Abstract
The CrFeCoNi coating was fabricated by the laser remelting method. The microstructure of the coating was detected. The corrosion behavior of coating at different temperatures was investigated by electrochemical measurements. Potentiodynamic polarization test results indicated that the corrosion current density increased with the increase in corrosion solution temperature in 3.5 wt% NaCl and 0.5 mol/L H2SO4 solutions, suggesting that the CrFeCoNi coating exhibited higher resistance to corrosion attack at lower solution temperature. The breakdown potential of the CrFeCoNi coating exhibited a decreasing trend with increasing solution temperature in 3.5 wt% NaCl solution. This indicated that the passive film formed at low temperatures had higher stability. The electrochemical impedance spectroscopy test indicated that the CrFeCoNi coating possessed higher charge transfer resistance at lower solution temperatures. The corrosion mechanisms of the coating at different temperatures were also revealed.
1. Introduction
High entropy alloys (HEA) are a new kind of alloy, which contain multiple principal elements with each atomic concentration in the range of 5–35 at% [1,2,3,4]. It has attracted considerable attention due to its excellent properties, such as excellent mechanical properties, remarkable fatigue resistance, good wear resistance, high-temperature stability, and hardness [5,6,7,8]. Additionally, the HEAs have been proposed as promising corrosion resistance materials due to their high content of corrosion resistance alloying elements and the formation of protective passivation film [9,10].
A large number of reports have investigated the corrosion properties of HEAs. These studies mainly concentrate on two aspects. On the one hand, much attention has been focused on the effect of alloying elements on the corrosion behavior of HEAs. Cui et al. [11] investigated the effect of Nb content on the corrosion behavior of Ni1.5CrCoFe0.5Mo0.1Nbx HEA. They pointed out that the performance of passivation film was improved with the increase in Nb content. Ji et al. [12] fabricated Al35Mg30−xZn30Cu5Six (x = 5, 10, 15) lightweight HEA. They indicated that increasing the Si/Mg content had a positive influence on the corrosion resistance of HEA. Wang et al. [13] reported that the addition of Mn in HEA alloy coatings would decrease the corrosion resistance of HEA. Qzturk et al. [14] investigated the effect of titanium addition on the corrosion behavior of CoCuFeNiMn HEA. They indicated that the corrosion resistance of CoCuFeNiMnTix HEA decreased with the addition of titanium. On the other hand, relentless effort has been performed to explore the effect of microstructure on the corrosion behavior of HEAs. Shuang et al. [15] investigated the effects of the size of eutectic structures on the corrosion behavior of FeCrNiCoNb0.5 HEA. They pointed out that microstructure refinement was beneficial in improving the corrosion resistance of FeCrNiCoNb0.5 HEA. Thota et al. [16] studied the effect of grain boundary engineering on the corrosion behavior of CoCrFeMnNi HEA. They found that the grain boundary engineering specimens exhibited higher corrosion resistance, which was attributed to their high fraction of low ∑CSL boundaries. Parakh et al. [17] researched the effect of crystal structure and grain size on the corrosion properties of AlCoCrFeNi HEA and found that an increase in grain size contributed to improving corrosion resistance.
All the above studies about the corrosion behavior of HEA were carried out at room temperature. However, limited reports on the corrosion behavior of HEA at higher temperatures can be found in published studies and works. It is well known that temperature plays an important role in the corrosion behavior of alloys. Therefore, it is necessary to investigate the effect of temperature on the corrosion behavior of HEA. High entropy alloy bulks are generally fabricated by a variety of methods, including conventional melting, forging, powder metallurgy, and additive manufacturing [18,19,20]. The high cost of bulk HEAs limits their application. Preparing the HEAs as coatings would enlarge its application range and reduce cost. Therefore, it is meaningful to study the effect of temperature on the corrosion behavior of HEA coating.
Numerous methods have been used to prepare HEA coating, including air plasma spray technology, magnetron sputtering, cold spraying, arc cladding, and laser cladding [2,4,9,10,21,22]. Laser cladding technology utilizes a high-energy density laser heat source to deposit a thin layer of the desired material on the substrate. It is considered a promising approach due to excellent coating cohesion, low thermal effect on the substrate, a controlled dilution ratio and coating thickness, easier automation, and high coating preparation efficiency [13,23,24]. Many researchers have successfully fabricated high entropy alloys using the laser cladding method. They found that laser-cladded coating exhibited high coating equality and good properties [9,13]. Therefore, this study applied the laser cladding method to fabricate HEA coating. Previous studies have indicated that CrFeCoNi HEA possessed excellent corrosion resistance [25]. Therefore, it is expected that CrFeCoNi HEA coating can be used in some industrial applications for corrosion protection, such as in accelerator-driven systems and light water reactors [26,27,28,29].
In this study, the CrFeCoNi HEA coating was fabricated by laser cladding technology. The microstructure of the coating was investigated. The electrochemical tests were carried out at different temperatures to reveal the effect of temperature on the corrosion behavior of the CrFeCoNi coating. The rationale behind the differences in corrosion resistance at different temperatures was discussed.
2. Materials and Methods
The CrFeCoNi high entropy alloy powder in equimolar ratio was used to prepare coatings on the X65 steel substrate. The equipment was a fiber laser machine equipped with a powder feeder. The laser cladding process was performed twice using the technological parameter of 2.5 kW power, 350 mm/s scanning rate, and 50% overlapping ratio. Afterward, the prepared coating was cut into 4 mm × 4 mm bulks for microstructure observation and electrochemical test.
