Research Progress of Corrosion Induced by Second-Phase Particles in Microalloyed High-Strength Rebars—Review
Abstract
:1. Introduction
2. The Role of Second-Phase Particles in Corrosion
2.1. The Role of Aluminum Oxides in Corrosion
2.1.1. The Role of Al2O3 Inclusions in Corrosion
2.1.2. Effect of Rare-Earth-Modified Al2O3 Inclusions in Corrosion
2.2. The Role of Carbonitride Precipitates in Corrosion
2.2.1. The Role of Carbide Precipitation in Corrosion
2.2.2. The Role of Nitride Precipitation in Corrosion
2.3. The Role of Sulfide Inclusions in Corrosion
2.3.1. The Role of CaS Inclusions in Corrosion
2.3.2. The Role of MnS Inclusions in Corrosion
3. Conclusions
- (1)
- At present, there are three main ways of local corrosion of microalloyed high-strength rebars caused by second-phase particles: pitting corrosion, crevice corrosion, and stress corrosion.
- (2)
- The research progressed on corrosion induced by Al2O3, (RE)-AlO3, CaS, MnS, carbonitride, and other second phase particles in microalloyed high-strength rebars is mainly expounded. The current research shows that sulfide inclusions are harmful to the corrosion resistance of microalloyed, high-strength rebars, and different characteristics of Al2O3 inclusions have different effects on the corrosion resistance of rebars. For this phenomenon, many researchers add alloying elements and rare earth elements to steel to modify inclusions, refine grains, and improve microstructures, so as to improve the corrosion resistance of microalloyed, high-strength rebars.
- (3)
- At present, the main shortcomings of the research on the corrosion induced by the second phase particles are that the researchers start from a single inclusion and the microscopic angle, and the lack of research on the relationship between the number, density, and distribution of inclusions in steel bars and the corrosion resistance of microalloyed, high-strength steel bars.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Sun, C.; Niu, D. A review of research on critical chloride ion concentration for steel corrosion. Silic. Bull. 2014, 33, 83–91. [Google Scholar] [CrossRef]
- Li, J.; Xiong, J.; Fan, Z.; Zheng, H.; Li, W. Research progress and prospect of macro cell corrosion of rebars in concrete. J. Silic. 2021, 49, 11. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T.; Shao, Y.; Meng, G.; Wang, F. New understanding of the effect of hydrostatic pressure on the corrosion of Ni–Cr–Mo–V high strength steel. Corros. Sci. 2013, 73, 250–261. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Wang, Z.; Zhang, S.; Wu, X.; Huang, Y.; Li, X. The effect of nanosized NbC precipitates on electrochemical corrosion behavior of high-strength low-alloy steel in 3.5% NaCl solution. Int. J. Hydrogen Energy 2017, 42, 22175–22184. [Google Scholar] [CrossRef]
