Optimization, Kinetic Studies of Tin Leaching from Waste Printed Circuit Boards and Selective Tin Recovery from Its Pregnant Solution
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Reagents
2.2. Leaching Tests
2.3. Design of Experiments
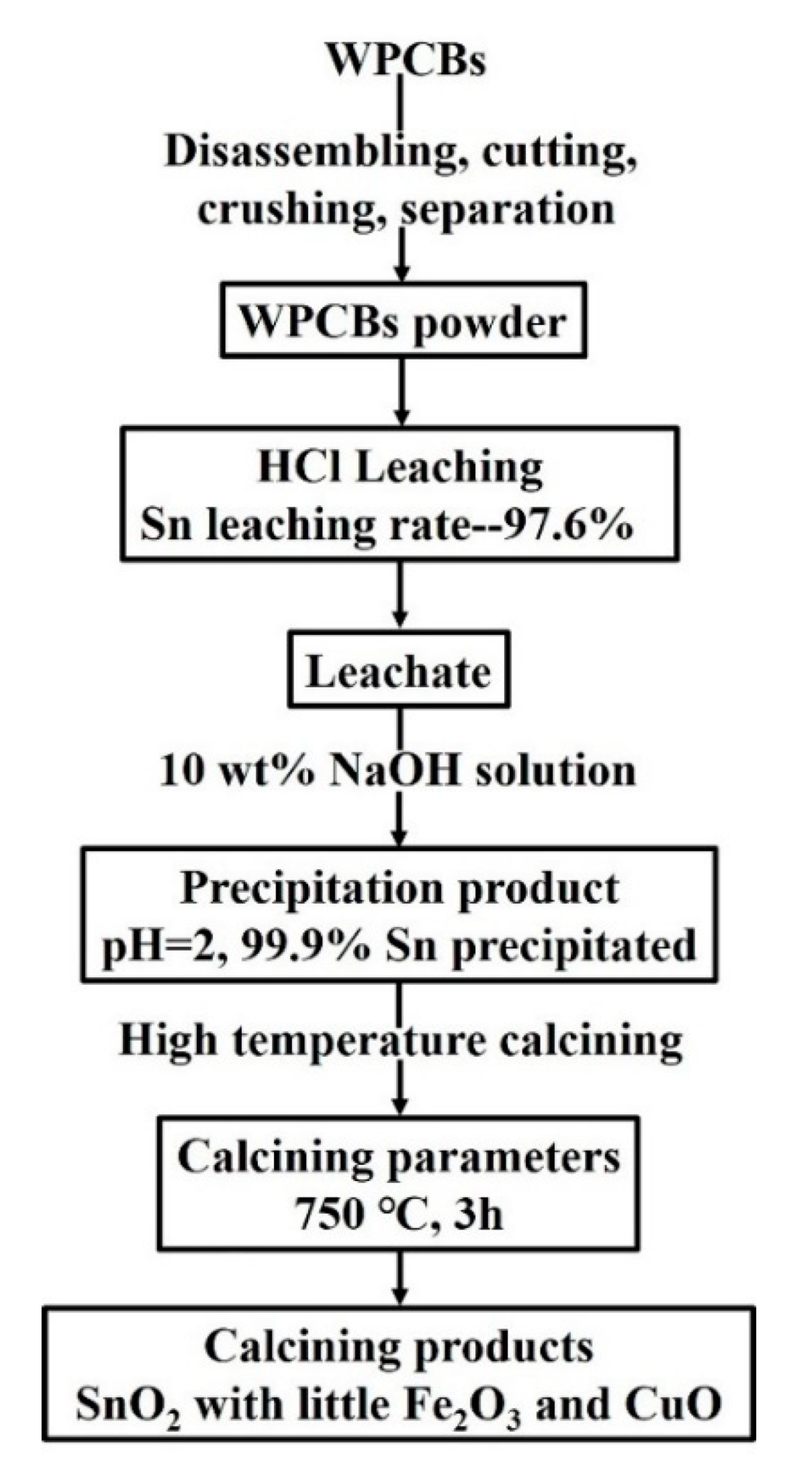
2.4. The Recovery of Sn from Pregnant Leach Solution
3. Results and Discussion
3.1. Effect of Temperature
3.2. Effect of HCl Concentration
3.3. Effect of Solid–Liquid Ratio
3.4. Effect of Stirring Speed