The microstructure of the CrFeCoNi coating was characterized by optical microscopy. The chemical composition of coating was analyzed by energy dispersive spectroscopy (EDS, IE 350). The electrochemical experiments were performed using a traditional three electrodes system, in which a platinum sheet acted as the counter electrode, a Hg/Hg2SO4 acted as the reference electrode, and the prepared coating samples served as the working electrode. The potentiodynamic polarization curve investigations were carried out in 3.5 wt% NaCl and 0.5 mol/L H2SO4 solution at different temperatures at a scanning rate of 0.0005 V/s. Prior to tests, the samples were immersed in the test solution to achieve relative stability of potential. Electrochemical impedance spectroscopy (EIS) tests were performed by exerting a 10 mV amplitude signal in the frequency range from 100 kHz to 0.01 Hz at different temperatures. Then the EIS data were fitted using the ZsimpWin software (https://www.ameteksi.com/products/software/zsimpwin, accessed on 4 May 2022).
3. Results and Discussion
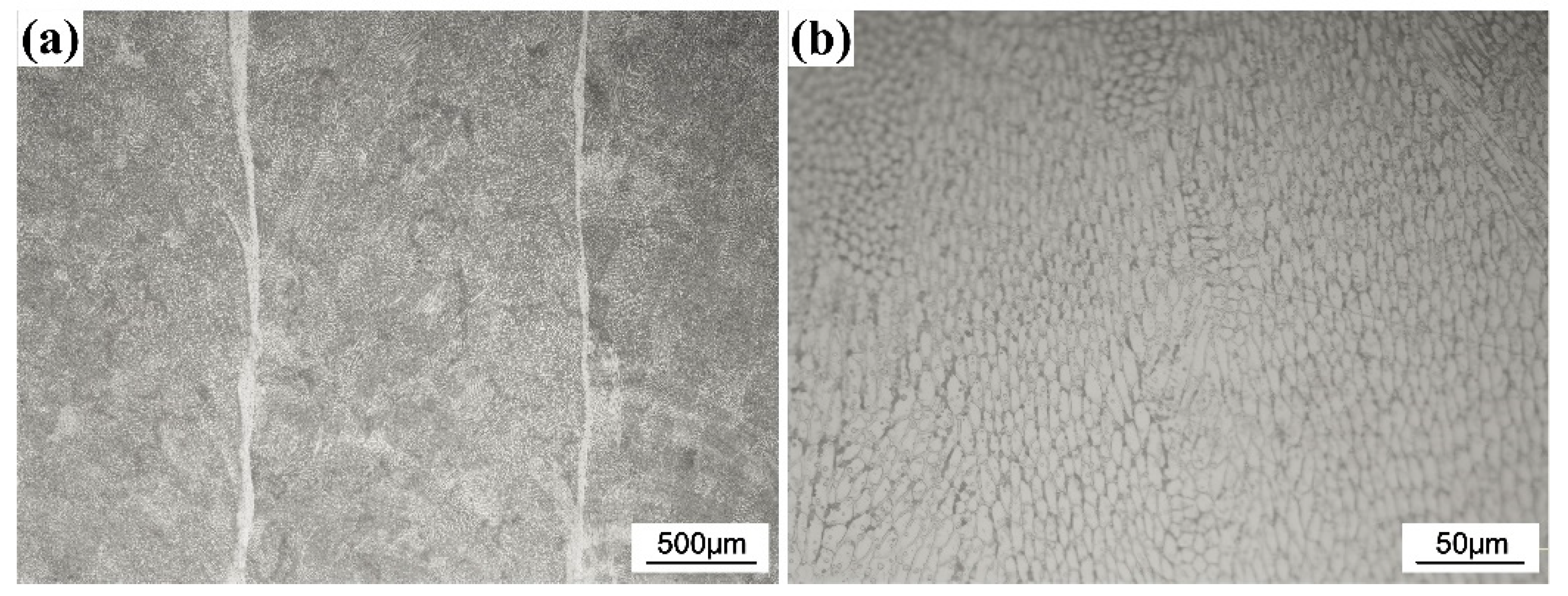
Figure 1 shows the cross-sectional optical micrographs of the CrFeCoNi coating. It can be found that the thickness of the coating is about 1000 μm. The substrate and coating exhibit metallurgical bonding. Figure 2 shows the optical microstructure of the CrFeCoNi coating on the surface. It was found that the coating is dense, and no obvious defect is found, indicating suitable machining parameters. The high magnified microstructure in Figure 2b shows that the CrFeCoNi coating is composed of numerous fine subgrains, which is consistent with the previous result [13]. The chemical composition of the coating is investigated using EDS, and the result is provided in Table 1. As shown in Table 1, the coating has higher Fe content than primary CrFeCoNi powder due to the dilution effect of the substrate.

Figure 1.
Cross-sectional optical microstructure of the CrFeCoNi coating.
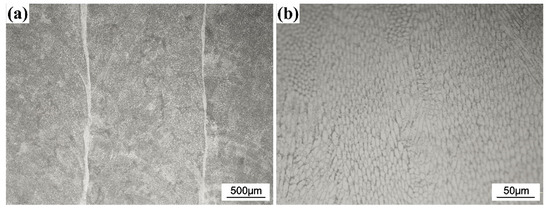
Figure 2.
Optical microstructure of the CrFeCoNi coating at low magnification (a) and high magnification (b).

Table 1.
The chemical composition of the prepared coating.