- Wang, L.; Tian, H.; Gao, H.; Xie, F.; Zhao, K.; Cui, Z. Electrochemical and XPS analytical investigation of the accelerative effect of bicarbonate/carbonate ions on AISI 304 in alkaline environment. Appl. Surf. Sci. 2019, 492, 792–807. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef]
- Lind, M.; Holappa, L. Transformation of Alumina Inclusions by Calcium Treatment. Met. Mater. Trans. A 2010, 41, 359–366. [Google Scholar] [CrossRef]
- Verma, N.; Pistorius, P.C.; Fruehan, R.J.; Potter, M.; Lind, M.; Story, S.R. Transient inclusion evolution during modification of alumina inclusions by calcium in liquid steel: Part I. Background, Ex-perimental Techniques and Analysis Methods. Met. Mater. Trans. B 2011, 42, 711–719. [Google Scholar] [CrossRef]
- Opiela, M.; Grajcar, A. Modification of Non-Metallic Inclusions by Rare-Earth Elements in Microalloyed Steels. Arch. Foundry Eng. 2012, 12, 129–134. [Google Scholar] [CrossRef]
- Wang, L.; Xin, J.; Cheng, L.; Zhao, K.; Sun, B.; Li, J.; Wang, X.; Cui, Z. Influence of inclusions on initiation of pitting corrosion and stress corrosion cracking of X70 steel in near-neutral pH environment. Corros. Sci. 2019, 147, 108–127. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Zhang, D.; Liu, Z.; Lutz, A.; Zhang, F.; Zhao, T.; Ma, H.; Li, X.; Terryn, H. Role of Al2O3 inclusions on the localized corrosion of Q460NH weathering steel in marine environment. Corros. Sci. 2018, 138, 96–104. [Google Scholar] [CrossRef]
- . Szklarska-Śmialowska, Z.; Lunarska, E. The effect of sulfide inclusions on the susceptibility of steels to pitting, stress corrosion cracking and hydrogen embrittlement. Mater. Corros. 1981, 32, 478–485. [Google Scholar] [CrossRef]
- Ha, H.; Park, C.; Kwon, H. Effects of misch metal on the formation of non-metallic inclusions and the associated resistance to pitting corrosion in 25% Cr duplex stainless steels. Scr. Mater. 2006, 55, 991–994. [Google Scholar] [CrossRef]
- Punckt, C.; Boelscher, M.; Rotermund, H.H.; Mikhailov, A.S.; Organ, L.; Budiansky, N.; Scully, J.R.; Hudson, J.L. Sudden Onset of Pitting Corrosion on Stainless Steel as a Critical Phenomenon. ChemInform 2004, 305, 1133–1136. [Google Scholar] [CrossRef]
- da Costa-Mattos, H.; Bastos, I.; Gomes, J. A simple model for slow strain rate and constant load corrosion tests of austenitic stainless steel in acid aqueous solution containing sodium chloride. Corros. Sci. 2008, 50, 2858–2866. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Du, C.; Lu, L.; Zhang, Y.; Cheng, Y. Effect of inclusions on initiation of stress corrosion cracks in X70 pipeline steel in an acidic soil environment. Corros. Sci. 2009, 51, 895–900. [Google Scholar] [CrossRef]
