Review on Grain Refinement of Metallic Materials to Regulate Cellular Behavior
Abstract
:1. Introduction
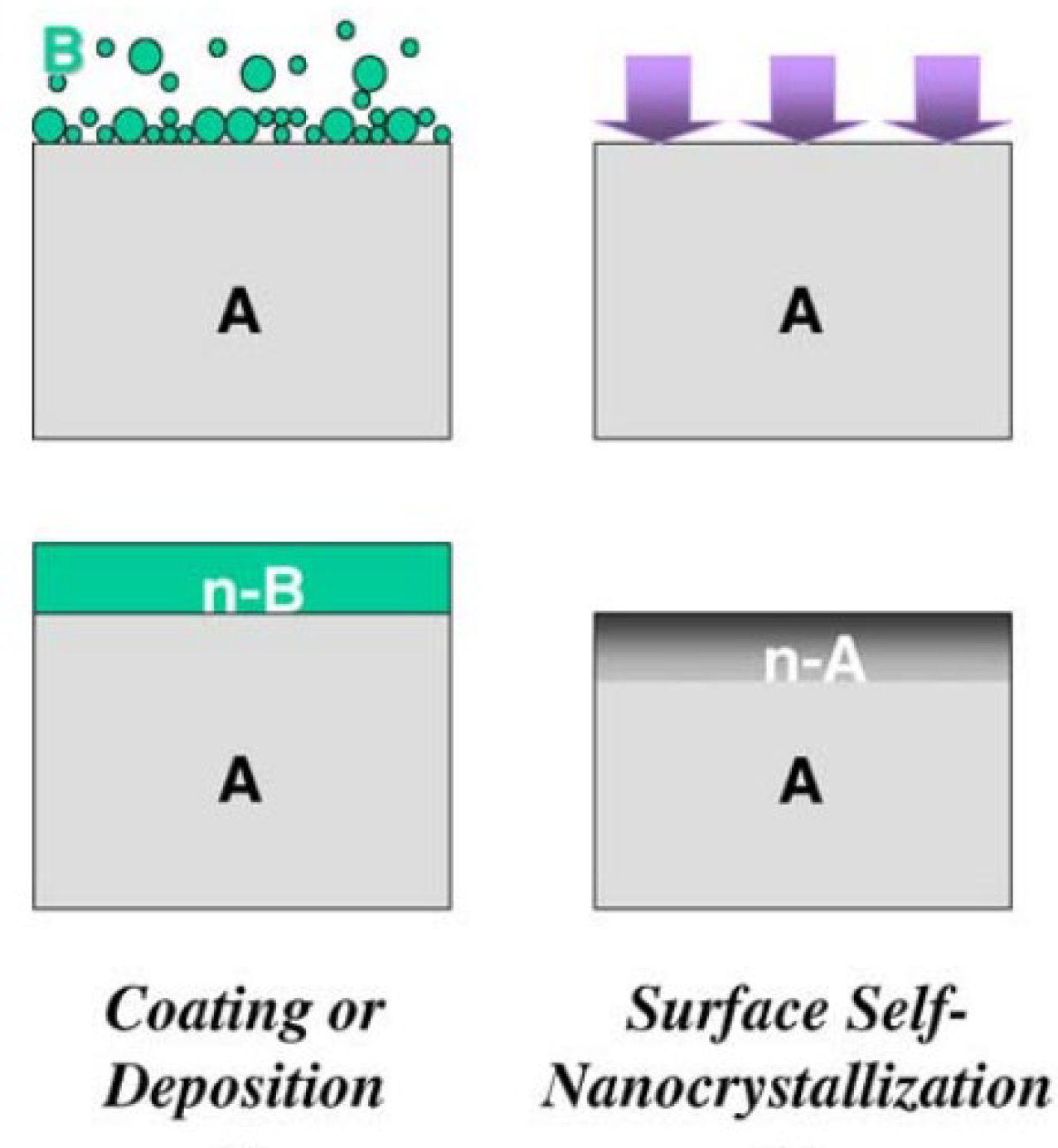
2. Two Grain Refinement Modes for Treating Metallic Materials
2.1. Surface Grain Refinement
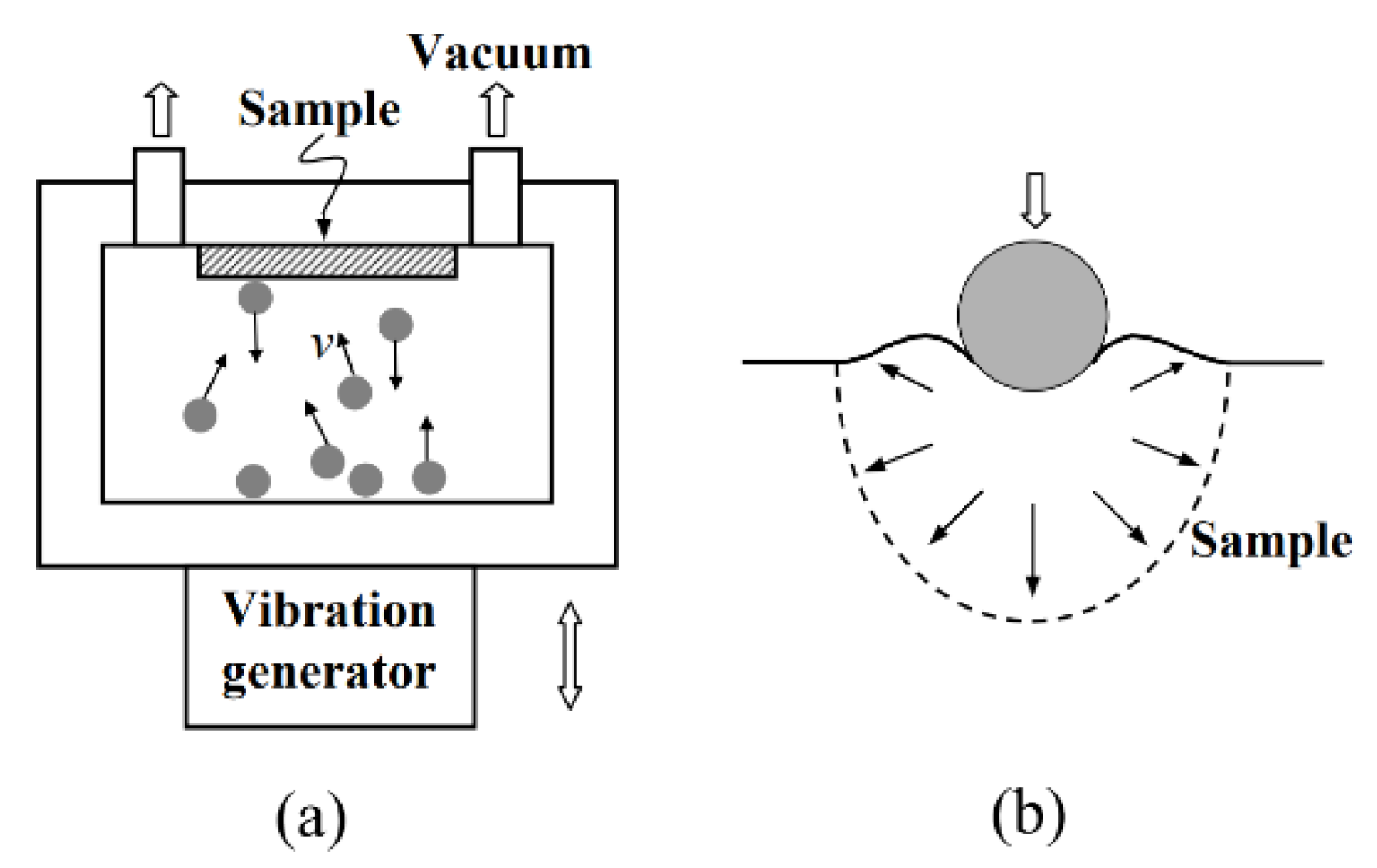
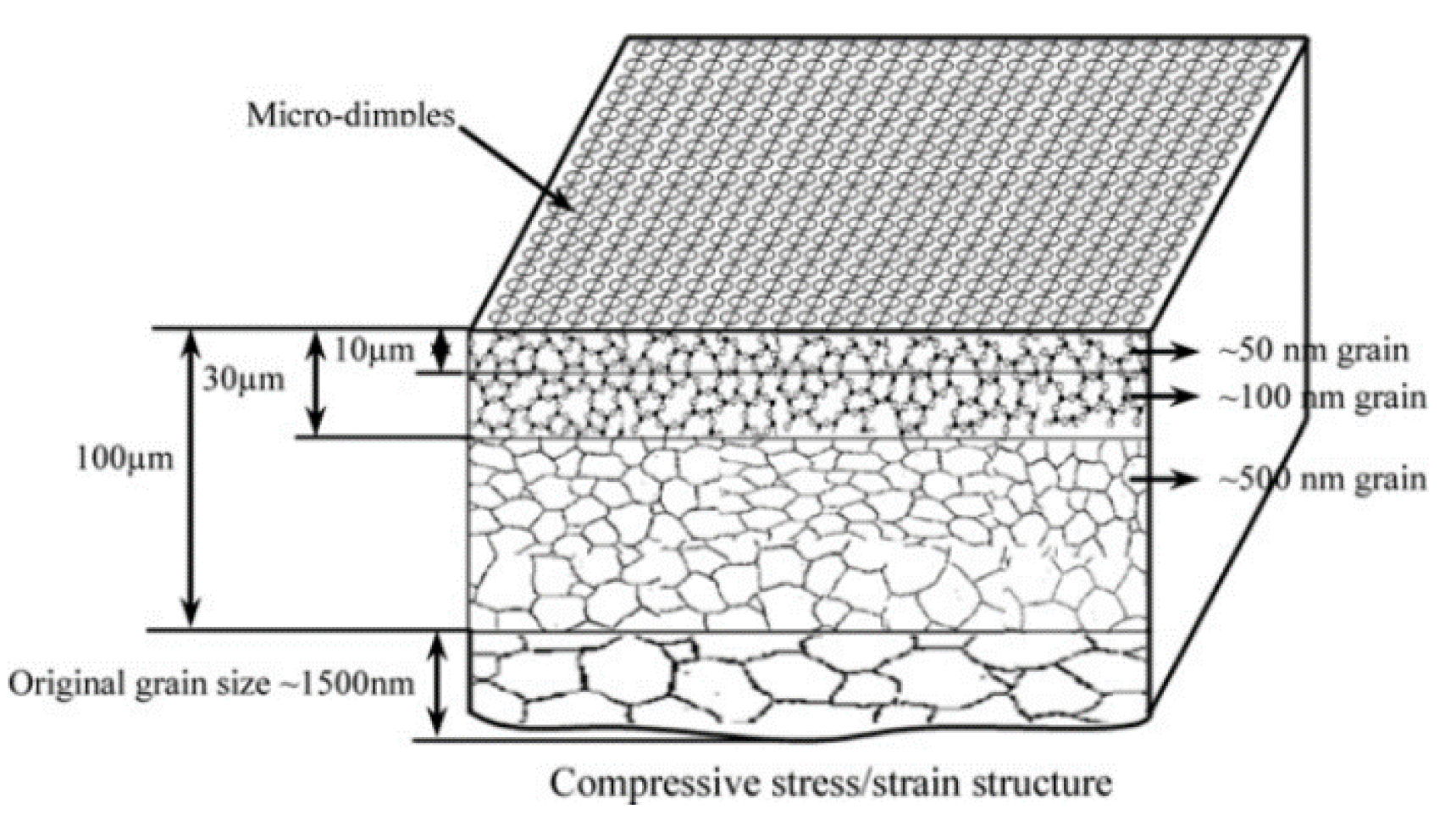
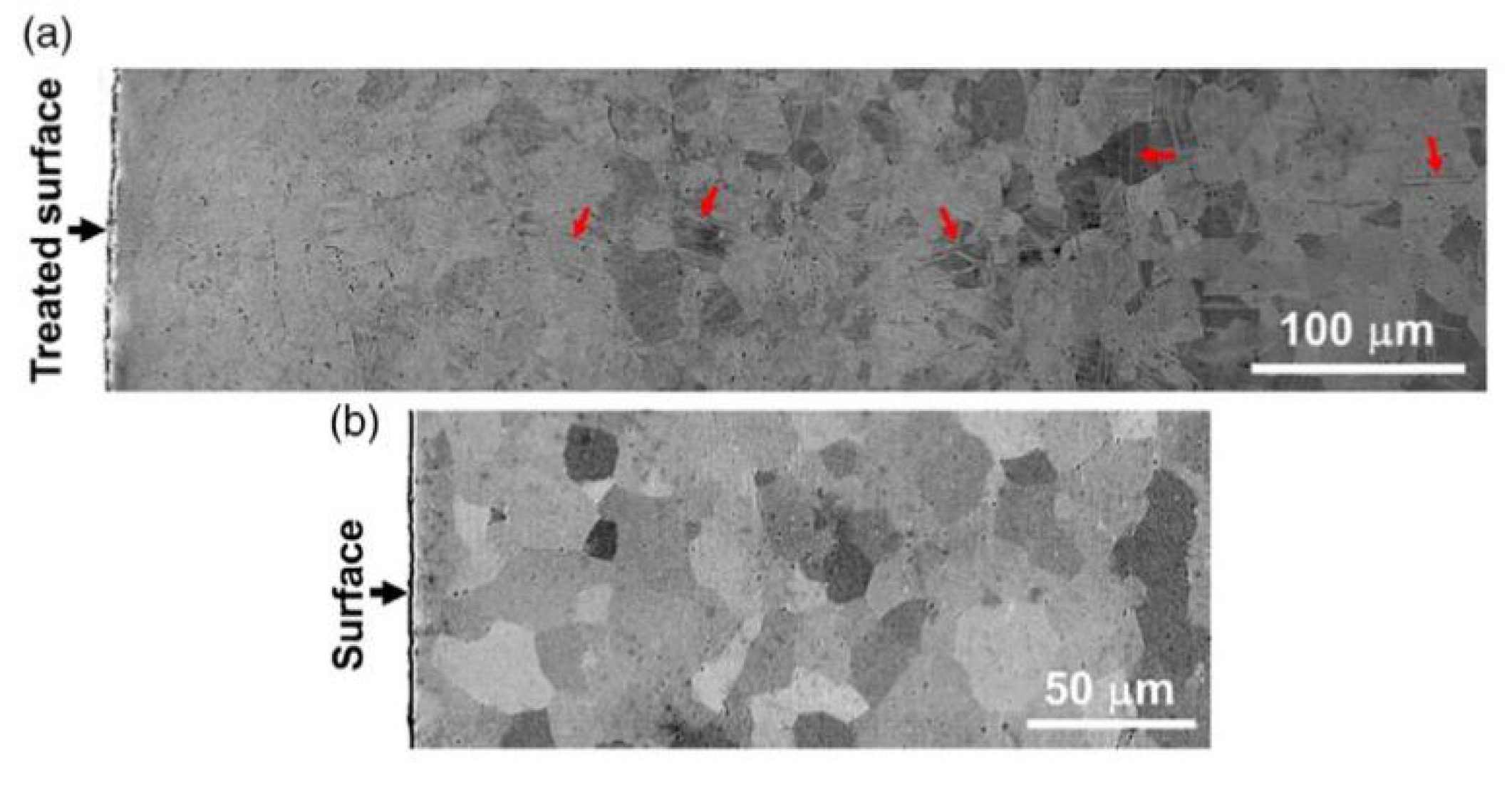
2.1.1. Surface Mechanical Attrition Treatment (SMAT)
2.1.2. Ultrasonic Shot Peening (USSP)
2.1.3. Laser Shock Peening (LSP)
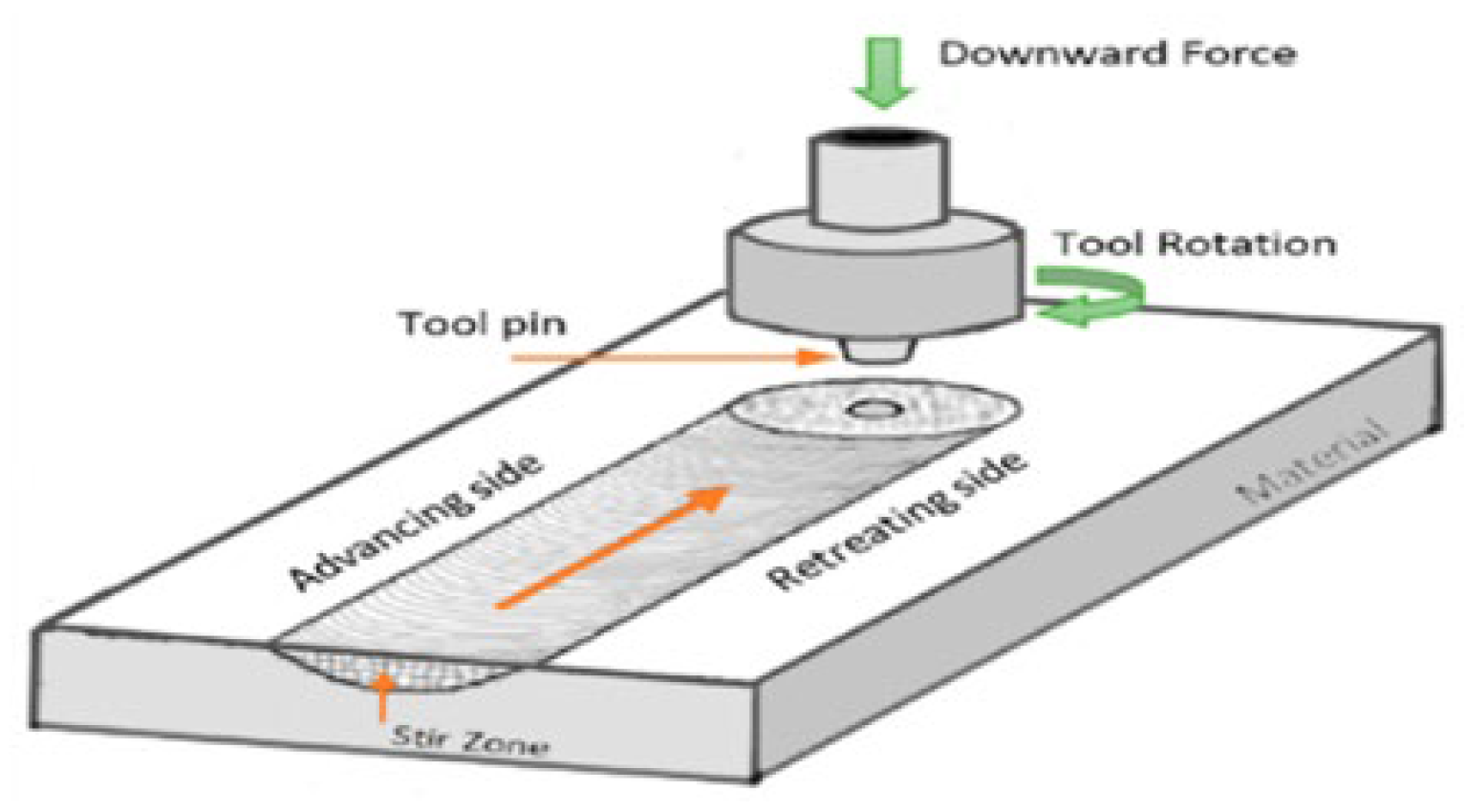
2.1.4. Friction Stir Processing (FSP)
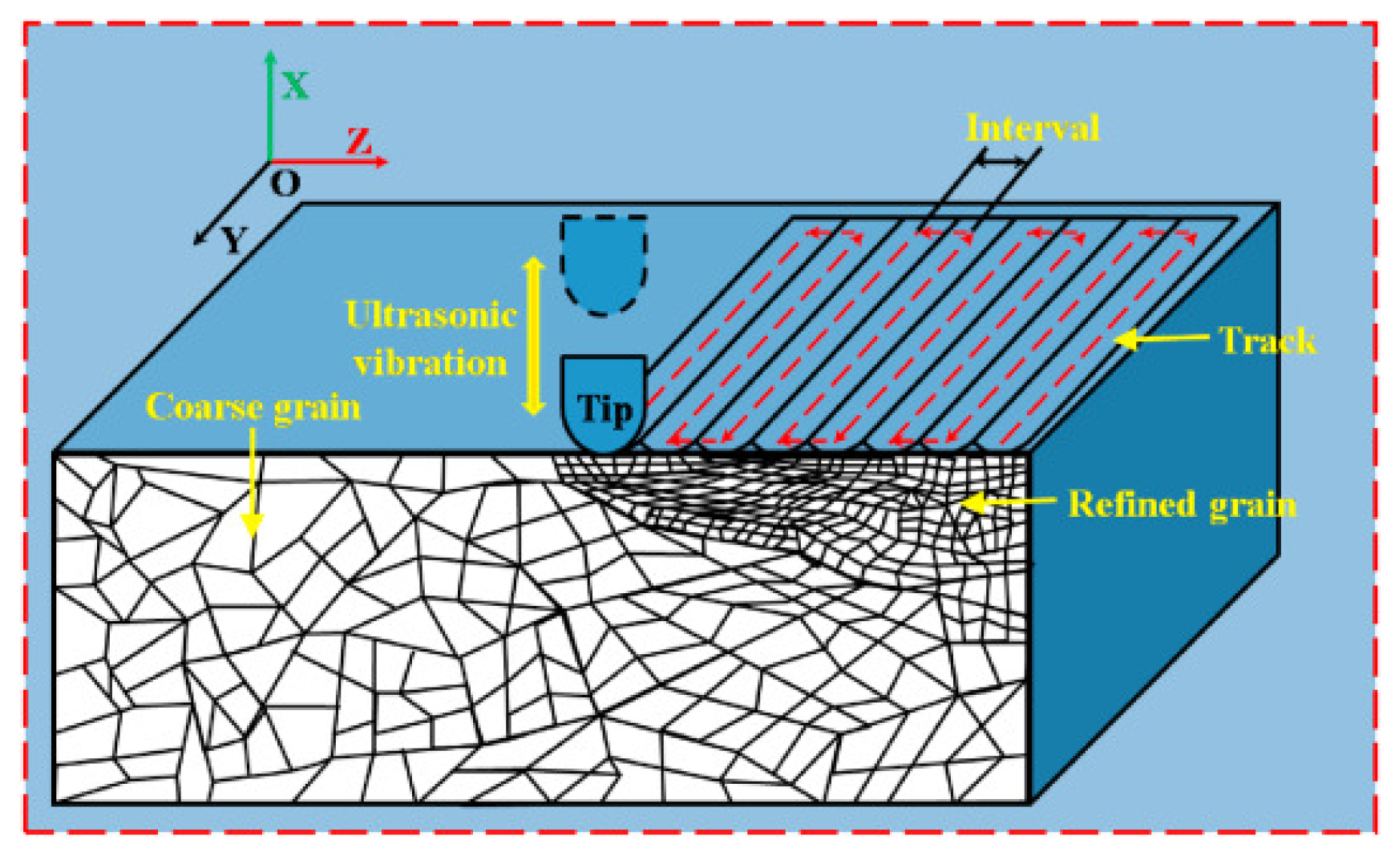
2.1.5. Ultrasonic Nanocrystal Surface Modification (UNSM)
2.2. Bulk Grain Refinement
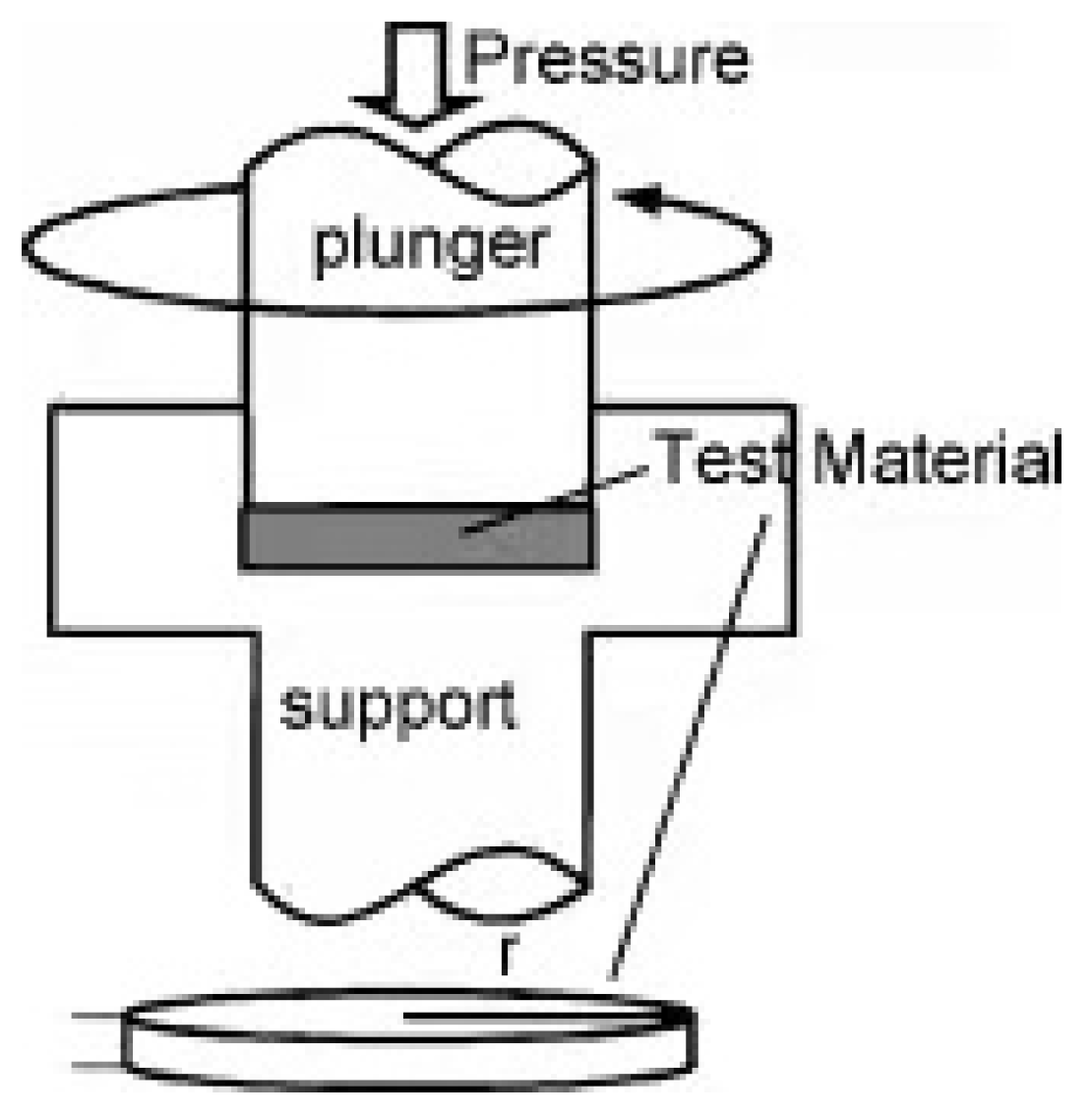
2.2.1. High-Pressure Torsion (HPT)
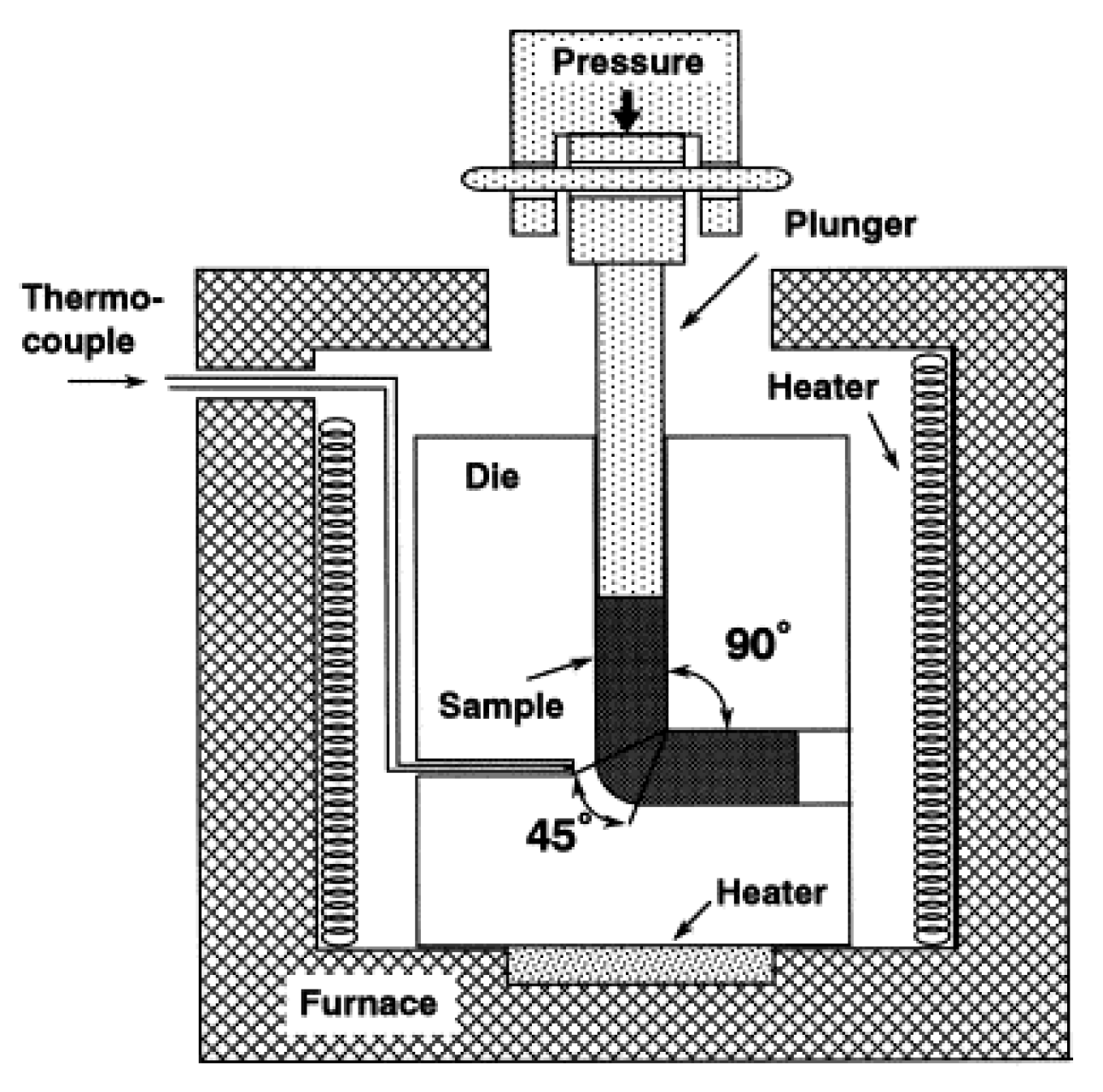
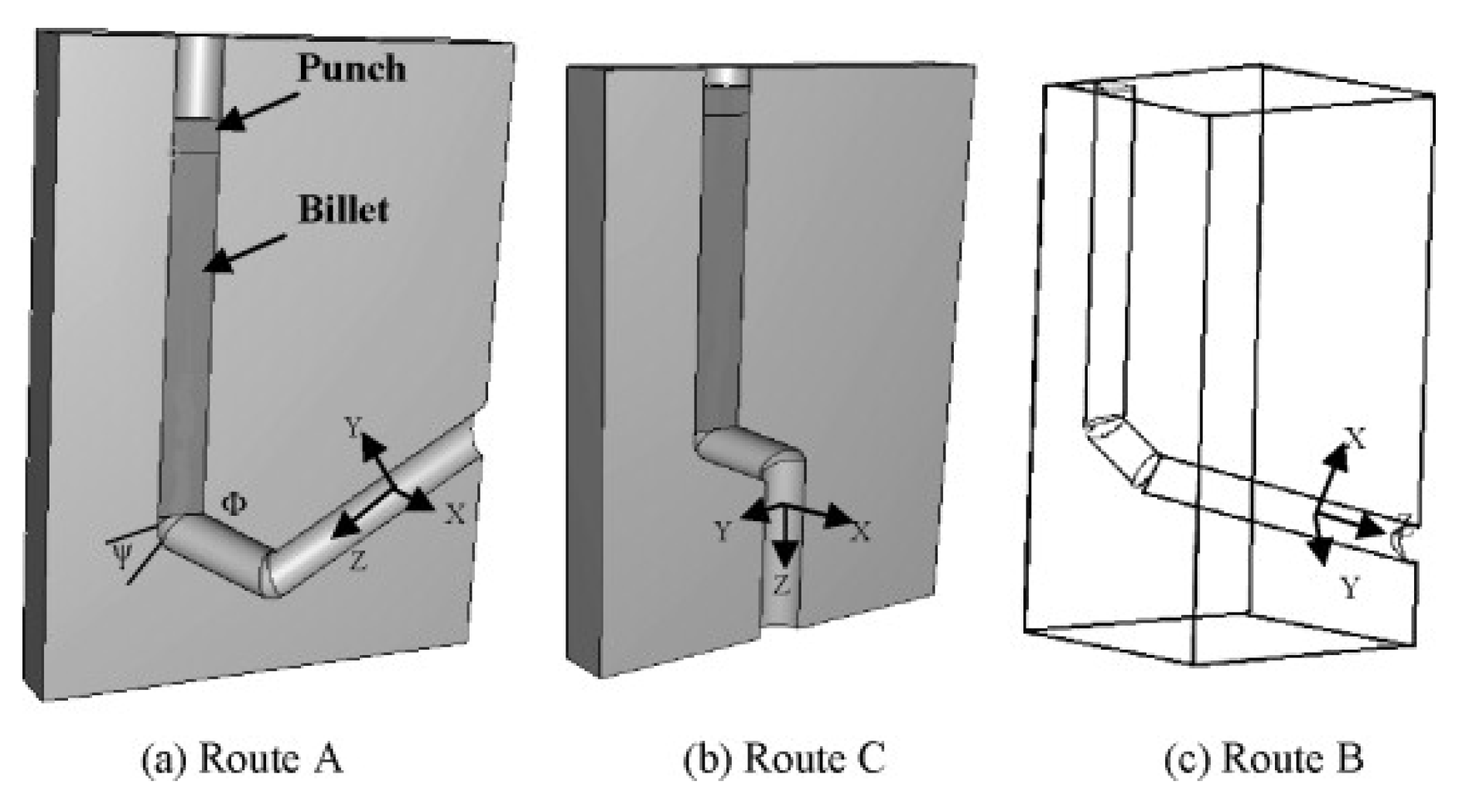
2.2.2. Equal Channel Angular Pressing (ECAP)
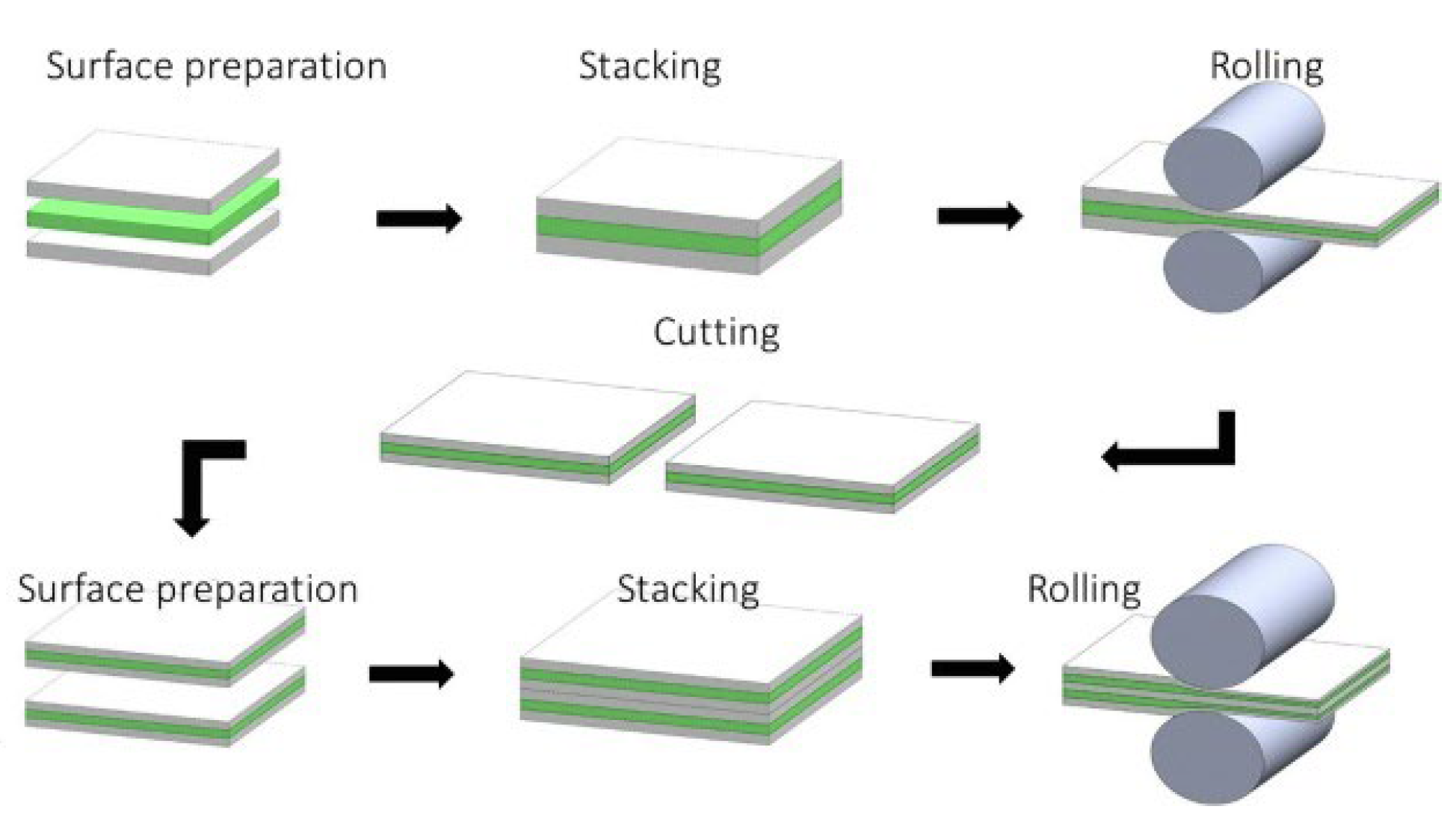
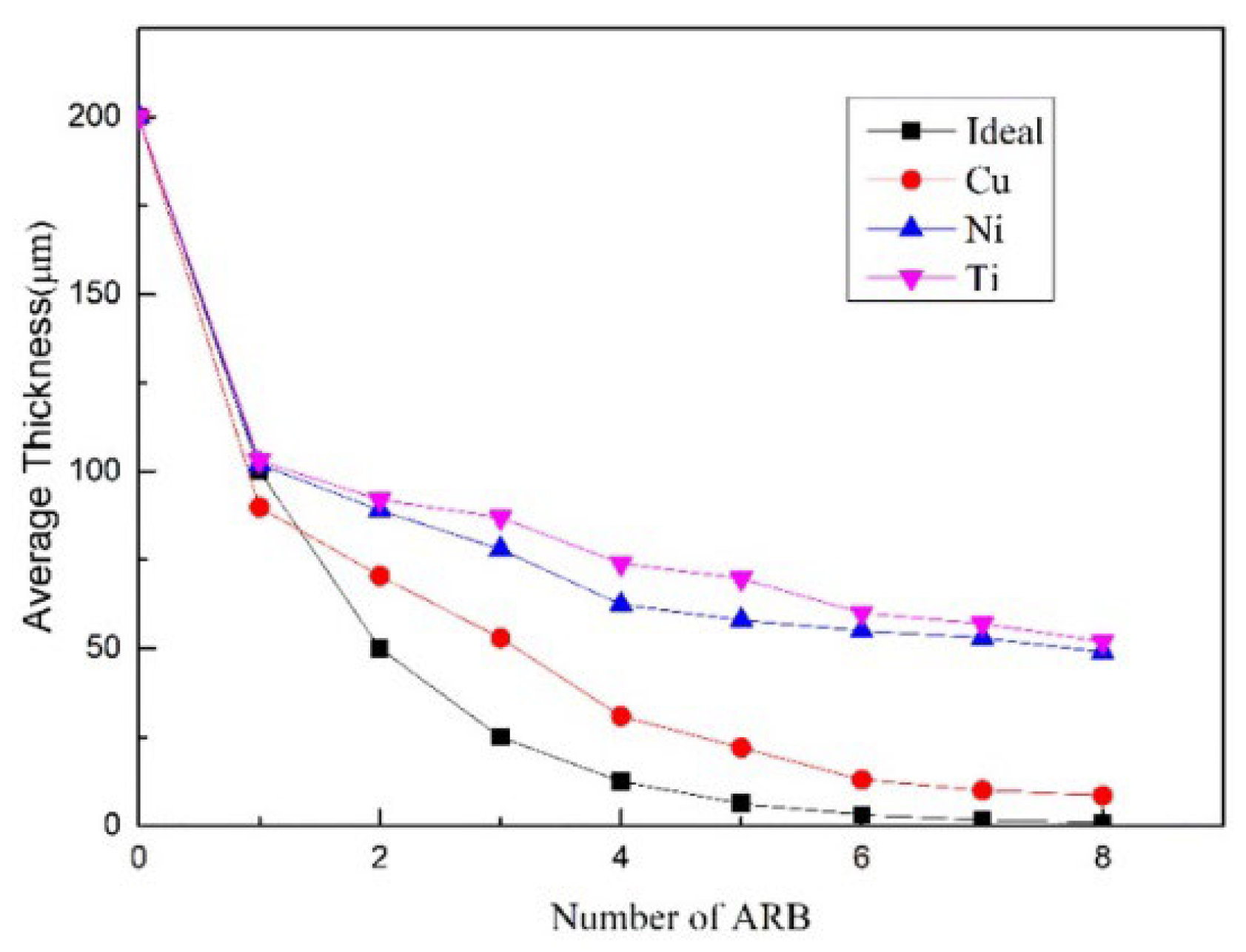
2.2.3. Accumulative Roll Bonding (ARB)
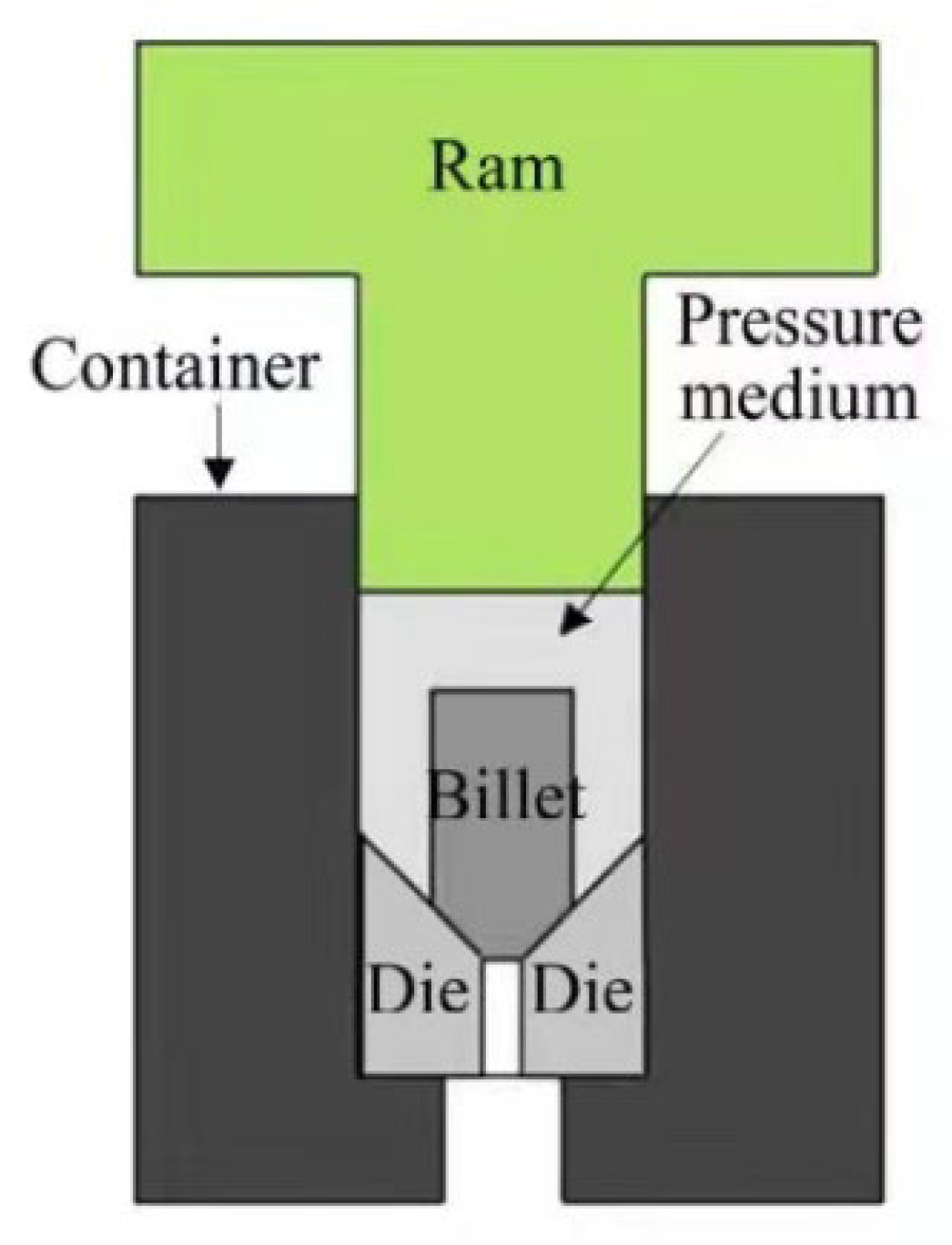
2.2.4. Hydrostatic Extrusion (HE)
2.2.5. Multi-Directional Forging (MDF)
3. Grain Refinement of Different Metallic Materials to Modulate Cell Response
3.1. Titanium and Its Alloys
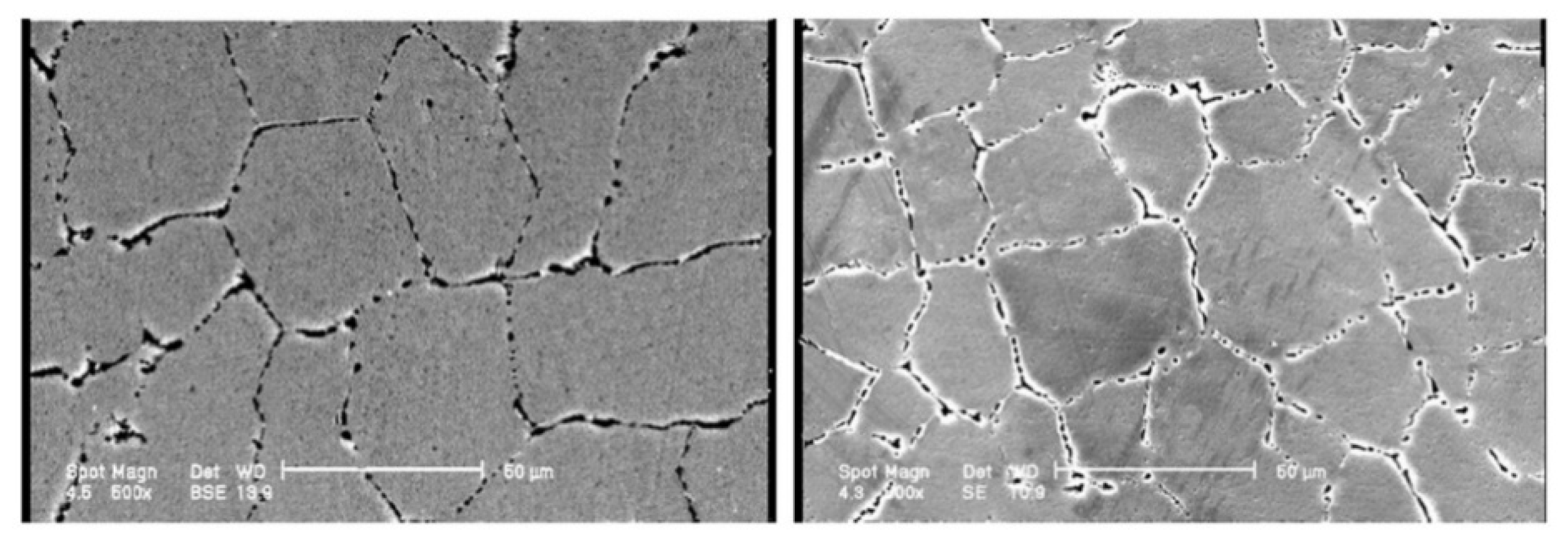
3.2. Stainless Steel
3.3. Magnesium and Its Alloys
3.4. NiTi Shape Memory Alloys
3.5. Other Metals
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
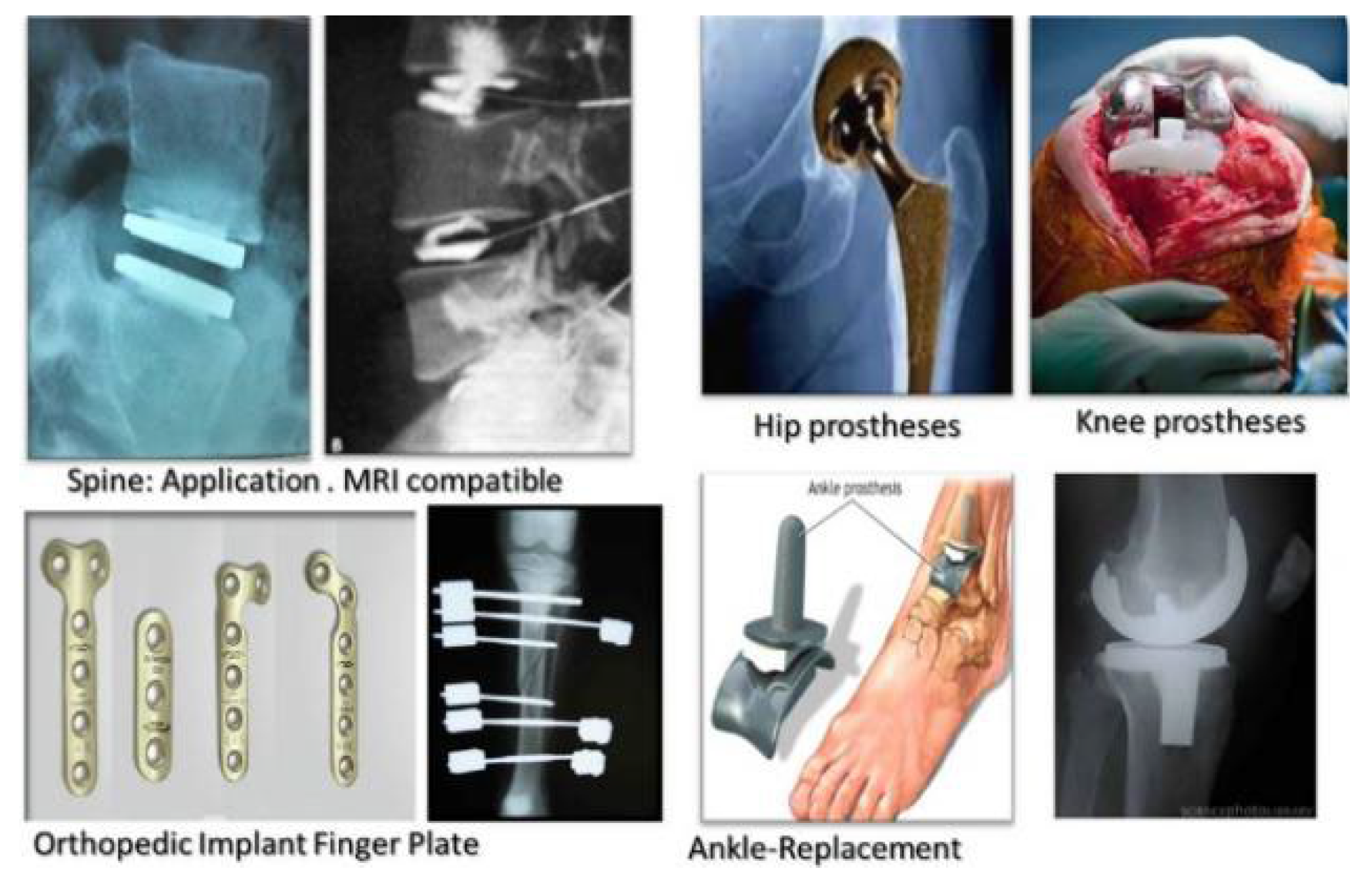
- Misra, S.; Raghuwanshi, S. Enhancing the mechanical and biological performance of a metallic biomaterial for orthopedic applications. In Fundamental Biomaterials: Metals; Woodhead Publishing: Duxford, UK, 2018; pp. 355–370. [Google Scholar]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109895. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.-Y.; Chin, W.-H.; Chien, C.-S.; Hsieh, Y.-H. Mechanical and biological properties of graded porous tantalum coatings deposited on titanium alloy implants by vacuum plasma spraying. Surf. Coat. Technol. 2019, 372, 399–409. [Google Scholar] [CrossRef]
- Lo, Y.S.; Chang, C.C.; Lin, P.C.; Lin, S.P.; Wang, C.L. Direct growth of structurally controllable hydroxyapatite coating on Ti-6Al-4V through a rapid hydrothermal synthesis. Appl. Surf. Sci. 2021, 556, 149672. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, S.; Zhang, X.; Lu, X.; Dong, Z. Construction of multi-layered Zn-modified TiO2 coating by ultrasound-auxiliary micro-arc oxidation: Microstructure and biological property. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112487. [Google Scholar] [CrossRef] [PubMed]
- Estrin, Y.; Kim, H.E.; Lapovok, R.; Ng, H.P.; Jo, J.H. Mechanical strength and biocompatibility of ultrafine-grained commercial purity titanium. Biomed. Res. Int. 2013, 2013, 914764. [Google Scholar] [CrossRef] [Green Version]
- Parfenov, E.V.; Parfenova, L.V.; Dyakonov, G.S.; Danilko, K.V.; Mukaeva, V.R.; Farrakhov, R.G.; Lukina, E.S.; Valiev, R.Z. Surface functionalization via PEO coating and RGD peptide for nanostructured titanium implants and their in vitro assessment. Surf. Coat. Technol. 2019, 357, 669–683. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Enhanced in vitro biocompatibility of ultrafine-grained titanium with hierarchical porous surface. Appl. Surf. Sci. 2011, 257, 5634–5640. [Google Scholar] [CrossRef]
- Edalati, K.; Bachmaier, A.; Beloshenko, V.A.; Beygelzimer, Y.; Blank, V.D.; Botta, W.J.; Bryła, K.; Čížek, J.; Divinski, S.; Enikeev, N.A.; et al. Nanomaterials by severe plastic deformation: Review of historical developments and recent advances. Mater. Res. Lett. 2022, 10, 163–256. [Google Scholar] [CrossRef]
- Kalantari, K.; Saleh, B.; Webster, T.J. Biological Applications of Severely Plastically Deformed Nano-Grained Medical Devices: A Review. Nanomaterials 2021, 11, 748. [Google Scholar] [CrossRef]
- Dobatkin, S.; Martynenko, N.; Anisimova, N.; Kiselevskiy, M.; Prosvirnin, D.; Terentiev, V.; Yurchenko, N.; Salishchev, G.; Estrin, Y. Mechanical Properties, Biodegradation, and Biocompatibility of Ultrafine Grained Magnesium Alloy WE43. Materials 2019, 12, 3627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, K.; Lu, J. Nanostructured surface layer on metallic materials induced by surface mechanical attrition treatment. Mater. Sci. Eng. A 2004, 375–377, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.K.; Pandey, V.; Barhanpurkar-Naik, A.; Wani, M.R.; Chattopadhyay, K.; Singh, V. Effect of ultrasonic shot peening duration on microstructure, corrosion behavior and cell response of cp-Ti. Ultrasonics 2020, 104, 106110. [Google Scholar] [CrossRef] [PubMed]
