Optimization of Iron Recovery from BOF Slag by Oxidation and Magnetic Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Methods
3. Results and Discussion
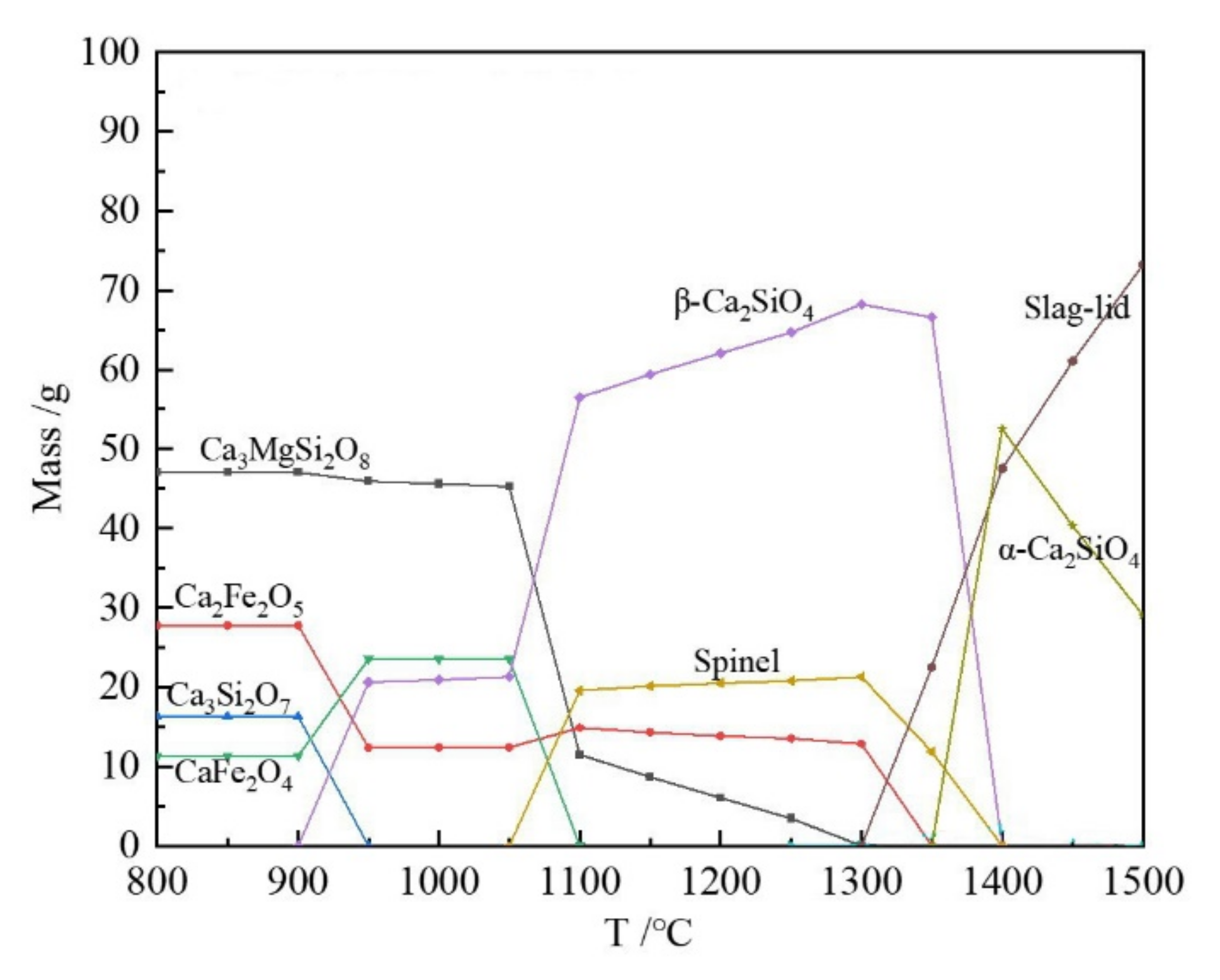
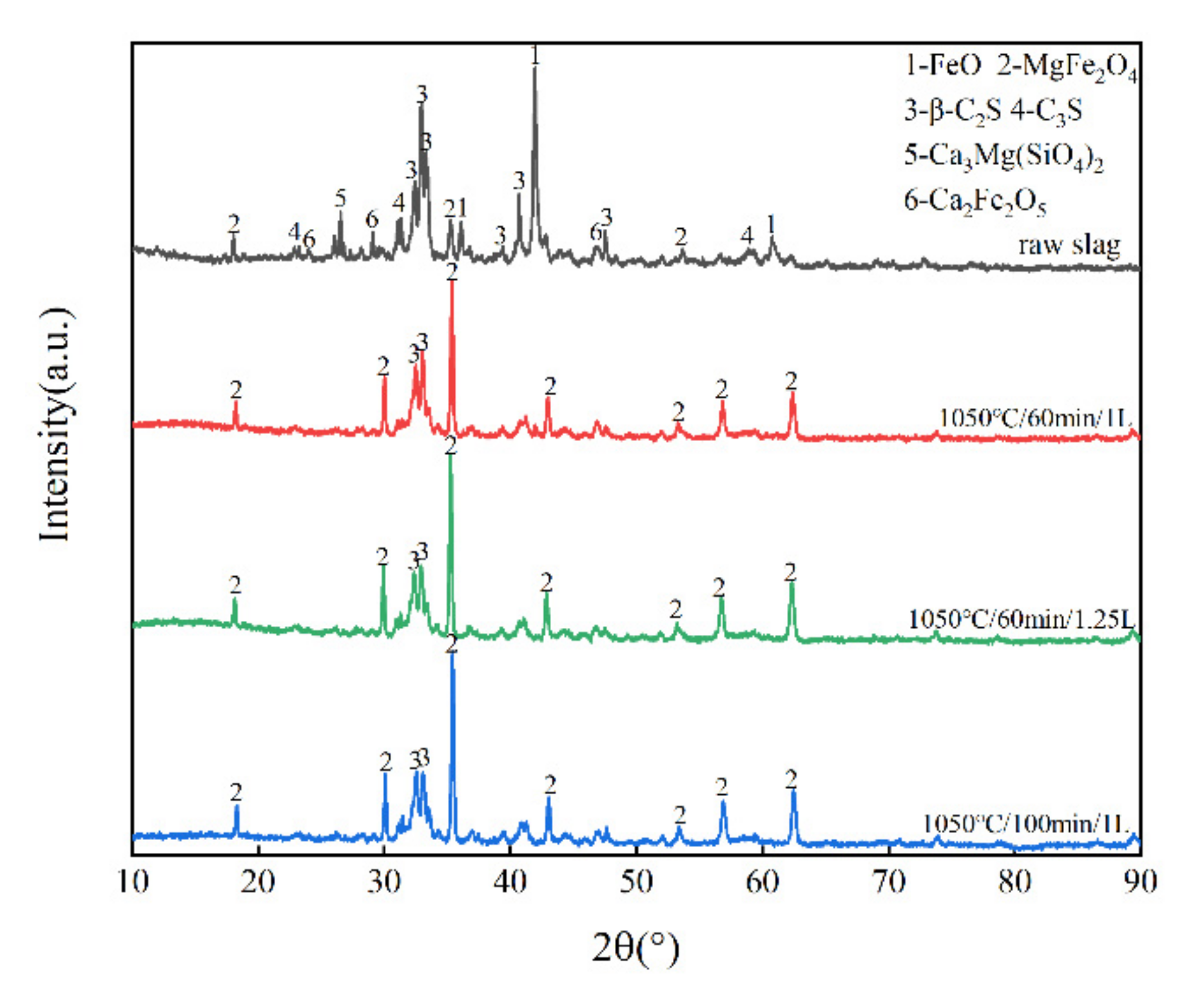
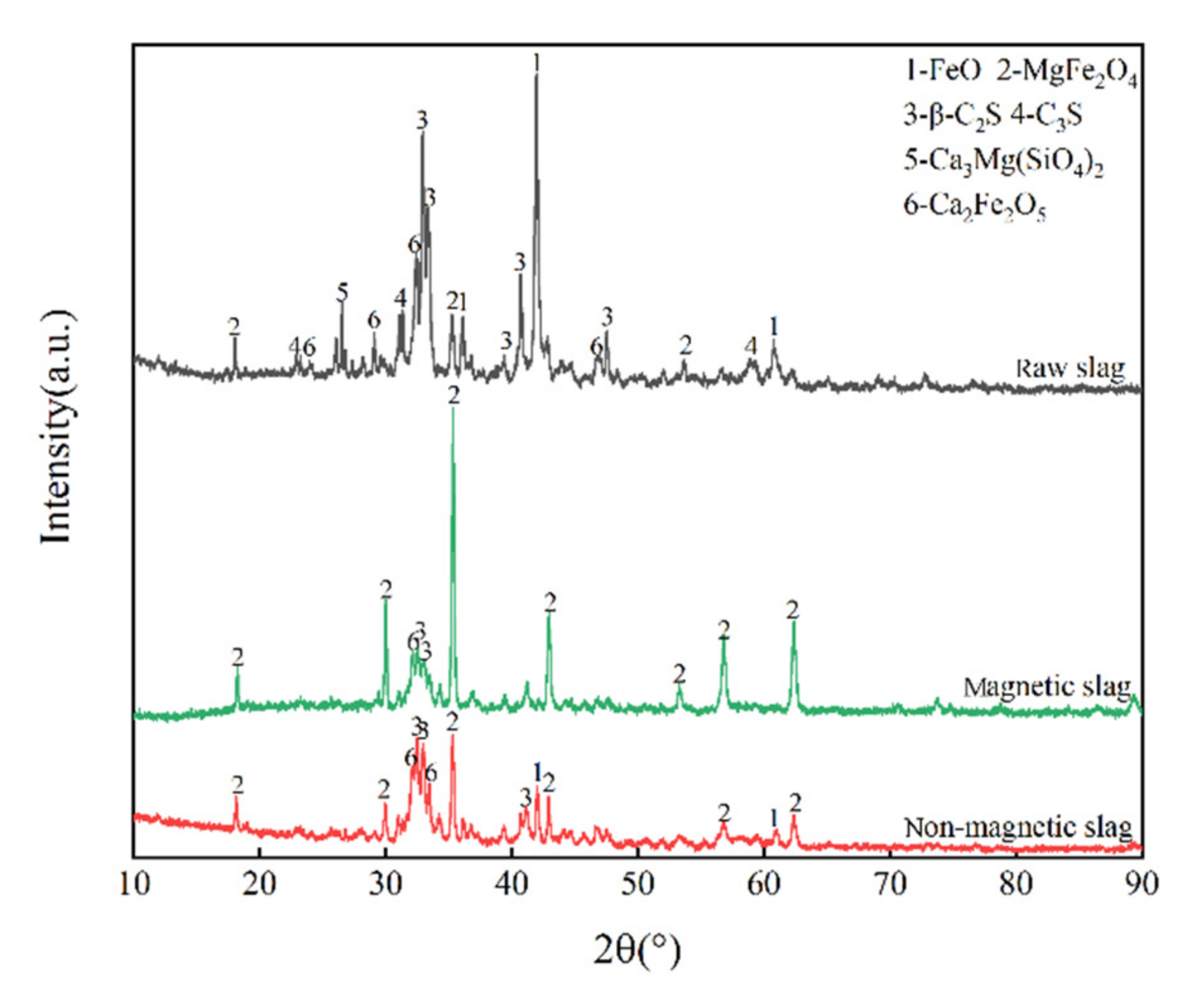
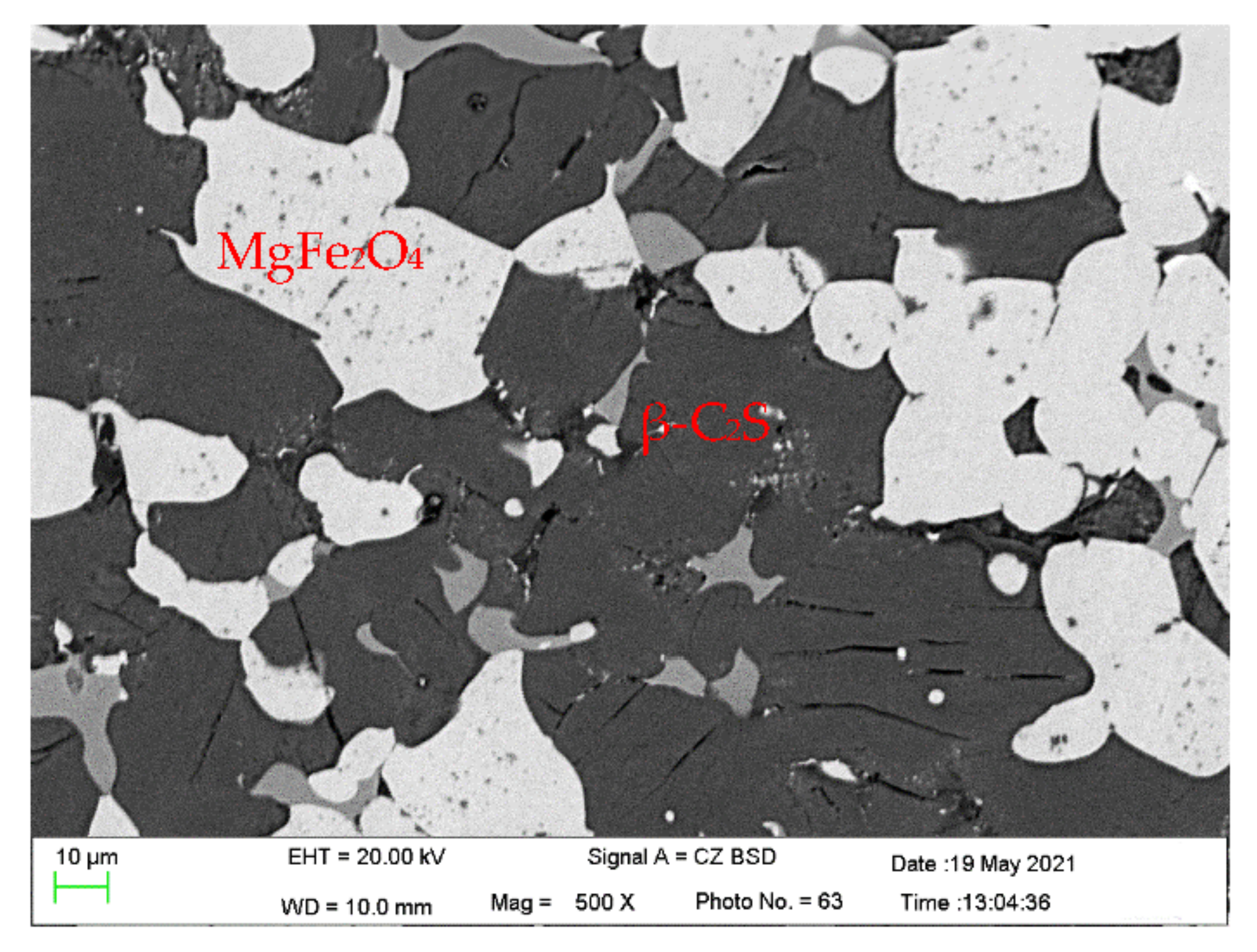
3.1. Phase Composition of Raw Slag, Oxide Slag and Magnetic Separation Slag
3.2. The Influence of Oxidation Temperature on the Effect of Magnetic Separation
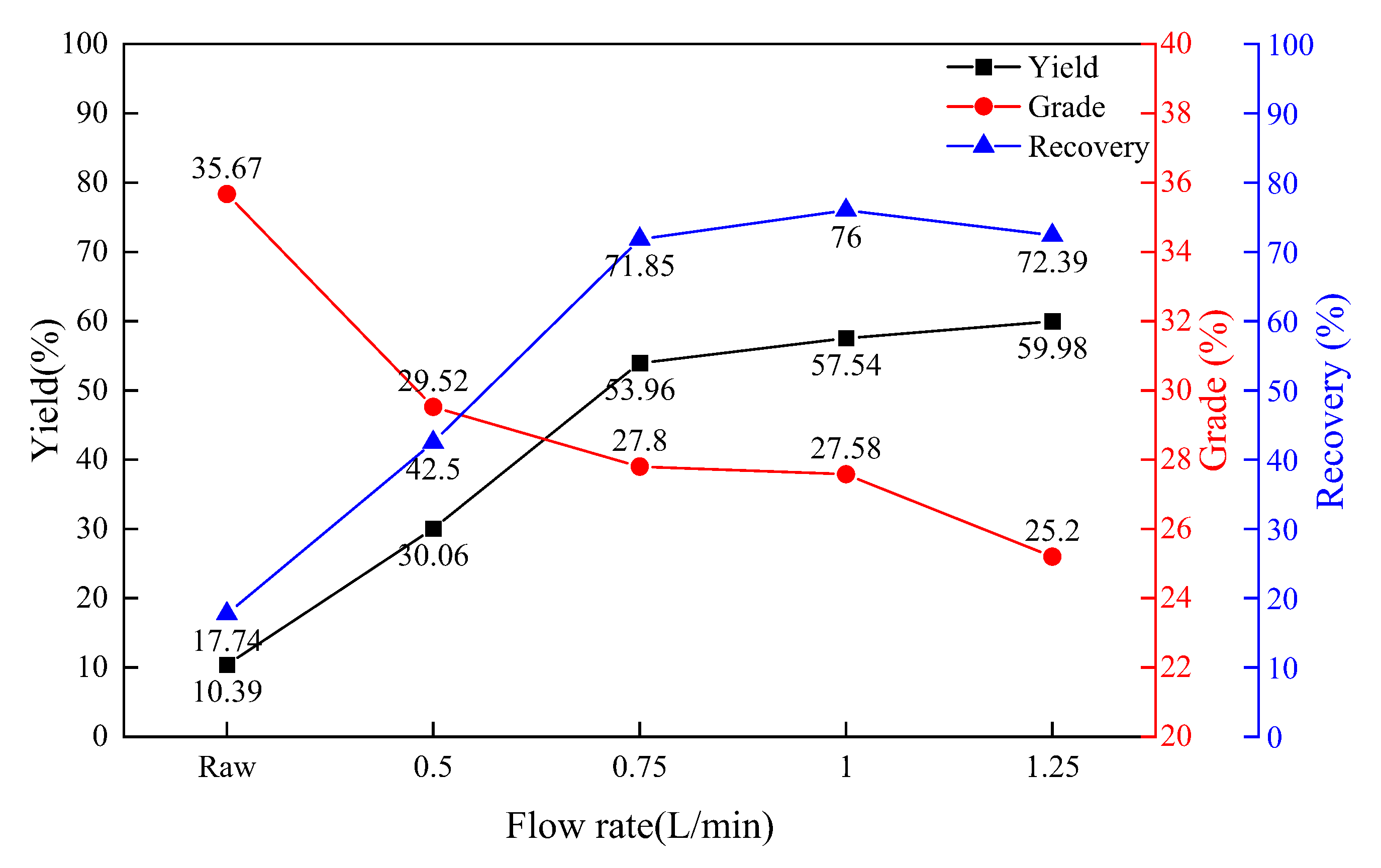
3.3. The Influence of Gas Flow Rate on the Effect of Magnetic Separation
3.4. The Influence of Oxidation Time on the Effect of Magnetic Separation
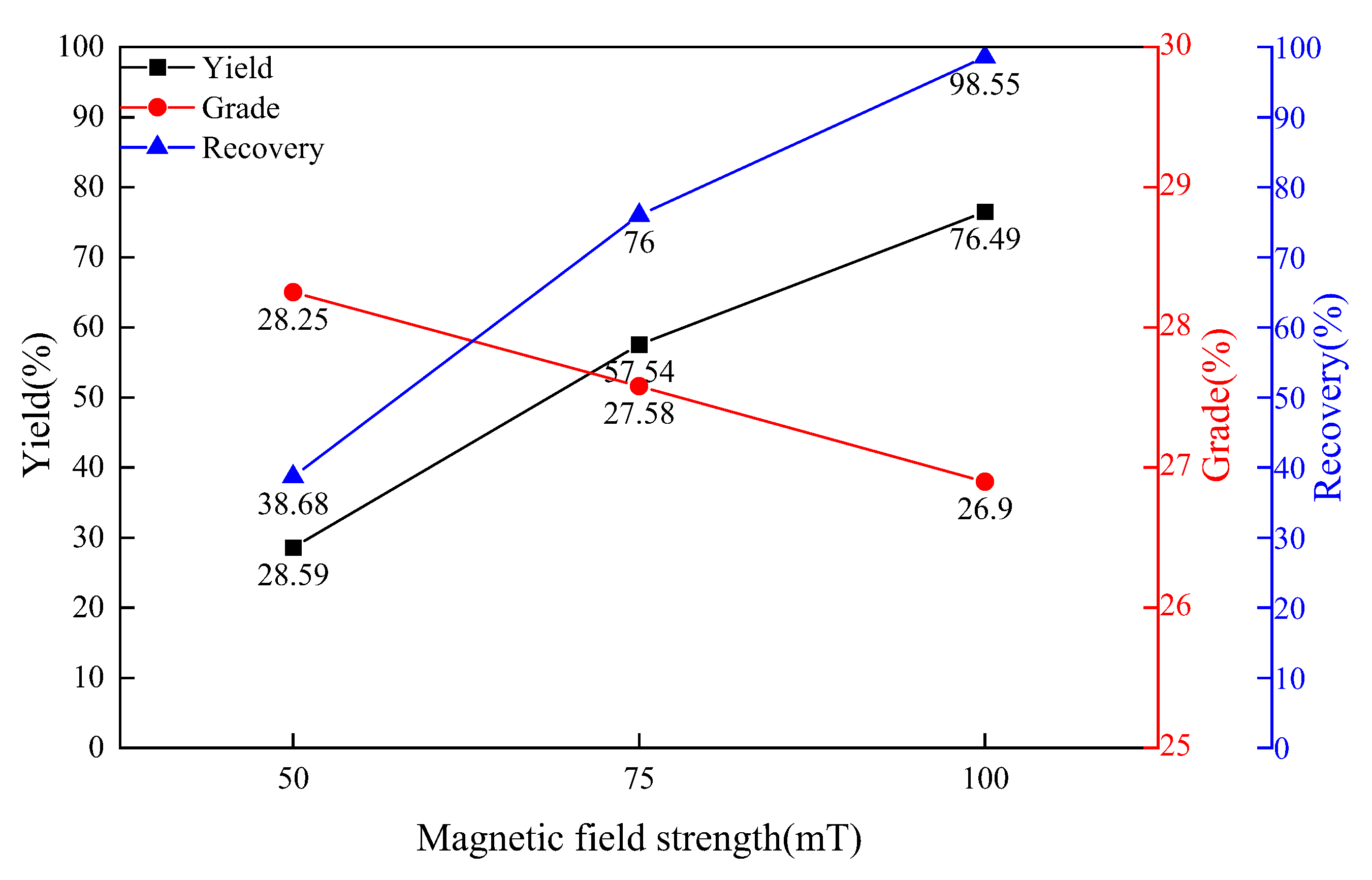
3.5. The Influence of Magnetic Field Strength on the Effect of Magnetic Separation
4. Conclusions
- (1)