- Jin, T.; Liu, Z.; Cheng, Y. Effect of non-metallic inclusions on hydrogen-induced cracking of API5L X100 steel. Int. J. Hydrogen Energy 2010, 35, 8014–8021. [Google Scholar] [CrossRef]
- Gu, B.; Yu, W.Z.; Luo, J.L.; Mao, X. Transgranular Stress Corrosion Cracking of X-80 and X-52 Pipeline Steels in Dilute Aqueous Solution with Near-Neutral pH. Corrosion 1999, 55, 312–318. [Google Scholar] [CrossRef]
- Reformatskaya, I.I.; Rodionova, I.G.; Beilin, Y.A.; Nisel’Son, L.A.; Podobaev, A.N. The Effect of Nonmetal Inclusions and Microstructure on Local Corrosion of Carbon and Low-alloyed Steels. Prot. Met. 2004, 40, 447–452. [Google Scholar] [CrossRef]
- Xie, Z.; Ma, X.; Shang, C.; Wang, X.; Subramanian, S. Nano-sized precipitation and properties of a low carbon niobium micro-alloyed bainitic steel. Mater. Sci. Eng. A 2015, 641, 37–44. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Q.; Ji, J.; Lan, D. New study concerning development of application of rare earth metals in steels. J. Alloy. Compd. 2006, 408–412, 384–386. [Google Scholar] [CrossRef]
- Wilson, W.G.; Kay, D.A.R.; Vahed, A. The use of thermodynamics and phase equilibria to predict the behavior of the rare earth elements in steel. JOM 1974, 26, 14–23. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kim, S.-K.; Kang, Y.-B.; Lee, H.-G. Compositional Evolution of Oxide Inclusions in Austenitic Stainless Steel during Continuous Casting. Steel Res. Int. 2015, 86, 284–292. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.; Li, J.; Ni, H.; Zhang, X. The Influence of FeO on the Reaction between Fe–Al–Ca Alloy and Al2O3–CaO–FeO Oxide during Heat Treatment at 1473 K. Metals 2017, 7, 129. [Google Scholar] [CrossRef]
- Arafin, M.; Szpunar, J. A new understanding of intergranular stress corrosion cracking resistance of pipeline steel through grain boundary character and crystallographic texture studies. Corros. Sci. 2009, 51, 119–128. [Google Scholar] [CrossRef]
- Torkkeli, J.; Saukkonen, T.; Hänninen, H. Effect of MnS inclusion dissolution on carbon steel stress corrosion cracking in fuel-grade ethanol. Corros. Sci. 2015, 96, 14–22. [Google Scholar] [CrossRef]
- Tewary, N.; Kundu, A.; Nandi, R.; Saha, J.; Ghosh, S. Microstructural characterisation and corrosion performance of old railway girder bridge steel and modern weathering structural steel. Corros. Sci. 2016, 113, 57–63. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Xiao, K.; Yu, Q.; Li, X. The Combined Effect of Chemical and Structural Factors on Pitting Corrosion Induced by MnS-(Cr, Mn, Al)O Duplex Inclusions. Corrosion 2017, 74, 312–325. [Google Scholar] [CrossRef]