- Bahl, S.; Aleti, B.T.; Suwas, S.; Chatterjee, K. Surface nanostructuring of titanium imparts multifunctional properties for orthopedic and cardiovascular applications. Mater. Des. 2018, 144, 169–181. [Google Scholar] [CrossRef]
- Sandeep Kranthi Kiran, A.; Sireesha, M.; Ramalingam, R.; Kizhakeyil, A.; Verma, N.K.; Lakshminarayanan, R.; Sampath Kumar, T.S.; Doble, M.; Ramakrishna, S. Modulation of biological properties by grain refinement and surface modification on titanium surfaces for implant-related infections. J. Mater. Sci. 2019, 54, 13265–13282. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Fu, Y.; Dunne, N.; Liang, C.; Luo, X.; Liu, K.; Li, X.; Pang, X.; Lu, K. Improved osteogenic differentiation of human amniotic mesenchymal stem cells on gradient nanostructured Ti surface. J. Biomed. Mater. Res. A 2020, 108, 1824–1833. [Google Scholar] [CrossRef]
- Tao, N.R.; Lu, J.; Lu, K. Surface Nanocrystallization by Surface Mechanical Attrition Treatment. Mater. Sci. Forum 2008, 579, 91–108. [Google Scholar] [CrossRef]
- Tao, N.R.; Wang, Z.B.; Tong, W.P.; Sui, M.L.; Lu, J.; Lu, K. An investigation of surface nanocrystallization mechanism in Fe induced by surface mechanical attrition treatment. Acta Mater. 2002, 50, 4603–4616. [Google Scholar] [CrossRef]
- Tao, N.R.; Sui, M.L.; Lu, J.; Lu, K. Surface nanocrystallization of iron induced by ultrasonic shot peening. Nanostructured Mater. 1999, 11, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Chaise, T.; Li, J.; Nélias, D.; Kubler, R.; Taheri, S.; Douchet, G.; Robin, V.; Gilles, P. Modelling of multiple impacts for the prediction of distortions and residual stresses induced by ultrasonic shot peening (USP). J. Mater. Processing Technol. 2012, 212, 2080–2090. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, B.; Swaroop, S. Review: Laser shock peening as post welding treatment technique. J. Manuf. Processes 2018, 32, 721–733. [Google Scholar] [CrossRef]
- Acharya, S.; Suwas, S.; Chatterjee, K. Review of recent developments in surface nanocrystallization of metallic biomaterials. Nanoscale 2021, 13, 2286–2301. [Google Scholar] [CrossRef] [PubMed]
- Perumal, G.; Ayyagari, A.; Chakrabarti, A.; Kannan, D.; Pati, S.; Grewal, H.S.; Mukherjee, S.; Singh, S.; Arora, H.S. Friction Stir Processing of Stainless Steel for Ascertaining Its Superlative Performance in Bioimplant Applications. ACS Appl. Mater. Interfaces 2017, 9, 36615–36631. [Google Scholar] [CrossRef] [PubMed]
- Węglowski, M.S. Friction stir processing—State of the art. Arch. Civ. Mech. Eng. 2018, 18, 114–129. [Google Scholar] [CrossRef]
- Merah, N.; Abdul Azeem, M.; Abubaker, H.M.; Al-Badour, F.; Albinmousa, J.; Sorour, A.A. Friction Stir Processing Influence on Microstructure, Mechanical, and Corrosion Behavior of Steels: A Review. Materials 2021, 14, 5023. [Google Scholar] [CrossRef]
- Mironov, S.; Sato, Y.S.; Kokawa, H. Friction-stir processing. In Nanocrystalline Titanium; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–69. [Google Scholar]
- Maleki, E.; Unal, O.; Guagliano, M.; Bagherifard, S. The effects of shot peening, laser shock peening and ultrasonic nanocrystal surface modification on the fatigue strength of Inconel 718. Mater. Sci. Eng. Struct. Mater. Prop. Microstruct. Processing 2021, 810, 141029. [Google Scholar] [CrossRef]
- Liu, R.Y.; Yuan, S.; Lin, N.M.; Zeng, Q.F.; Wang, Z.H.; Wu, Y.C. Application of ultrasonic nanocrystal surface modification (UNSM) technique for surface strengthening of titanium and titanium alloys: A mini review. J. Mater. Res. Technol. JmrT 2021, 11, 351–377. [Google Scholar] [CrossRef]
- Liang, Y.; Qin, H.F.; Mehra, N.; Zhu, J.H.; Yang, Z.N.; Doll, G.L.; Ye, C.; Dong, Y.L. Controllable hierarchical micro/nano patterns on biomaterial surfaces fabricated by ultrasonic nanocrystalline surface modification. Mater. Des. 2018, 137, 325–334. [Google Scholar] [CrossRef]
- Amanov, A.; Cho, I.S.; Pyoun, Y.S.; Lee, C.S.; Park, I.G. Micro-dimpled surface by ultrasonic nanocrystal surface modification and its tribological effects. Wear 2012, 286, 136–144. [Google Scholar] [CrossRef]
- Moon, J.H.; Baek, S.M.; Lee, S.G.; Seong, Y.; Amanov, A.; Lee, S.; Kim, H.S. Effects of residual stress on the mechanical properties of copper processed using ultrasonic-nanocrystalline surface modification. Mater. Res. Lett. 2018, 7, 97–102. [Google Scholar] [CrossRef]
