Abstract
A dispersive solid-phase microextraction (DSPME) sorbent consisting of poly(1,6-hexanediol diacrylate)-based polymer microspheres, with embedded graphene microparticles (poly(HDDA)/graphene), was synthesized by microfluidic emulsification/photopolymerization and characterized by optical microscopy and X-ray fluorescence spectrometry. This sorbent was applied for simple, fast, and sensitive vortex-assisted DSPME of rare earth elements (RREs) in coal fly ash (CFA) leachate, prior to their quantification by inductively coupled plasma mass spectrometry (ICP-MS). Among nine DSPME variables, the Plackett–Burman screening design (PBD), followed by the central composite optimization design (CCD) using the Derringer desirability function (D), identified the eluent type as the most influencing DSPME variable. The optimum conditions with maximum D (0.65) for the chelating agent di-(2-ethylhexyl) phosphoric acid (D2EHPA) amount, the sorbent amount, the eluting solvent, the extraction temperature, the centrifuge speed, the vortexing time, the elution time, the centrifugation time, and pH, were set to 60 μL, 30 mg, 2 M HNO3, 25 °C, 6000 rpm, 1 min, 1 min, 5 min, and 4.2, respectively. Analytical validation of the DSPME method for 16 REEs (Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) in CFA leachate samples estimated the detection limits at the low ppt level, the recovery range 43–112%, and relative standard deviation within ± 22%. This method was applied to a water extraction procedure (EP) and acetic acid toxicity characteristic leaching procedure (TCLP) for leachate of CFA, from five different coal-fired thermoelectric power plants. The most abundant REEs in leachate (20 ÷ 1 solid-to-liquid ratio) are Ce, Y, and La, which were found in the range of 22–194 ng/L, 35–105 ng/L, 48–95 ng/L, and 9.6–51 μg/L, 7.3–22 μg/L, 2.4–17 μg/L, for EP and TCLP leachate, respectively. The least present REE in TCLP leachate was Lu (42–125 ng/L), which was not detected in EP leachate.
1. Introduction
Rare earth elements (REEs) have recently gained an important role in various applications in hi-tech devices, specific catalysts, superconductors, telecommunications, laser technologies, etc. [1,2]. They are quite valuable due to their high conductivity and magnetism, which enable various engineering solutions.
In addition to ores, waste materials and by-products are increasingly being considered alternative sources for obtaining REEs [3,4]. Coal fly ash (CFA) is a promising source of REEs, whose potential as a source of REEs is being intensively studied [5]. REEs are found in CFA in various forms [6], so their recovery is very complex [7]. The major phase components of the CFA are quartz, mullite, hematite and amorphous glass. The rare earth elements are captured in this structure [8,9]. Therefore, the extraction of rare earth elements is very difficult.
The technology of obtaining REEs from CFA consists of several stages: mechanical grinding, magnetic separation, leaching, extraction, and refining [10]. Alkali-acid leaching is a common practice for conventional REEs recovery, while the chelating solvent extraction is usually used to separate REEs from CFA leachate. Finally, the refining stage involves electrolysis, zone melting, etc.
An important part of the REEs production from CFA is monitoring wastewater originating from CFA leaching, from both the recovery process and landfill. Two widely accepted standardized leaching testing procedures are the EN-12457-2 aqueous extraction procedure (EP) and US EPA 1311 toxicity characteristic leaching procedure (TCLP) [11].
The quantification of REEs in CFA leachate is quite challenging, consisting of several steps. Instrumental measurements for determining REEs somewhat converge towards ICP-MS [12,13]. However, even if ICP-MS is a powerful technique, it suffers from being not sensitive enough for some REEs present at a very low level [14,15] or interferences from high-matrix aqueous samples [16]. Therefore, the separation and preconcentration of REEs from a matrix solution are usually needed prior to an instrumental ICP-MS measurement [17].
Traditional methods for separating REEs include liquid–liquid extraction, ion-exchange, co-precipitation, and dry digestion [18,19,20,21]. Even if there have been tremendous advances in developing new solvents [22,23] and hybrid sorbents [24,25] for trace elements separations, these methods are somehow inconvenient, such as being time consuming, quite expensive, and not environmentally friendly [26].
One of the popular directions of research into improving the method of sample preparation is the introduction of microextraction in the field of analytical determination of trace elements [27,28]. Several microextraction sample preparation and preconcentration techniques for REEs prior to the instrumental measurements by ICP-OES and ICP-MS [29,30] have been investigated. REEs were subjected to preconcentration from groundwater by dispersive liquid–liquid microextraction (DLLME) followed by ICP-MS [31]. A few studies dealt with dispersive solid-phase microextraction (DSPME) of REEs, in which ICP-MS quantification was performed [16,32].
In most cases, the optimization of the microextraction procedure was conducted with the traditional “one-variable-at-a-time” (OVAT) approach. OVAT is an optimization technique in which one variable is changed while keeping all other variables constant. A more advanced chemometric approach using the design of experiments (DOE) enables optimization by changing all variables simultaneously. In addition to identifying the critical variables, it can also be used to achieve the desired response.
Although the chemometric optimization has been applied to the simultaneous preconcentration of several metals by microextraction prior to ICP-OES [33,34], only one study [16] has undertaken the optimization of DSPME of REEs in drinking water by using response surface methods. The chemometric approach can also be applied as a two-step optimization, consisting of a screening design followed by the response surface methodology. All cited works dealt with water samples or diluted aqueous solutions. However, microextraction from high-matrix CFA leachate could be much more difficult.
In this work, synthesized poly(HDDA)/graphene monodispersed particles were used as the sorbent in DSPME for the REEs separation from CFA leachate prior to their analysis by ICP-MS. Furthermore, since many variables in a DSPME process exist, a chemometric optimization of the experimental DSPME variables was conducted.
