Large-Arc Discharge Mechanism and Avoidance in Micro-Arc Oxidation of Magnesium Alloy
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Electrolyte
2.2. MAO Coatings Preparation
2.3. Characterization of MAO Coatings
3. Results
3.1. Large-Arc Discharge Characterization of MAO Coatings
3.1.1. Arc Discharge Evolution
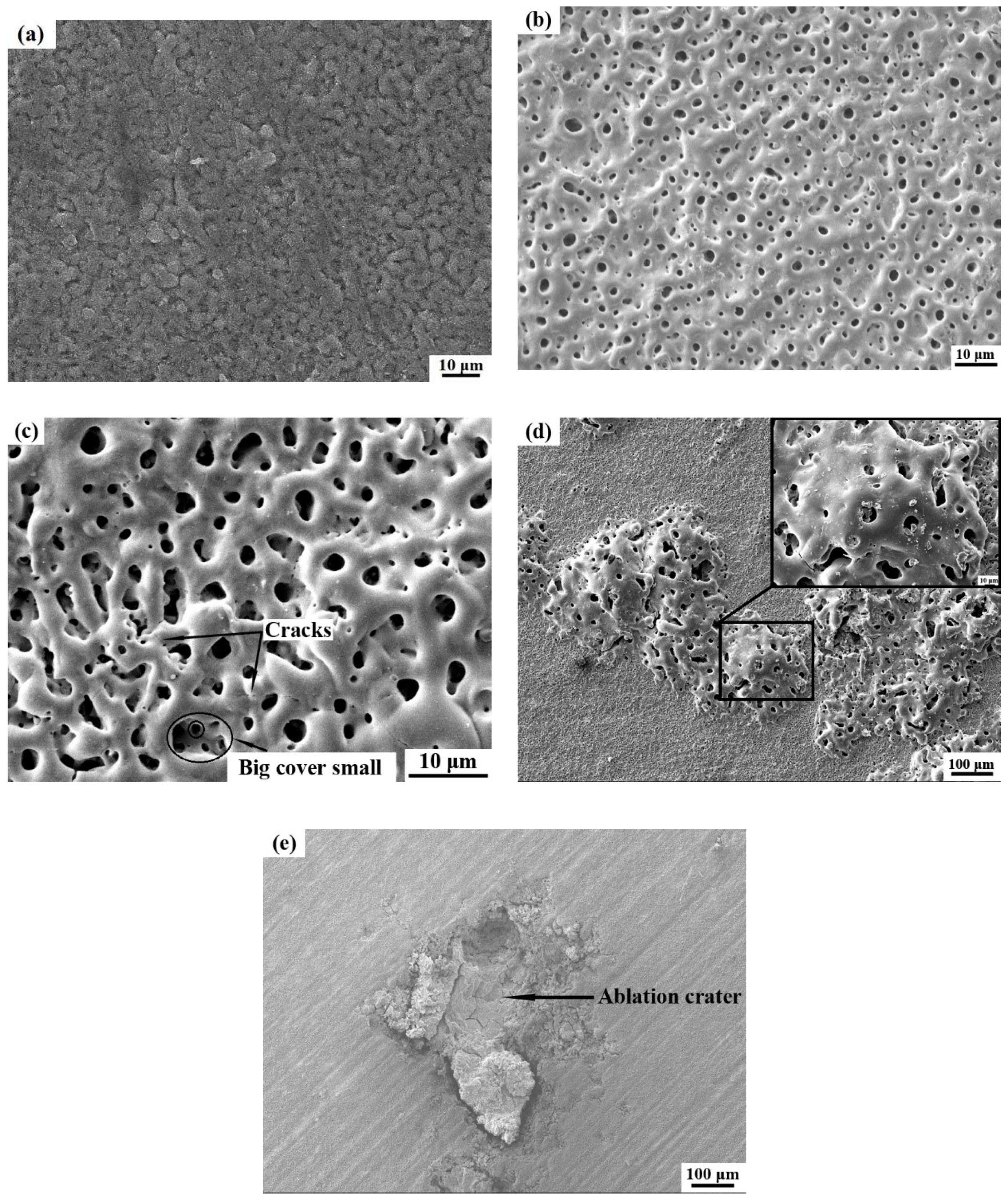
3.1.2. Surface Morphology Evolution of Ceramic Coatings
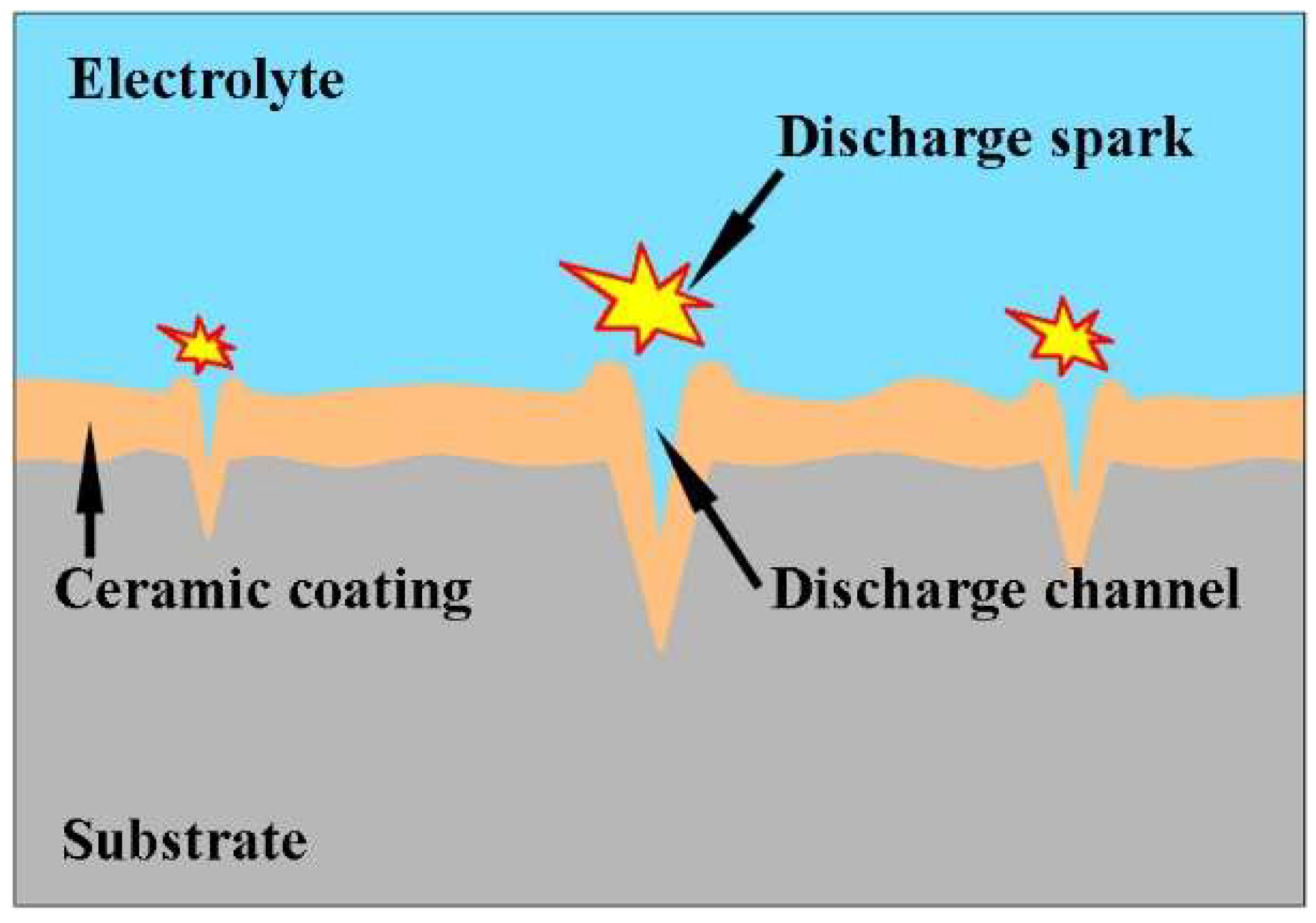
3.2. Formation Mechanism of Large-Arc Discharge
3.2.1. Phase Structure Evolution of Ceramic Coatings
3.2.2. Analysis of Load Characteristics
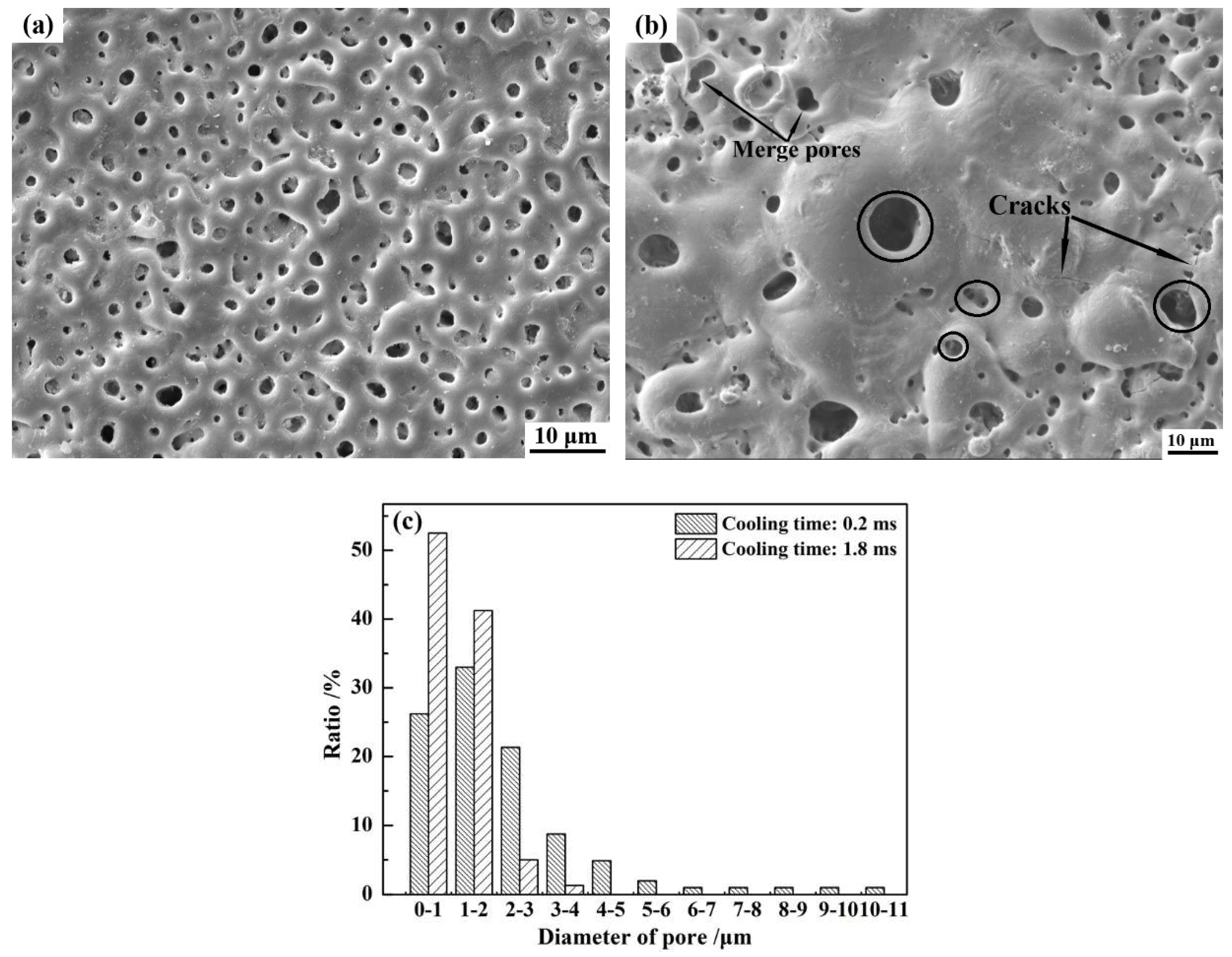
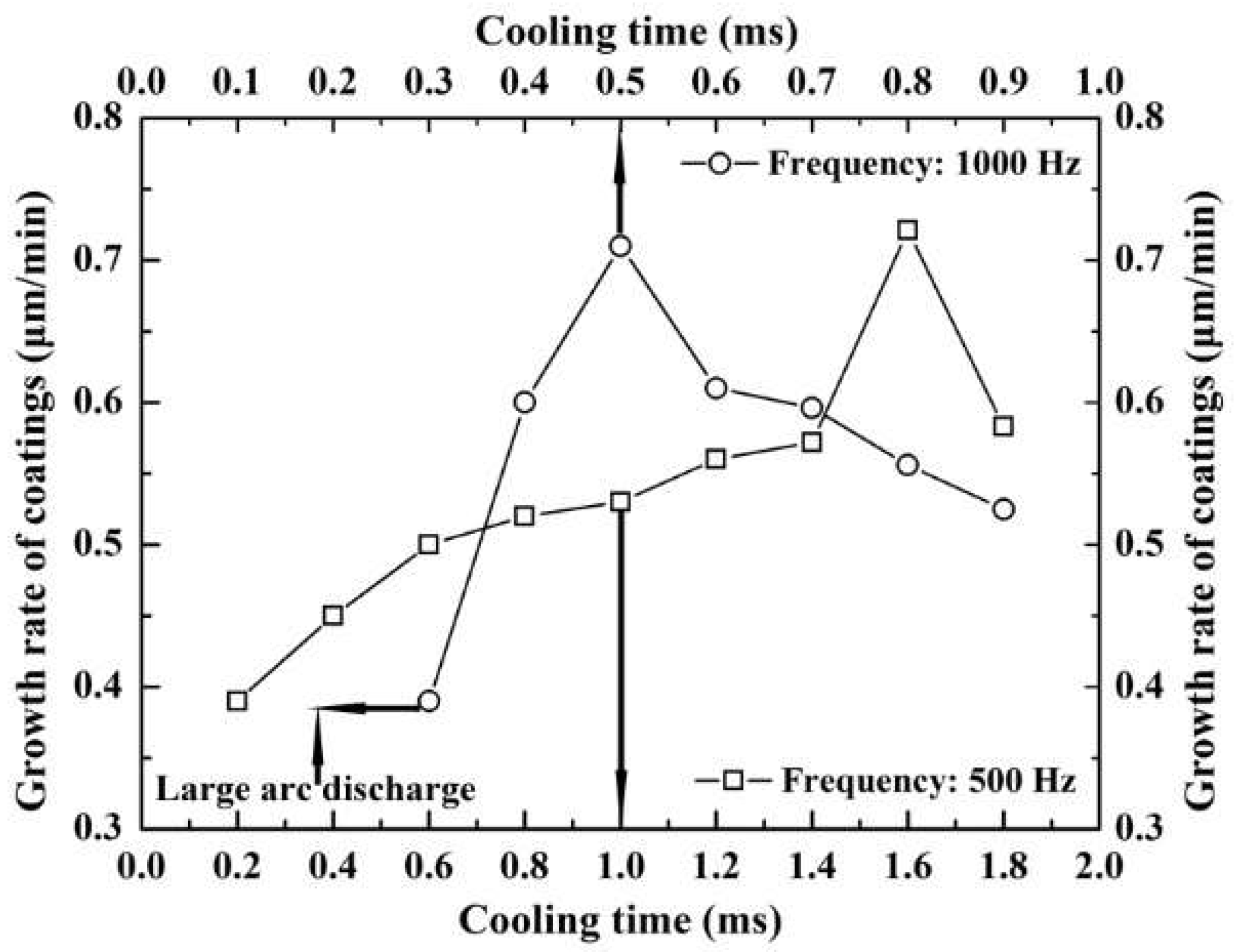
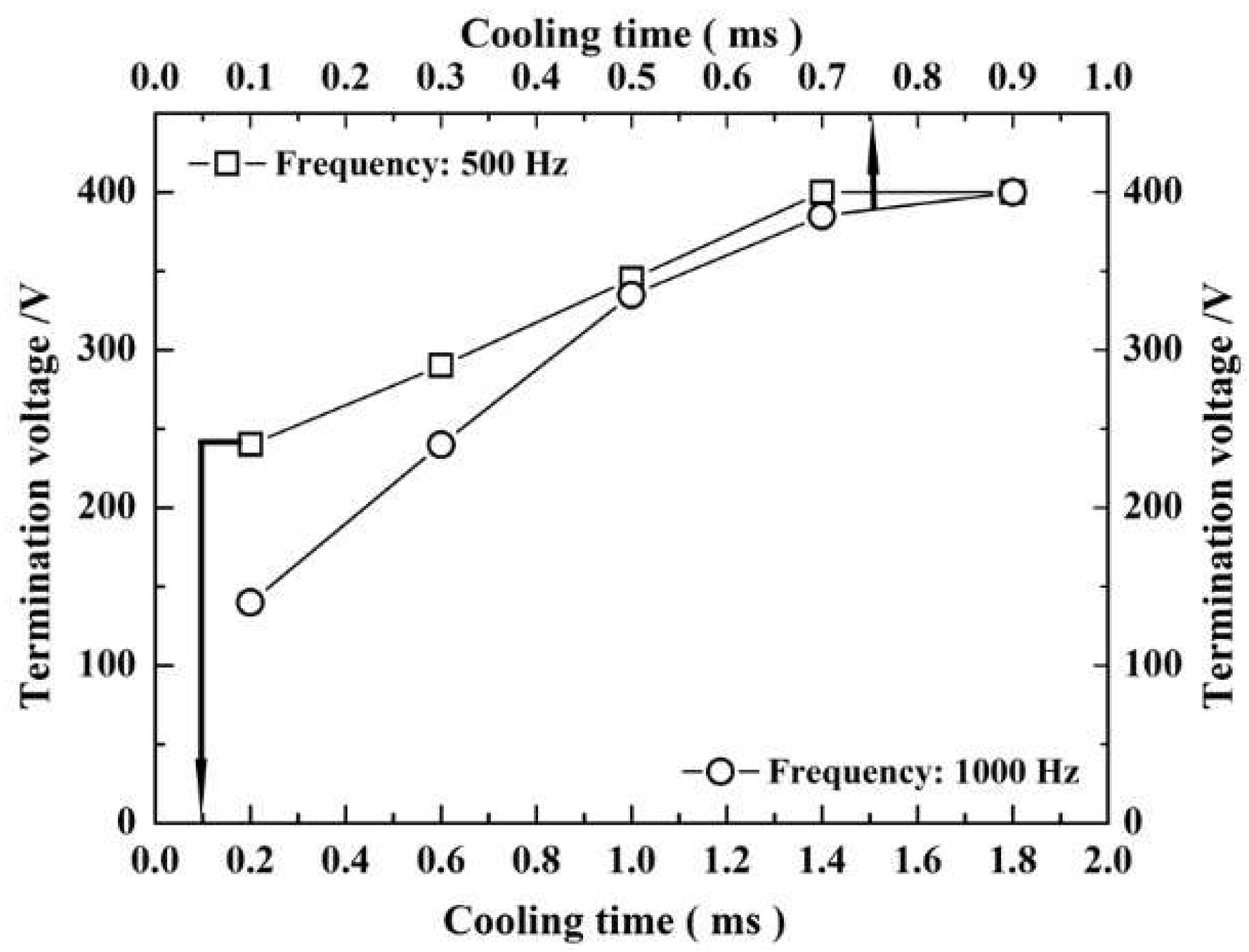
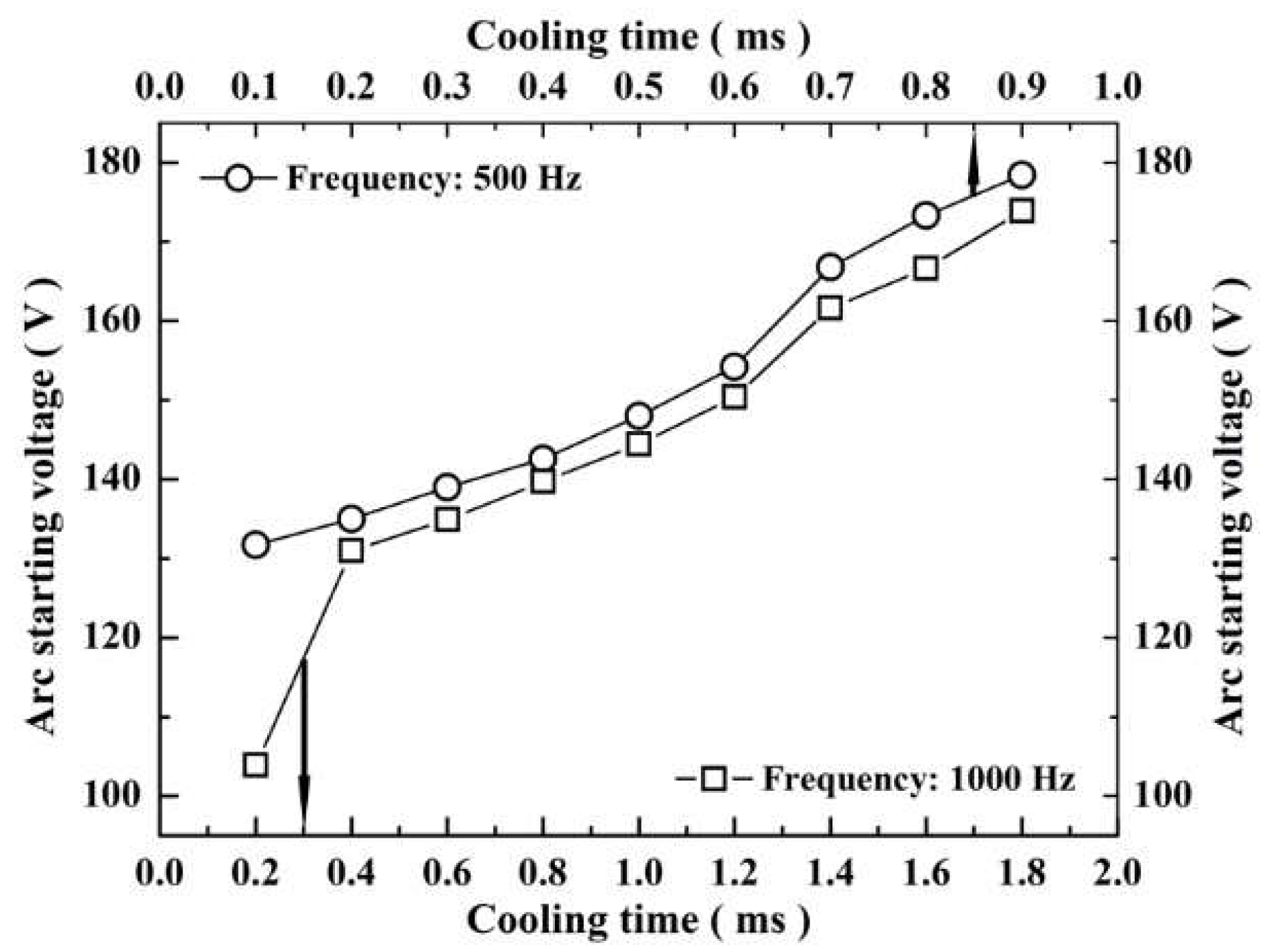
3.3. Effect of Cooling Time
3.3.1. Surface Morphology of Coatings
3.3.2. Growth Rate of Coatings
3.3.3. Tendency of Large-Arc Discharge
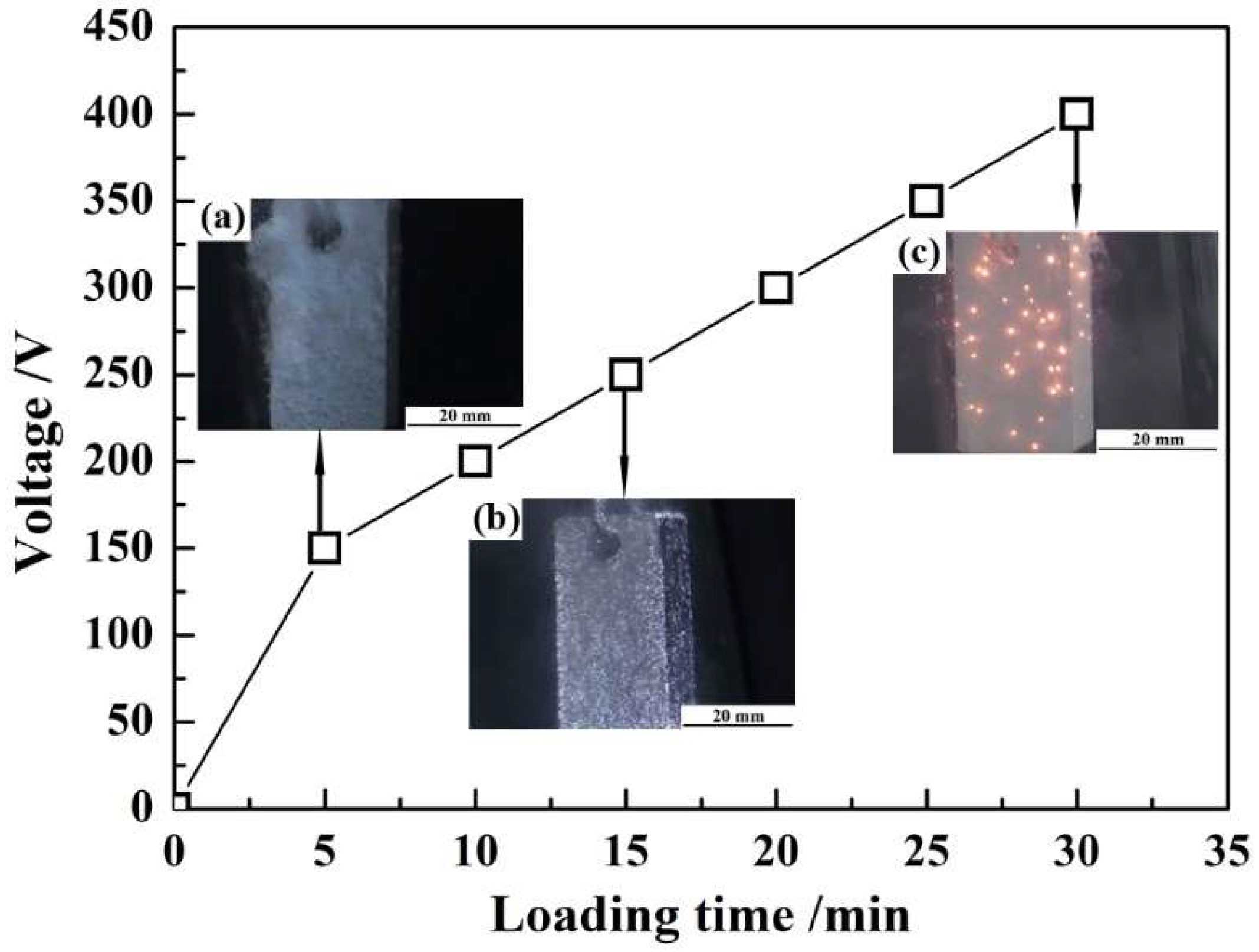
3.3.4. The Arc Starting Voltage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, Y.; Pang, X.; Jiang, B.; Jahed, H. Influence of Micro-arc Oxidation Coatings on Corrosion Performances of AZ80 cast alloy. Int. J. Electrochem. Sci. 2018, 13, 7265–7281. [Google Scholar] [CrossRef]
