A New Strategy for Dissimilar Material Joining between SiC and Al Alloys through Use of High-Si Al Alloys
Abstract
:1. Introduction
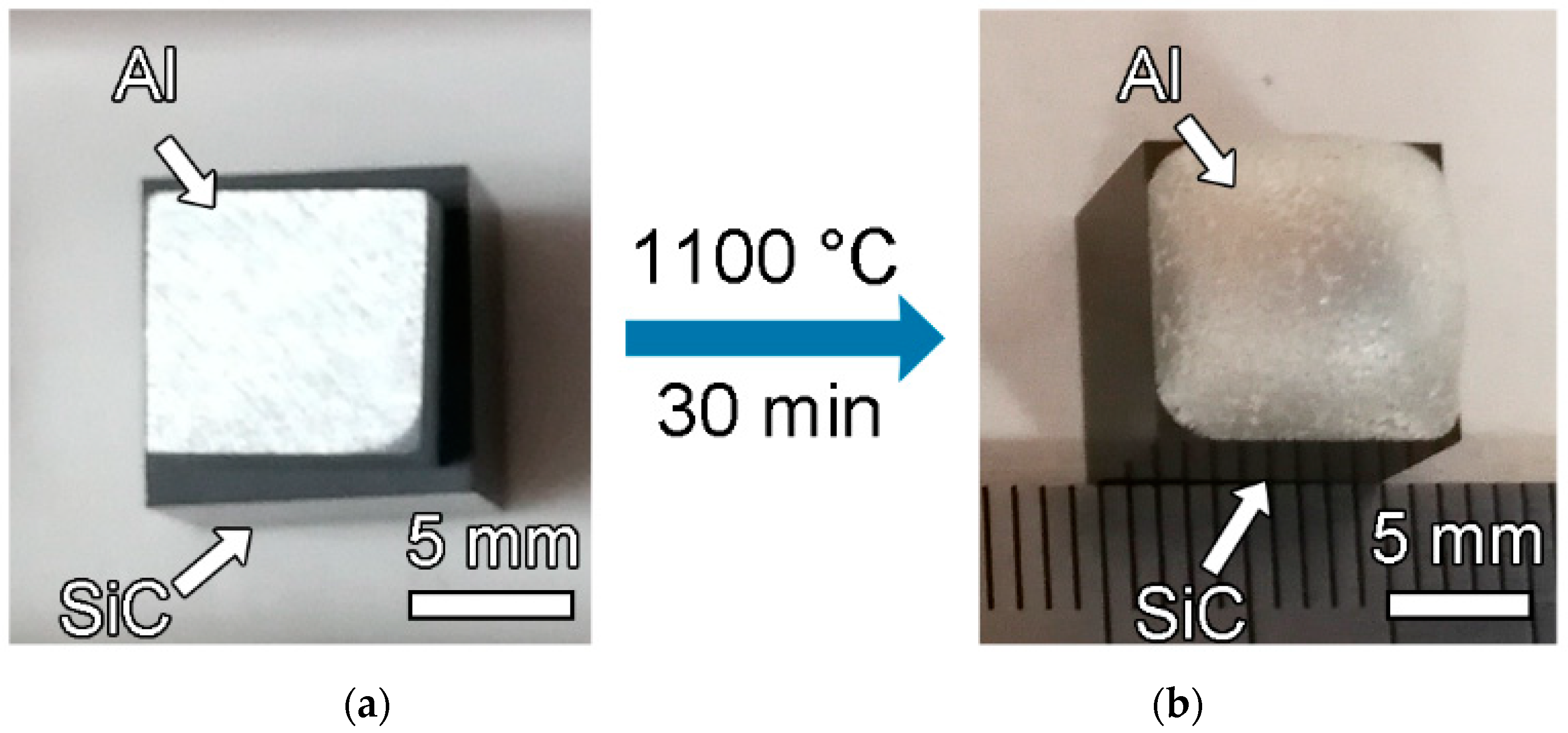