3.5. Modeling of Sn Leaching Rate
3.6. Kinetics Analysis
3.6.1. Leaching Kinetics of Sn
3.6.2. Leaching Regular Patterns under Different Parameters
3.6.3. Kinetic Equations of Sn Leaching
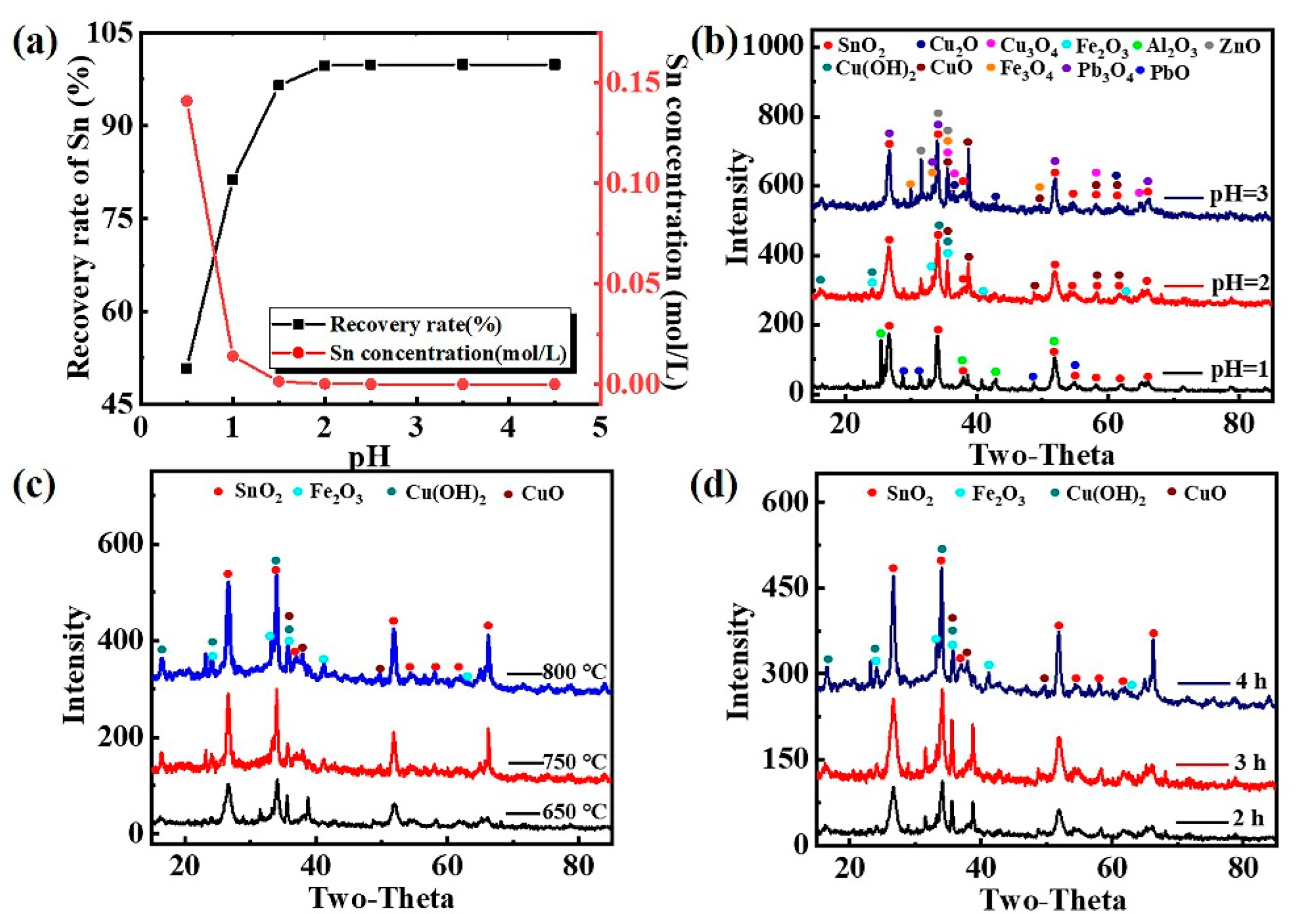
3.7. The Recovery of Sn
4. Conclusions
- (1)
- During the leaching processes, the leaching rates of Sn increased with the increases in HCl concentration, temperature, and stirring speed, but decreased with the increase in solid–liquid ratio.