- Xue, Y.; Pang, X.; Karparvarfard, S.M.H.; Jahed, H.; Luo, S.; Shen, Y. Corrosion Protection of ZK60 Wrought Magnesium Alloys by Micro-Arc Oxidation. Metals 2022, 12, 449. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, T.; Yu, X.; Sun, X.; Yang, H. Functionalization treatment of micro-arc oxidation coatings on magnesium alloys: A review. J. Alloys Compd. 2021, 879, 160453. [Google Scholar] [CrossRef]
- Muhaffel, F.; Cimenoglu, H. Development of corrosion and wear resistant micro-arc oxidation coating on a magnesium alloy. Surf. Coat. Technol. 2019, 357, 822–832. [Google Scholar] [CrossRef]
- Jin, S.; Ma, X.; Wu, R.; Wang, G.; Zhang, J.; Krit, B.; Betsofen, S.; Liu, B. Advances in micro-arc oxidation coatings on Mg-Li alloys. Appl. Surf. Sci. Adv. 2022, 8, 100219. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Li, Q.; Yang, W.; Liu, C.; Wang, D.; Liang, J. Correlations between the growth mechanism and properties of micro-arc oxidation coatings on titanium alloy: Effects of electrolytes. Surf. Coat. Technol. 2017, 316, 162–170. [Google Scholar] [CrossRef]