2. Materials and Methods
2.1. Chemicals and Reagents
The REEs’ analytical standards were prepared from a mixed multi-element ICP-MS standard PE-MECAL2-ASL-1 (Accustandard Inc., New Haven, CT, USA) containing 10 μg/mL each of all REEs. This solution was also used to make the spiked samples. Single element stocks from Merck Co. (Darmstadt, Germany) for the elements Si, Al, Fe, Ca, Na, and Cl, were added to the spiked samples to give higher concentration levels for these major elements, similar to the CFA leachate matrix. The internal standard (ISTD) solution ICP-MS-IS-IN-1 (Accustandard Inc., New Haven, CT, USA) containing 115In was used to control the instrument stability. Deionized Milli-Q water (Millipore, Burlington, USA) was used to prepare all solutions. All other chemicals used were purchased from Merck Co. (Darmstadt, Germany). TCLP extraction fluid consisted of 5.7 mL/L glacial acetic acid. Di-2-ethylhexylphosphoric acid (D2EHPA) was diluted to a concentration of 10% (v/v) in hexane. Composite polymer/graphene microspheres were produced using Darocur 1173 (2-hydroxy-2-methylpropiophenone) as a photo initiator, HDDA (1,6 hexanediol diacrylate) as a UV-curable monomer, and graphene oxide as a nanofiller used to increase the adsorption capacity of the particles, all from Sigma-Aldrich (Gillingham, UK).
2.2. Leaching of CFA Samples
A portion of 1.0 g CFA sample was mixed with a volume (20 mL) of EP or TCLP leaching fluid in a polyethylene bottle and rotary agitated at room temperature for 24 h. Then, the leachate was centrifuged at 6000 rpm for 10 min, and the supernatant was decanted. The decanted leachate was used for DSPME experiments. Since CFA is an alkaline solid, an unbuffered acetic acid (pH = 2.88) was used as the TCLP extraction fluid. Deionized water (18.2 MΩ∙cm) was used to make the EP leachate.
2.3. Synthesis of Poly(HDDA)/Graphene
Graphene-embedded polymer microspheres were fabricated in a two-phase glass capillary microfluidic device. Emulsion droplets were first produced, followed by on-the-fly photopolymerization to solidify the droplets and form poly(HDDA)/graphene microspheres. The procedure of fabricating the microsphere used in this work is described in detail elsewhere [35]. Morphological investigation of these particles was performed by OMAX (Kent, WA, USA) model OM349P polarizing microscope, while chemical purity was checked by a Thermo Niton XL3t Goldd+ X-ray fluorescence spectrometer (Thermo Fisher Scientific, Waltham, MA, USA).
2.4. Factorial Design of DSPME
A Thermo mode Orion 3 pH-meter (Thermo Fisher Scientific, Chelmsford, MA, USA), Radwag analytical microbalance model MYA 5-3Y (Radwag, Radom, Poland), Centrifuge model LACE16 (Colo lab Expert, Novo Mesto, Slovenia), Lauda model RM-6 water bath (Lauda-Brinkmann, Delran, NJ, USA), and Vortex model IKA MS2 (IKA-Werke, Staufen, Germany) were used in the DSPME experiments.
The following DSPME procedure was used: 25 mL of a spiked sample or CFA leachate was taken in a 50 mL centrifuge tube, and its pH was adjusted with HNO3 or NaOH. Then, an accurately weighted mass of poly(HDDA)/graphene sorbent and a volume of D2EHPA solution were added to form a chelating complex with REEs in the solution. Then, an emulsion was produced by vortexation. Next, the DSPME sorbent containing chelated REEs complexes was separated using centrifugation. Afterwards, nitric acid was added to the residue to release REEs. The final volume was made up to 2.5 mL with deionized water and further diluted prior to ICP-MS measurement. The experimental DSPME variables that were optimized are listed in Table 1.

Table 1.
The variables and their coded values (−1, +1) for the Plackett–Burman design.
2.5. ICP-MS Measurements
A Thermo Scientific ICP-MS instrument (Thermo Fisher Scientific, Waltham, MA, USA) model iCAP Q, equipped with a Cetac ASX-520 autosampler and controlled via Qtegra software was used in this work to measure the content of REEs. The sample introduction system includes a standard Peltier-cooled quartz vortex spray chamber, PFA nebulizer with removable quartz rectangular central tube (0.25 mm id) and standard nickel sampling and skimmer cones. The instrument runs in single kinetic energy discrimination (KED) collision cell mode, using pure helium as the collision gas.
Table 2 presents the ICP-MS instrument parameters and isotopes with potentially interfering masses for each REE.

Table 2.
ICP-MS instrument setup and isotopes (interference) of each REE.
A mixed-matrix-matched ICP-MS standard solution of 16 REEs was diluted with 1% nitric acid to a concentration of 0.1 ng/L to 50 μg/L. Each standard solution was spiked to contain 10 mg/L Si, 5.0 mg/L Al, 2.0 mg/L Fe, and 1.0 mg/L Ca. These standard spiked solutions were used to test the linearity and recovery.