- Edalati, K.; Horita, Z. A review on high-pressure torsion (HPT) from 1935 to 1988. Mater. Sci. Eng. A 2016, 652, 325–352. [Google Scholar] [CrossRef]
- Azushima, A.; Kopp, R.; Korhonen, A.; Yang, D.Y.; Micari, F.; Lahoti, G.D.; Groche, P.; Yanagimoto, J.; Tsuji, N.; Rosochowski, A.; et al. Severe plastic deformation (SPD) processes for metals. Cirp Ann. Manuf. Technol. 2008, 57, 716–735. [Google Scholar] [CrossRef]
- Figueiredo, R.B.; Langdon, T.G. Processing Magnesium and Its Alloys by High-Pressure Torsion: An Overview. Adv. Eng. Mater. 2019, 21, 1801039. [Google Scholar] [CrossRef] [Green Version]
- Faraji, G.; Kim, H.S.; Kashi, H.T. Severe Plastic Deformation Methods for Bulk Samples. In Severe Plastic Deformation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–112. [Google Scholar]
- Sadasivan, N.; Balasubramanian, M.; Rameshbapu, B.R. A comprehensive review on equal channel angular pressing of bulk metal and sheet metal process methodology and its varied applications. J. Manuf. Processes 2020, 59, 698–726. [Google Scholar] [CrossRef]
- Yamashita, A.; Horita, Z.; Langdon, T.G. Improving the mechanical properties of magnesium and a magnesium alloy through severe plastic deformation. Mater. Sci. Eng. A 2001, 300, 142–147. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.A.; Chen, X.Y. Research progress of ultrafine grained magnesium alloy prepared by equal channel angular pressing. Mater. Res. Express 2021, 8, 022001. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk nanostructured materials from severe plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Suo, T.; Li, Y.; Deng, Q.; Liu, Y. Optimal pressing route for continued equal channel angular pressing by finite element analysis. Mater. Sci. Eng. A 2007, 466, 166–171. [Google Scholar] [CrossRef]
- Stolyarov, V.V.; Zhu, Y.T.; Alexandrov, I.V.; Lowe, T.C.; Valiev, R.Z. Influence of ECAP routes on the microstructure and properties of pure Ti. Mater. Sci. Eng. A 2001, 299, 59–67. [Google Scholar] [CrossRef]
- Elias, C.N.; Meyers, M.A.; Valiev, R.Z.; Monteiro, S.N. Ultrafine grained titanium for biomedical applications: An overview of performance. J. Mater. Res. Technol. 2013, 2, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Mora-Sanchez, H.; Sabirov, I.; Monclus, M.A.; Matykina, E.; Molina-Aldareguia, J.M. Ultra-fine grained pure Titanium for biomedical applications. Mater. Technol. 2016, 31, 756–771. [Google Scholar] [CrossRef]
- Verlinden, B. Severe plastic deformation of metals. Metal. J. Metall. 2005, 11, 165–182. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, F.; Farzin, M. Finite element analysis of ultrasonic-assisted equal channel angular pressing. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2013, 228, 1859–1868. [Google Scholar] [CrossRef]
- Ahmadi, F.; Farzin, M.; Meratian, M.; Loeian, S.M.; Forouzan, M.R. Improvement of ECAP process by imposing ultrasonic vibrations. Int. J. Adv. Manuf. Technol. 2015, 79, 503–512. [Google Scholar] [CrossRef]
- Saito, Y.; Utsunomiya, H.; Tsuji, N.; Sakai, T. Novel ultra-high straining process for bulk materials—development of the accumulative roll-bonding (ARB) process. Acta Mater. 1999, 47, 579–583. [Google Scholar] [CrossRef]
- Rao, G.N.M.; Kumar, V.R.M. A review on recent advances in accumulative roll bonding of similar, dissimilar and metal matrix composites. Mater. Today Proc. 2021, 56, A13–A18. [Google Scholar] [CrossRef]
- Ye, N.; Ren, X.; Liang, J. Microstructure and mechanical properties of Ni/Ti/Al/Cu composite produced by accumulative roll bonding (ARB) at room temperature. J. Mater. Res. Technol. 2020, 9, 5524–5532. [Google Scholar] [CrossRef]
- McCabe, R.J.; Nizolek, T.J.; Li, N.; Zhang, Y.F.; Coughlin, D.R.; Miller, C.; Carpenter, J.S. Evolution of microstructures and properties leading to layer instabilities during accumulative roll bonding of Fe-Cu, Fe-Ag, and Fe-Al. Mater. Des. 2021, 212, 110204. [Google Scholar] [CrossRef]
- Kadkhodaee, M.; Babaiee, M.; Manesh, H.D.; Pakshir, M.; Hashemi, B. Evaluation of corrosion properties of Al/nanosilica nanocomposite sheets produced by accumulative roll bonding (ARB) process. J. Alloy. Compd. 2013, 576, 66–71. [Google Scholar] [CrossRef]