- Rakoch, A.; Monakhova, E.; Khabibullina, Z.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.; Gladkova, A. Plasma electrolytic oxidation of AZ31 and AZ91 magnesium alloys: Comparison of coatings formation mechanism. J. Magnes. Alloy. 2020, 8, 587–600. [Google Scholar] [CrossRef]
- Zhao, Z.; Pan, Q.; Yan, J.; Ye, J.; Liu, Y. Direct current micro-arc oxidation coatings on Al-Zn-Mg-Mn-Zr extruded alloy with tunable structures and properties templated by discharge stages. Vacuum 2018, 150, 155–165. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Zhang, S.; Zhao, R.; Zhang, R.; Li, X.; Chen, C. Investigation on entrance mechanism of calcium and magnesium into micro-arc oxidation coatings developed on Ti-6Al-4V alloy. Surf. Coat. Technol. 2019, 378, 124951. [Google Scholar] [CrossRef]
- Cengiz, S.; Azakli, Y.; Tarakci, M.; Stanciu, L.; Gencer, Y. Microarc oxidation discharge types and bio properties of the coating synthesized on zirconium. Mater. Sci. Eng. C 2017, 77, 374–383. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Cai, Z.-B.; Cui, Y.; Liu, J.-H.; Zhu, M.-H. Effect of oxidation time on the impact wear of micro-arc oxidation coating on aluminum alloy. Wear 2019, 426–427, 285–295. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, Q.; Mei, D.; Tong, Y.; Zheng, Y.; Li, L.; Zhong, Z. Microstructure, corrosion resistance and formation mechanism of alumina micro-arc oxidation coatings on sintered NdFeB permanent magnets. Surf. Coat. Technol. 2017, 309, 621–627. [Google Scholar] [CrossRef]
- Nashrah, N.; Kamil, M.; Yoon, D.; Kim, Y.; Ko, Y. Formation mechanism of oxide layer on AZ31 Mg alloy subjected to micro-arc oxidation considering surface roughness. Appl. Surf. Sci. 2019, 497, 143772. [Google Scholar] [CrossRef]
- Wang, W.; Feng, S.; Li, Z.; Chen, Z.; Zhao, T. Microstructure and properties of micro-arc oxidation ceramic films on AerMet100 steel. J. Mater. Res. Technol. 2020, 9, 6014–6027. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Zeng, R.-C.; Guan, S.-K.; Qi, W.; Zhang, F.; Li, S.-Q.; Han, E.-H. Degradation mechanism of micro-arc oxidation coatings on biodegradable Mg-Ca alloys: The influence of porosity. J. Alloys Compd. 