3. Results and Discussion
3.1. Characterization of Poly(HDDA)/Graphene Particles
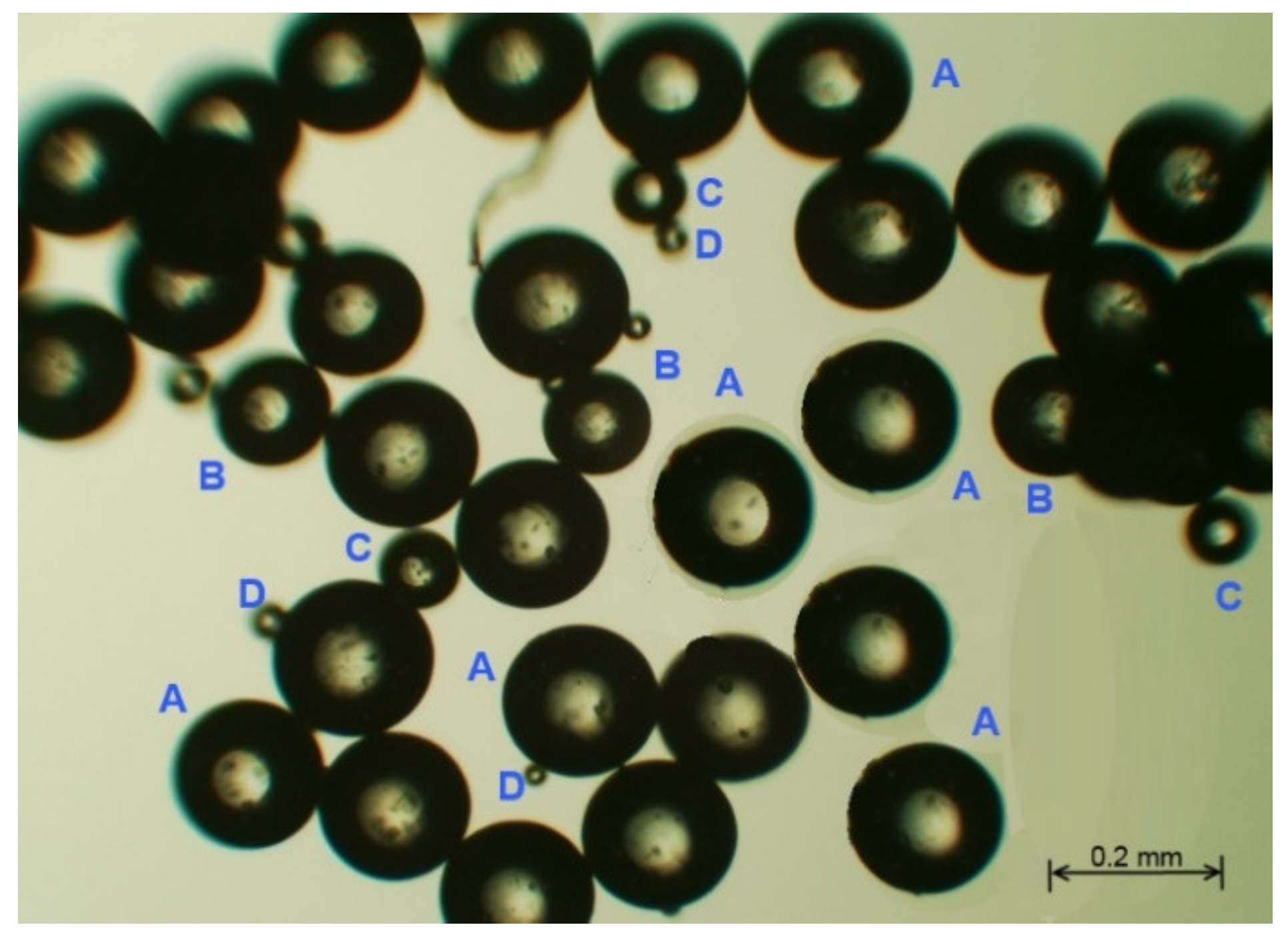
Optical microscopy measurements clearly indicated spherical poly(HDDA)/graphene particles (Figure 1). Four groups of particles with different diameters (A, B, C, D) are identified. It is also noticeable that there is an agglomeration of particles, in which a single particle is attracted to a neighboring one. This property is beneficial in the DSPME process, in which an aqueous solution is to be separated from particles.
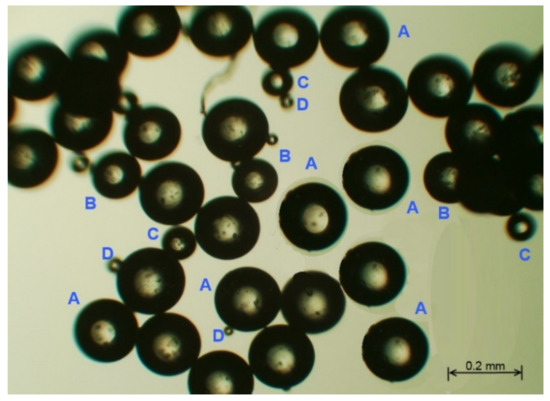
Figure 1.
Optical microscopy image of the fabricated poly(HDDA)/graphene microspheres.
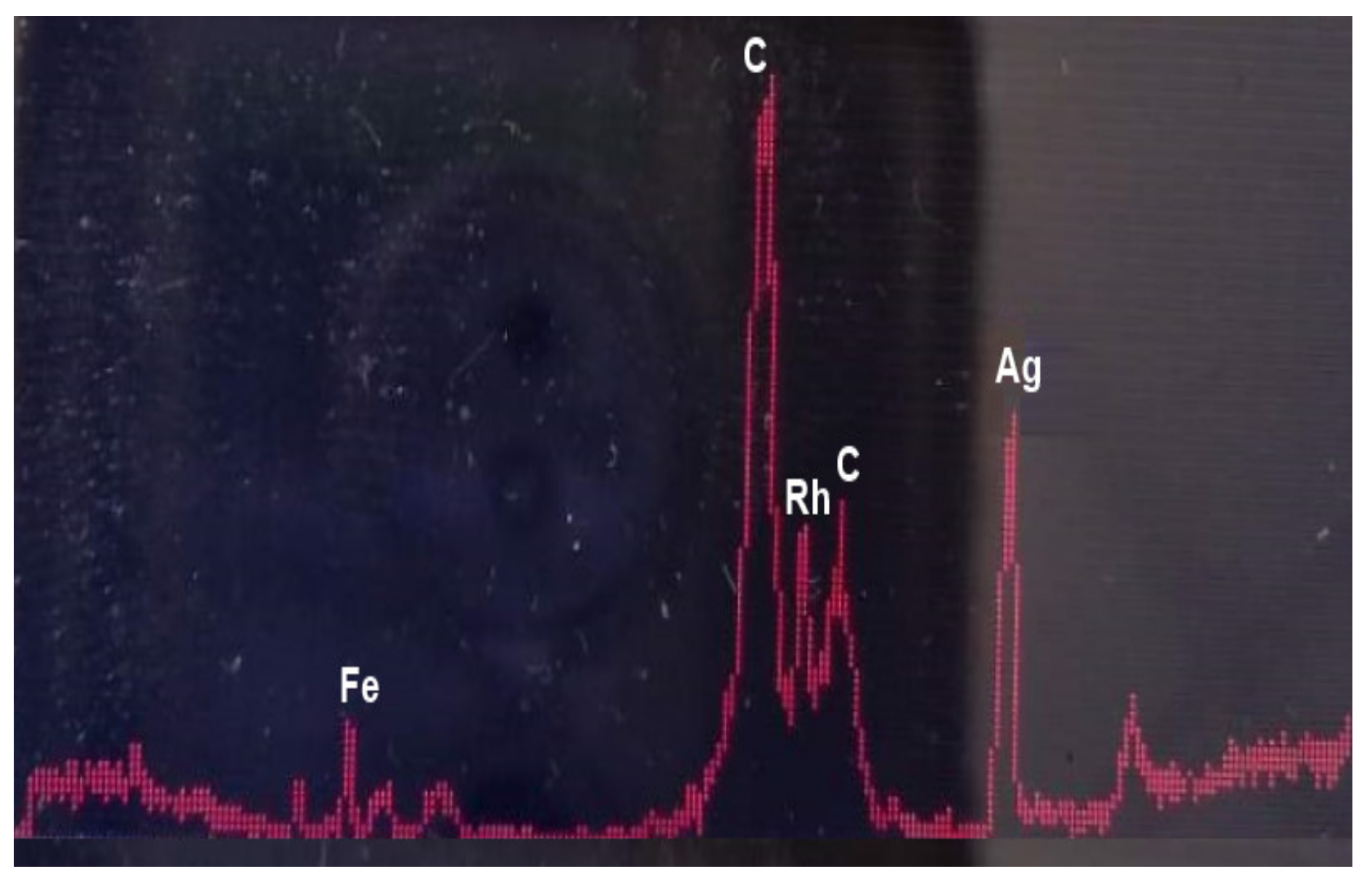
A critical characteristic of a DSPME sorbent is chemical purity. In particular, the absence of trace elements in DSPME sorbent is a must when determining trace elements. Thus, the presence of elements in the sorbent, even if they are not analytes, can lead to various isobaric and polyatomic interferences in an ICP-MS measurement. Therefore, the DSPME sorbent used was checked for the presence of trace elements by x-ray fluorescence spectrometry (XRF) before use. Figure 2 shows an XRF spectrum of poly(HDDA)/graphene particles. It is obvious that no metal elements were detected. Just to note, the peaks in the spectrum belong to the instrumental blank.
