An Improved Approach to Manufacture Carbon Nanotube Reinforced Magnesium AZ91 Composites with Increased Strength and Ductility
Abstract
:1. Introduction
2. Experimental Procedure and Results
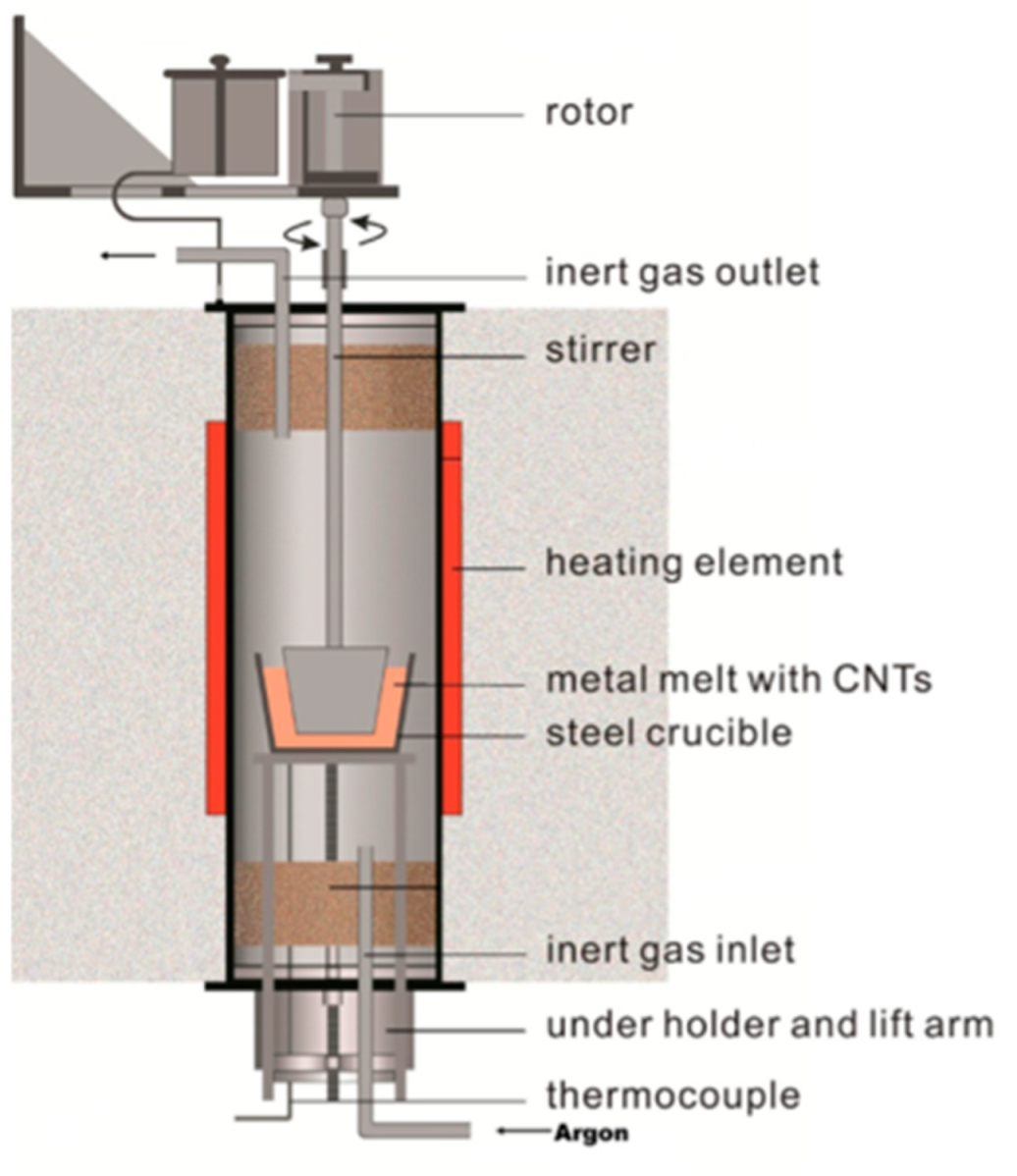
2.1. Synthesis of Mg AZ91 Composite Reinforced with Pt-Coated MWCNTs
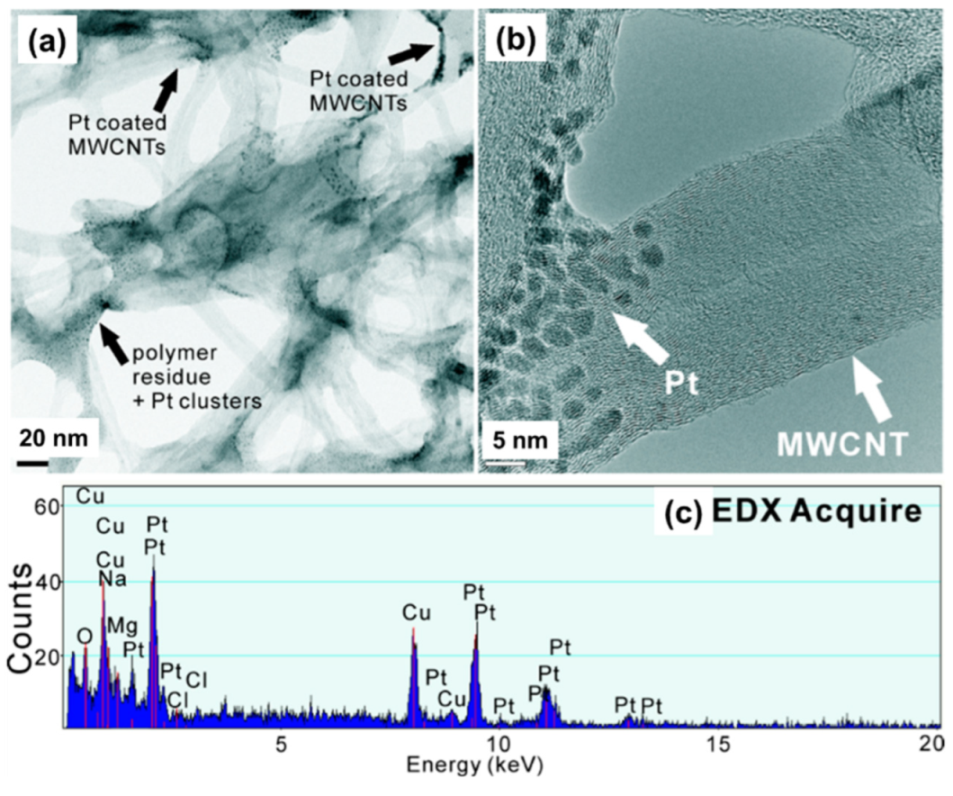
2.2. TEM Imaging and EDX Analysis of Pt-Coated MWCNTs
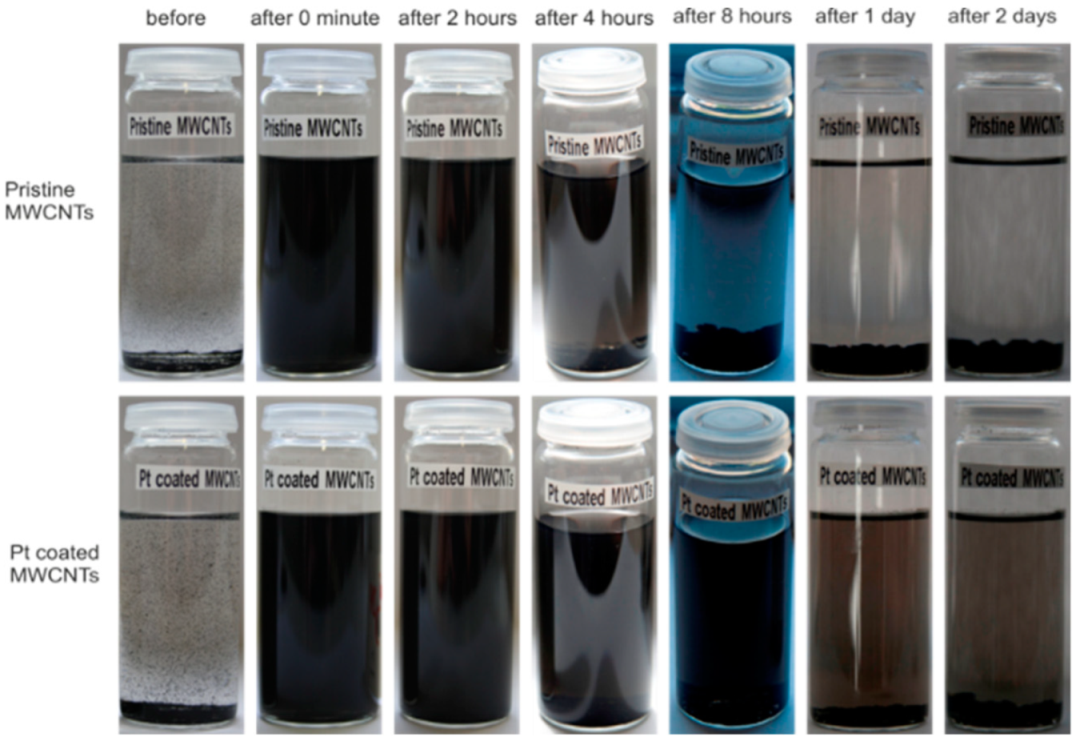
2.3. Dispersion of Pt Coated MWCNTs
2.4. Compression Tests of Composite
3. Atomistic Simulation Procedure and Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sammalkorpi, M.; Krasheninnikov, A.; Kuronen, A.; Nordlund, K.; Kaski, K. Mechanical properties of carbon nanotubes with vacancies and related defects. Phys. Rev. B 2004, 70, 245416. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, S.; Zaiser, M. Rupture of graphene sheets with randomly distributed defects. AIMS Mater. Sci. 2016, 3, 1340–1349. [Google Scholar] [CrossRef]
- Khare, R.; Mielke, S.L.; Paci, J.T.; Zhang, S.; Ballarini, R.; Schatz, G.C.; Belytschko, T. Coupled quantum mechanical/molecular mechanical modeling of the fracture of defective carbon nanotubes and graphene sheets. Phys. Rev. B 2007, 75, 075412. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Deng, L.; Young, R.J.; Kinloch, I.A.; Sun, R.; Zhang, G.; Noé, L.; Monthioux, M. Coefficient of thermal expansion of carbon nanotubes measured by Raman spectroscopy. Appl. Phys. Lett. 2014, 104, 051907. [Google Scholar] [CrossRef] [Green Version]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569. [Google Scholar] [CrossRef] [Green Version]