- (2)
- Based on the three-dimensional response surface plots, the interactive relationships between each of the following pairs of A (temperature)-B (stirring speed), A (temperature)-C (solid–liquid ratio), and A (temperature)-D (HCl concentration) showed significant effects on the Sn leaching rate.
- (3)
- The optimal leaching parameters obtained by RSM were 74.1 °C, 495.5 rpm, 0.08 g/mL, and 4.94 mol/L HCl, and 97.6% of Sn was leached at these optimization leaching conditions.
- (4)
- In the HCL solution, Sn leaching process is controlled by mixed control reaction with apparent activation energies of 20.3 kJ/mol. Meanwhile, as shown in Equation (17), a macroscopic kinetic equation of the Sn leaching process was established and could suggest the effect of various factors comprehensively.
- (5)
- Sn in the pregnant leach solution can be precipitated selectively through adjusting pH value of leach solution as 2, and almost all Sn was recovered. Then, the precipitation product was calcinated and SnO2 with good crystallinity can be obtained by calcining at 750 °C for 3 h.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Xu, Y.; Tian, T.; Wang, T.; Liu, Y. The Impacts of China’s Fund Policy on Waste Electrical and Electronic Equipment Utilization. J. Clean. Prod. 2020, 251, 119582. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Ghorbani, Y.; Chong, M.N.; Herath, G.; Moyo, T.; Petersen, J. E-Waste in the International Context—A Review of Trade Flows, Regulations, Hazards, Waste Management Strategies and Technologies for Value Recovery. Waste Manag. 2018, 82, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Barnwal, A.; Dhawan, N. Evaluation of Fluidization Process for Recovery of Metals from Discarded Printed Circuit Boards. J. Sustain. Metall. 2019, 5, 519–527. [Google Scholar] [CrossRef]
- Jian-Guang, Y.; Jie, L.; Si-Yao, P.; Yuan-Lu, L.; Wei-Qiang, S. A New Membrane Electro-Deposition Based Process for Tin Recovery from Waste Printed Circuit Boards. J. Hazard. Mater. 2016, 304, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.; Flerus, B.; Nagraj, S.; Bokelmann, K.; Stauber, R.; Friedrich, B. Comparative Analysis About Degradation Mechanisms of Printed Circuit Boards (PCBs) in Slow and Fast Pyrolysis: The Influence of Heating Speed. J. Sustain. Metall. 2018, 4, 205–221. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, J.C.; Lee, K.S.; Kim, B.S.; Kim, M.S.; Kim, S.K.; Pandey, B.D. Recovery of Sn, Ag and Cu from Waste Pb-Free Solder Using Nitric Acid Leaching. Mater. Trans. 2012, 53, 2175–2180. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.; Park, I.; Yoo, K.; Ryu, H. The Effects of Temperature and Agitation Speed on the Leaching Behaviors of Tin and Bismuth from Spent Lead Free Solder in Nitric Acid Leach Solution. Geosystem Eng. 2015, 18, 213–218. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Choubey, P.K.; Lee, J.C.; Kumar, V.; Jeong, J. Leaching of Lead from Solder Material of Waste Printed Circuit Boards (PCBs). Hydrometallurgy 2012, 121–124, 28–34. [Google Scholar] [CrossRef]