- Shakouri, S.; Eghbali, B. Characterization of Microstructure and Mechanical Properties of Multilayer Al/Cu/Mg/Ni Composite Produced through Accumulative Roll Bonding. Phys. Met. Metallogr. 2019, 120, 796–805. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, H.; Park, S. Effect of extrusion ratios on hardness, microstructure, and crystal texture anisotropy in pure niobium tubes subjected to hydrostatic extrusion. Trans. Nonferrous Met. Soc. China 2021, 31, 1689–1699. [Google Scholar] [CrossRef]
- Sillekens, W.H.; Bohlen, J. Hydrostatic extrusion of magnesium alloys. In Advances in Wrought Magnesium Alloys; Woodhead Publishing: Duxford, UK, 2012; pp. 323–345. [Google Scholar]
- Garbacz, H.; Topolski, K.; Motyka, M. Hydrostatic extrusion. In Nanocrystalline Titanium; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–53. [Google Scholar]
- Sakai, T.; Belyakov, A.; Kaibyshev, R.; Miura, H.; Jonas, J.J. Dynamic and post-dynamic recrystallization under hot, cold and severe plastic deformation conditions. Prog. Mater. Sci. 2014, 60, 130–207. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lin, F.; Zhang, L.; Wang, X. Experimental study and finite element analysis on the frame of multi-directional forging press. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2016, 231, 2112–2122. [Google Scholar] [CrossRef]
- Shahriyari, F.; Shaeri, M.H.; Dashti, A.; Zarei, Z.; Noghani, M.T.; Cho, J.H.; Djavanroodi, F. Evolution of mechanical properties, microstructure and texture and of various brass alloys processed by multi-directional forging. Mater. Sci. Eng. A 2022, 831, 142149. [Google Scholar] [CrossRef]
- Perks, C.; Mudd, G. Titanium, zirconium resources and production: A state of the art literature review. Ore Geol. Rev. 2019, 107, 629–646. [Google Scholar] [CrossRef]
- Weiss, L.; Nessler, Y.; Novelli, M.; Laheurte, P.; Grosdidier, T. On the Use of Functionally Graded Materials to Differentiate the Effects of Surface Severe Plastic Deformation, Roughness and Chemical Composition on Cell Proliferation. Metals 2019, 9, 1344. [Google Scholar] [CrossRef] [Green Version]
- Kheradmandfard, M.; Kashani-Bozorg, S.F.; Lee, J.S.; Kim, C.-L.; Hanzaki, A.Z.; Pyun, Y.-S.; Cho, S.-W.; Amanov, A.; Kim, D.-E. Significant improvement in cell adhesion and wear resistance of biomedical β-type titanium alloy through ultrasonic nanocrystal surface modification. J. Alloy. Compd. 2018, 762, 941–949. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Parfenov, E.V.; Parfenova, L.V. Developing Nanostructured Metals for Manufacturing of Medical Implants with Improved Design and Biofunctionality. Mater. Trans. 2019, 60, 1356–1366. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Liu, L.; Li, B.; Qin, L.; Huang, L.; Yeung, K.W.K.; Han, Y. Nanograins on Ti-25Nb-3Mo-2Sn-3Zr alloy facilitate fabricating biological surface through dual-ion implantation to concurrently modulate the osteogenic functions of mesenchymal stem cells and kill bacteria. J. Mater. Sci. Technol. 2021, 73, 31–44. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, L.; Huang, L.; Zhu, J. Enhanced in-vitro osteoblastic functions on beta-type titanium alloy using surface mechanical attrition treatment. Mater. Sci. Eng. C Mater Biol. Appl. 2019, 97, 688–697. [Google Scholar] [CrossRef]
- Zhao, C.; Cao, P.; Ji, W.; Han, P.; Zhang, J.; Zhang, F.; Jiang, Y.; Zhang, X. Hierarchical titanium surface textures affect osteoblastic functions. J. Biomed. Mater. Res. A 2011, 99, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hu, B.; Tang, C.; Wu, Y.; Sun, P.; Zhang, X.; Jia, Y. Increased osteoblast function in vitro and in vivo through surface nanostructuring by ultrasonic shot peening. Int. J. Nanomed. 2015, 10, 4593–4603. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Yao, Q.T.; Zhang, Y.H.; Du, X.D.; Wu, Y.C.; Tong, W.P. Simultaneously improving surface mechanical properties and in vitro biocompatibility of pure titanium via surface mechanical attrition treatment combined with low-temperature plasma nitriding. Surf. Coat. Technol. 2017, 309, 382–389. [Google Scholar] [CrossRef]
- Luo, X.; Liang, C.; Li, N.; Zhu, Y.H.; Cao, N.J.; Wang, J.; Liu, K.D.; Zhao, H.W.; Wang, Z.B.; Wang, W. Effect of Gradient Nanostructured Ti on Behaviours of MG63 Cells In Vitro. J. Nanomater. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Zhao, C.; Han, P.; Ji, W.; Zhang, X. Enhanced mechanical properties and in vitro cell response of surface mechanical attrition treated pure titanium. J. Biomater. Appl. 2012, 27, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.E.; Neumann, A.; Ng, H.P.; Lapovok, R.; Kasper, C.; Lowe, T.C.; Anumalasetty, V.N.; Estrin, Y. Combined effect of grain refinement and surface modification of pure titanium on the attachment of mesenchymal stem cells and osteoblast-like SaOS-2 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Estrin, Y.; Ivanova, E.P.; Michalska, A.; Truong, V.K.; Lapovok, R.; Boyd, R. Accelerated stem cell attachment to ultrafine grained titanium. Acta Biomater. 2011, 7, 900–906. [Google Scholar] [CrossRef]