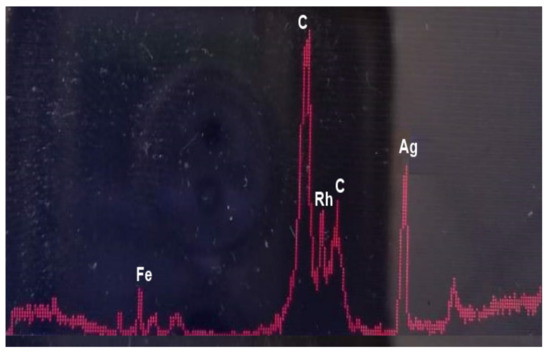
Figure 2.
EDXRF spectrum of poly(HDDA)/graphene microspheres (C—Compton peaks; Rh—Kα + Kβ Rayleigh).
3.2. Factorial Optimization of DSPME
3.2.1. Plackett–Burman Screening
PBD design was used to screen nine independent variables. The Derringer [36] desirability function (D) derived from recoveries was used as a response variable. D is obtained from individual desirabilities, i.e., recoveries, using the geometric mean and is calculated according to the following equation:
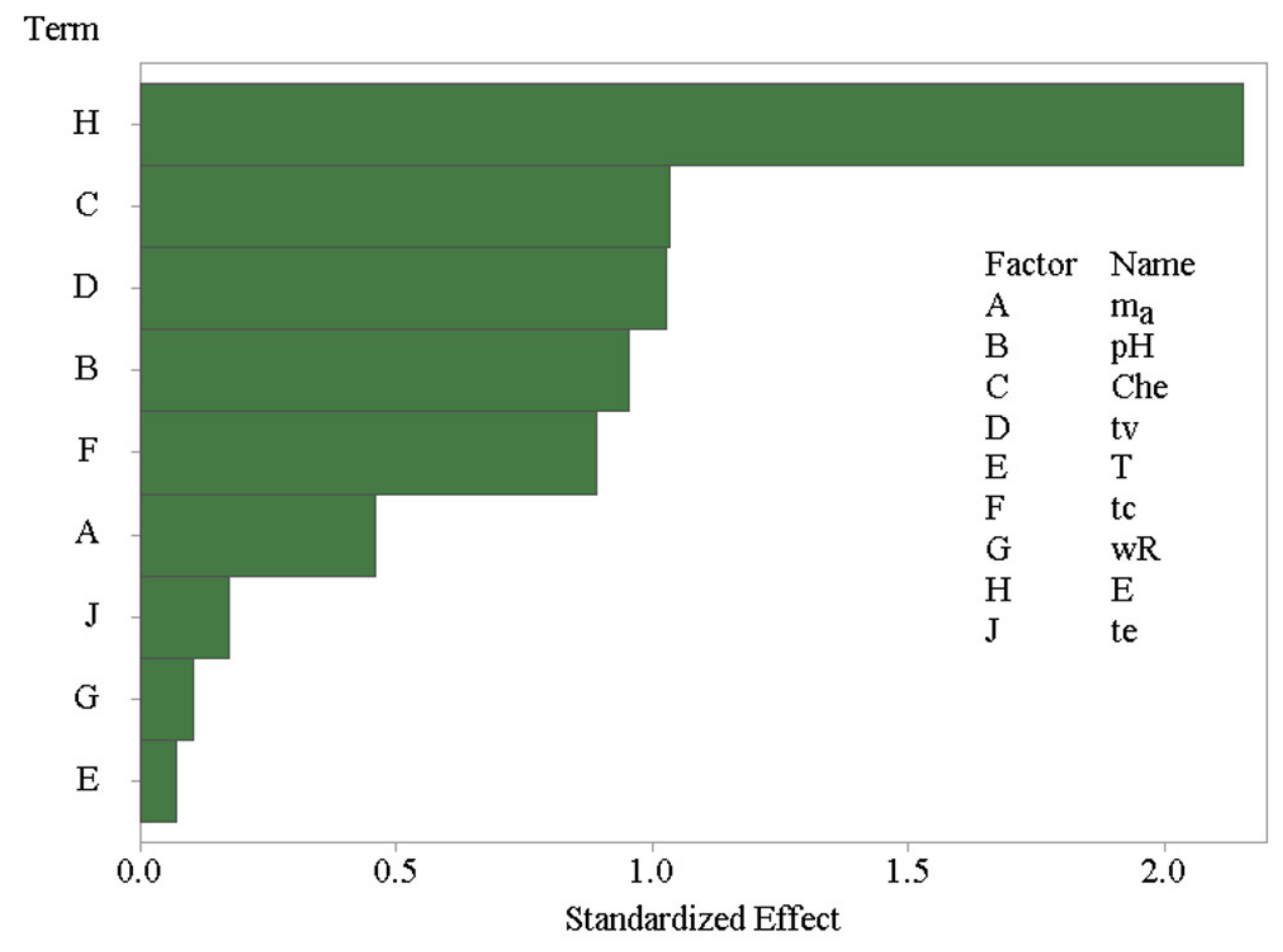
where di represents individual desirabilities, n is the number of REEs, and ri is the coefficient of the importance of the variable compared to other variables. This coefficient can vary, but in this case, it was assumed that all coefficients are of equal importance, so that no weights were assigned to different REEs. The result of the PBD design analysis is shown in the form of a Pareto plot in Figure 3.