- Esawi, A.M.K.; Borady, M.A.E. Carbon nanotube-reinforced aluminium strips. Compos. Sci. Technol. 2008, 68, 486–492. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Agarwal, A. An analysis of the factors affecting strengthening in carbon nanotube reinforced aluminum composites. Carbon 2011, 49, 533–544. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. Carbon Nanotubes (CNTs)-Reinforced Magnesium-Based Matrix Composites: A Comprehensive Review. Materials 2020, 13, 4421. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Gan, W.; Hu, X.; Wu, K.; Wang, X. Investigation into the influence of carbon nanotubes addition on residual stresses and mechanical properties in the CNTs@SiCp/Mg-6Zn hybrid composite using neutron diffraction method. Mater. Sci. Eng. A 2020, 797, 140105. [Google Scholar] [CrossRef]
- Nam, D.H.; Kim, Y.K.; Cha, S.I.; Hong, S.H. Effect of CNTs on precipitation hardening behavior of CNT/Al–Cu composites. Carbon 2012, 50, 4809–4814. [Google Scholar] [CrossRef]
- Chen, B.; Shen, J.; Ye, X.; Jia, L.; Li, S.; Umeda, J.; Takahashi, M.; Kondoh, K. Length effect of carbon nanotubes on the strengthening mechanisms in metal matrix composites. Acta Mater. 2017, 140, 317–325. [Google Scholar] [CrossRef]
- Li, B.Q.; Sui, M.L.; Li, B.; Ma, E.; Mao, S.X. Reversible twinning in pure aluminum. Phys. Rev. Lett. 2009, 102, 205504. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Li, S.; Imai, H.; Jia, L.; Umeda, J.; Takahashi, M.; Kondoh, K. Load transfer strengthening in carbon nanotubes reinforced metal matrix composites via in-situ tensile tests. Compos. Sci. Technol. 2015, 113, 1–8. [Google Scholar] [CrossRef]
- Chen, B.; Kondoh, K.; Umeda, J.; Li, S.; Jia, L.; Li, J. Interfacial in-situ Al2O3 nanoparticles enhance load transfer in carbon nanotube (CNT)-reinforced aluminum matrix composites. J. Alloy. Compd. 2019, 789, 25–29. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Yeom, M.S.; Shin, J.W.; Kim, H.; Cui, Y.; Kysar, J.W.; Hone, J.; Jung, Y.; Jeon, S.; et al. Strengthening effect of single-atomic-layer graphene in metal-graphene nanolayered composites. Nat. Commun. 2013, 4, 2114. [Google Scholar] [CrossRef]