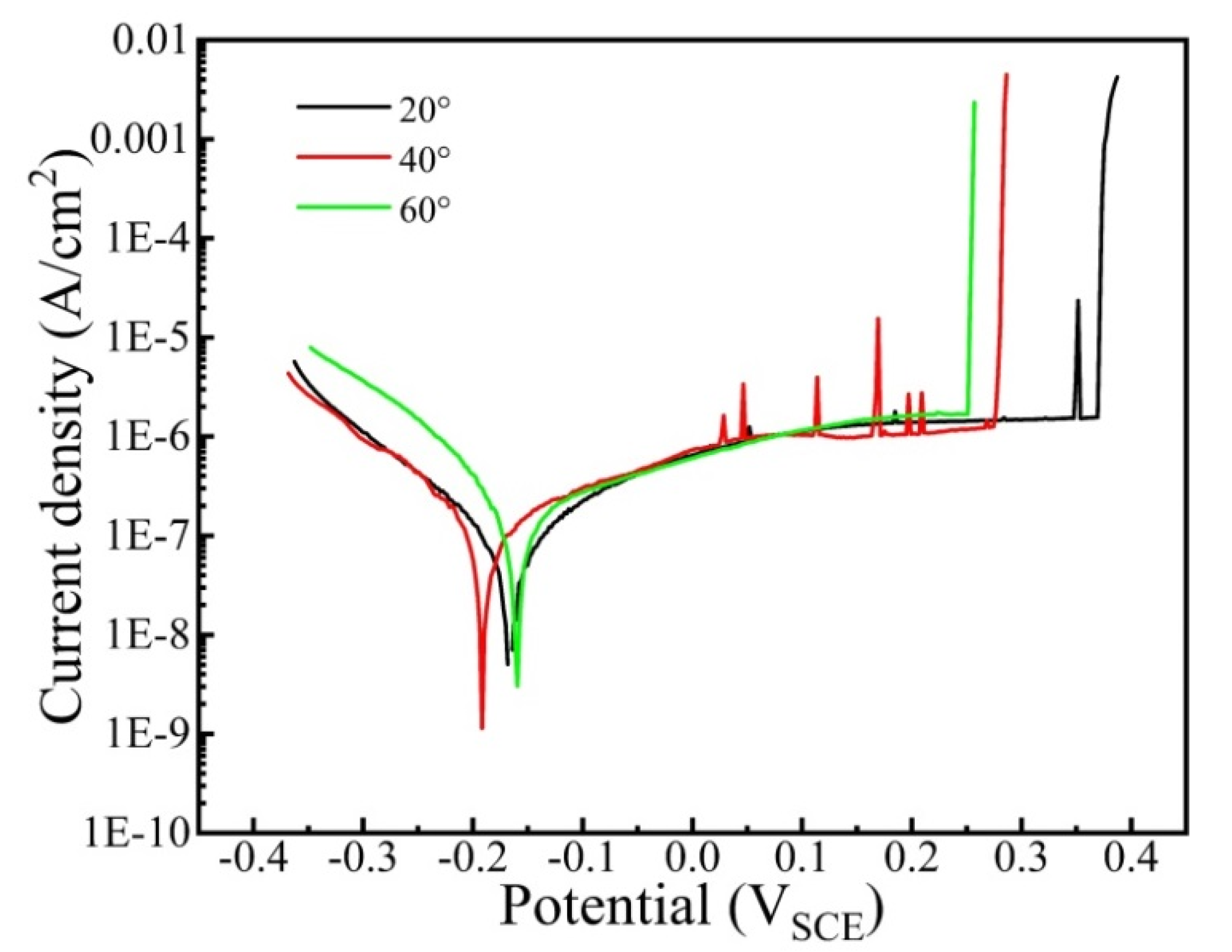
Figure 3 shows the potentiodynamic polarization curve of the CrFeCoNi coating in 3.5 wt% NaCl solution at different temperatures. As shown in Figure 3, the cathode regions of all the curves obey Tafel linearity. At the anode region, all the curves show immediate passivation. However, the passivation regions exhibit evident differences. It can be found that the passivation regions of the coating gradually turn wider with the decreasing corrosion solution temperature. The corresponding electrochemical parameters are calculated in Table 2. It is found that the corrosion current density of the coating gradually increases from 7.15 × 10−8 A/cm2 to 2.99 × 10−7 A/cm2 as the solution temperature rises from 20 °C to 60 °C. This indicates that the corrosion resistance of coating decreases with the rising corrosion temperature. The breakdown potential of coating presents a decreasing trend with the increase in corrosion temperature, suggesting that the forming passivation film at low temperature possesses higher stability.
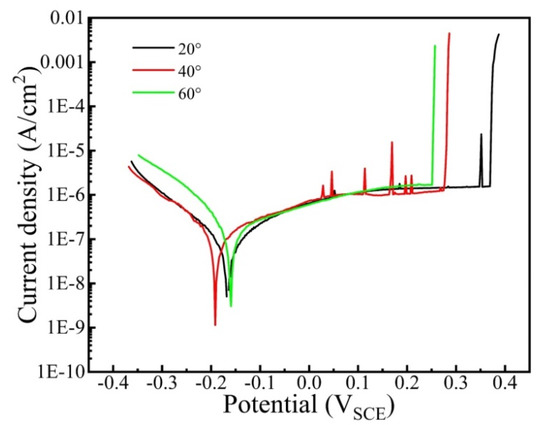
Figure 3.
Potentiodynamic polarization curve of the CrFeCoNi coating in 3.5 wt% NaCl solution at different temperatures.

Table 2.
The electrochemical parameters of the CrFeCoNi coating in 3.5 wt% NaCl solution at different tepmeratures.