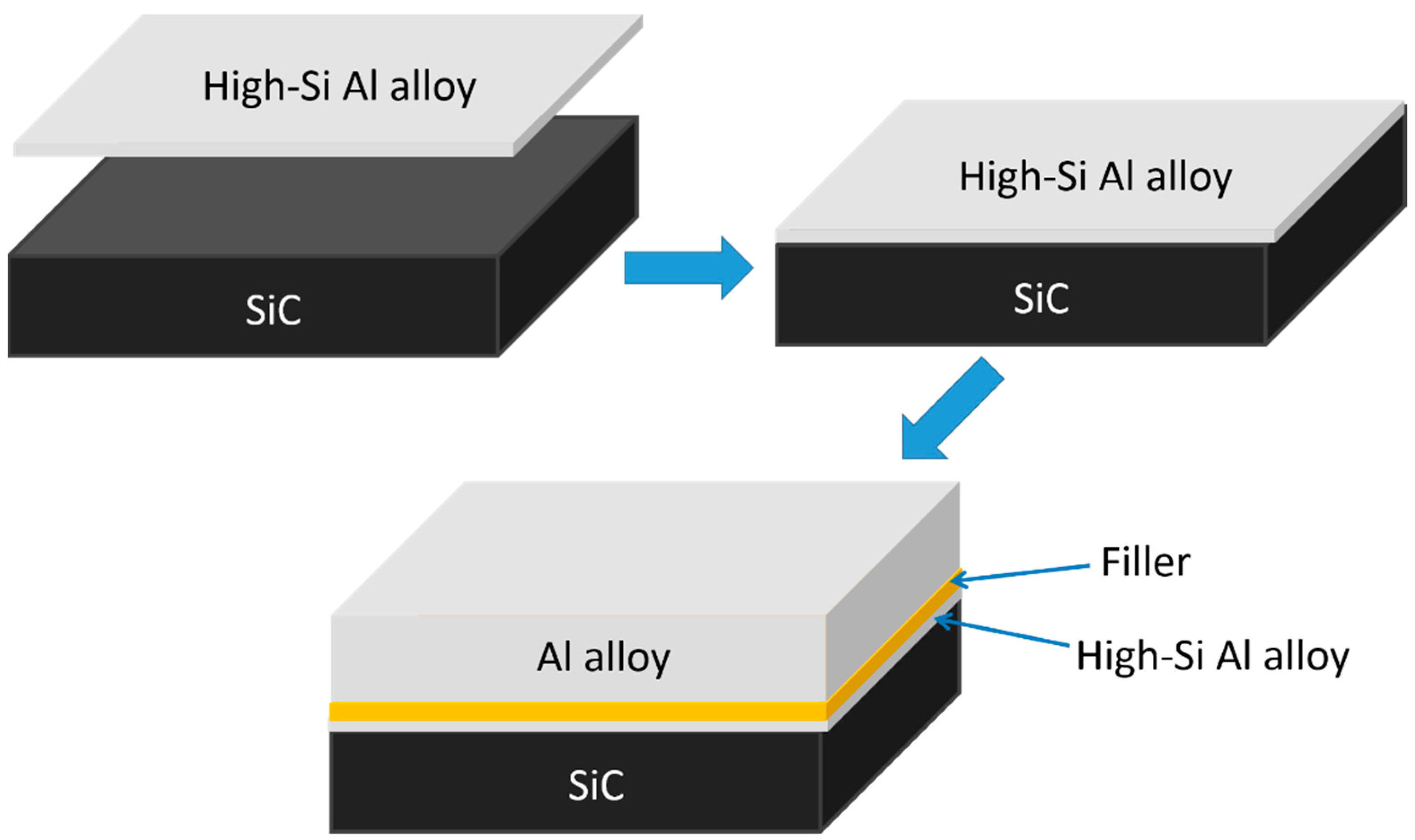
2. Materials and Methods
3. Results and Discussion
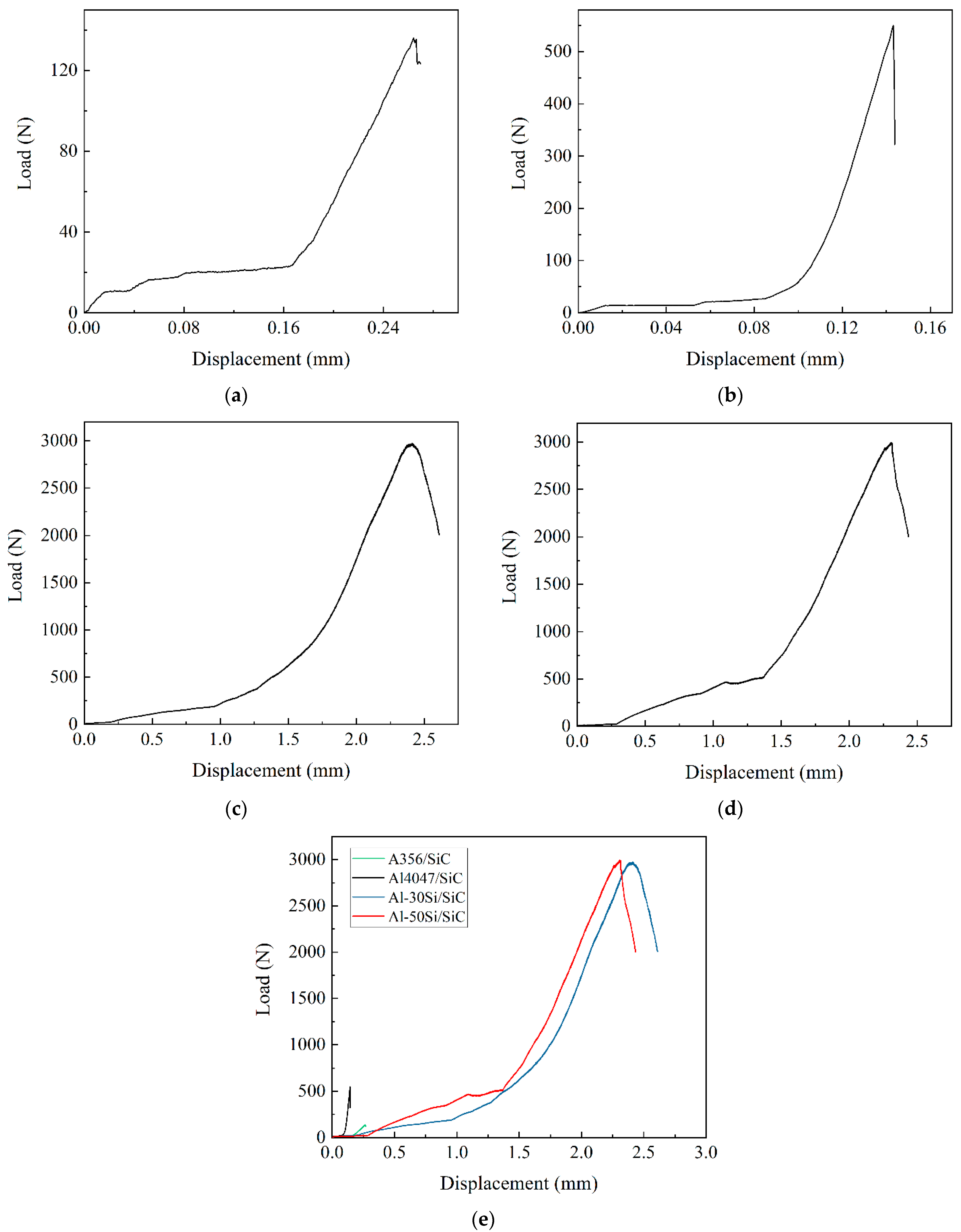