- Ito, Y.; Hoshi, N.; Hayakawa, T.; Ohkubo, C.; Miura, H.; Kimoto, K. Mechanical properties and biological responses of ultrafine-grained pure titanium fabricated by multi-directional forging. Mater. Sci. Eng. B 2019, 245, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Gurau, C.; Gurau, G.; Mitran, V.; Dan, A.; Cimpean, A. The Influence of Severe Plastic Deformation on Microstructure and In Vitro Biocompatibility of the New Ti-Nb-Zr-Ta-Fe-O Alloy Composition. Materials 2020, 13, 4853. [Google Scholar] [CrossRef]
- Yilmazer, H.; Şen, M.; Niinomi, M.; Nakai, M.; Huihong, L.; Cho, K.; Todaka, Y.; Shiku, H.; Matsue, T. Developing biomedical nano-grained β-type titanium alloys using high pressure torsion for improved cell adherence. RSC Adv. 2016, 6, 7426–7430. [Google Scholar] [CrossRef]
- Faghihi, S.; Azari, F.; Zhilyaev, A.P.; Szpunar, J.A.; Vali, H.; Tabrizian, M. Cellular and molecular interactions between MC3T3-E1 pre-osteoblasts and nanostructured titanium produced by high-pressure torsion. Biomaterials 2007, 28, 3887–3895. [Google Scholar] [CrossRef]
- Attarilar, S.; Salehi, M.T.; Al-Fadhalah, K.J.; Djavanroodi, F.; Mozafari, M. Functionally graded titanium implants: Characteristic enhancement induced by combined severe plastic deformation. PLoS ONE 2019, 14, e0221491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubacka, D.; Yamamoto, A.; Wieciński, P.; Garbacz, H. Biological behavior of titanium processed by severe plastic deformation. Appl. Surf. Sci. 2019, 472, 54–63. [Google Scholar] [CrossRef]
- Kim, T.N.; Balakrishnan, A.; Lee, B.C.; Kim, W.S.; Dvorankova, B.; Smetana, K.; Park, J.K.; Panigrahi, B.B. In vitro fibroblast response to ultra fine grained titanium produced by a severe plastic deformation process. J. Mater. Sci. Mater. Med. 2008, 19, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Wojtas, D.; Mzyk, A.; Kawalko, J.; Imbir, G.; Trembecka-Wojciga, K.; Marzec, M.; Jarzebska, A.; Maj, L.; Wierzbanowski, K.; Chulist, R.; et al. Texture-Governed Cell Response to Severely Deformed Titanium. ACS Biomater. Sci. Eng. 2021, 7, 114–121. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface modification of stainless steel for biomedical applications: Revisiting a century-old material. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 1073–1089. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R-Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, P.; Wu, L.H.; Song, Q.N.; Wang, D.; Xiao, B.L.; Ma, Z.Y. Effect of grain ultra-refinement on corrosion behavior of ultra-high strength high nitrogen stainless steel. Corros. Sci. 2020, 174, 108847. [Google Scholar] [CrossRef]
- Perumal, G.; Chakrabarti, A.; Grewal, H.S.; Pati, S.; Singh, S.; Arora, H.S. Enhanced antibacterial properties and the cellular response of stainless steel through friction stir processing. Biofouling 2019, 35, 187–203. [Google Scholar] [CrossRef]
- Perumal, G.; Grewal, H.S.; Arora, H.S. Enhanced durability, bio-activity and corrosion resistance of stainless steel through severe surface deformation. Colloids Surf. B Biointerfaces 2020, 194, 111197. [Google Scholar] [CrossRef]
- Bahl, S.; Shreyas, P.; Trishul, M.A.; Suwas, S.; Chatterjee, K. Enhancing the mechanical and biological performance of a metallic biomaterial for orthopedic applications through changes in the surface oxide layer by nanocrystalline surface modification. Nanoscale 2015, 7, 7704–7716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, R.D.; Thein-Han, W.W.; Pesacreta, T.C.; Somani, M.C.; Karjalainen, L.P. Biological significance of nanograined/ultrafine-grained structures: Interaction with fibroblasts. Acta Biomater. 2010, 6, 3339–3348. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.D.; Thein-Han, W.W.; Pesacreta, T.C.; Hasenstein, K.H.; Somani, M.C.; Karjalainen, L.P. Cellular response of preosteoblasts to nanograined/ultrafine-grained structures. Acta Biomater. 2009, 5, 1455–1467. [Google Scholar] [CrossRef]
- Gong, N.; Hu, C.; Hu, B.; An, B.; Misra, R.D.K. On the mechanical behavior of austenitic stainless steel with nano/ultrafine grains and comparison with micrometer austenitic grains counterpart and their biological functions. J. Mech. Behav. Biomed. Mater. 2020, 101, 103433. [Google Scholar] [CrossRef] [PubMed]