- Shibaeva, T.V.; Laurinavichyute, V.; Tsirlina, G.; Arsenkin, A.M.; Grigorovich, K.V. The effect of microstructure and non-metallic inclusions on corrosion behavior of low carbon steel in chloride containing solutions. Corros. Sci. 2014, 80, 299–308. [Google Scholar] [CrossRef]
- Vignal, V.; Krawiec, H.; Heintz, O.; Oltra, R. The use of local electrochemical probes and surface analysis methods to study the electrochemical behaviour and pitting corrosion of stainless steels. Electrochimica Acta 2007, 52, 4994–5001. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Oltra, R. Use of the electrochemical microcell technique and the SVET for monitoring pitting corrosion at MnS inclusions. Electrochem. Commun. 2004, 6, 655–660. [Google Scholar] [CrossRef]
- Xue, H.; Cheng, Y. Characterization of inclusions of X80 pipeline steel and its correlation with hydrogen-induced cracking. Corros. Sci. 2011, 53, 1201–1208. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Heintz, O.; Oltra, R. Influence of the dissolution of MnS inclusions under free corrosion and potentiostatic conditions on the composition of passive films and the electrochemical behaviour of stainless steels. Electrochimica Acta 2006, 51, 3235–3243. [Google Scholar] [CrossRef]
- Suter, T.; Webb, E.G.; Böhni, H.; Alkire, R.C. Pit Initiation on Stainless Steels in 1 M NaCl With and Without Mechanical Stress. J. Electrochem. Soc. 2001, 148, B174–B185. [Google Scholar] [CrossRef]
- Jin, T.; Cheng, Y. In situ characterization by localized electrochemical impedance spectroscopy of the electrochemical activity of microscopic inclusions in an X100 steel. Corros. Sci. 2011, 53, 850–853. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Z.; Zhao, J.; Cheng, X.; Liu, Z.; Zhang, D.; Li, X. Influence of rare earth metals on mechanisms of localised corrosion induced by inclusions in Zr-Ti deoxidised low alloy steel. Corros. Sci. 2020, 166, 108461–108463. [Google Scholar] [CrossRef]
- Brossia, C.S.; Kelly, R.G. Influence of Alloy Sulfur Content and Bulk Electrolyte Composition on Crevice Corrosion Initiation of Austenitic Stainless Steel. Corrosion 1998, 54, 145–154. [Google Scholar] [CrossRef]
- Wei, W.-Z.; Wu, K.-M.; Liu, J.; Cheng, L.; Zhang, X. Initiation and propagation of localized corrosion induced by (Zr, Ti, Al)-Ox inclusions in low-alloy steels in marine environment. J. Iron Steel Res. Int. 2020, 28, 453–463. [Google Scholar] [CrossRef]
- Villavicencio, J.; Ulloa, N.; Lozada, L.; Moreno, M.; Castro, L. The role of non-metallic Al2O3 inclusions, heat treatments and microstructure on the corrosion resistance of an API 5L X42 steel. J. Mater. Res. Technol. 2020, 9, 5894–5911. [Google Scholar] [CrossRef]