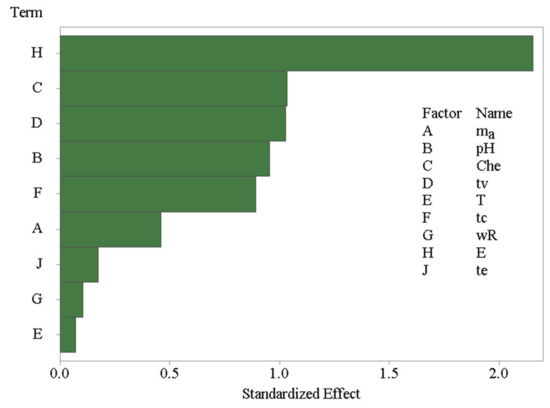
Figure 3.
Pareto plot for the Plackett–Burman screening experiments.
It is obvious that the eluting solvent used to extract REEs from the DSPME sorbent was the most influencing variable. The addition of organic solvents, such as methanol or acetone, was found to have a strong negative effect on the REEs recoveries. The variation in the amount of D2EHPA chelating agent, the vortexation time, pH, and centrifugation time were also found to be significant variables. An increase in the D2EHPA amount and pH negatively affect the DSPME process, while the decrease has an opposite effect. The remaining variables are negligible. Thus, the poly(HDDA)/graphene amount, the extraction temperature, and the centrifuge speed were set to their middle values in the experimental domain of 30 mg, 25 °C, and 6000 rpm, respectively. Vortexing and elution time were minimized to 1 min, but centrifugation time was set to the maximum (5 min). Two variables, pH and the D2EHPA amount, were selected for the subsequent step in the DSPME optimization by response surface methodology.
3.2.2. Central Composite Design Optimization
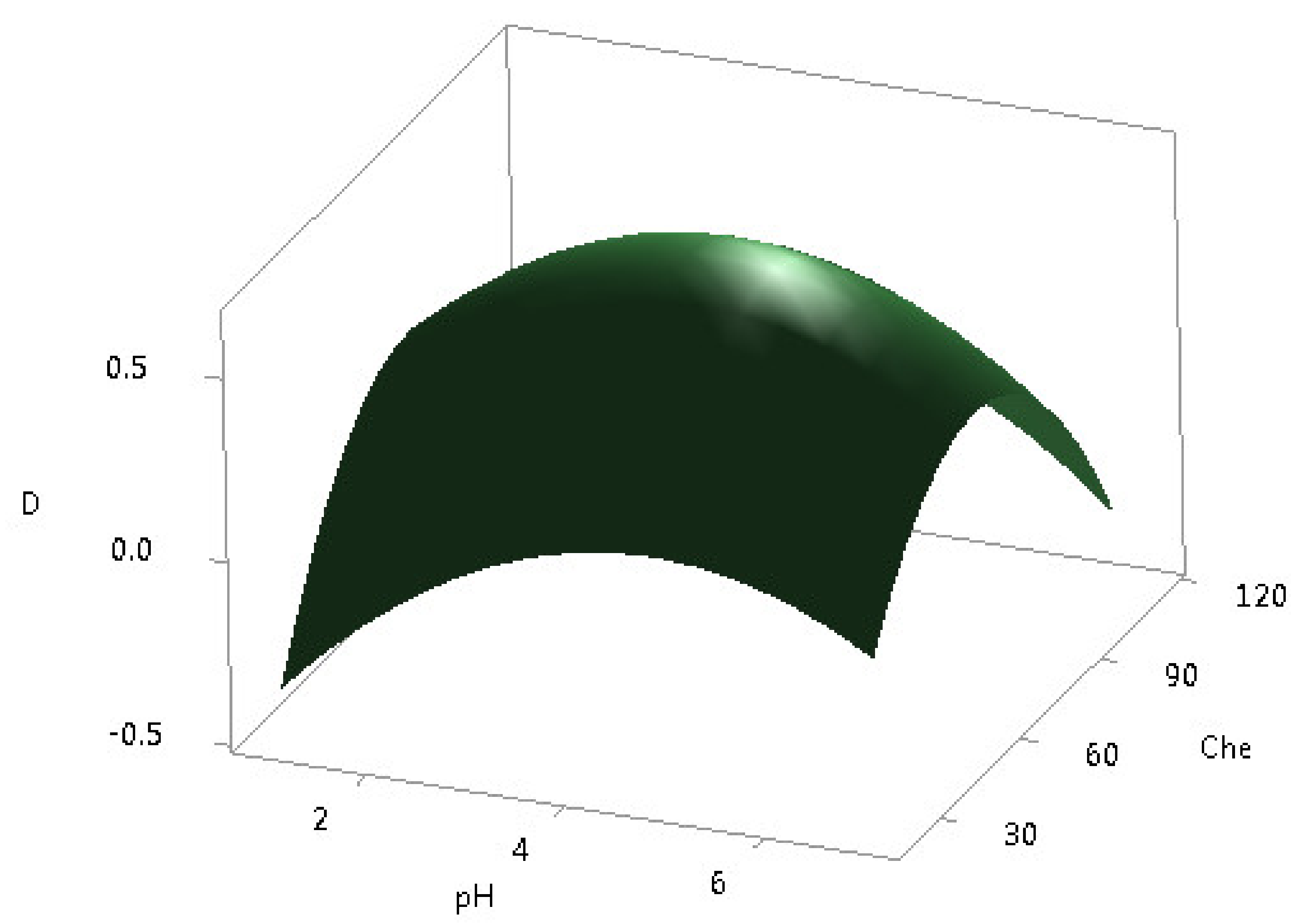
According to the CCD experiments, 13 runs were carried out, and the results of the response procedures in five different levels of the two independent variables are summarized. The effect of the amount of chelating agent D2EHPA, in the range of 20–110 μL and the pH values from 1.0 to 6.0, was investigated by unblocked CCD with axial points. Derringer aggregate response for all 16 REEs was maximized during the optimization. The response surface plot is shown in Figure 4. These data were fitted by a second-order polynomial expression model, including linear, polynomial, and cross terms, in Equation (2) for the pH values and the D2EHPA amounts.