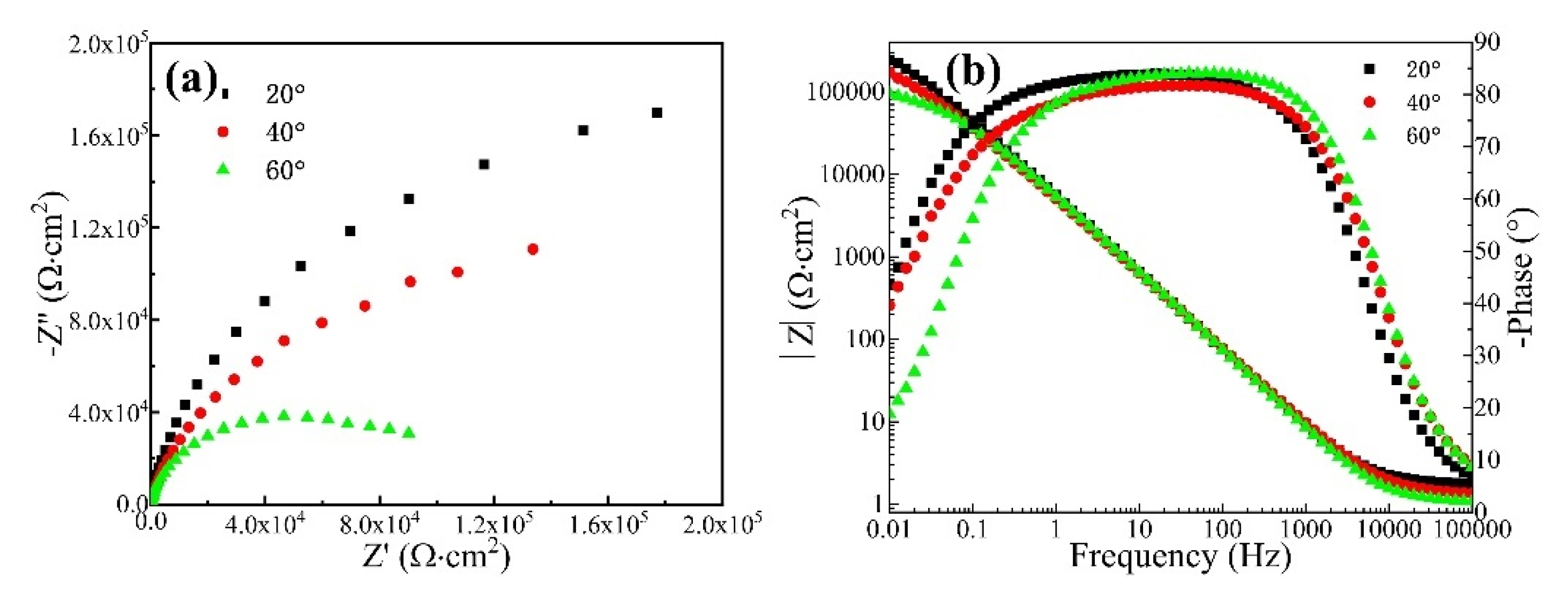
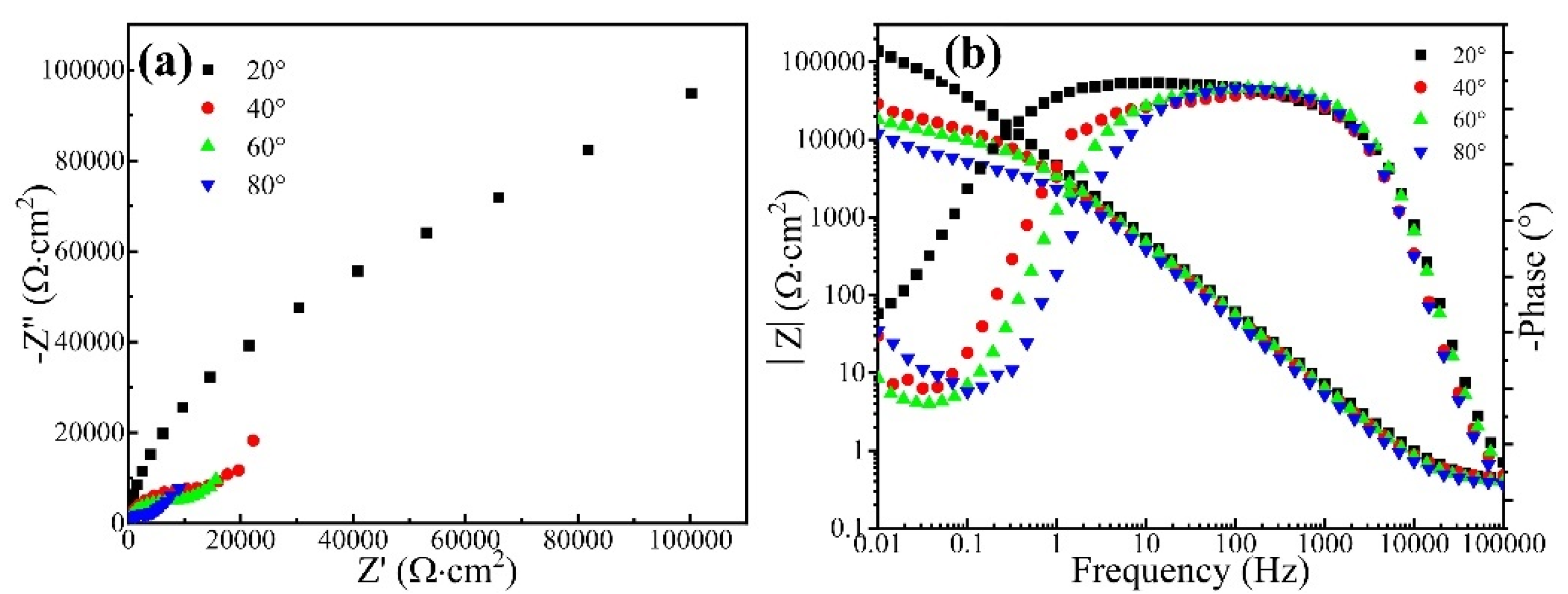
Figure 4 shows the Nyquist diagram and Bode plot of the CrFeCoNi coating in 3.5 wt% NaCl solution at different temperatures. It can be seen that all the Nyquist diagrams of the coating at different temperatures present an incomplete semicircle. Moreover, as the corrosion solution temperature increases, the diameter of the capacitive arc gradually decreases. This indicates that increasing solution temperature would result in the decreasing corrosion resistance of the CrFeCoNi coating. Figure 4b shows the Bode plot of the CrFeCoNi coating in 3.5 wt% NaCl solution at different temperatures. In the high-frequency region, the impedance modulus exhibits a straight horizontal line, which corresponds to solution resistance. At the frequency range of 0.1–1000 Hz, the log|Z| and logf keep a linear relation, and the slope is close to −1. The impedance modulus in the low-frequency region represents polarization resistance [30,31]. It can be seen that with the increase in solution temperature, the impedance modulus of the coating decreases in the low-frequency region. This indicates that the corrosion resistance of the coating decreases with the increase in solution temperature, which is consistent with the result of the potentiodynamic polarization curve.