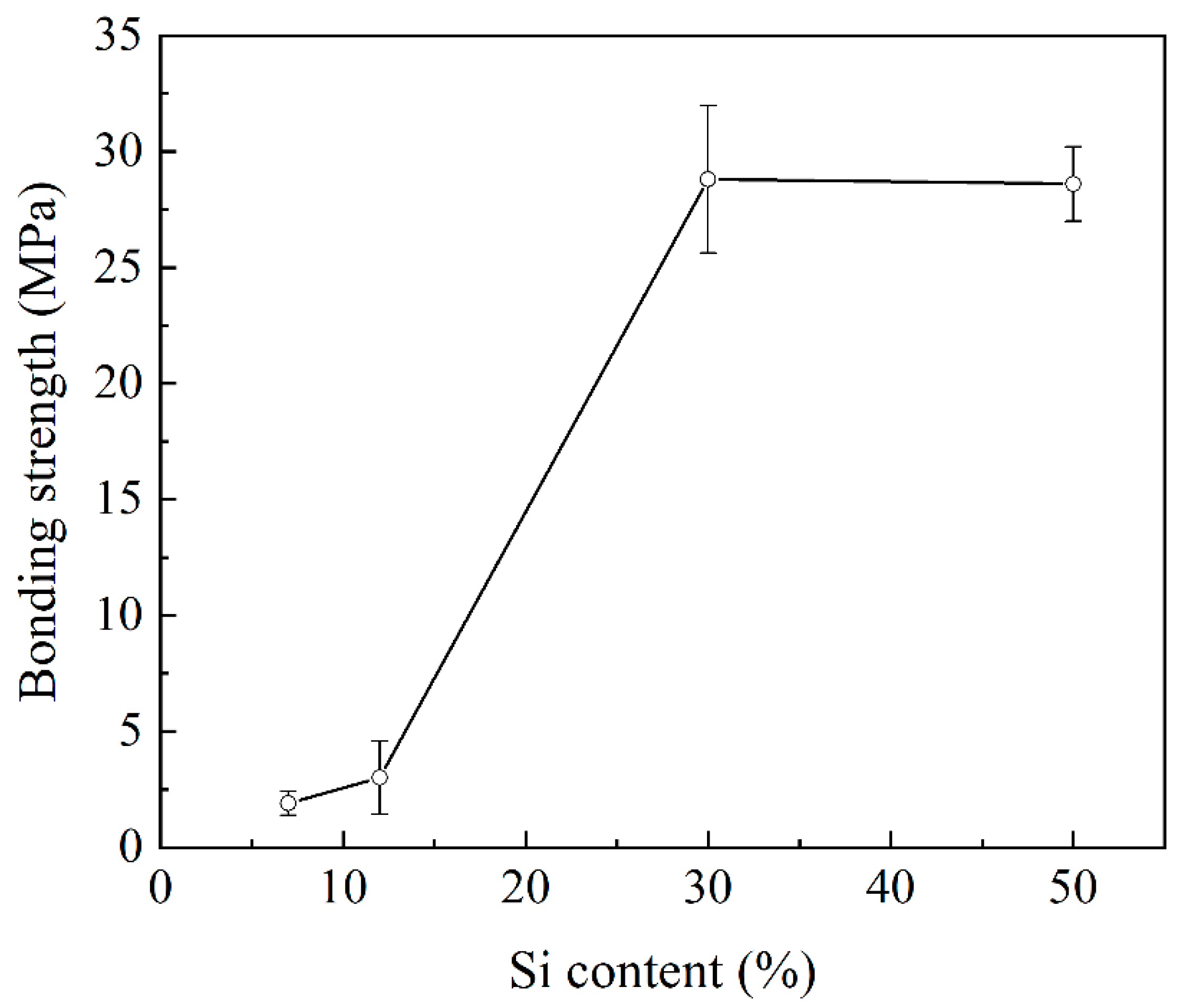
3.1. Effect of Si Content on Bonding Strength
3.2. Effect of Si Content on CTE
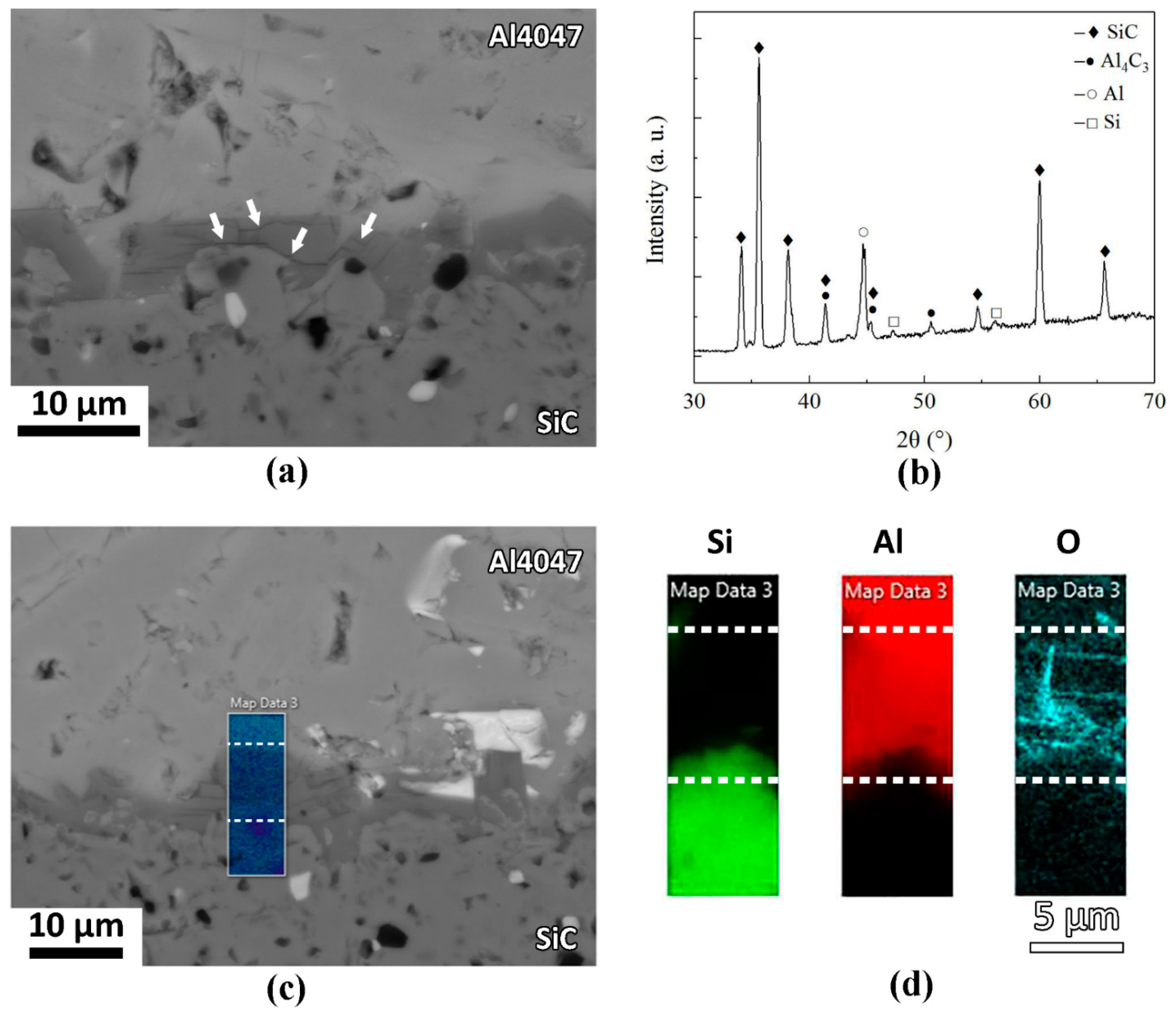
3.3. Effect of Si Content on Interfacial Reactions
3.4. Perspectives
4. Conclusions
- (1)
- The bonding strength of the joints was low when using low-Si Al alloys (A356 and Al4047) to bond with SiC. A significant improvement in bonding strength was found in high-Si Al/SiC joints (Al-30Si/SiC and Al-50Si/SiC).