D = −1.30 + 0.368⋅pH + 0.0357⋅Che − 0.0367⋅pH2 − 0.000251⋅Che2 − 0.00085⋅pH⋅Che
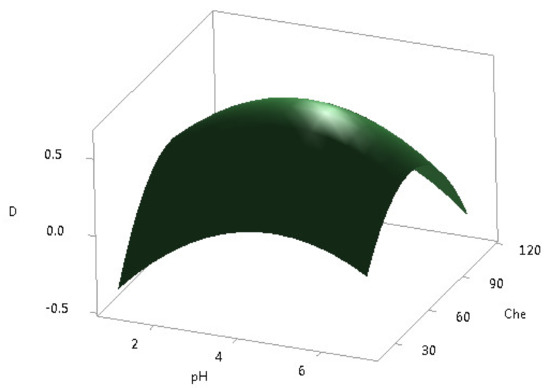
Figure 4.
Response surface plot for DSPME optimization.
The maximum D value of 0.65 was reached for the D2EHPA amount of 60 μL and pH = 4.2. Finally, the overall optimum for the DSPME process was obtained at 30 mg, 25 °C, and 6000 rpm, 1 min, 1 min, 5 min, 60 μL, 2 M HNO3, and 4.2, for the sorbent amount, the extraction temperature, the centrifuge speed, the vortexing time, the elution time, the centrifugation time, the D2EHPA amount, the eluting solvent, and pH, respectively. These optimized values were further used for validation study and the application of the DSPME method to real CFA leachate samples.
3.3. Analytical Characteristics
In order to assess the validity of the developed method, spiked aqueous solutions with increased Si, Al, Fe, and Ca content were examined by determining the limit of detection (LOD), the linear correlation coefficients (R2), average recovery (R), and relative standard deviation (RSD). Each standard, spike, and CFA leachate sample was spiked with an internal standard used to correct for shifts in signal intensity.
Good linearity in the method was proved in the range of 0.1 ng/L–50 μg/L of REEs in the diluted spiked solutions. This range covers the LOD levels for all REEs studied at which they can be found in CFA leachates. LOD, average recovery, and RSD for each RRE are all presented in Table 3. One can see that LODs are between 0.6 and 83 ng/L, the recovery ranges from 43 and 112%, while RSD values are within ±22%. Thus, the method based on a combination of DSPME with ICP-MS may be considered acceptable to determine the REEs concentrations in these high-matrix aqueous samples.

Table 3.
Analytical characteristics of DSPME-ICP-MS of REEs.
3.4. Analytical Applications
A recent study on the extraction of REEs from CFA by sequential extraction [37] showed that most REEs are in the residual fraction, so it is necessary to use strong mineral acids for the efficient leaching of REEs. On the other hand, a significant portion of the REEs is trapped in alumina matrices, which may be more easily leached by alkaline agents [38]. Therefore, the CFA leachate from the REEs recovery process, produced by alkaline roasting [39] and followed by acid leaching [40], is likely to contain a high level of matrix elements.
The next step in the REEs recovery from CFA leachate includes removing the matrix elements (Al, Si, and Fe) with some of the separation techniques, and finally, REEs separation. These separations rely on precipitation, adsorption, ion exchange, and chelating extraction [41]. Unfortunately, these processes are characterized by high consumption of energy and reagents. Therefore, continued research is underway to make the REE recovery more economical.
From the ecological point of view, a significant amount of the CFA recovery process leachate, accompanied by the CFA landfill leachate, ending in wastewater streams, causes serious concern and needs to be monitored. In this study, the proposed DSPME-ICP-MS method was used to analyze the EP and TCLP leachates of CFA from five different coal-fired thermal power plants in Serbia (Power plants: A—Tent A; B—Tent B; C—Kolubara; D—Morava; E—Kostolac). Table 4 shows the REEs content in CFA leachates of 20 ÷ 1 liquid-to-solid (L/S) ratio. The following order of REEs in the decreasing content was observed: Ce > Y > La > Nd > Dy > Gd > Sm > Er > Pr > Tb > Eu > Ho > Yb > Tm > Sc,Lu (n.d.) for aqueous leachate, and Ce > La > Y > Nd > Er > Gd > Sm > Dy > Pr > Tm > Ho > Tb > Eu > Sc > Yb > Lu for acetic acid leachate.

Table 4.
REEs content (ng/L, except for ΣREEs is μg/L) in CFA leachate (20 ÷ 1 L/S). Leaching agents: EP—water; TCLP—acetic acid. Samples from coal-fired power plants: A—Tent A; B—Tent B; C—Kolubara; D—Morava; E—Kostolac.
The most abundant REE in studied CFA aqueous leachates was Ce (22–194 ng/L), followed by Y (35–105 ng/L) or La (48–95 ng/L). In contrast, the lowest concentrations were found for Lu (0.048–0.084 ng/L).
It is seen that the samples (CEP and CTCLP) from the Kolubara power plant have a higher REE content, while REEs’ concentrations in the Kostolac power plant samples are at the lowest level. These differences can be attributed to different coals used in the power plants.
Note that the present method detected no Sc and Lu in aqueous leachate. On the other hand, the content of REEs in TCLP leachate, by two orders of magnitude, is higher compared to the aqueous leachate. In this case, the concentrations range from 42 ng/L (Lu) to 51 μg/L (Ce). In this study, the ratio of the concentrations of REEs in the TCLP leachate to aqueous extracts ranged from 104 to 352.