- da Silva, M.S.B.; de Melo, R.A.C.; Lopes-Moriyama, A.L.; Souza, C.P. Electrochemical Extraction of Tin and Copper from Acid Leachate of Printed Circuit Boards Using Copper Electrodes. J. Environ. Manag. 2019, 246, 410–417. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, P.; Liu, W.; Chen, L.; Zhang, D. Recovery of Tin from Metal Powders of Waste Printed Circuit Boards. Waste Manag. 2017, 68, 449–457. [Google Scholar] [CrossRef]
- Han, J.; Liang, C.; Liu, W.; Qin, W.; Jiao, F.; Li, W. Pretreatment of Tin Anode Slime Using Alkaline Pressure Oxidative Leaching. Sep. Purif. Technol. 2017, 174, 389–395. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Qin, H.; Liu, Y.; Tian, Q.; Li, D. Recovery of Metal Values from Waste Printed Circuit Boards Using an Alkali Fusion–Leaching–Separation Process. Hydrometallurgy 2015, 156, 199–205. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, K.; Kumar, M.; Lee, J. Separation of Sn from Waste Pb-Free Sn—Ag—Cu Solder in Hydrochloric Acid Solution with Ferric Chloride. Hydrometallurgy 2015, 157, 184–187. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Yoo, K. Leaching of Tin from Waste Pb-Free Solder in Hydrochloric Acid Solution with Stannic Chloride. Hydrometallurgy 2016, 165, 143–147. [Google Scholar] [CrossRef]
- Jeon, S.; Yoo, K.; Diaz, R. Separation of Sn, Bi, Cu from Pb-Free Solder Paste by Ammonia Leaching Followed by Hydrochloric Acid Leaching. Hydrometallurgy 2017, 169, 26–30. [Google Scholar] [CrossRef]
- Jo, J.H.; Lee, D.S.; Park, D.; Choe, W.S.; Park, J.M. Optimization of Key Process Variables for Enhanced Hydrogen Production by Enterobacter Aerogenes Using Statistical Methods. Bioresour. Technol. 2008, 99, 2061–2066. [Google Scholar] [CrossRef]
- Bao, S.; Chen, Q.; Zhang, Y.; Tian, X. Optimization of Preparation Conditions of Composite Electrodes for Selective Adsorption of Vanadium in CDI by Response Surface Methodology. Chem. Eng. Res. Des. 2021, 168, 37–45. [Google Scholar] [CrossRef]
- Ghafarzadeh, M.; Abedini, R.; Rajabi, R. Optimization of Ultrasonic Waves Application in Municipal Wastewater Sludge Treatment Using Response Surface Method. J. Clean. Prod. 2017, 150, 361–370. [Google Scholar] [CrossRef]
- Rincón, J.; Camarillo, R.; Martín, A. Solubility of Aluminum Sulfate in Near-Critical and Supercritical Water. J. Chem. Eng. Data 2012, 57, 2084–2094. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.C.; Huynh, T.H.; Jeong, J.; Pandey, B.D. Optimizing the Thiosulfate Leaching of Gold from Printed Circuit Boards of Discarded Mobile Phone. Hydrometallurgy 2014, 149, 118–126. [Google Scholar] [CrossRef]