- Rybalchenko, O.V.; Anisimova, N.Y.; Kiselevsky, M.V.; Belyakov, A.N.; Tokar, A.A.; Terent’ev, V.F.; Prosvirnin, D.V.; Rybalchenko, G.V.; Raab, G.I.; Dobatkin, S.V. The influence of ultrafine-grained structure on the mechanical properties and biocompatibility of austenitic stainless steels. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Liao, H.B.; Mo, L.L.; Zhou, X.; Zhao, B.; Du, J. Grain refinement and improvement of mechanical properties of AZ31 magnesium alloy inoculated by in-situ oxidation process. J. Mater. Res. Technol. JmrT 2021, 12, 807–817. [Google Scholar] [CrossRef]
- Patel, V.; Li, W.; Andersson, J.; Li, N. Enhancing grain refinement and corrosion behavior in AZ31B magnesium alloy via stationary shoulder friction stir processing. J. Mater. Res. Technol. 2022, 17, 3150–3156. [Google Scholar] [CrossRef]
- Nayak, S.; Bhushan, B.; Jayaganthan, R.; Gopinath, P.; Agarwal, R.D.; Lahiri, D. Strengthening of Mg based alloy through grain refinement for orthopaedic application. J. Mech. Behav. Biomed. Mater. 2016, 59, 57–70. [Google Scholar] [CrossRef]
- Hou, X.; Qin, H.; Gao, H.; Mankoci, S.; Zhang, R.; Zhou, X.; Ren, Z.; Doll, G.L.; Martini, A.; Sahai, N.; et al. A systematic study of mechanical properties, corrosion behavior and biocompatibility of AZ31B Mg alloy after ultrasonic nanocrystal surface modification. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1061–1071. [Google Scholar] [CrossRef]
- Prithivirajan, S.; Nyahale, M.B.; Naik, G.M.; Narendranath, S.; Prabhu, A.; Rekha, P.D. Bio-corrosion impacts on mechanical integrity of ZM21 Mg for orthopaedic implant application processed by equal channel angular pressing. J. Mater. Sci. Mater. Med. 2021, 32, 65. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Nanocrystalline Ti49.2Ni50.8 shape memory alloy as orthopaedic implant material with better performance. J. Mater. Sci. Technol. 2019, 35, 2156–2162. [Google Scholar] [CrossRef]
- Zhang, R.; Mankoci, S.; Walters, N.; Gao, H.; Zhang, H.; Hou, X.; Qin, H.; Ren, Z.; Zhou, X.; Doll, G.L.; et al. Effects of laser shock peening on the corrosion behavior and biocompatibility of a nickel-titanium alloy. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.Y.; Nie, F.L.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Valiev, R.Z. Enhanced in vitro biocompatibility of ultrafine-grained biomedical NiTi alloy with microporous surface. Appl. Surf. Sci. 2011, 257, 9086–9093. [Google Scholar] [CrossRef]
- Saldana, L.; Mendez-Vilas, A.; Jiang, L.; Multigner, M.; Gonzalez-Carrasco, J.L.; Perez-Prado, M.T.; Gonzalez-Martin, M.L.; Munuera, L.; Vilaboa, N. In vitro biocompatibility of an ultrafine grained zirconium. Biomaterials 2007, 28, 4343–4354. [Google Scholar] [CrossRef]
- Rodríguez-Espinoza, B.L.; García-Pastor, F.A.; Martínez-Poveda, B.; Quesada, A.R.; Lopez-Crespo, P. High-strength low-modulus biocompatible Nb-1Zr alloy processed by accumulative roll bonding. Mater. Sci. Eng. A 2020, 797, 140226. [Google Scholar] [CrossRef]
- Huo, W.T.; Zhao, L.Z.; Yu, S.; Yu, Z.T.; Zhang, P.X.; Zhang, Y.S. Significantly enhanced osteoblast response to nano-grained pure tantalum. Sci. Rep. 2017, 7, 40868. [Google Scholar] [CrossRef] [Green Version]
- van Wachem, P.; Hogt, A.; Beugeling, T.; Feijen, J.; Bantjes, A.; Detmers, J.; van Aken, W. Adhesion of cultured human endothelial cells onto methacrylate polymers with varying surface wettability and charge. Biomaterials 1987, 8, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Misra, R.D.; Nune, C.; Pesacreta, T.C.; Somani, M.C.; Karjalainen, L.P. Understanding the impact of grain structure in austenitic stainless steel from a nanograined regime to a coarse-grained regime on osteoblast functions using a novel metal deformation-annealing sequence. Acta Biomater. 2013, 9, 6245–6258. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Olshanska, N.; de Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. A 2006, 76, 323–334. [Google Scholar] [CrossRef]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Dheda, S.S.; Kim, Y.K.; Melnyk, C.; Liu, W.; Mohamed, F.A. Corrosion and in vitro biocompatibility properties of cryomilled-spark plasma sintered commercially pure titanium. J. Mater. Sci. Mater. Med. 2013, 24, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Gu, J.F.; Liu, G.; Wang, Z.B.; Lu, J. Surface evolution of a gradient structured Ti in hydrogen peroxide solution. Appl. Surf. Sci. 2008, 254, 2905–2910. [Google Scholar] [CrossRef]