- Dong, C.; Liu, Z.; Li, X.; Cheng, Y. Effects of hydrogen-charging on the susceptibility of X100 pipeline steel to hydrogen-induced cracking. Int. J. Hydrogen Energy 2009, 34, 9879–9884. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, X.; Dai, Z.; Liu, R.; Li, Z.; Cui, L.; Chen, M.; Ke, L. Synergistic Effect of Al2O3 Inclusion and Pearlite on the Localized Corrosion Evolution Process of Carbon Steel in Marine Environment. Materials 2018, 11, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Li, C.; Qi, Y.; Chen, L.; Chen, C. Mechanism of (Mg,Al,Ca)-oxide inclusion-induced pitting corrosion in 316L stainless steel exposed to sulphur environments containing chloride ion. Corros. Sci. 2013, 67, 20–31. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, S.; Zhao, M.; Zhu, L.; Li, J. Pitting Corrosion of Steel Induced by Al2O3 Inclusions. Metals 2017, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Yue, L.; Wang, L.; Han, J. Effects of rare earth on inclusions and corrosion resistance of 10PCuRE weathering steel. J. Rare Earths 2010, 28, 952–956. [Google Scholar] [CrossRef]
- Hirata, H.; Isobe, K. Steel Having Finely Dispersed Inclusions. U.S. Patemt US10547303[P], 20 July 2006. [Google Scholar]
- Kim, S.-T.; Jeon, S.-H.; Lee, I.-S.; Park, Y.-S. Effects of rare earth metals addition on the resistance to pitting corrosion of super duplex stainless steel—Part 1. Corros. Sci. 2010, 52, 1897–1904. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Kim, S.-T.; Choi, M.-S.; Kim, J.-S.; Kim, K.-T.; Park, Y.-S. Effects of cerium on the compositional variations in and around inclusions and the initiation and propagation of pitting corrosion in hyperduplex stainless steels. Corros. Sci. 2013, 75, 367–375. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Kim, S.-T.; Lee, I.-S.; Park, Y.-S. Effects of sulfur addition on pitting corrosion and machinability behavior of super duplex stainless steel containing rare earth metals: Part 2. Corros. Sci. 2010, 52, 3537–3547. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hwang, W.S.; Su, Y.H. Effect of Adding Cerium on Microstructure and Morphology of Ce-Based Inclusions Formed in Low-Carbon Steel. Sci. Rep. 2017, 7, 46503. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Qiu, S.; Xiang, L. Effect of Ce on the Evolution of Recrystallization Texture in a 1.2%Si-0.4%Al Non-oriented Electrical Steel. ISIJ Int. 2016, 56, 1256–1261. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Revilla, R.I.; Liu, Z.; Zhang, D.; Li, X.; Terryn, H. Effect of inclusions modified by rare earth elements (Ce, La) on localized marine corrosion in Q460NH weathering steel. Corros. Sci. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Afshar, F.N.; de Wit, J.; Terryn, H.; Mol, J. Scanning Kelvin probe force microscopy as a means of predicting the electrochemical characteristics of the surface of a modified AA4xxx/AA3xxx (Al alloys) brazing sheet. Electrochimica Acta 2013, 88, 330–339. [Google Scholar] [CrossRef]