- Yazici, E.Y.; Deveci, H. Extraction of Metals from Waste Printed Circuit Boards (WPCBs) in H2SO4-CuSO4-NaCl Solutions. Hydrometallurgy 2013, 139, 30–38. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, X.; Zhao, Z. Kinetics of Nickel Leaching from Low-Nickel Matte in Sulfuric Acid Solution under Atmospheric Pressure. Hydrometallurgy 2020, 194, 105353. [Google Scholar] [CrossRef]
- Crundwell, F.K. The Dissolution and Leaching of Minerals: Mechanisms, Myths and Misunderstandings. Hydrometallurgy 2013, 139, 132–148. [Google Scholar] [CrossRef]
- Luo, M.J.; Liu, C.L.; Xue, J.; Li, P.; Yu, J.G. Leaching Kinetics and Mechanism of Alunite from Alunite Tailings in Highly Concentrated KOH Solution. Hydrometallurgy 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Moosakazemi, F.; Ghassa, S.; Mohammadi, M.R.T. Environmentally Friendly Hydrometallurgical Recovery of Tin and Lead from Waste Printed Circuit Boards: Thermodynamic and Kinetics Studies. J. Clean. Prod. 2019, 228, 185–196. [Google Scholar] [CrossRef]
- Saleh, S.M.; Said, S.A.; El-shahawi, M.S. Extraction and Recovery of Au, Sb and Sn from Electrorefined Solid Waste. Anal. Chim. Acta 2001, 436, 69–77. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Luo, T.; Chen, Y. Comprehensive Recovery of Sn-Cu Bearing Residue and Preparation of High Purity SnO2 and CuSO4⋅5H2O. Sep. Purif. Technol. 2021, 257, 117826. [Google Scholar] [CrossRef]
- Seo, E.Y.; Cheong, Y.W.; Yim, G.J.; Min, K.W.; Geroni, J.N. Recovery of Fe, Al and Mn in Acid Coal Mine Drainage by Sequential Selective Precipitation with Control of PH. Catena 2017, 148, 11–16. [Google Scholar] [CrossRef]
- Balladares, E.; Jerez, O.; Parada, F.; Baltierra, L.; Hernández, C.; Araneda, E.; Parra, V. Neutralization and Co-Precipitation of Heavy Metals by Lime Addition to Effluent from Acid Plant in a Copper Smelter. Miner. Eng. 2018, 122, 122–129. [Google Scholar] [CrossRef]
- Comel, J.; Meux, E.; Leclerc, N.; Diliberto, S. Use of Phytic Acid for Selective Precipitation of Undesirable Metals (Al, Fe, Pb) Contained in the Leachates from Hydrometallurgical Processes. J. Environ. Chem. Eng. 2021, 9, 105450. [Google Scholar] [CrossRef]
- Gao, J.M.; Zhang, M.; Cheng, F.; Guo, M. Process Development for Selective Precipitation of Valuable Metals and Simultaneous Synthesis of Single-Phase Spinel Ferrites from Saprolite-Limonite Laterite Leach Liquors. Hydrometallurgy 2017, 173, 98–105. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical Precipitation of Heavy Metals from Acid Mine Drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Experimental Design Methods for Fermentative Hydrogen Production: A Review. Int. J. Hydrogen Energy 2009, 34, 235–244. [Google Scholar] [CrossRef]