2017, 695, 2464–2476. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Qiao, L.; Zhao, R.; Zhang, S.; Zhang, R.; Chen, C.; Li, X.; Zhao, Y. Preparation and formation mechanism of copper incorporated micro-arc oxidation coatings developed on Ti-6Al-4V alloys. Surf. Coat. Technol. 2019, 375, 74–85. [Google Scholar] [CrossRef]
- Li, X.-J.; Zhang, M.; Wen, S.; Mao, X.; Huo, W.-G.; Guo, Y.-Y.; Wang, Y.-X. Microstructure and wear resistance of micro-arc oxidation ceramic coatings prepared on 2A50 aluminum alloys. Surf. Coat. Technol. 2020, 394, 125853. [Google Scholar] [CrossRef]
- Ling, K.; Mo, Q.; Lv, X.; Qin, G.; Yang, W.; Li, L.; Li, W. Growth characteristics and corrosion resistance of micro-arc oxidation coating on Al–Mg composite plate. Vacuum 2022, 195, 110640. [Google Scholar] [CrossRef]
- Yao, J.-T.; Wang, S.; Zhou, Y.; Dong, H. Effects of the Power Supply Mode and Loading Parameters on the Characteristics of Micro-Arc Oxidation Coatings on Magnesium Alloy. Metals 2020, 10, 1452. [Google Scholar] [CrossRef]