- Nasiri, S.; Zaiser, M. Effects of elasticity and dislocation core structure on the interaction of dislocations with embedded CNTs in aluminium: An atomistic simulation study. Materialia 2022, 21, 101347. [Google Scholar] [CrossRef]
- Cheng, K.; He, D.; Peng, T.; Lv, H.; Pan, M.; Mu, S. Porous graphene supported Pt catalysts for proton exchange membrane fuel cells. Electrochim. Acta 2014, 132, 356–363. [Google Scholar] [CrossRef]
- Choi, H.-J.; Jung, S.-M.; Seo, J.-M.; Chang, D.W.; Dai, L.; Baek, J.-B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- George, R.; Kashyap, K.T.; Rahul, R.; Yamdagni, S. Strengthening in carbon nanotube/aluminium (CNT/Al) composites. Scr. Mater. 2005, 53, 1159–1163. [Google Scholar] [CrossRef]
- Li, Q.; Viereckl, A.; Rottmair, C.A.; Singer, R.F. Improved processing of carbon nanotube/magnesium alloy composites. Compos. Sci. Technol. 2009, 69, 1193–1199. [Google Scholar] [CrossRef]
- Choi, B.K.; Yoon, G.H.; Lee, S. Molecular dynamics studies of CNT-reinforced aluminum composites under uniaxial tensile loading. Compos. Part B Eng. 2016, 91, 119–125. [Google Scholar] [CrossRef]
- Park, D.M.; Kim, J.H.; Lee, S.J.; Yoon, G.H. Analysis of geometrical characteristics of CNT-Al composite using molecular dynamics and the modified rule of mixture (MROM). J. Mech. Sci. Technol. 2018, 32, 5845–5853. [Google Scholar] [CrossRef]
- Xiang, J.; Xie, L.; Meguid, S.A.; Pang, S.; Yi, J.; Zhang, Y.; Liang, R. An atomic-level understanding of the strengthening mechanism of aluminum matrix composites reinforced by aligned carbon nanotubes. Comput. Mater. Sci. 2017, 128, 359–372. [Google Scholar] [CrossRef]