- Through oxidation treatment in an air atmosphere, and under suitable conditions of reaction temperature, time and air flow rate, the magnetic iron oxides in the steel slag can be transformed into ferromagnetic magnesium-iron spinel;
- (2)
- When the reaction temperature is controlled between 1050 and 1100 °C, the oxidation time is controlled between 40 and 60 min, the air flow rate is controlled at 0.75–1 L/min and the magnetic field strength is 75 mT, the yield of modified BOF slag is 46–57.54%, the iron grade can reach 27.58–29.10%, and the iron recovery can reach 64.12–76.00%, which is the best process parameter range for the magnetic separation experiment;
- (3)
- The magnetic field strength has a great influence on the results of magnetic separation. The effect is best when the magnetic separation strength is 75 mT. After 75 mT, the magnetic separation yield will rise sharply, the grade of the concentrate will decrease, and the magnetic separation effect does not change significantly with the increase of the magnetic field strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, K.; Wang, L.; Li, X. Review of the energy consumption and production structure of China’s steel industry: Current situation and future development. Metals 2020, 10, 302. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Trofimov, E.; Chumanov, I.; Dildin, A.; Samoylova, O. On expediency of the preliminary heat treatment for liquid-phase reduction of waste steelmaking slag. Am. J. Appl. Sci. 2015, 12, 952–961. [Google Scholar] [CrossRef]
- Che Ghani, S.A.; Lim, J.W.; Chew, L.H.; Choong, T.S.Y.; Tezara, C.; Yazdi, M.H.; Alias, A.; Mamat, R.; Rahman, M.M. Overview of steel slag application and utilization. MATEC Web Conf. 2016, 74, 26. [Google Scholar]