- Dai, W.; Li, C.; He, D.; Jia, D.; Zhang, Y.; Tan, Z. Influence of duty cycle on fatigue life of AA2024 with thin coating fabricated by micro-arc oxidation. Surf. Coat. Technol. 2019, 360, 347–357. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Y.; Zhao, X.; Ma, R.; Du, A.; Cao, X. Influence of duty cycle on the growth behavior and wear resistance of micro-arc oxidation coatings on hot dip aluminized cast. Surf. Coat. Technol. 2018, 337, 141–149. [Google Scholar] [CrossRef]
- Ly, X.; Yang, S.; Nguyen, T. Effect of equal channel angular pressing as the pretreatment on microstructure and corrosion behavior of micro-arc oxidation (MAO) composite coating on biodegradable Mg-Zn-Ca alloy. Surf. Coat. Technol. 2020, 395, 125923. [Google Scholar] [CrossRef]
- Bai, L.; Dong, B.; Chen, G.; Xin, T.; Wu, J.; Sun, X. Effect of positive pulse voltage on color value and corrosion property of magnesium alloy black micro-arc oxidation ceramic coating. Surf. Coat. Technol. 2019, 374, 402–408. [Google Scholar] [CrossRef]
- Yilmaz, M.S.; Sahin, O. Applying high voltage cathodic pulse with various pulse durations on aluminium via micro-arc oxidation (MAO). Surf. Coat. Technol. 2018, 347, 278–285. [Google Scholar] [CrossRef]