- Sathirachinda, N.; Pettersson, R.; Wessman, S.; Pan, J. Study of nobility of chromium nitrides in isothermally aged duplex stainless steels by using SKPFM and SEM/EDS. Corros. Sci. 2010, 52, 179–186. [Google Scholar] [CrossRef]
- Williams, D.E.; Zhu, Y.Y. Explanation for Initiation of Pitting Corrosion of Stainless Steels at Sulfide Inclusions. J. Electrochem. Soc. 2000, 147, 1763–1766. [Google Scholar] [CrossRef]
- Suter, T.; Böhni, H. A new microelectrochemical method to study pit initiation on stainless steels. Electrochimica Acta 1997, 42, 3275–3280. [Google Scholar] [CrossRef]
- Stewart, J.; Williams, D.E. The initiation of pitting corrosion on austenitic stainless steel: On the role and importance of sulphide inclusions. Corros. Sci. 1992, 33, 457–474. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Z.; Fan, E.; Wu, X.; Huang, Y.; Li, X. Effects of Nanosized Nb Carbide Precipitates on the Corrosion Behavior of High-Strength Low-Alloy Steel in Simulated Seawater. Int. J. Electrochem. Sci. 2017, 12, 7989–7996. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Sun, B.; Liao, Q.; Lu, H.; Jian, B.; Mohrbacher, H.; Zhang, W.; Guo, A.; Zhang, Y. Effect of Nb on hydrogen-induced delayed fracture in high strength hot stamping steels. Mater. Sci. Eng. A 2015, 626, 136–143. [Google Scholar] [CrossRef]
- Ejaz, A.; Lu, Z.; Chen, J.; Xiao, Q.; Ru, X.; Han, G.; Shoji, T. The effects of hydrogen on anodic dissolution and passivation of iron in alkaline solutions. Corros. Sci. 2015, 101, 165–181. [Google Scholar] [CrossRef]
- Rehrl, J.; Mraczek, K.; Pichler, A.; Werner, E. The Impact of Nb, Ti, Zr, B, V, and Mo on the Hydrogen Diffusion in Four Different AHSS/UHSS Microstructures. Steel Res. Int. 2014, 85, 336–346. [Google Scholar] [CrossRef]
- Wallaert, E.; DePover, T.; Arafin, M.; Verbeken, K. Thermal Desorption Spectroscopy Evaluation of the Hydrogen-Trapping Capacity of NbC and NbN Precipitates. Met. Mater. Trans. A 2014, 45, 2412–2420. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Gao, K.; He, X. Corrosion Resistance of low alloy weathering steel in chloride-containing environment. J. Mater. Heat Treat. 2008, 29, 170–175. [Google Scholar] [CrossRef]
- Guo, J.; Yang, S.; Shang, C.; Wang, Y.; He, X. Influence of carbon content and microstructure on corrosion behaviour of low alloy steels in a Cl− containing environment. Corros. Sci. 2009, 51, 242–251. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Li, J.; Yang, L.; Xie, D. Effect of Nb on the corrosion behavior of X80 steel welding heat affected zone in simulated seawater. Chin. J. Corros. Prot. 2016, 36, 7. [Google Scholar] [CrossRef]