- (2)
- Microstructure characterization revealed that a reaction layer with a thickness of a few micrometers occurred at the interface in the Al4047/SiC joint. The reaction layer consisted of brittle Al4C3 phase, resulting in a lower bonding strength of the joints.
- (3)
- The remarkable improvement in bonding strength in high-Si Al/SiC joints could be attributed to the reduced thermal stresses and the suppression of brittle reaction products at the interface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.C.; McGinn, P.J.; Mukasyan, A.S. High temperature rapid reactive joining of dissimilar materials: Silicon carbide to an aluminum alloy. J. Eur. Ceram. Soc. 2012, 32, 3809–3818. [Google Scholar] [CrossRef]
- Südmeyer, I.; Hettesheimer, T.; Rohde, M. On the shear strength of laser brazed SiC-steel joints: Effects of braze metal fillers and surface patterning. Ceram. Int. 2010, 36, 1083–1090. [Google Scholar] [CrossRef]
- Zhong, Z.; Hinoki, T.; Jung, H.C.; Park, Y.H.; Kohyama, A. Microstructure and mechanical properties of diffusion bonded SiC/steel joint using W/Ni interlayer. Mater. Des. 2010, 31, 1070–1076. [Google Scholar] [CrossRef]
- Zhong, Z.; Hinoki, T.; Kohyama, A. Microstructure and mechanical strength of diffusion bonded joints between silicon carbide and F82H steel. J. Nucl. Mater. 2011, 417, 395–399. [Google Scholar] [CrossRef]
- Zhu, M.; Chung, D.D.L. Active brazing alloy containing carbon fibers for metal-ceramic joining. J. Am. Ceram. Soc. 1994, 77, 2712–2720. [Google Scholar] [CrossRef]
- Huang, B.; Shu, Z.; Li, T.; Li, H.; Zhang, D.; Yi, F. Effect of different interlayers on microstructure and mechanical properties of diffusion-bonded joints between SiC and 316LN. Philos. Mag. 2022, 102, 137–152. [Google Scholar] [CrossRef]
- Song, Y.; Liu, D.; Song, X.; Hu, S.; Cao, J. In-situ synthesis of TiC nanoparticles during joining of SiC ceramic and GH99 superalloy. J. Am. Ceram. Soc. 2019, 102, 6529–6541. [Google Scholar] [CrossRef]
- Xiong, H.P.; Mao, W.; Xie, Y.H.; Guo, W.L.; Li, X.H.; Cheng, Y.Y. Brazing of SiC to a wrought nickel-based superalloy using CoFeNi(Si, B)CrTi filler metal. Mater. Lett. 2007, 61, 4662–4665. [Google Scholar] [CrossRef]
- Shi, H.; Chai, Y.; Li, N.; Yan, J.; Peng, H.; Zhang, R.; Li, M.; Bai, D.; Chen, K.; Liu, Z.; et al. Investigation of interfacial reaction mechanism between SiC and Inconel 625 superalloy using thermodynamic calculation. J. Eur. Ceram. Soc. 2021, 41, 3960–3969. [Google Scholar] [CrossRef]
- Sozhamannan, G.G.; Prabu, S.B. Evaluation of interface bonding strength of aluminum/silicon carbide. Int. J. Adv. Manuf. Technol. 2009, 44, 385–388. [Google Scholar] [CrossRef]
- Sozhamannan, G.G.; Prabu, S.B. Influence of interface compounds on interface bonding characteristics of aluminium and silicon carbide. Mater. Charact. 2009, 60, 986–990. [Google Scholar] [CrossRef]