- Nasiri, S.; Wang, K.; Yang, M.; Guénolé, J.; Li, Q.; Zaiser, M. Atomistic aspects of load transfer and fracture in CNT-reinforced aluminium. Materialia 2022, 22, 101376. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, P.; Shi, L.; Jiang, Q. Wetting of B4C, TiC and graphite substrates by molten Mg. Mater. Chem. Phys. 2011, 130, 665–671. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Development of novel carbon nanotube reinforced magnesium nanocomposites using the powder metallurgy technique. Nanotechnology 2005, 17, 7–12. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Simultaneous enhancement in strength and ductility by reinforcing magnesium with carbon nanotubes. Mater. Sci. Eng. A 2006, 423, 153–156. [Google Scholar] [CrossRef]
- Zhou, S.-M.; Zhang, X.-B.; Ding, Z.-P.; Min, C.-Y.; Xu, G.-L.; Zhu, W.-M. Fabrication and tribological properties of carbon nanotubes reinforced Al composites prepared by pressureless infiltration technique. Compos. Part A Appl. Sci. Manuf. 2007, 38, 301–306. [Google Scholar] [CrossRef]
- Deng, C.F.; Wang, D.Z.; Zhang, X.X.; Li, A.B. Processing and properties of carbon nanotubes reinforced aluminum composites. Mater. Sci. Eng. A 2007, 444, 138–145. [Google Scholar] [CrossRef]
- Li, Q.; Rottmair, C.A.; Singer, R.F. CNT reinforced light metal composites produced by melt stirring and by high pressure die casting. Compos. Sci. Technol. 2010, 70, 2242–2247. [Google Scholar] [CrossRef] [Green Version]
- Ip, S.W.; Sridhar, R.; Toguri, J.M.; Stephenson, T.F.; Warner, A.E.M. Wettability of nickel coated graphite by aluminum. Mater. Sci. Eng. A 1998, 244, 31–38. [Google Scholar] [CrossRef]
- Chu, H.Y.; Lin, J.F. Experimental analysis of the tribological behavior of electroless nickel-coated graphite particles in aluminum matrix composites under reciprocating motion. Wear 2000, 239, 126–142. [Google Scholar] [CrossRef]
- Guo, M.L.T.; Tsao, C.-Y.A. Tribological behavior of aluminum/SiC/nickel-coated graphite hybrid composites. Mater. Sci. Eng. A 2002, 333, 134–145. [Google Scholar]