- Cai, K.; Lai, M.; Yang, W.; Hu, R.; Xin, R.; Liu, Q.; Sung, K.L. Surface engineering of titanium with potassium hydroxide and its effects on the growth behavior of mesenchymal stem cells. Acta Biomater. 2010, 6, 2314–2321. [Google Scholar] [CrossRef]
- Shen, X.; Ma, P.; Hu, Y.; Xu, G.; Zhou, J.; Cai, K. Mesenchymal stem cell growth behavior on micro/nano hierarchical surfaces of titanium substrates. Colloids Surf. B Biointerfaces 2015, 127, 221–232. [Google Scholar] [CrossRef]
- Motemani, Y.; Greulich, C.; Khare, C.; Lopian, M.; Buenconsejo, P.J.S.; Schildhauer, T.A.; Ludwig, A.; Koller, M. Adherence of human mesenchymal stem cells on Ti and TiO2 nano-columnar surfaces fabricated by glancing angle sputter deposition. Appl. Surf. Sci. 2014, 292, 626–631. [Google Scholar] [CrossRef]
- Wen, M.; Wen, C.; Hodgson, P.; Li, Y. Improvement of the biomedical properties of titanium using SMAT and thermal oxidation. Colloids Surf. B Biointerfaces 2014, 116, 658–665. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Wang, G.S.; Liu, Z.Q.; Huang, Y.; Wang, H.W. Morphologically modified surface with hierarchical micro-/nano-structures for enhanced bioactivity of titanium implants. J. Mater. Sci. 2018, 53, 12679–12691. [Google Scholar] [CrossRef]






| Material | Process | Grain Size | Cell Type | Cellular Behavior | Ref. |
|---|---|---|---|---|---|
| Ti-Nb alloy | SMAT, 5 min, 20 kHz | <100 nm | hMSC | Adhesion is enhanced | [61] |
| CpTi | USSP, 30–120 s, 20 kHz | 20.23–23.80 nm | hMSC | Viability and proliferation are enhanced | [14] |
| CpTi (Grade 2) | SMAT, 30 min, 25 Hz | 40 ± 15 nm | hMSC | Adhesion and proliferation are enhanced | [15] |
| CpTi plates | Ultrasonic-assisted SMAT, 20 min, 20 kHz | ~56 nm | hMSC | Adhesion, proliferation, and osteogenic differentiation are enhanced | [17] |
| TLM alloy (Ti-25Nb-3Mo-2Sn3Zr) | SMAT, 30 min, 50 Hz | 30–40 nm | Rabbit bone marrow mesenchymal stem cell | Adhesion, proliferation, osteogenic differentiation, and mineralization are enhanced | [64] |
| TLM alloy | SMAT, 60 min, 50 Hz | 26 ± 5 nm | hFOB1.19 | Adhesion, proliferation, osteogenic differentiation, and mineralization are enhanced | [65] |
| TLM alloy | SMAT, 20 kHz | 29.7 nm | Osteoblastic cell | Adhesion, spreading, viability, and osteogenic differentiation are enhanced | [66] |
| TNTZ alloy (Ti-29Nb-13Ta-4.6Zr) | UNSM, 20 kHz | 40–200 nm | Murine pre-osteoblast (MC3T3 cell) | Adhesion, spreading, and proliferation are enhanced | [62] |
| Pure Ti | USSP, 30 min, 50 kHz | 57–88 nm | Human osteoblast-like cell (MG63) | Adhesion, proliferation, ALP activity, and calcium deposition are enhanced | [67] |
| CpTi | SMAT, 60 min | 10 nm | Osteoblast cell (MG63 cell) | Adhesion, proliferation, and osteogenic differentiation are enhanced | [68] |
| CpTi | Ultrasonic-assisted SMAT, 20 min, 20 kHz | <100 nm | MG63 cell | Enhanced cell adhesion and proliferation, and alleviated cell apoptosis | [69] |
| CpTi | SMAT, 50 Hz | 25.2 nm | Saos-2 cell | Enhanced adhesion and viability, and promotion of the progression of cells into the S phase | [70] |
| Material | Process | Grain Size | Cell Type | Cellular Behavior | Ref. |
|---|---|---|---|---|---|
| CpTi (Grade 2 and Grade 4) | ECAP, pressing at 300 °C with a 90° angle channel | 230 nm | Primary human adipose-derived mesenchymal stem cell | Mineralization and viability are enhanced | [71] |
| CpTi (Grade 2) | ECAP, pressing at 350 °C with a 90° angle channel, 8 passes | 170–200 nm | hMSC | Adhesion and spreading are enhanced | [72] |
| CpTi (Grade 2) | ECAP, pressing at 400 °C with a 120° angle channel, 3 passes | 500–700 nm | Human fetal osteoblast cell (hFOB1.19) | Adhesion, spreading, and viability are enhanced | [16] |
| CpTi (Grade 2) | MDF | <100 nm | MC3T3-E1 cell | Proliferation is enhanced | [73] |
| Ti-31.5Nb-3.1Zr-3.1Ta-0.9Fe-0.16O alloy | HPT, 1–2 GPa, 100–2000 rpm | <1000 nm | MC3T3-E1 cell | Adhesion, proliferation, and osteogenic differentiation are enhanced | [74] |
| TNTZ alloy | HPT | 285 nm × 35 nm α needle and 12 nm β particle | Human osteoblast cell (hOB) | Attachment and proliferation are enhanced | [75] |
| CpTi | HPT, 6 GPa | 10–50 nm | MC3T3-E1 cell | Attachment and spreading are enhanced | [76] |
| CpTi (Grade 2) | ECAP (pressing at 450 °C with a 90° angle channel, 4 passes) + SMAT (2 h, 6 Hz) | ~420 nm | Homo sapiens human osteosarcoma cell (G292 cell) | Attachment, viability, and ALP activity are enhanced | [77] |