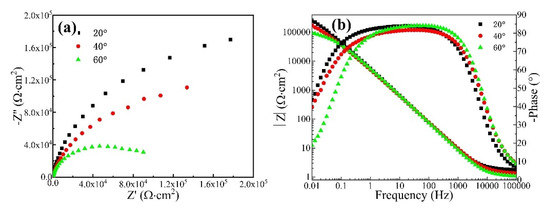
Figure 4.
Nyquist diagram (a) and Bode plot (b) of the CrFeCoNi coating in 3.5 wt% NaCl solution at different temperatures.
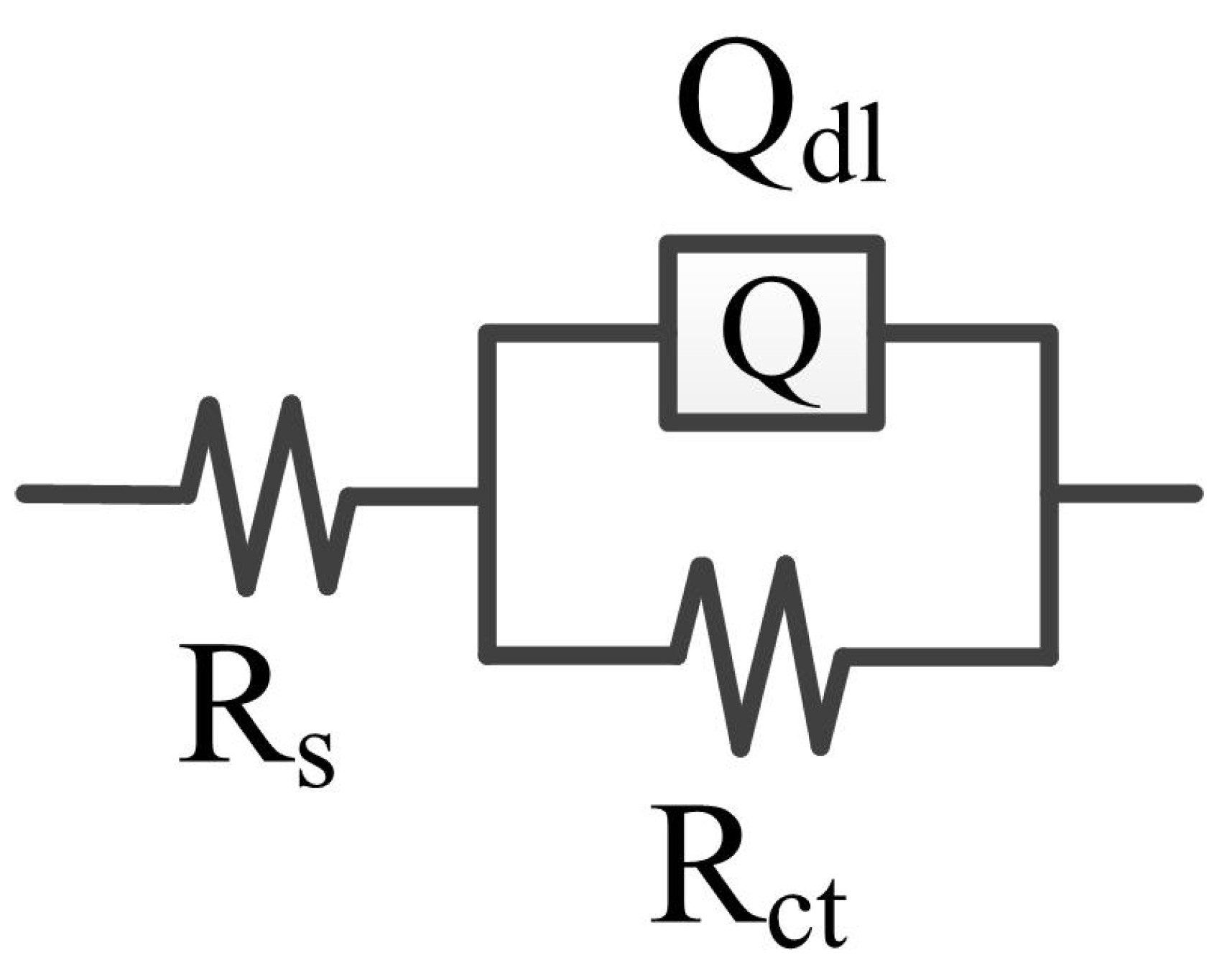
Figure 5 shows the electrical equivalent circuit (EEC) for fitting the CrFeCoNi coating in 3.5 wt% NaCl solution at different temperatures. In the EEC, Rs represents solution resistance, Qdl refers to double layer capacitance, and the Rct is the charge transfer resistance [32,33]. The fitting results are shown in Table 3. As shown in Table 3, the values of charge transfer resistance decrease from 3.51 × 105 to 3.46 × 104 Ω·cm2 with increasing solution temperature. This means that with the decrease in solution temperature, the passivation film formed on the CrFeCoNi coating has higher stability.