- Song, H.-Y.; Zha, X.-W. Influence of nickel coating on the interfacial bonding characteristics of carbon nanotube–aluminum composites. Comput. Mater. Sci. 2010, 49, 899–903. [Google Scholar] [CrossRef]
- Duan, K.; Li, L.; Hu, Y.; Wang, X. Enhanced interfacial strength of carbon nanotube/copper nanocomposites via Ni-coating: Molecular-dynamics insights. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 88, 259–264. [Google Scholar] [CrossRef]
- Nasiri, S.; Wang, K.; Yang, M.; Li, Q.; Zaiser, M. Nickel coated carbon nanotubes in aluminum matrix composites: A multiscale simulation study. Eur. Phys. J. B 2019, 92, 186. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.Z.; Zhang, X.B.; Xiong, W.Q.; Liu, F.; Huang, W.Z.; Sun, Y.L.; Tu, J.P.; Chen, X.W. Continuous Ni-layer on multiwall carbon nanotubes by an electroless plating method. Surf. Coat. Technol. 2002, 155, 33–36. [Google Scholar] [CrossRef]
- Jafri, R.I.; Sujatha, N.; Rajalakshmi, N.; Ramaprabhu, S. Au–MnO2/MWNT and Au–ZnO/MWNT as oxygen reduction reaction electrocatalyst for polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energy 2009, 34, 6371–6376. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Liu, Y.; Shen, G.; Yu, R. Platinum nanoparticles-doped sol–gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens. Bioelectron. 2006, 21, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Day, T.M.; Unwin, P.R.; Wilson, N.R.; Macpherson, J.V. Electrochemical templating of metal nanoparticles and nanowires on single-walled carbon nanotube networks. J. Am. Chem. Soc. 2005, 127, 10639–10647. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Dai, L. Substrate-enhanced electroless deposition of metal nanoparticles on carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 10806–10807. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Zhang, H.; Wu, P.; Yu, J.; Yang, D. A general approach for uniform coating of a metal layer on MWCNTs via layer-by-layer assembly. J. Phys. Chem. C 2009, 113, 17387–17391. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Nasiri, S.; Greff, C.; Wang, K.; Yang, M.; Li, Q.; Moretti, P.; Zaiser, M. Multilayer Structures of Graphene and Pt Nanoparticles: A Multiscale Computational Study. Adv. Eng. Mater. 2020, 22, 2000207. [Google Scholar] [CrossRef]
- Khomyakov, P.A.; Giovannetti, G.; Rusu, P.C.; Brocks, G.V.; Van den Brink, J.; Kelly, P.J. First-principles study of the interaction and charge transfer between graphene and metals. Phys. Rev. B 2009, 79, 195425. [Google Scholar] [CrossRef] [Green Version]
- Schneider, W.B.; Benedikt, U.; Auer, A.A. Interaction of platinum nanoparticles with graphitic carbon structures: A computational study. ChemPhysChem 2013, 14, 2984–2989. [Google Scholar] [CrossRef]
- Ramos-Sanchez, G.; Balbuena, P. Interactions of platinum clusters with a graphite substrate. Phys. Chem. Chem. Phys. 2013, 15, 11950–11959. [Google Scholar] [CrossRef]
- Gan, Y.; Sun, L.; Banhart, F. One-and Two-Dimensional Diffusion of Metal Atoms in Graphene. Small 2008, 4, 587–591. [Google Scholar] [CrossRef]
- Hippmann, S.; Li, Q.; Addinal, R.; Volk, W. Carbon nanotubes–reinforced copper matrix composites produced by melt stirring. Proc. Inst. Mech. Eng. Part N J. Nanoeng. Nanosyst. 2013, 227, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Everitt, B. Statistical Models in S; Chambers, J.M., Hastie, T.J., Eds.; Book reviews; Statistical Methods in Medical Research; Wadsworth and Brooks/Cole: Los Angeles, CA, USA, 1992; Volume 1, pp. 220–221. ISBN 0-534-16765-9. [Google Scholar]