- Xue, W.; Li, Z.; Xiao, K.; Yu, W.; Song, J.; Chen, J.; Dong, C.; Li, X. Initial microzonal corrosion mechanism of inclusions associated with the precipitated (Ti, Nb)N phase of Sb-containing weathering steel. Corros. Sci. 2020, 163, 108232. [Google Scholar] [CrossRef]
- Yue, Y.; Tang, D.; Wu, H.; Liang, J.; Ju, B. Effect of Nb on corrosion properties of low-alloy steel in strongly acidic solution environment with high Cl-. Mater. Eng. 2015, 43, 14–20. [Google Scholar] [CrossRef]
- Tyurin, A.G.; Pyshmintsev, I.Y.; Kostitsyna, I.V.; Zubkova, I.M. Thermodynamics of chemical and electrochemical stability of corrosion active nonmetal inclusions. Prot. Met. 2007, 43, 34–44. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Influence of Sulfide Inclusions on the Pitting Corrosion of Steels. Corrosion 1972, 28, 388–396. [Google Scholar] [CrossRef]
- Wranglen, G. Pitting and sulphide inclusions in steel. Corros. Sci. 1974, 14, 331–349. [Google Scholar] [CrossRef]
- Williams, D.E.; Kilburn, M.; Cliff, J.; Waterhouse, G. Composition changes around sulphide inclusions in stainless steels, and implications for the initiation of pitting corrosion. Corros. Sci. 2010, 52, 3702–3716. [Google Scholar] [CrossRef]
- Avci, R.; Davis, B.; Wolfenden, M.; Beech, I.; Lucas, K.; Paul, D. Mechanism of MnS-mediated pit initiation and propagation in carbon steel in an anaerobic sulfidogenic media. Corros. Sci. 2013, 76, 267–274. [Google Scholar] [CrossRef]
- Ke, R.; Alkire, R. ChemInform Abstract: Initiation of Corrosion Pits at Inclusions on 304 Stainless Steel. ChemInform 1996, 27, 4056–4062. [Google Scholar] [CrossRef]
- Ke, R.; Alkire, R. Surface Analysis of Corrosion Pits Initiated at MnS Inclusions in 304 Stainless Steel. J. Electrochem. Soc. 1992, 139, 1573–1580. [Google Scholar] [CrossRef]
- Park, I.-J.; Lee, S.-M.; Kang, M.; Lee, S.; Lee, Y.-K. Pitting corrosion behavior in advanced high strength steels. J. Alloy. Compd. 2015, 619, 205–210. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Zheng, S.; Zhang, B.; Ma, X. Strain-induced preferential dissolution at the dislocation emergences in MnS: An atomic scale study. Philos. Mag. 2015, 95, 2365–2375. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J.; Ke, W.; He, X. Influence of Inclusions on Early Corrosion Development of Ultra-Low Carbon Bainitic Steel in NaCl Solution. Corrosion 2015, 71, 1467–1480. [Google Scholar] [CrossRef]
- Al-Negheimish, A.; Alhozaimy, A.; Hussain, R.R.; Al-Zaid, R.; Singh, J.; Singh, D. Role of Manganese Sulfide Inclusions in Steel Rebar in the Formation and Breakdown of Passive Films in Concrete Pore Solutions. Corrosion 2014, 70, 74–86. [Google Scholar] [CrossRef]