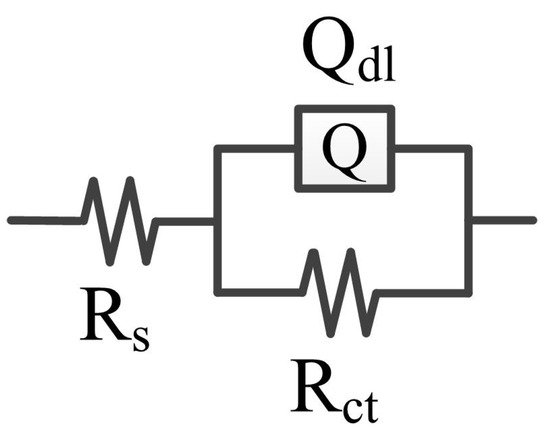
Figure 5.
EEC for fitting the CrFeCoNi coating in 3.5 wt% NaCl solution at different temperatures.

Table 3.
EEC fitting parameters for the EIS of the CrFeCoNi coating in 3.5 wt% NaCl solution at different temperatures.
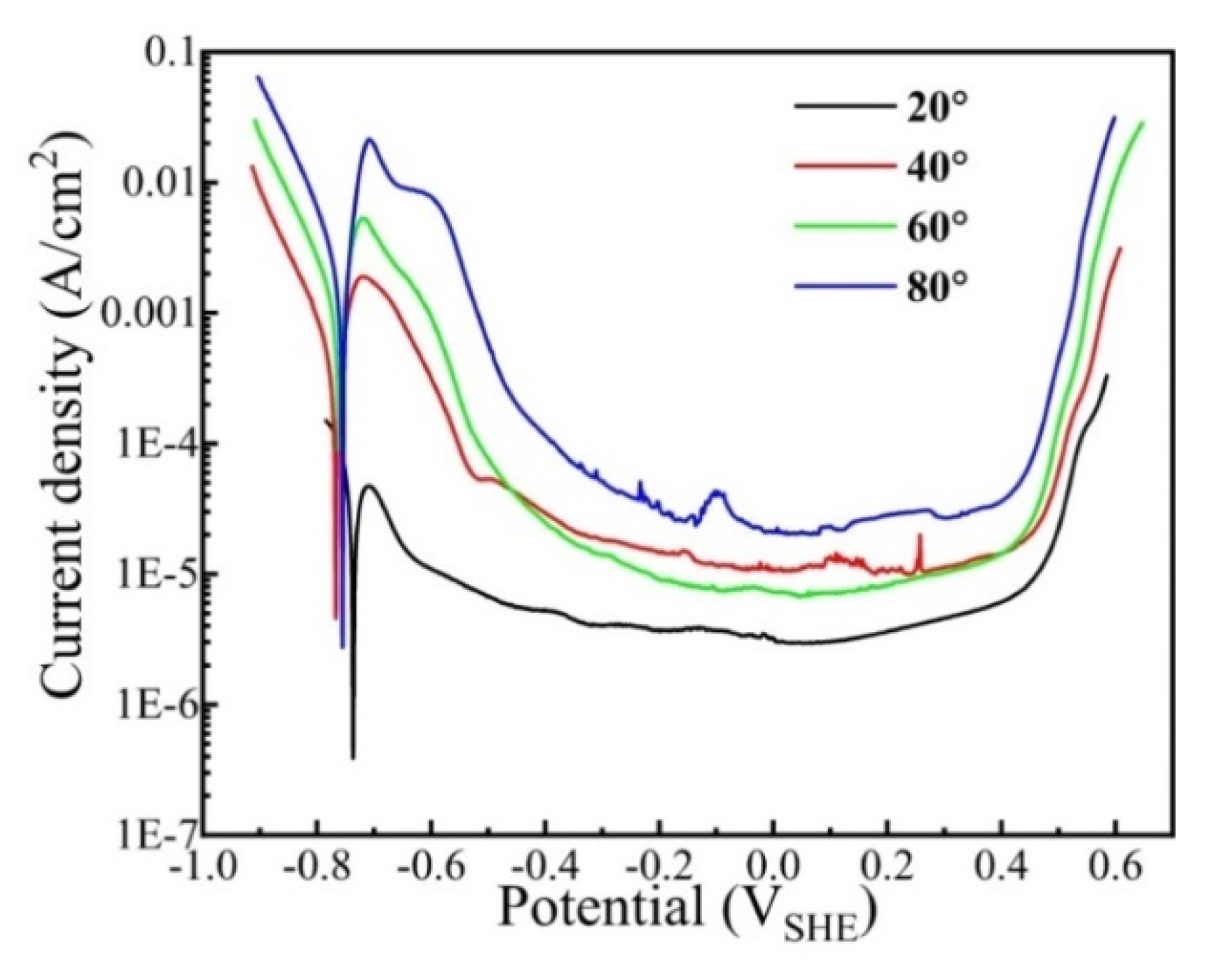
Figure 6 shows the potentiodynamic polarization curves of the CrFeCoNi coating in 0.5 mol/L H2SO4 solution at different temperatures. As shown in Figure 6, all the cathodic polarization curves satisfy the Tafel linear relation. At the anode region, all the curves exhibit active-passivation characteristics. The peak current density of the coating obviously increases with the increase in solution temperature, indicating that the increasing solution temperature accelerates the anodic process. The anodic peak is due to the transition from active dissolution to passivation of alloying elements. The electrochemical parameters of the coating at different solution temperatures could be obtained by the Tafel extrapolation method, and the results are presented in Table 4. It is found that the corrosion current density evidently increases from 7.07 × 10−5 A/cm2 to 3.32 × 10−3 A/cm2 with increasing solution temperature from 20 °C to 80 °C. The transpassivation potential (Et) is ascertained as the potential when the current density reaches 100 μA/cm2 [34]. As the solution temperature rises, the transpassivation potential gradually decreases. This suggests that the passivation film formed at higher temperatures has higher defects and is much easier to break down.