- Teng, P.; Li, X.; Hua, P.; Liu, H.; Wang, G. Effect of metallization temperature on brazing joints of SiC ceramics and 2219 aluminum alloy. Int. J. Appl. Ceram. Technol. 2022, 19, 498–507. [Google Scholar] [CrossRef]
- Xu, R.; Indacochea, J.E. Silicon nitride-stainless steel braze joining with an active filler metal. J. Mater. Sci. 1994, 29, 6287–6294. [Google Scholar] [CrossRef]
- Bissig, V.; Galli, M.; Janczak-Rusch, J. Comparison of three different active filler metals used for brazing ceramic-to-ceramic and ceramic-to-metal. Adv. Eng. Mater. 2006, 8, 191–196. [Google Scholar] [CrossRef]
- An, Q.; Cong, X.S.; Shen, P.; Jiang, Q.C. Roles of alloying elements in wetting of SiC by Al. J. Alloys Compd. 2019, 784, 1212–1220. [Google Scholar] [CrossRef]
- Fernie, J.A.; Drew, R.A.L.; Knowles, K.M. Joining of engineering ceramics. Int. Mater. Rev. 2009, 54, 283–331. [Google Scholar] [CrossRef]
- Cong, X.S.; Shen, P.; Wang, Y.; Jiang, Q. Wetting of polycrystalline SiC by molten Al and Al-Si alloys. Appl. Surf. Sci. 2014, 317, 140–146. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, X.; Wang, T.; Liu, G.; Shao, H.; Wan, Y.; Qiao, G. Effects of Pd ion implantation and Si addition on wettability of Al/SiC system. Surf. Coat. Technol. 2018, 335, 198–204. [Google Scholar] [CrossRef]
- Lee, J.C.; Park, S.B.; Seok, H.K.; Oh, C.S.; Lee, H.I. Prediction of Si contents to suppress the interfacial reaction in the SiCp/2014 Al composite. Acta Mater. 1998, 46, 2635–2643. [Google Scholar] [CrossRef]
- Chien, C.W.; Lee, S.L.; Lin, J.C.; Jahn, M.T. Effects of Sip size and volume fraction on properties of Al/Sip composites. Mater. Lett. 2002, 52, 334–341. [Google Scholar] [CrossRef]
- Bhowmik, A.; Yang, Y.; Zhou, W.; Chew, Y.; Bi, G. On the heterogeneous cooling rates in laser-clad Al-50Si alloy. Surf. Coat. Technol. 2021, 408, 126780. [Google Scholar] [CrossRef]
- Fan, D.; Huang, J.; Wang, Y.; Chen, S.; Zhao, X. Active brazing of carbon fiber reinforced SiC composite and 304 stainless steel with Ti-Zr-Be. Mater. Sci. Eng. A 2014, 617, 66–72. [Google Scholar] [CrossRef]
- Nunes, R.; Adams, J.H.; Ammons, M.; Avery, H.S.; Barnhurst, R.J.; Bean, J.C.; Beaudry, B.J. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Novelty, OH, USA, 1990; Volume 2, ISBN 978-1-62708-162-7. [Google Scholar]
- Haque, A.; Shekhar, S.; Narayana Murty, S.V.S.; Ramkumar, J.; Kar, K.; Mondal, K. Fabrication of controlled expansion Al-Si composites by pressureless and spark plasma sintering. Adv. Powder Technol. 2018, 29, 3427–3439. [Google Scholar] [CrossRef]
- Controlled Expansion (CE) Alloy Products—Sandvik Materials Technology. Available online: https://www.materials.sandvik/en/products/ce-alloys/ (accessed on 12 October 2020).