- Semykina, A.; Shatokha, V.; Iwase, M.; Seetharaman, S. Kinetics of oxidation of divalent iron to trivalent state in liquid FeO-CaO-SiO2 slags. Metall. Mater. Trans. B 2010, 41, 1230–1239. [Google Scholar] [CrossRef]
- Semykina, A. The kinetics of oxidation of liquid FeO-MnO-CaO-SiO2 slags in air. Metall. Mater. Trans. B 2011, 43, 56–63. [Google Scholar] [CrossRef]
- Li, P.; Guo, H.; Gao, J.; Min, J.; Yan, B.; Chen, D.; Seetharaman, S. Novel concept of steam modification towards energy and iron recovery from steel slag: Oxidation mechanism and process evaluation. J. Clean. Prod. 2020, 254, 119952. [Google Scholar] [CrossRef]
- Xue, P.; He, D.; Xu, A.; Gu, Z.; Yang, Q.; Engström, F.; Björkman, B. Modification of industrial BOF slag: Formation of MgFe2O4 and recycling of iron. J. Alloys Compd. 2017, 712, 640–648. [Google Scholar] [CrossRef]
- He, Z.; Lan, M.; Hu, X.; Xue, X.; Chou, K.-C. Study on oxidation behavior of industrial basic oxygen furnace slag and recovery of magnetic iron-containing phase. Steel Res. Int. 2022, 92, 2100558. [Google Scholar] [CrossRef]
- Calmon, J.L.; Tristão, F.A.; Giacometti, M.; Meneguelli, M.; Moratti, M.; Teixeira, J.E.S.L. Effects of BOF steel slag and other cementitious materials on the rheological properties of self-compacting cement pastes. Constr. Build Mater. 2013, 40, 1046–1053. [Google Scholar] [CrossRef]
- Rashad, A.M. A synopsis manual about recycling steel slag as a cementitious material. J. Mater. Res. Technol. 2019, 8, 4940–4955. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Y.; Geng, R.; Zhu, T.; Li, B. Simultaneous removal of SO2 and NOx using steel slag slurry combined with ozone oxidation. ACS Omega 2021, 6, 28804–28812. [Google Scholar] [CrossRef] [PubMed]