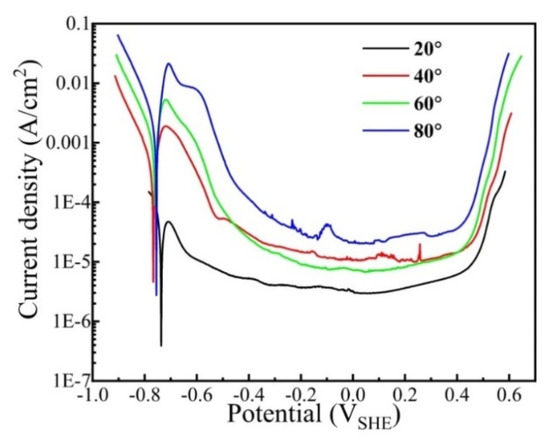
Figure 6.
Potentiodynamic polarization curves of the CrFeCoNi coating in 0.5 mol/L H2SO4 solution at different temperatures.

Table 4.
Electrochemical parameters of the CrFeCoNi coating in 0.5 mol/L H2SO4 solution at different temperatures.
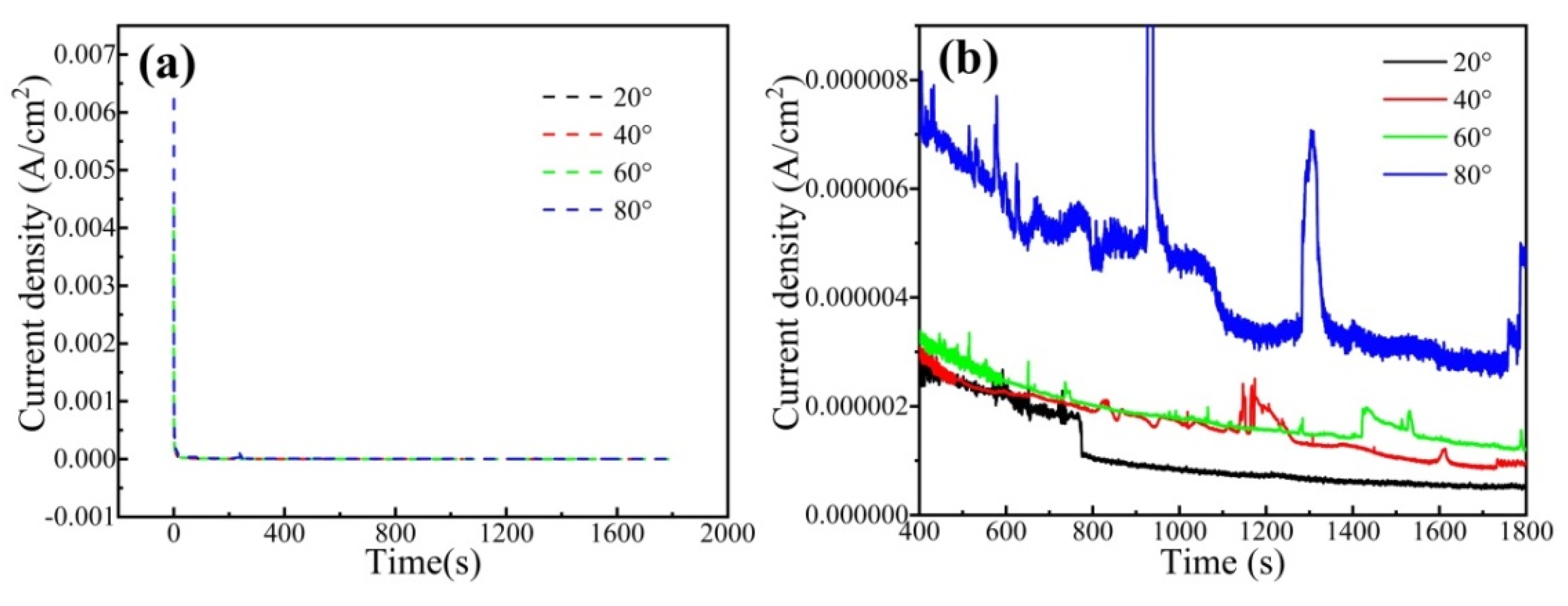
Figure 7 shows the current-time transients for the CrFeCoNi coating at an applied potential of 0 Vref in 0.5 mol/L H2SO4 solution. In Figure 7a, the current density of the coating at different temperatures drops sharply at the beginning stage of polarization, which is due to the rapid nucleation and growth of passivation film. Subsequently, the current density keeps relatively stable, which is due to the equilibrium of growth and decomposition of passivation film [35]. Figure 7b shows the detailed current-time transients for the CrFeCoNi coating. Some obvious current peaks could be found in Figure 7b. Moreover, the current peak value is obviously higher at the solution temperature of 80 °C, indicating that the passivation film formed at this temperature is more unstable. The passivation current density of the coating gradually increases with the increase in solution temperature.