- Songur, F.; Dikici, B.; Niinomi, M.; Arslan, E. The plasma electrolytic oxidation (PEO) coatings to enhance in-vitro corrosion resistance of Ti–29Nb–13Ta–4.6Zr alloys: The combined effect of duty cycle and the deposition frequency. Surf. Coat. Technol. 2019, 374, 345–354. [Google Scholar] [CrossRef]


| Composition | Al | Zn | Mn | Si | Cu | Fe | Ni | Mg |
|---|---|---|---|---|---|---|---|---|
| Weight percentage | 8.5~9.5 | 0.5~0.9 | 0.17~0.4 | ≤0.05 | ≤0.015 | ≤0.004 | ≤0.001 | Rest |
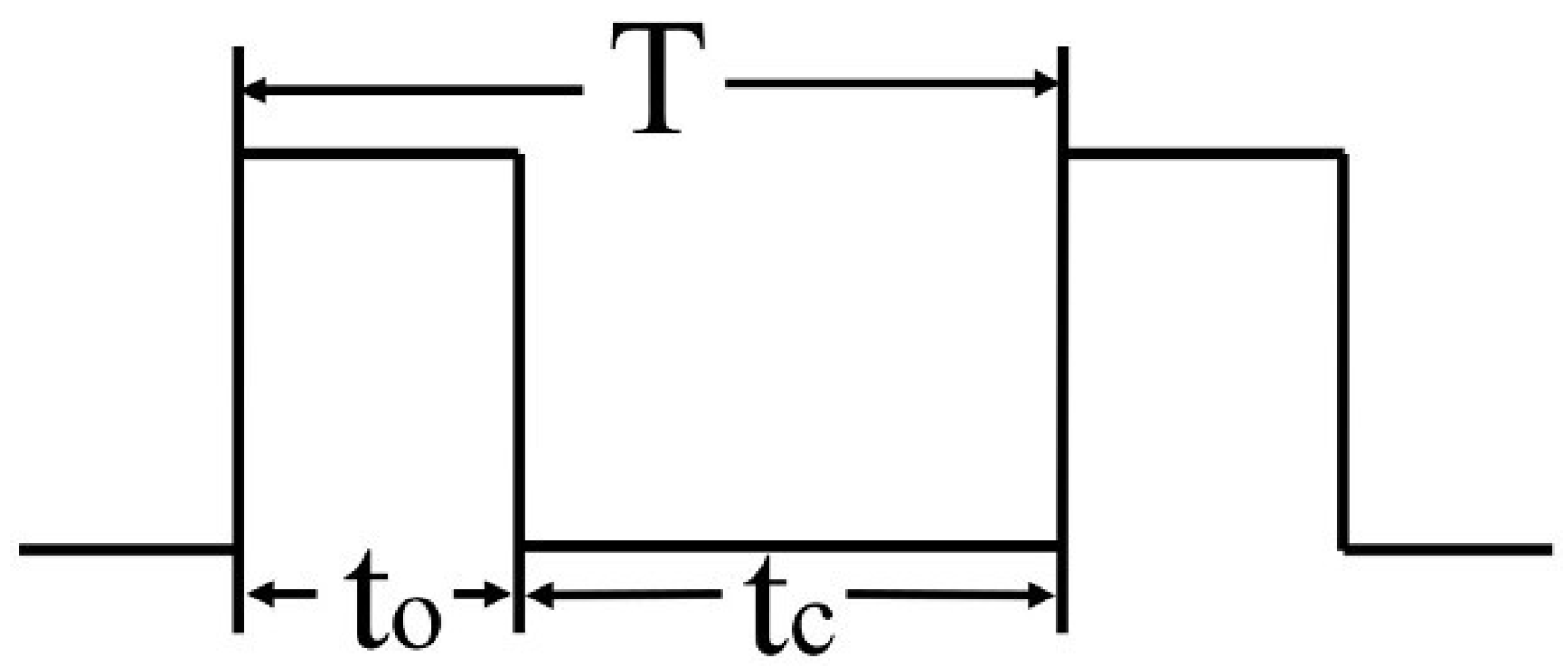
| Duty Cycle (δ/%) | Frequency (f/Hz) | ||||||
|---|---|---|---|---|---|---|---|
| 400 | 444 | 500 | 571 | 667 | 800 | 1000 | |
| 10 | 2.25 ms | 2.03 ms | 1.8 ms | 1.57 ms | 1.35 ms | 1.25 ms | 0.9 ms |
| 20 | 2.0 ms | 1.80 ms | 1.6 ms | 1.40 ms | 1.19 ms | 1.0 ms | 0.8 ms |
| 30 | 1.75 ms | 1.58 ms | 1.4 ms | 1.22 ms | 1.04 ms | 0.87 ms | 0.7 ms |
| 40 | 1.50 ms | 1.35 ms | 1.2 ms | 1.05 ms | 0.9 ms | 0.75 ms | 0.6 ms |
| 50 | 1.25 ms | 1.13 ms | 1.0 ms | 0.88 ms | 0.75 ms | 0.62 ms | 0.5 ms |
| 60 | 1.0 ms | 0.9 ms | 0.8 ms | 0.7 ms | 0.6 ms | 0.5 ms | 0.4 ms |