4. Conclusions
A new DSPME sorbent, consisting of spherical particles of poly(HDDA) and graphene, was synthesized by microfluidic emulsification, characterized, and applied in the DSPME of REEs prior to ICP-MS. The proposed DSPME-ICP-MS method is fast, has a low-consuming sorbent, and is specifically green. The main advantage of the DSPME technique is that it provides an extensive interface between poly(HDDA)/graphene particles and the aqueous phase after a cloudy solution formation. The separation factors for REEs were efficiently maximized by applying a two-step optimization using Plackett–Burman design, central composite designs, and Derringer desirability aggregate response function. Analytical characteristics and the method robustness are acceptable for most of the studied REEs for the analysis of coal fly ash leachate for REEs. Several leachate samples from CFA from different coal-fired power plants were analyzed by the proposed method. Cerium, La, and Y were found to be the most abundant REEs in CFA leachates. A significant difference between CFA leachate samples, in terms of the REEs content, was attributed to the coal properties.
Author Contributions
Investigation, writing—original draft preparation, L.S.-B.; formal analysis, project administration, L.I.; validation, resources, G.B.; data curation, software, A.S.; visualization, D.M.; conceptualization, methodology, G.V.; supervision, writing—review and editing, A.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Science Fund of the Republic of Serbia (Grant No. 7743343 SIW4SE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stopic, S.; Friedrich, B. Advances in Understanding of the Application of Unit Operations in Metallurgy of Rare Earth Elements. Metals 2021, 11, 978. [Google Scholar] [CrossRef]
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Jyothi, R.K.; Thenepalli, T.; Ahn, J.W.; Parhi, P.K.; Chung, K.W.; Lee, J.-Y. Review of Rare Earth Elements Recovery from Secondary Resources for Clean Energy Technologies: Grand Opportunities to Create Wealth from Waste. J. Clean. Prod. 2020, 267, 122048. [Google Scholar] [CrossRef]
- Palaparthi, J.; Chakrabarti, R.; Banerjee, S.; Guin, R.; Ghosal, S.; Agrahari, S.; Sengupta, D. Economically Viable Rare Earth Element Deposits along Beach Placers of Andhra Pradesh, Eastern Coast of India. Arab. J. Geosci. 2017, 10, 201. [Google Scholar] [CrossRef]
- Vilakazi, A.Q.; Ndlovu, S.; Chipise, L.; Shemi, A. The Recycling of Coal Fly Ash: A Review on Sustainable Developments and Economic Considerations. Sustainability 2022, 14, 1958. [Google Scholar] [CrossRef]
- Fu, B.; Hower, J.C.; Zhang, W.; Luo, G.; Hu, H.; Yao, H. A Review of Rare Earth Elements and Yttrium in Coal Ash: Content, Modes of Occurrences, Combustion Behavior, and Extraction Methods. Prog. Energy Combust. Sci. 2022, 88, 100954. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. A Comprehensive Review of Rare Earth Elements Recovery from Coal-Related Materials. Minerals 2020, 10, 451. [Google Scholar] [CrossRef]
- Keller, V.; Stopić, S.; Xakalashe, B.; Ma, Y.; Ndlovu, S.; Mwewa, B.; Simate, G.S.; Friedrich, B. Effectiveness of Fly Ash and Red Mud as Strategies for Sustainable Acid Mine Drainage Management. Minerals 2020, 10, 707. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Xakalashe, B.; Ndlovu, S.; Forsberg, K.; Friedrich, B. A Cleaner Approach for Recovering Al and Ti from Coal Fly Ash via Microwave-Assisted Baking, Leaching, and Precipitation. Hydrometallurgy 2021, 206, 105754. [Google Scholar] [CrossRef]
- Wen, Z.; Zhou, C.; Pan, J.; Cao, S.; Hu, T.; Ji, W.; Nie, T. Recovery of Rare-Earth Elements from Coal Fly Ash via Enhanced Leaching. Int. J. Coal Prep. Util. 2020, 284, 124725. [Google Scholar] [CrossRef]
- Tsiridis, V.; Samaras, P.; Kungolos, A.; Sakellaropoulos, G.P. Application of Leaching Tests for Toxicity Evaluation of Coal Fly Ash. Environ. Toxicol. 2006, 21, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hong, W.; Zhang, B.; Yang, C.; Wang, J.; Gao, J.; Mi, F.; Zhang, H.; Zhao, X.; Li, Q. Study on the Determination of Rare Earth Elements in Coal Ash by ICP-MS. Integr. Ferroelectr. 2019, 198, 116–121. [Google Scholar] [CrossRef]
- Wysocka, I. Determination of Rare Earth Elements Concentrations in Natural Waters—A Review of ICP-MS Measurement Approaches. Talanta 2021, 221, 121636. [Google Scholar] [CrossRef] [PubMed]
- Hann, S.; Boeck, K.; Koellensperger, G. Immunoaffinity Assisted LC-ICP-MS—a Versatile Tool in Biomedical Research. J. Anal. Spectrom. 2010, 25, 18–20. [Google Scholar] [CrossRef]
- Fisher, A.; Kara, D. Determination of Rare Earth Elements in Natural Water Samples—A Review of Sample Separation, Preconcentration and Direct Methodologies. Anal. Chim. Acta 2016, 935, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Manousi, N.; Gomez-Gomez, B.; Madrid, Y.; Deliyanni, E.A.; Zachariadis, G.A. Determination of Rare Earth Elements by Inductively Coupled Plasma-Mass Spectrometry after Dispersive Solid Phase Extraction with Novel Oxidized Graphene Oxide and Optimization with Response Surface Methodology and Central Composite Design. Microchem. J. 2020, 152, 104428. [Google Scholar] [CrossRef]
- Ebihara, M.; Hayano, K.; Shirai, N. Determination of Trace Rare Earth Elements in Rock Samples Including Meteorites by ICP-MS Coupled with Isotope Dilution and Comparison Methods. Anal. Chim. Acta 2020, 1101, 81–89. [Google Scholar] [CrossRef]
- Milicic, L.; Terzic, A.; Pezo, L.; Mijatovic, N.; Brceski, I.; Vukelic, N. Assessment of Efficiency of Rare Earth Elements Recovery from Lignite Coal Combustion Ash via Five-Stage Extraction. Sci. Sinter. 2021, 53, 169–185. [Google Scholar] [CrossRef]