- Ma, B.; Wang, J.; Lee, T.H.; Dorris, S.E.; Wen, J.; Balachandran, U. Microstructural characterization of Al4C3 in aluminum–graphite composite prepared by electron-beam melting. J. Mater. Sci. 2018, 53, 10173–10180. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, Z.M. Casting of SiC reinforced metal matrix composites. J. Mater. Process. Technol. 1997, 63, 358–363. [Google Scholar] [CrossRef]
- Iseki, T.; Maruyama, T.; Kameda, T. Interfacial reactions between SiC and aluminium during joining. J. Mater. Sci. 1984, 19, 1692–1698. [Google Scholar] [CrossRef]
- Ferro, A.C.; Derby, B. Wetting behaviour in the Al-Si/SiC system: Interface reactions and solubility effects. Acta Metall. Mater. 1995, 43, 3061–3073. [Google Scholar] [CrossRef]
- Viala, J.C.; Fortier, P.; Bouix, J. Stable and metastable phase equilibria in the chemical interaction between aluminium and silicon carbide. J. Mater. Sci. 1990, 25, 1842–1850. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Q.; Shi, L.; Zhang, X.; Yang, T.; Yan, J.; Zhou, X.; Chen, S. Ultrarapid formation of multi-phase reinforced joints of hypereutectic Al-Si alloys via an ultrasound-induced liquid phase method using Sn-51In interlayer. Mater. Sci. Eng. A 2018, 711, 94–98. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, L.; Chen, X.; Yan, J.; Xie, R.; Li, P.; Wang, Z.; Wang, Z.; Li, Y.; Zhou, X. Si particulate-reinforced Zn-Al based composites joints of hypereutectic Al-50Si alloys by ultrasonic-assisted soldering. Mater. Des. 2016, 107, 41–46. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Zhu, L.; Yan, J.; Lai, Z.; Zhao, P.; Bao, J.; Lv, G.; You, C.; Zhou, X.; et al. Rapid ultrasound-induced transient-liquid-phase bonding of Al-50Si alloys with Zn interlayer in air for electrical packaging application. Ultrason. Sonochem. 2017, 34, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, H.; Yang, C. Microstructure and Properties of the Al-27Si/Cu/Al-50Si Joint Brazed by the Partial Transient Liquid Phase Bonding. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2018, 49, 933–938. [Google Scholar] [CrossRef]
- Xu, L.; Wang, H.; Sun, Q.; Wan, W.; Chen, H. Effect of bonding temperature on microstructure and shear strength of the dissimilar Al/Al-27Si joints bonded by partial transient liquid phase bonding. J. Manuf. Process. 2019, 41, 297–306. [Google Scholar] [CrossRef]


| Al Type | Al | Si | Fe | Cu | Mg | Zn | Mn | Ni |
|---|---|---|---|---|---|---|---|---|
| A356 | balance | 7 | 0.2 | 0.2 | 0.3 | 0.1 | 0.1 | - |
| Al4047 | balance | 12 | 0.3 | 0.8 | 1.0 | - | 0.1 | 0.8 |
| Al-30Si | balance | 30 | ≤0.1 | - | - | - | - | - |
| Al-50Si | balance | 50 | ≤0.1 | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Bhowmik, A.; Tan, J.L.; Du, Z.; Zhou, W. A New Strategy for Dissimilar Material Joining between SiC and Al Alloys through Use of High-Si Al Alloys. Metals 2022, 12, 887. https://doi.org/10.3390/met12050887
Yang Y, Bhowmik A, Tan JL, Du Z, Zhou W. A New Strategy for Dissimilar Material Joining between SiC and Al Alloys through Use of High-Si Al Alloys. Metals. 2022; 12(5):887. https://doi.org/10.3390/met12050887
Chicago/Turabian StyleYang, Yongjing, Ayan Bhowmik, Jin Lee Tan, Zehui Du, and Wei Zhou. 2022. "A New Strategy for Dissimilar Material Joining between SiC and Al Alloys through Use of High-Si Al Alloys" Metals 12, no. 5: 887. https://doi.org/10.3390/met12050887
APA StyleYang, Y., Bhowmik, A., Tan, J. L., Du, Z., & Zhou, W. (2022). A New Strategy for Dissimilar Material Joining between SiC and Al Alloys through Use of High-Si Al Alloys. Metals, 12(5), 887. https://doi.org/10.3390/met12050887