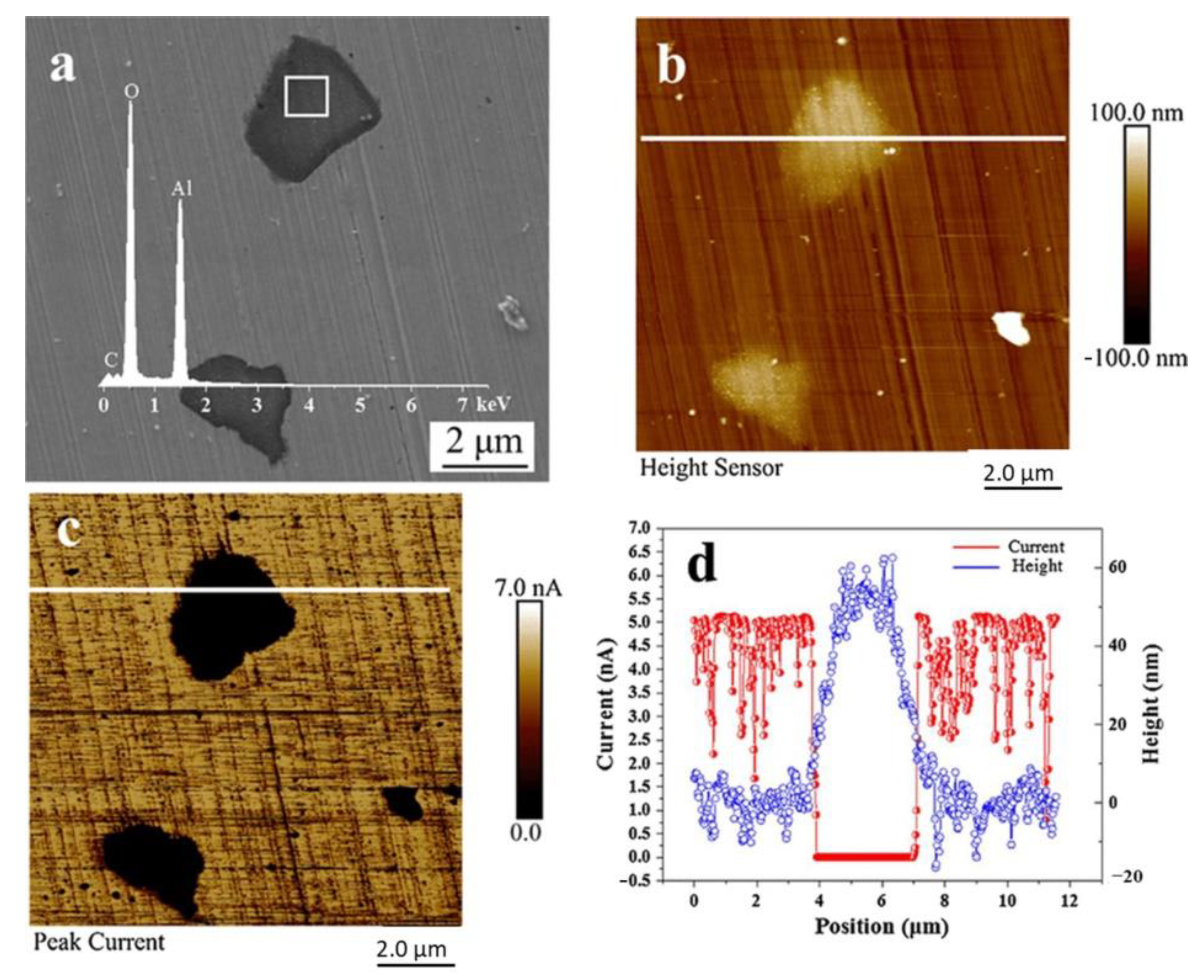
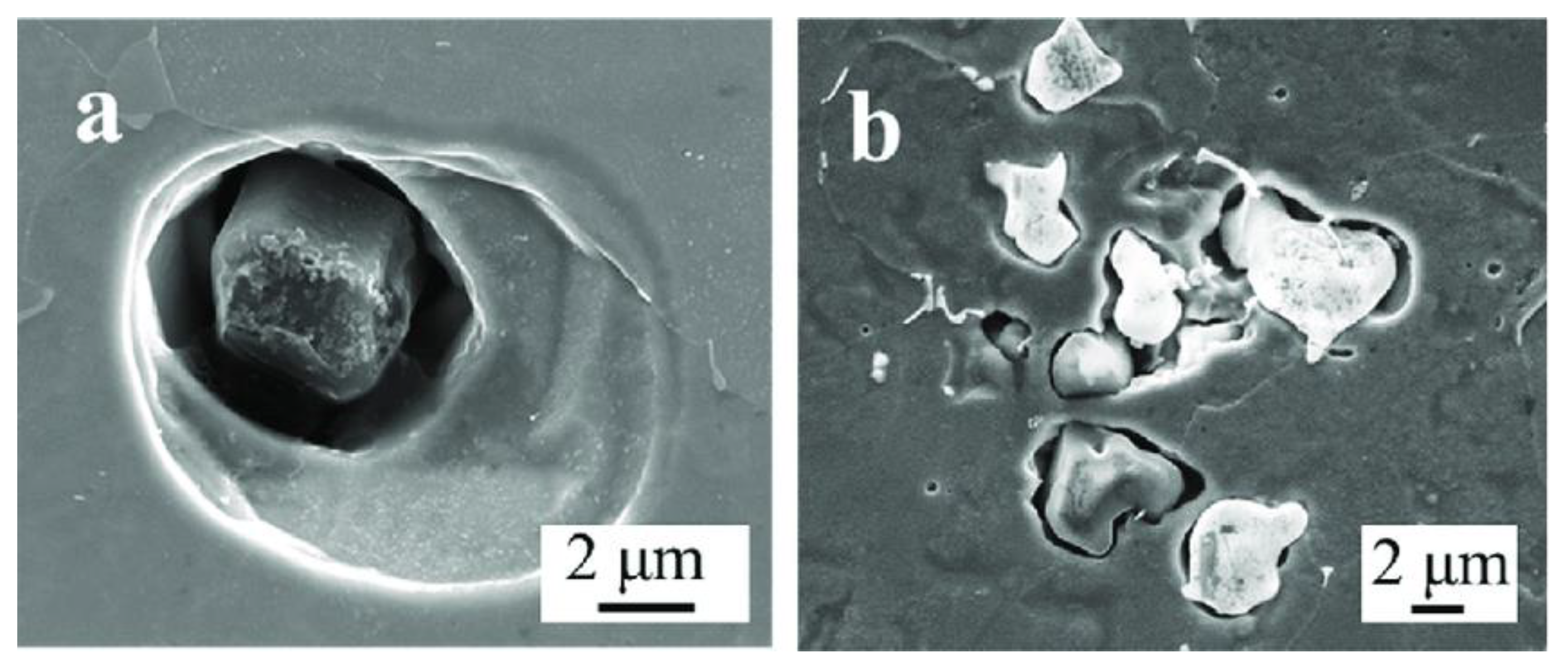
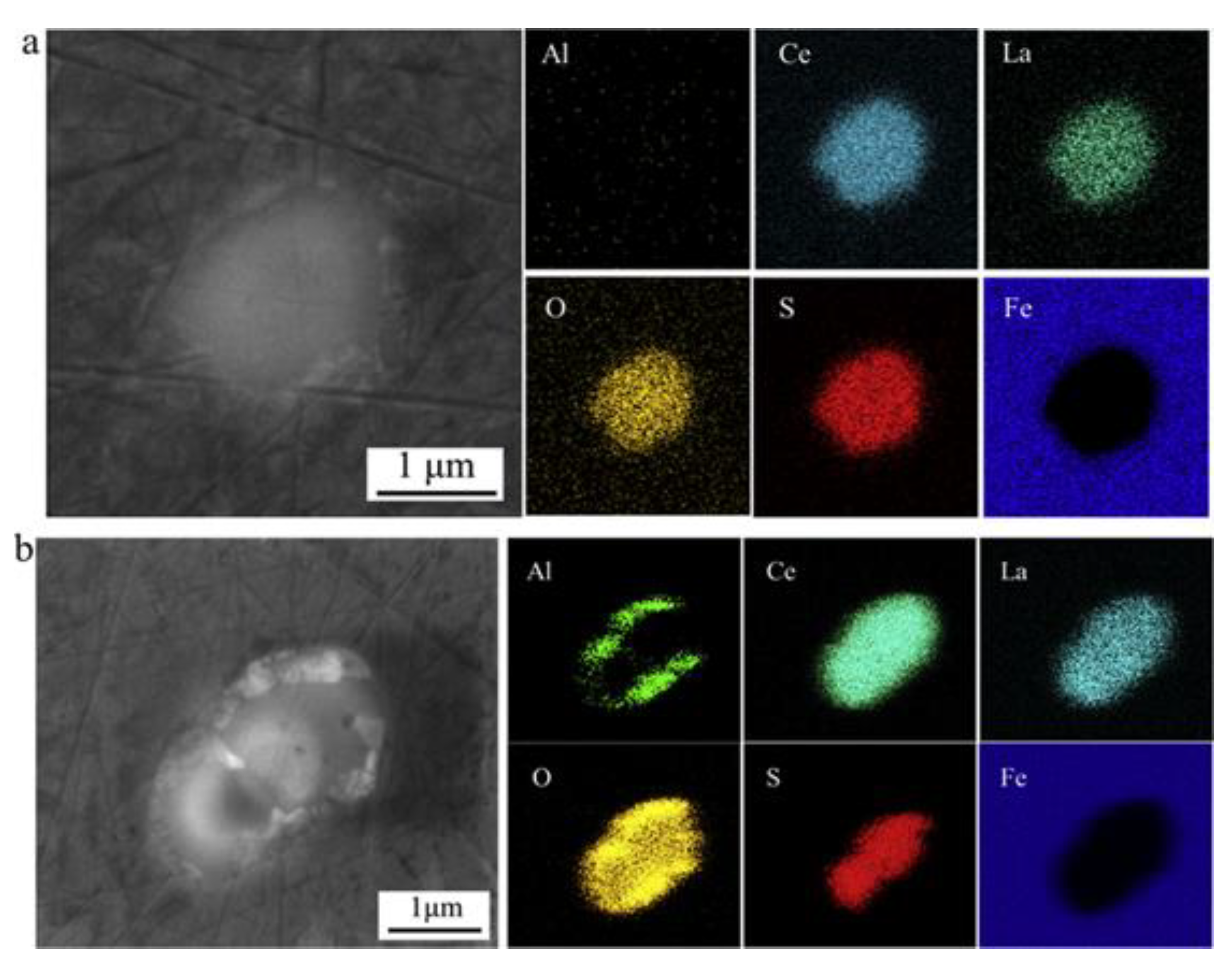
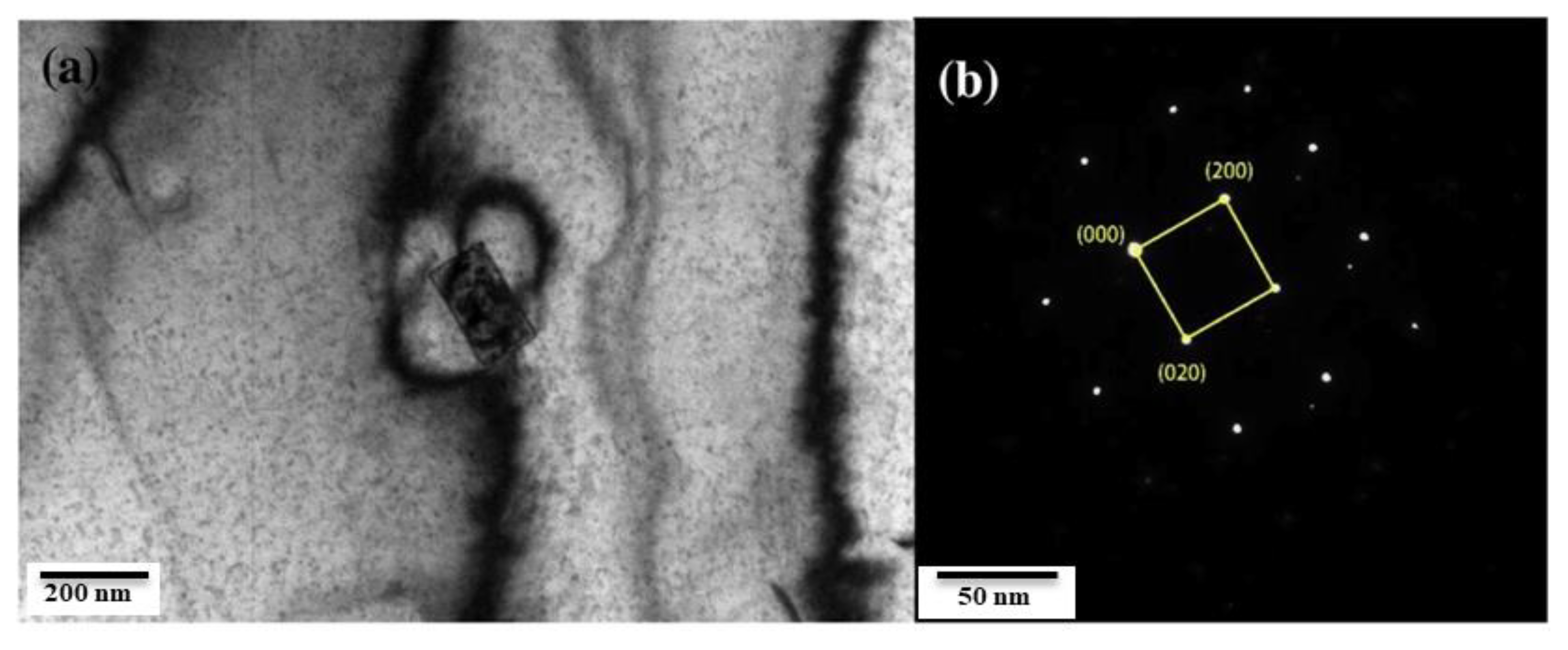
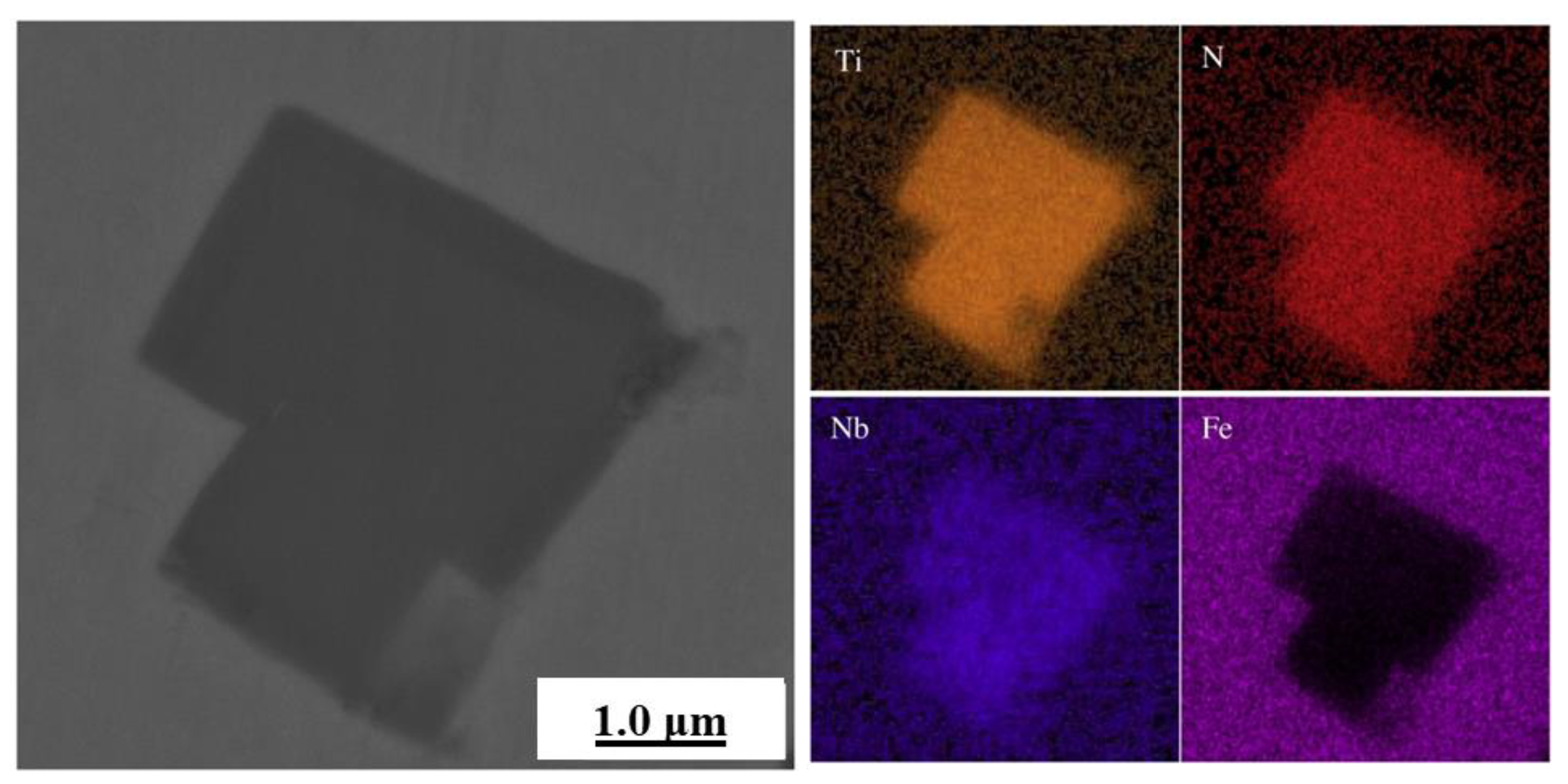

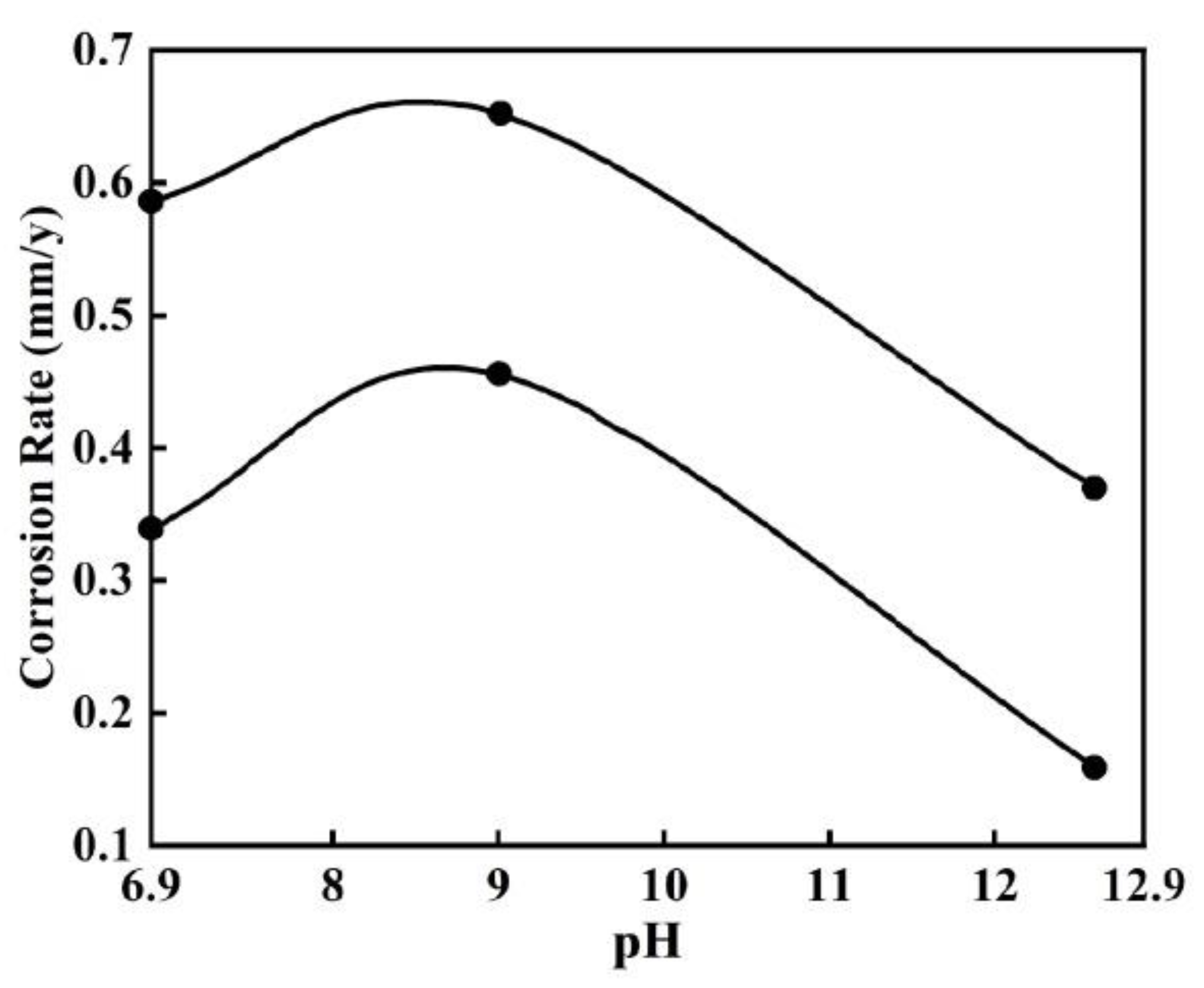
| Corrosion Time/h | Corrosion Rate of Different Steel Samples | |||||
|---|---|---|---|---|---|---|
| Q235 | 0# | 1# | 2# | 5# | 6# | |
| 70 | 5.47 | 3.30 | 2.87 | 3.09 | 3.00 | 2.74 |
| 140 | 3.90 | 2.56 | 2.26 | 2.26 | 2.24 | 2.21 |
| 240 | 3.31 | 2.21 | 2.02 | 1.89 | 1.76 | 1.88 |
| Second-Phase Particles | Can a Galvanic Couple Be Formed? | Corrosive Behavior | Anode or Cathode | Preferential Dissolution Region | Remark | Reference |
|---|---|---|---|---|---|---|
| Al2O3 | No | Crevice corrosion, Stress corrosion | \ | Interface between inclusion and matrix |
| [13,15,16,21,27,28,37,39] |
| Rare earth modified Al2O3 | No | Pitting corrosion | Anode | (RE)2O2S- (RE)xSy | Adding (RE) elements can modify Al2O3 inclusions, effectively control the size, shape, and quantity of Al2O3 inclusions, and improve the corrosion resistance of steel. | [45,48,50,51,52,53] |
| Carbide | Yes | Galvanic corrosion | Cathode | Steel matrix | Adding an appropriate amount of Nb, V, Ti can:
| [58,59,60,65] |
| Nitride | Yes | Galvanic corrosion | Cathode | Steel matrix | [4,65,66,67] | |
| CaS | No | Pitting corrosion | Anode | CaS inclusion | CaS dissolves preferentially, lowering the pH of the pore solution and further promoting corrosion. | [9,12,20,68] |
| MnS | No | Pitting corrosion | Anode | MnS inclusion |
| [71,72,75,76,77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, C.; Zeng, Z.; Zhuang, C.; Huang, S.; You, J. Research Progress of Corrosion Induced by Second-Phase Particles in Microalloyed High-Strength Rebars—Review. Metals 2022, 12, 925. https://doi.org/10.3390/met12060925
Li S, Li C, Zeng Z, Zhuang C, Huang S, You J. Research Progress of Corrosion Induced by Second-Phase Particles in Microalloyed High-Strength Rebars—Review. Metals. 2022; 12(6):925. https://doi.org/10.3390/met12060925
Chicago/Turabian StyleLi, Shiwang, Changrong Li, Zeyun Zeng, Changling Zhuang, Sheng Huang, and Jingtian You. 2022. "Research Progress of Corrosion Induced by Second-Phase Particles in Microalloyed High-Strength Rebars—Review" Metals 12, no. 6: 925. https://doi.org/10.3390/met12060925
APA StyleLi, S., Li, C., Zeng, Z., Zhuang, C., Huang, S., & You, J. (2022). Research Progress of Corrosion Induced by Second-Phase Particles in Microalloyed High-Strength Rebars—Review. Metals, 12(6), 925. https://doi.org/10.3390/met12060925