- Rubinos, D.A.; Barral, M.T. Sorptive Removal of Hg II by Red Mud (Bauxite Residue) in Contaminated Landfill Leachate. J. Environ. Sci. Health Part A 2017, 52, 84–98. [Google Scholar] [CrossRef]
- Balaram, V.; Subramanyam, K.S.V. Sample Preparation for Geochemical Analysis: Strategies and Significance. Adv. Sample Prep. 2022, 1, 100010. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Gronen, L.; Milivojevic, M.; Obradovic, S.; Friedrich, B. Neural Network Modeling for the Extraction of Rare Earth Elements from Eudialyte Concentrate by Dry Digestion and Leaching. Metals 2018, 8, 267. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Peng, G.; He, Q.; Zhu, H.; Al-Hamadani, S.M.Z.F. Dispersive Liquid–Liquid Microextraction Based on the Solidification of Floating Organic Drop Followed by ICP-MS for the Simultaneous Determination of Heavy Metals in Wastewaters. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 140, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Taggart, R.K.; Hower, J.C.; Wiesner, M.R.; Hsu-Kim, H. Selective Recovery of Rare Earth Elements from Coal Fly Ash Leachates Using Liquid Membrane Processes. Environ. Sci. Technol. 2019, 53, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, V.; Peric-Grujic, A.; Ristic, M.; Marinkovic, A.; Markovic, R.; Onjia, A.; Sljivic-Ivanovic, M. Selenate Adsorption from Water Using the Hydrous Iron Oxide-Impregnated Hybrid Polymer. Metals 2020, 10, 1630. [Google Scholar] [CrossRef]
- Suručić, L.; Tadić, T.; Janjić, G.; Marković, B.; Nastasović, A.; Onjia, A. Recovery of Vanadium (V) Oxyanions by a Magnetic Macroporous Copolymer Nanocomposite Sorbent. Metals 2021, 11, 1777. [Google Scholar] [CrossRef]
- Peiravi, M.; Ackah, L.; Guru, R.; Mohanty, M.; Liu, J.; Xu, B.; Zhu, X.; Chen, L. Chemical Extraction of Rare Earth Elements from Coal Ash. Miner. Metall. Process. 2017, 34, 170–177. [Google Scholar] [CrossRef]
- Aguirre, M.Á.; Baile, P.; Vidal, L.; Canals, A. Metal Applications of Liquid-Phase Microextraction. TrAC Trends Anal. Chem. 2019, 112, 241–247. [Google Scholar] [CrossRef]
- Rajakovic, L.; Todorovic, Z.; Rajakovic-Ognjanovic, V.; Onjia, A. Analytical Methods for Arsenic Speciation Analysis. J. Serb. Chem. Soc. 2013, 78, 1461–1479. [Google Scholar] [CrossRef] [Green Version]
- Sajid, M.; Asif, M.; Ihsanullah, I. Dispersive Liquid–Liquid Microextraction of Multi-Elements in Seawater Followed by Inductively Coupled Plasma-Mass Spectrometric Analysis and Evaluation of Its Greenness. Microchem. J. 2021, 169, 106565. [Google Scholar] [CrossRef]
- Labutin, T.A.; Lednev, V.N.; Ilyin, A.A.; Popov, A.M. Femtosecond Laser-Induced Breakdown Spectroscopy. J. Anal. At. Spectrom. 2016, 31, 90–118. [Google Scholar] [CrossRef]
- Krishnan Chandrasekaran, S.; Dheram Karunasagar, K.; Jayaraman Arunachalam, G. Dispersive Liquid-Liquid Micro-Extraction for Simultaneous Preconcentration of 14 Lanthanides at Parts per Trillion Levels from Groundwater and Determination Using a Micro-Flow Nebulizer in Inductively Coupled Plasma-Quadrupole Mass Spectrometry. J. Anal. Spectrom. 2010, 25, 18–20. [Google Scholar] [CrossRef]
- Chen, S.; Yan, J.; Li, J.; Lu, D. Magnetic ZnFe2O4 Nanotubes for Dispersive Micro Solid-Phase Extraction of Trace Rare Earth Elements Prior to Their Determination by ICP-MS. Microchim. Acta 2019, 186, 228. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Khojeh, V.; Samadi, S. Optimization of Dispersive Liquid–Liquid Microextraction Coupled with Inductively Coupled Plasma-Optical Emission Spectrometry with the Aid of Experimental Design for Simultaneous Determination of Heavy Metals in Natural Waters. Talanta 2011, 83, 885–890. [Google Scholar] [CrossRef]
- Pinheiro, F.C.; Aguirre, M.Á.; Nóbrega, J.A.; González-Gallardo, N.; Ramón, D.J.; Canals, A. Dispersive Liquid-Liquid Microextraction Based on Deep Eutectic Solvent for Elemental Impurities Determination in Oral and Parenteral Drugs by Inductively Coupled Plasma Optical Emission Spectrometry. Anal. Chim. Acta 2021, 1185, 339052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Moshtaghibana, S.; Zhu, T.; Fayemiwo, K.A.; Price, A.; Vladisavljević, G. Microfluidic Fabrication of Novel Polymeric Core-shell Microcapsules for Storage of CO2 Solvents and Organic Chelating Agents. J. Polym. Sci. 2022, pol.20210959. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Lim, Y.; Yu, J.; Chen, S.; Woo, S.W.; Yoon, S.; Bae, S.; Kim, H.S. Characterization of Rare Earth Elements Present in Coal Ash by Sequential Extraction. J. Hazard. Mater. 2021, 402, 123760. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, S.; Zou, J.; French, D.; Graham, I.T. Rare Earth Elements and Yttrium in Coal Ash from the Luzhou Power Plant in Sichuan, Southwest China: Concentration, Characterization and Optimized Extraction. Int. J. Coal Geol. 2019, 203, 1–14. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, C.; Pan, J.; Zhang, N.; Liu, C.; Cao, S.; Hu, T.; Ji, W. Study on Extraction of Rare Earth Elements from Coal Fly Ash through Alkali Fusion—Acid Leaching. Miner. Eng. 2019, 136, 36–42. [Google Scholar] [CrossRef]
- King, J.F.; Taggart, R.K.; Smith, R.C.; Hower, J.C.; Hsu-Kim, H. Aqueous Acid and Alkaline Extraction of Rare Earth Elements from Coal Combustion Ash. Int. J. Coal Geol. 2018, 195, 75–83. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A. Characteristics of Some Selected Methods of Rare Earth Elements Recovery from Coal Fly Ashes. Metals 2021, 11, 142. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).