- Yandell, B.S. Practical Data Analysis for Designed Experiments; Taylor & Francis: Abingdon, UK, 1997. [Google Scholar]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.W.; Johnson, R.A.; Wadley, H.N.G. Misfit-energy-increasing dislocations in vapor-deposited CoFe/NiFe multilayers. Phys. Rev. B 2004, 69, 144113. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Song, S.; Li, L.; Zhang, R. Molecular dynamics simulation for mechanical properties of magnesium matrix composites reinforced with nickel-coated single-walled carbon nanotubes. J. Compos. Mater. 2016, 50, 191–200. [Google Scholar] [CrossRef]
- Evans, D.J.; Holian, B.L. The Nose–Hoover thermostat. J. Chem. Phys. 1985, 83, 4069–4074. [Google Scholar] [CrossRef]
- Vanin, M.; Mortensen, J.J.; Kelkkanen, A.K.; Garcia-Lastra, J.M.; Thygesen, K.S.; Jacobsen, K.W. Graphene on metals: A van der Waals density functional study. Phys. Rev. B 2010, 81, 081408. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Lee, G.; Shan, B.; Vogel, E.M.; Wallace, R.M.; Cho, K. First-principles study of metal–graphene interfaces. J. Appl. Phys. 2010, 108, 123711. [Google Scholar] [CrossRef]
- Fampiou, I.; Ramasubramaniam, A. Binding of Pt nanoclusters to point defects in graphene: Adsorption, morphology, and electronic structure. J. Phys. Chem. C 2012, 116, 6543–6555. [Google Scholar] [CrossRef]
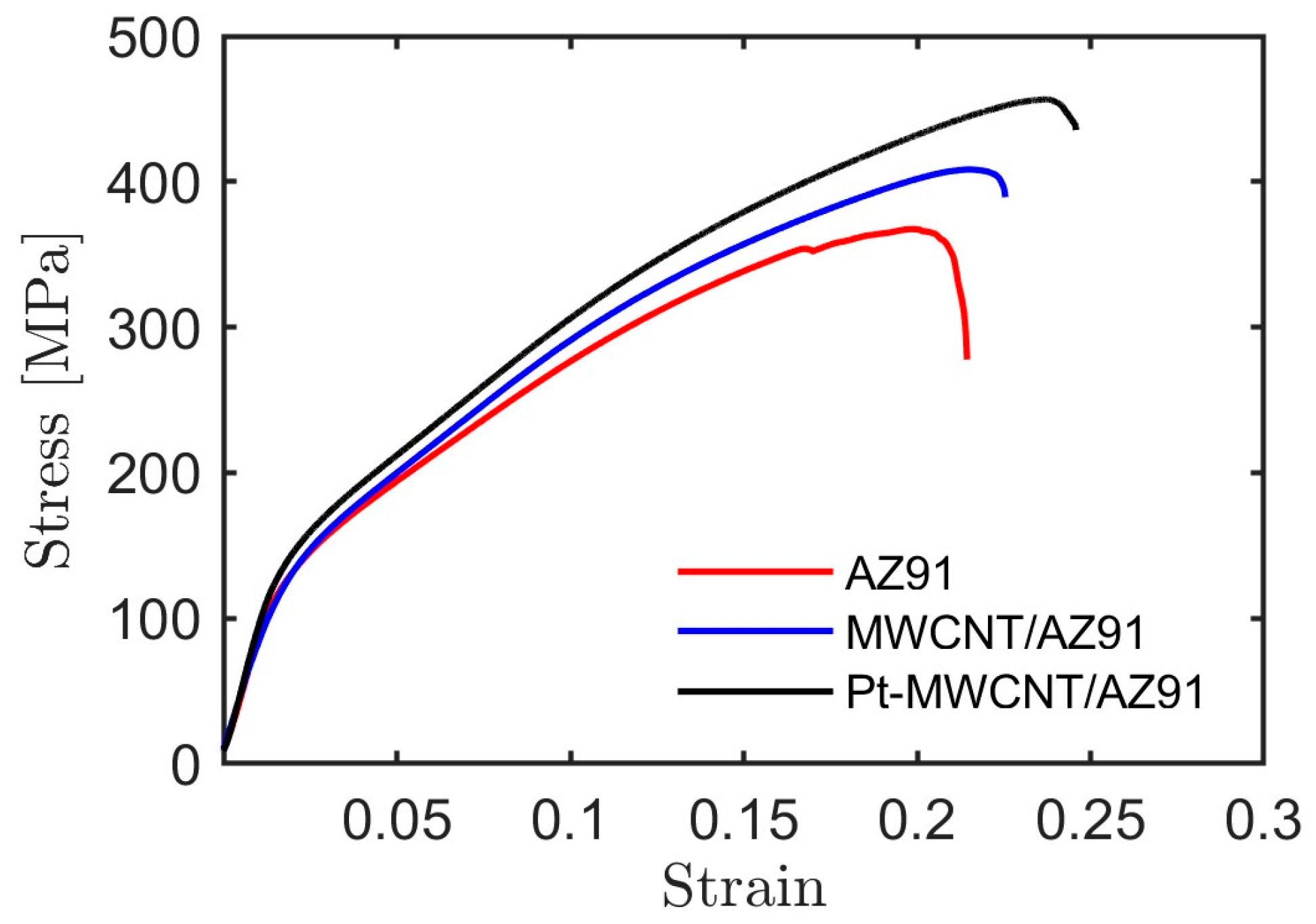
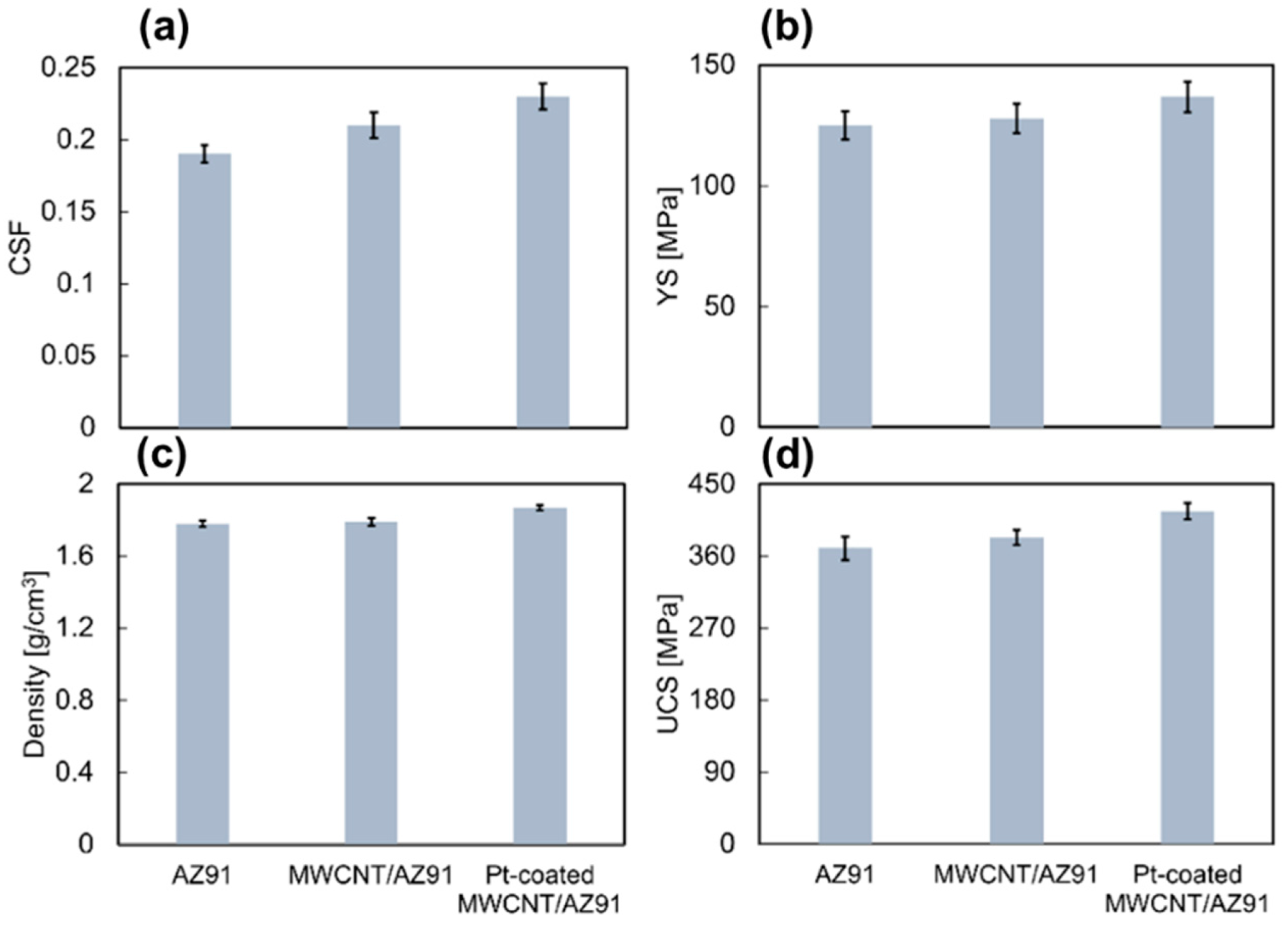


| Sample | 2% YS [MPa] | UCS [Mpa] | CSF [%] | Density [g/cm3] |
|---|---|---|---|---|
| AZ91 | 125 ± 5.6 | 370 ± 14.4 | 19 ± 0.6 | 1.78 ± 0.01 |
| MWCNT/AZ91 | 128 ± 5.8 | 383 ± 9.3 | 21 ± 0.9 | 1.79 ± 0.02 |
| Pt-MWCNT/AZ91 | 137 ± 7.0 | 416 ± 10.0 | 23 ± 0.9 | 1.87 ± 0.01 |
| 2% YS | |||||
|---|---|---|---|---|---|
| Factor | Degree of Freedom | Sum of Squares | Mean Square | F Value | Pr Value |
| Different samples (Pure AZ91, MWCNT/AZ91, Pt-MWCNT/AZ91) | 2 | 949 | 474.5 | 5.344 | 6.08 × 10−3 |
| Residuals | 34 | 3019 | 88.8 | ||
| USC | |||||
| Different samples | 2 | 15,767 | 7883 | 34.73 | 6.08 × 10−9 |
| Residuals | 34 | 7717 | 227 | ||
| CSF | |||||
| Different samples | 2 | 47.28 | 23.64 | 29.16 | 4.21 × 10−8 |