| CpTi (Grade 2 and Grade 4) | ECAP, pressing at 300 °C with a 90° angle channel | 230 nm | Human osteosarcoma cell (Saos-2 cell) | Viability is enhanced | [71] |
| CpTi (Grade 2) | HE, 10 stages | 87 nm | Saos-2 cell | Adhesion and proliferation are enhanced | [78] |
| CpTi (Grade 2) | ECAP, Route Bc | 0.238 ± 0.05 µm | Mouse fibroblast cell | Adhesion and proliferation are enhanced | [79] |
| CpTi (Grade 2) | HE, 4 stages | ~0.60 µm | Human umbilical vein endothelial cell (HUVEC) | (101(__)0) crystallographic plane favors cell attachment | [80] |
| Material | Process | Grain Size | Cell Type | Cellular Behavior | Ref. |
|---|---|---|---|---|---|
| 316L stainless steel | FSP, 1800 rpm | 0.9 µm | Primary human dermal fibroblast | Spreading and proliferation are enhanced | [84] |
| 316L stainless steel | FSP, 388 rpm | 0.6 µm | Primary human dermal fibroblast | Viability is decreased | [85] |
| 316L stainless steel | FSP, 1800 rpm | 0.8 µm | MDCK-1 cell and HepG2 | Enhanced cell attachment and proliferation, and restrained platelet and fibrinogen adhesion | [24] |
| 316L stainless steel | SMAT, 50 Hz, 15 min | <50 nm | MC3T3-E1 cell | Viability and spreading are enhanced | [86] |
| Material | Process | Grain Size | Cell Type | Cellular Behavior | Ref. |
|---|---|---|---|---|---|
| 316L stainless steel | Rolling, several passes | <1000 nm | Mouse fibroblast cell | Adhesion, viability, and proliferation are enhanced | [87] |
| 316L stainless steel | Rolling, several passes | <1000 nm | MC3T3-E1 cell | Enhanced vinculin signals and actin stress fibers in the outer region of the cells | [88] |
| Austenitic stainless steel | MDF | 200–400 nm | Pre-osteoblast cell | Adhesion and growth are enhanced | [89] |
| 316L stainless steel | ECAP, pressing at 400 °C with a 120° angle channel, 8 passes | 176 ± 10 nm | Multipotent mesenchymal stromal/stem cell (MMSC) | Enhanced cell proliferation and suppressed cell apoptosis | [90] |
| Material | Process | Grain Size | Cell Type | Cellular Behavior | Ref. |
|---|---|---|---|---|---|
| Mg-3Zn alloy | Rolling, 10 passes | <40 µm | MG63 | Viability is decreased | [94] |
| AZ31B Mg alloy | UNSM, 20 kHz | <(40–100) µm | Adipose-derived stem cell (ADSC) | Adhesion is not compromised | [95] |
| ZM21 Mg alloy | ECAP, pressing at 220 °C with a 90° angle channel, 4 passes | ~5.4 µm | MG63 cell | Viability is not compromised | [96] |
| Material | Process | Grain Size | Cell Type | Cellular Behavior | Ref. |
|---|---|---|---|---|---|
| Ni50.8Ti49.2 alloy | ECAP, 8 passes | 150–250 nm | Murine fibroblast cell line (L-929) | Viability, adhesion, proliferation, ALP activity, and mineralization are promoted | [97] |
| Ni50.3Ti49.7 alloy | LSP, laser with a wavelength of 1064 nm and intensity of 4 GW/cm2 | <1000 nm | Adipose-derived stem cell (ADSC) | Viability, growth, and spread are enhanced | [98] |
| Ni50.8Ti49.2 alloy | ECAP | 200–300 nm | MG63 cell | Attachment and proliferation are boosted | [99] |
| Material | Process | Grain Size | Cell Type | Cellular Behavior | Ref. |
|---|---|---|---|---|---|
| Pure Zr | Rolling, 1 pass | ~240 nm | Saos-2 cell and hMSC | Attachment, spreading, viability of Saos-2 cells, and ALP activity, mineralization nodule formation of hMSC are all unchanged | [100] |
| Nb-1Zr alloy | Accumulative Roll Bonding, 5 cycles | ~800 nm | Mouse fibroblast (L-929), primary gingival fibroblast (HGF), and human osteoblast-like cell (U-2 OS) | Survival is not compromised | [101] |
| Pure Ta | Sliding Friction Treatment, 100 cycle | ≤20 nm | hFOB1.19 | Adhesion, proliferation, osteogenic differentiation, and maturation are enhanced | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Huang, R.; Hao, Y. Review on Grain Refinement of Metallic Materials to Regulate Cellular Behavior. Metals 2022, 12, 829. https://doi.org/10.3390/met12050829
Gu Y, Huang R, Hao Y. Review on Grain Refinement of Metallic Materials to Regulate Cellular Behavior. Metals. 2022; 12(5):829. https://doi.org/10.3390/met12050829
Chicago/Turabian StyleGu, Yingjian, Run Huang, and Yufei Hao. 2022. "Review on Grain Refinement of Metallic Materials to Regulate Cellular Behavior" Metals 12, no. 5: 829. https://doi.org/10.3390/met12050829
APA StyleGu, Y., Huang, R., & Hao, Y. (2022). Review on Grain Refinement of Metallic Materials to Regulate Cellular Behavior. Metals, 12(5), 829. https://doi.org/10.3390/met12050829