| 70 | 0.75 ms | 0.67 ms | 0.6 ms | 0.52 ms | 0.44 ms | 0.37 ms | 0.3 ms |
| 80 | 0.50 ms | 0.45 ms | 0.4 ms | 0.35 ms | 0.3 ms | 0.25 ms | 0.2 ms |
| 90 | 0.25 ms | 0.22 ms | 0.2 ms | 0.17 ms | 0.15 ms | 0.12 ms | 0.1 ms |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, K.-X.; Yao, J.-T.; Wang, L.-S.; Dong, H. Large-Arc Discharge Mechanism and Avoidance in Micro-Arc Oxidation of Magnesium Alloy. Metals 2022, 12, 949. https://doi.org/10.3390/met12060949
Guan K-X, Yao J-T, Wang L-S, Dong H. Large-Arc Discharge Mechanism and Avoidance in Micro-Arc Oxidation of Magnesium Alloy. Metals. 2022; 12(6):949. https://doi.org/10.3390/met12060949
Chicago/Turabian StyleGuan, Ke-Xin, Jian-Tao Yao, Li-Shuang Wang, and Hui Dong. 2022. "Large-Arc Discharge Mechanism and Avoidance in Micro-Arc Oxidation of Magnesium Alloy" Metals 12, no. 6: 949. https://doi.org/10.3390/met12060949
APA StyleGuan, K.-X., Yao, J.-T., Wang, L.-S., & Dong, H. (2022). Large-Arc Discharge Mechanism and Avoidance in Micro-Arc Oxidation of Magnesium Alloy. Metals, 12(6), 949. https://doi.org/10.3390/met12060949