| Residuals | 34 | 27.56 | 0.811 | ||
| 2% YS | ||||
|---|---|---|---|---|
| Sample | Difference of Means | Lwr | Upr | Adjusted p-Value |
| AZ91 vs. MWCNT/AZ91 | 3.056250 | −8.488996 | 14.60150 | 0.7943670 |
| AZ91 vs. Pt-MWCNT/AZ91 | 11.547798 | 1.954288 | 21.14131 | 0.0153950 |
| Pt-MWCNT/AZ91 vs. MWCNT/AZ91 | 8.491548 | −1.101962 | 18.08506 | 0.0912189 |
| UCS | ||||
| AZ91 vs. MWCNT/AZ91 | 13.1500 | −5.30850 | 31.60850 | 0.2032903 |
| AZ91 vs. Pt-MWCNT/AZ91 | 47.31845 | 31.98038 | 62.65652 | 0 |
| Pt-MWCNT/AZ91 vs. MWCNT/AZ91 | 34.16845 | 18.83038 | 49.50652 | 0.0000128 |
| CSF | ||||
| AZ91 vs. MWCNT/AZ91 | 0.637500 | −0.465587 | 1.740587 | 0.3440493 |
| AZ91 vs. Pt-MWCNT/AZ91 | 2.560952 | 1.644343 | 3.477561 | 0.0000002 |
| Pt-MWCNT/AZ91 vs. MWCNT/AZ91 | 1.923452 | 1.006843 | 2.840061 | 0.0000328 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiri, S.; Yang, G.; Spiecker, E.; Li, Q. An Improved Approach to Manufacture Carbon Nanotube Reinforced Magnesium AZ91 Composites with Increased Strength and Ductility. Metals 2022, 12, 834. https://doi.org/10.3390/met12050834
Nasiri S, Yang G, Spiecker E, Li Q. An Improved Approach to Manufacture Carbon Nanotube Reinforced Magnesium AZ91 Composites with Increased Strength and Ductility. Metals. 2022; 12(5):834. https://doi.org/10.3390/met12050834
Chicago/Turabian StyleNasiri, Samaneh, Guang Yang, Erdmann Spiecker, and Qianqian Li. 2022. "An Improved Approach to Manufacture Carbon Nanotube Reinforced Magnesium AZ91 Composites with Increased Strength and Ductility" Metals 12, no. 5: 834. https://doi.org/10.3390/met12050834
APA StyleNasiri, S., Yang, G., Spiecker, E., & Li, Q. (2022). An Improved Approach to Manufacture Carbon Nanotube Reinforced Magnesium AZ91 Composites with Increased Strength and Ductility. Metals, 12(5), 834. https://doi.org/10.3390/met12050834