- Dhoble, Y.N.; Ahmed, S. Review on the innovative uses of steel slag for waste minimization. J. Mater. Cycles Waste Manag. 2018, 20, 1373–1382. [Google Scholar] [CrossRef]
- Iizuka, A.; Yamasaki, A.; Yanagisawa, Y. Desulfurization performance of sorbent derived from waste concrete. J. Chem. Eng. Jpn. 2011, 44, 746–749. [Google Scholar] [CrossRef]
- Blackman, L.F.C. On the formation of Fe2+ in the system MgO-Fe2O3-MgFe2O4 at high temperatures. J. Am. Ceram. Soc. 1959, 42, 143–145. [Google Scholar] [CrossRef]
- Phillips, B.; Muan, A. Phase equilibria in the system CaO-iron oxide in air and at 1 atm. O2 pressure. J. Am. Ceram. Soc. 1958, 41, 445–454. [Google Scholar] [CrossRef]
- Yadav, U.S.; Pandey, B.D.; Das, B.K.; Jena, D.N. Influence of magnesia on sintering characteristics of iron ore. Ironmak. Steelmak. 2013, 29, 91–95. [Google Scholar] [CrossRef]
- Shu, Q.F.; Liu, Y. Effects of basicity, MgO and MnO on mineralogical phases of CaO–FeOx–SiO2–P2O5 slag. Ironmak. Steelmak. 2017, 45, 363–370. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Annersten, H.; Virgo, D. The temperature dependence of the cation distribution in magnesioferrite (MgFe2O4) from powder XRD structural refinements and Mössbauer spectroscopy. Am. Mineral. 1992, 77, 725–740. [Google Scholar]


| Composition | CaO | SiO2 | MgO | Al2O3 | MnO | P2O5 | TiO2 | FeO | TFe |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt %) | 41.40 | 16.80 | 6.53 | 4.87 | 3.76 | 1.91 | 1.43 | 20.60 | 20.88 |
| Procedure | Temp. (°C) | Time (min) | Flow Rate (L/min) | Magnetic Field (mT) | |
|---|---|---|---|---|---|
| Temp. (°C) | 950 | - | 60 | 1 | |
| 1000 | |||||
| 1050 | 50, 75, 100 | ||||
| 1100 | |||||
| Time (min) | 20 | 1050 | - | 1 | |
| 40 | |||||
| 60 | 50, 75, 100 | ||||
| 100 | |||||
| Flow rate (L/min) | 0.5 | 1050 | 60 | - | |
| 0.75 | |||||
| 1 | 50, 75, 100 | ||||
| 1.25 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, M.; He, Z.; Hu, X. Optimization of Iron Recovery from BOF Slag by Oxidation and Magnetic Separation. Metals 2022, 12, 742. https://doi.org/10.3390/met12050742
Lan M, He Z, Hu X. Optimization of Iron Recovery from BOF Slag by Oxidation and Magnetic Separation. Metals. 2022; 12(5):742. https://doi.org/10.3390/met12050742
Chicago/Turabian StyleLan, Mo, Zhanwei He, and Xiaojun Hu. 2022. "Optimization of Iron Recovery from BOF Slag by Oxidation and Magnetic Separation" Metals 12, no. 5: 742. https://doi.org/10.3390/met12050742
APA StyleLan, M., He, Z., & Hu, X. (2022). Optimization of Iron Recovery from BOF Slag by Oxidation and Magnetic Separation. Metals, 12(5), 742. https://doi.org/10.3390/met12050742