- Shawabkeh, R.A. Hydrometallurgical Extraction of Zinc from Jordanian Electric Arc Furnace Dust. Hydrometallurgy 2010, 104, 61–65. [Google Scholar] [CrossRef]
- Yamasaki, N.; Lan, X.; Feng, Q.I. Kinetics of Hydrothermal Oxidation of Granular Pb Metal to Pbo Powder in Sodium Hydroxide Solutions. Metall. Mater. Trans. B Process Metall. Mater. Processing Sci. 1997, 28, 33–37. [Google Scholar] [CrossRef]
- Levenspiel, O. Experimental Search for a Simple Rate Equation to Describe Deactivating Porous Catalyst particles. J. Catal. 1972, 25, 265–272. [Google Scholar] [CrossRef]
- Abrantes, L.M.; Costa, M.C. Electro-Oxidation as a Pre-Treatment for Gold Recovery. Hydrometallurgy 1996, 40, 99–110. [Google Scholar] [CrossRef]
- Gao, G.; Li, D.; Zhou, Y.; Sun, X.; Sun, W. Kinetics of High-Sulphur and High-Arsenic Refractory Gold Concentrate Oxidation by Dilute Nitric Acid under Mild Conditions. Miner. Eng. 2009, 22, 111–115. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, T.; Wen, J.; Gao, H.; Wang, J.; Xue, X. Leaching Kinetics of Mechanically Activated Boron Concentrate in a NaOH Solution. Hydrometallurgy 2018, 179, 60–72. [Google Scholar] [CrossRef]
- Supothina, S.; Rattanakam, R.; Vichaphund, S.; Thavorniti, P. Effect of Synthesis Condition on Morphology and Yield of Hydrothermally Grown SnO2 Nanorod Clusters. J. Eur. Ceram. Soc. 2011, 31, 2453–2458. [Google Scholar] [CrossRef]
- Fang, B.; Yu, J.; Ge, X.; Yang, C. Ionic Liquid Assisted Hydrothermal Synthesis of Monodispersed Mesoporous SnO2 Nanospheres. Mater. Lett. 2012, 73, 229–231. [Google Scholar] [CrossRef]
- Yang, H. Enhanced Photoluminescence Property of SnO2 Nanoparticles Contained in Mesoporous Silica Synthesized with Leached Talc as Si Source. Microporous Mesoporous Mater. 2007, 102, 204–211. [Google Scholar] [CrossRef]






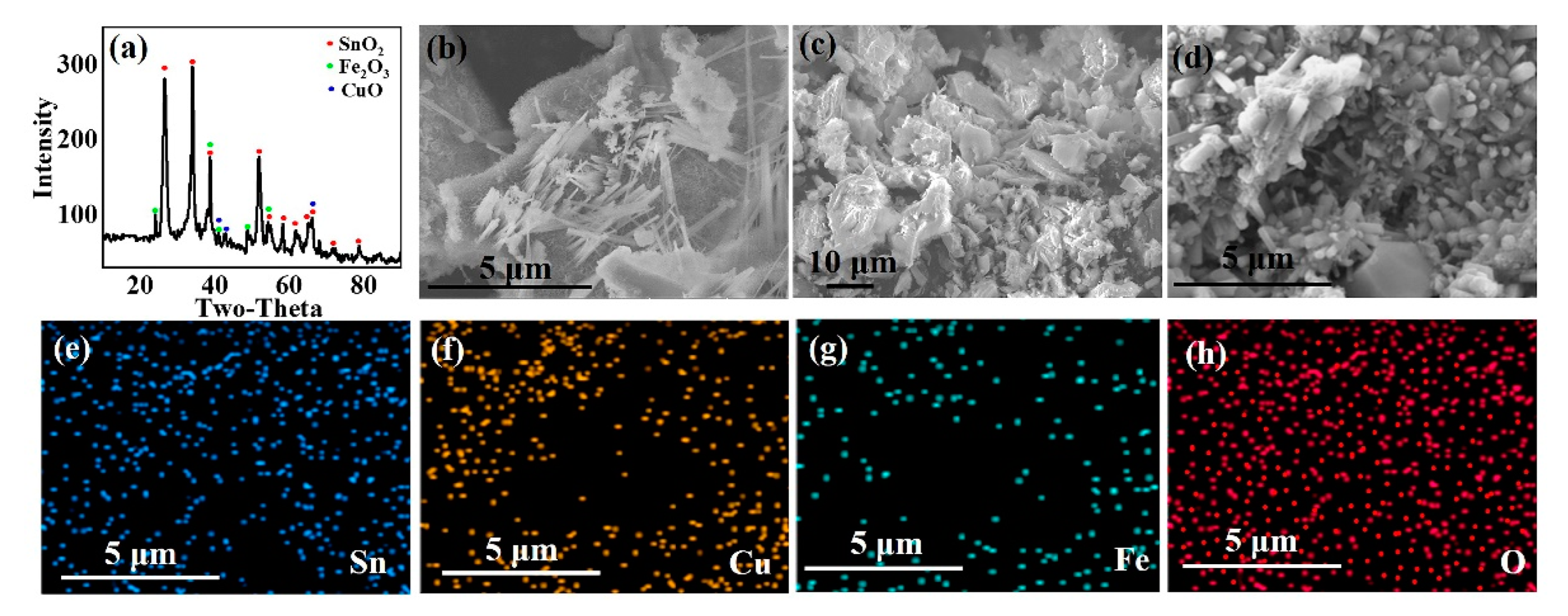
| Elements | Au | Ag | Cu | Fe | Ni | Zn | Sn | Pb | Al | Cr | Co |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/g) | 0.08 | 0.62 | 422.96 | 5.09 | 8.40 | 8.15 | 24.9 | 2.80 | 0.76 | BDL | BDL |
| Parameters | Levels | |||
|---|---|---|---|---|
| Low | Median | High | ||
| −1 | 0 | 1 | ||
| A | Temperature (°C) | 45 | 60 | 75 |
| B | Agitation speed (rpm) | 300 | 400 | 500 |
| C | Solid–liquid ratio (g/mL) | 0.05 | 0.1 | 0.15 |
| D | HCl concentration (mol/L) | 3 | 4 | 5 |
| No | A | B | C | D | Recovery (%) |
|---|---|---|---|---|---|
| Sn | |||||
| 1 | 75 | 300 | 0.10 | 4 | 90 |
| 2 | 60 | 500 | 0.05 | 4 | 73.27 |
| 3 | 45 | 400 | 0.10 | 5 | 58.95 |
| 4 | 60 | 300 | 0.05 | 4 | 73.7 |
| 5 | 60 | 400 | 0.05 | 5 | 80.56 |
| 6 | 60 | 500 | 0.10 | 5 | 75.3 |
