Su, Y.; Wang, L.; Ritchie, R.O.; Guo, E.; Li, T.; Yang, H.; Huang, H.; et al. Annealed microstructure dependent corrosion behavior of Ti-6Al-3Nb-2Zr-1Mo alloy. J. Mater. Sci. Technol. 2021, 62, 234–248. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Wang, J.; Zheng, M.; Guo, P.; Zhang, Y.; Ren, Y.; Liu, J.; Huang, W. Effect of stress-relief annealing on anodic dissolution behaviour of additive manufactured Ti-6Al-4V via laser solid forming. Corros. Sci. 2019, 153, 314–326. [Google Scholar] [CrossRef]
- Yen, C.-C.; Lu, H.-N.; Tsai, M.-H.; Wu, B.-W.; Lo, Y.-C.; Wang, C.-C.; Chang, S.-Y.; Yen, S.-K. Corrosion mechanism of annealed equiatomic AlCoCrFeNi tri-phase high-entropy alloy in 0.5 M H2SO4 aerated aqueous solution. Corros. Sci. 2019, 157, 462–471. [Google Scholar] [CrossRef]
- Liao, W.-B.; Zhang, H.; Liu, Z.-Y.; Li, P.-F.; Huang, J.-J.; Yu, C.-Y.; Lu, Y. High Strength and Deformation Mechanisms of Al0.3CoCrFeNi High-Entropy Alloy Thin Films Fabricated by Magnetron Sputtering. Entropy 2019, 21, 146. [Google Scholar] [CrossRef] [Green Version]
- Meghwal, A.; Anupam, A.; Luzin, V.; Schulz, C.; Hall, C.; Murty, B.; Kottada, R.S.; Berndt, C.C.; Ang, A.S.M. Multiscale mechanical performance and corrosion behaviour of plasma sprayed AlCoCrFeNi high-entropy alloy coatings. J. Alloys Compd. 2021, 854, 157140. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high-entropy alloy coatings on aluminum by laser cladding. Opt. Laser Technol. 2021, 134, 106632. [Google Scholar] [CrossRef]
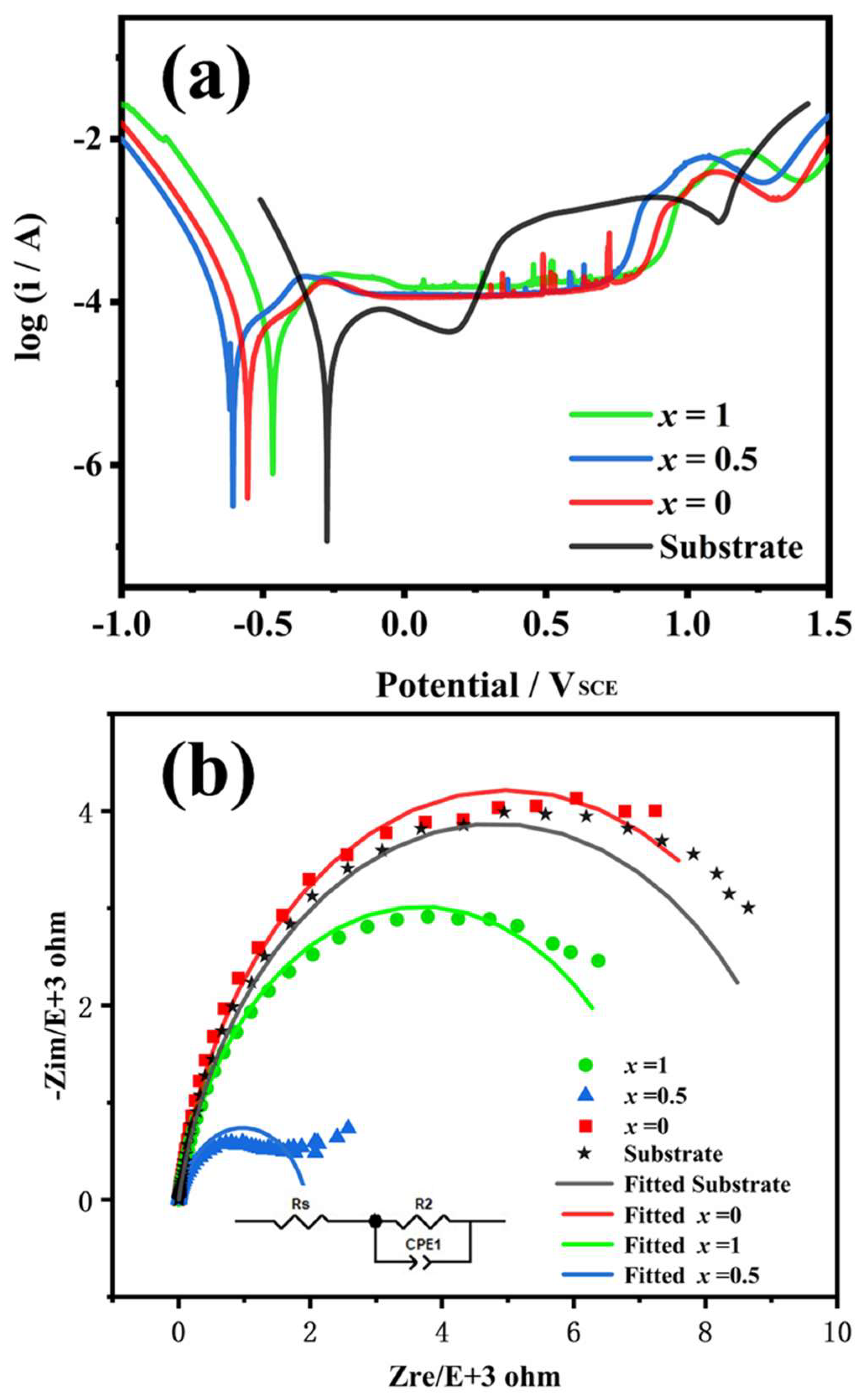

| Electrochemical Parameters | X = 0 | X = 0.5 | X = 1 | Substrate |
|---|---|---|---|---|
| Ecorr/mVSCE | −553.7 | −605.0 | −468.8 | −273.0 |
| Icorr/μA cm−2 | 31.41 | 35.65 | 44.77 | 33.34 |
| Epit/mVSCE | 838.8 | 756.7 | 898.6 | 170.8 |
| Epit − Ecorr/mVSCE−2 | 1392.5 | 1361.7 | 1367.4 | 443.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, S.; Guo, Y.; Liu, Q. [(AlxTi1−x)-(FeCoNi)12](AlxTi1−x)0.5Cr2.5 High-Entropy Alloy Coating by Laser Cladding. Metals 2022, 12, 740. https://doi.org/10.3390/met12050740
Bo S, Guo Y, Liu Q. [(AlxTi1−x)-(FeCoNi)12](AlxTi1−x)0.5Cr2.5 High-Entropy Alloy Coating by Laser Cladding. Metals. 2022; 12(5):740. https://doi.org/10.3390/met12050740
Chicago/Turabian StyleBo, Shenghong, Yaxiong Guo, and Qibin Liu. 2022. "[(AlxTi1−x)-(FeCoNi)12](AlxTi1−x)0.5Cr2.5 High-Entropy Alloy Coating by Laser Cladding" Metals 12, no. 5: 740. https://doi.org/10.3390/met12050740