| 7 | 60 | 400 | 0.15 | 5 | 35.79 |
| 8 | 60 | 500 | 0.10 | 3 | 62.29 |
| 9 | 60 | 300 | 0.15 | 4 | 28.36 |
| 10 | 75 | 400 | 0.10 | 5 | 96 |
| 11 | 60 | 400 | 0.05 | 3 | 64.98 |
| 12 | 60 | 300 | 0.10 | 3 | 62.8 |
| 13 | 45 | 400 | 0.05 | 4 | 56.55 |
| 14 | 45 | 500 | 0.10 | 4 | 53.52 |
| 15 | 60 | 500 | 0.15 | 4 | 33.22 |
| 16 | 75 | 400 | 0.10 | 3 | 70 |
| 17 | 75 | 400 | 0.05 | 4 | 94 |
| 18 | 75 | 400 | 0.15 | 4 | 60 |
| 19 | 45 | 300 | 0.10 | 4 | 50.1 |
| 20 | 45 | 400 | 0.10 | 3 | 46.15 |
| 21 | 45 | 400 | 0.15 | 4 | 22.71 |
| 22 | 60 | 400 | 0.15 | 3 | 20.64 |
| 23 | 75 | 500 | 0.10 | 4 | 90 |
| 24 | 60 | 300 | 0.10 | 5 | 76.95 |
| 25 | 60 | 400 | 0.10 | 4 | 69.56 |
| 26 | 60 | 400 | 0.10 | 4 | 72.67 |
| 27 | 60 | 400 | 0.10 | 4 | 73.36 |
| 28 | 60 | 400 | 0.10 | 4 | 73.25 |
| 29 | 60 | 400 | 0.10 | 4 | 73.75 |
| Source | Sum of Squares | Mean Quares | p-Value |
|---|---|---|---|
| Model | 11,543.99 | 824.57 | <0.0001 |
| A-Temperature | 3746.26 | 3746.26 | <0.0001 |
| B-Stirring speed | 2.7 | 2.7 | 0.6731 |
| C-Solid–liquid ratio | 4894.2 | 4894.2 | <0.0001 |
| D-HCl concentration | 778.89 | 778.89 | <0.0001 |
| AB | 2.92 | 2.92 | 0.6609 |
| AC | 0.0062 | 0.0062 | 0.9839 |
| AD | 43.59 | 43.59 | 0.1055 |
| BC | 7.00 | 7.00 | 0.4992 |
| BD | 0.3247 | 0.3247 | 0.8834 |
| CD | 0.05 | 0.046 | 0.956 |
| A2 | 3.61 | 3.61 | 0.6264 |
| B2 | 15.89 | 15.89 | 0.3137 |
| C2 | 1936.66 | 1936.66 | <0.0001 |
| D2 | 101.26 | 101.26 | 0.0195 |
| Error | 2.45 | 0.61 | |
| Residual | 203.73 | 14.55 | |
| Lack of fit | 192.18 | 19.22 | 0.065 |
| Total | 11,747.72 |
| Elements | Sn | Cu | Fe | Zn | Ni | Al |
|---|---|---|---|---|---|---|
| Content (g/L) | 2.43 | 1.31 | 0.48 | 0.81 | 0.71 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Wang, X.; Wang, Y.; Guo, F.; Wu, Y. Optimization, Kinetic Studies of Tin Leaching from Waste Printed Circuit Boards and Selective Tin Recovery from Its Pregnant Solution. Metals 2022, 12, 954. https://doi.org/10.3390/met12060954
Hao J, Wang X, Wang Y, Guo F, Wu Y. Optimization, Kinetic Studies of Tin Leaching from Waste Printed Circuit Boards and Selective Tin Recovery from Its Pregnant Solution. Metals. 2022; 12(6):954. https://doi.org/10.3390/met12060954
Chicago/Turabian StyleHao, Juanjuan, Xiaolu Wang, Yishu Wang, Fu Guo, and Yufeng Wu. 2022. "Optimization, Kinetic Studies of Tin Leaching from Waste Printed Circuit Boards and Selective Tin Recovery from Its Pregnant Solution" Metals 12, no. 6: 954. https://doi.org/10.3390/met12060954
APA StyleHao, J., Wang, X., Wang, Y., Guo, F., & Wu, Y. (2022). Optimization, Kinetic Studies of Tin Leaching from Waste Printed Circuit Boards and Selective Tin Recovery from Its Pregnant Solution. Metals, 12(6), 954. https://doi.org/10.3390/met12060954