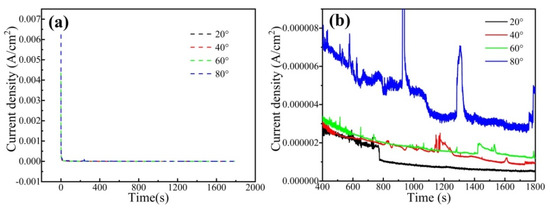
Figure 7.
Current-time transients for the CrFeCoNi coating at applied potential of 0 Vref in 0.5 mol/L H2SO4 solution. (a) Current-time transients, (b) The detailed current-time transients.
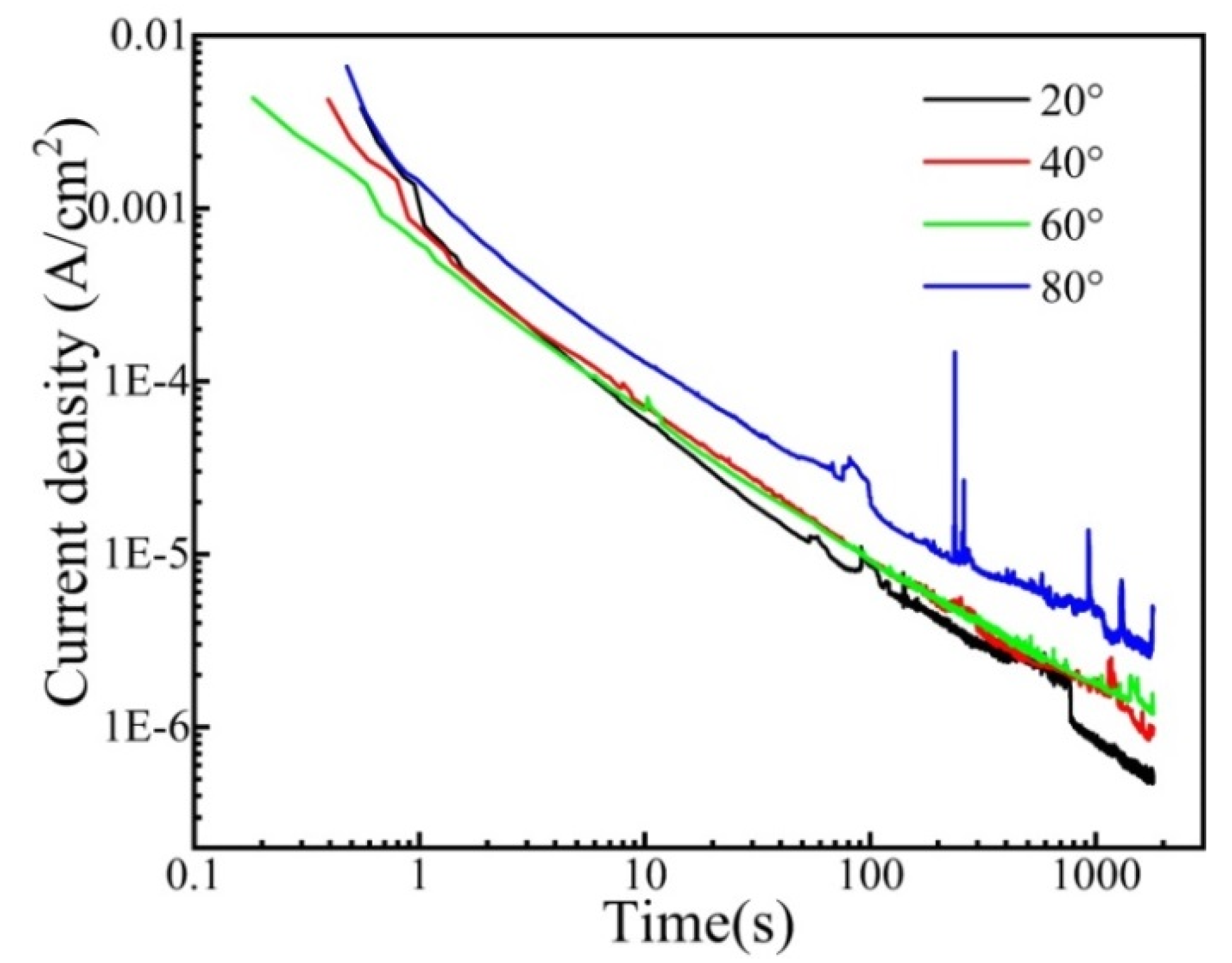
Figure 8 shows the logi–logt plots of CrFeCoNi coating at 0 VRef at different temperatures in 0.5 mol/L H2SO4 solution. The coating at different temperatures exhibits similar decay dynamics. The current density decreases with the increase in polarization time, suggesting that the formation of passivation film dominates the process of potentiostatic polarization. A previous formula illustrated the relationship between the current and time [35].
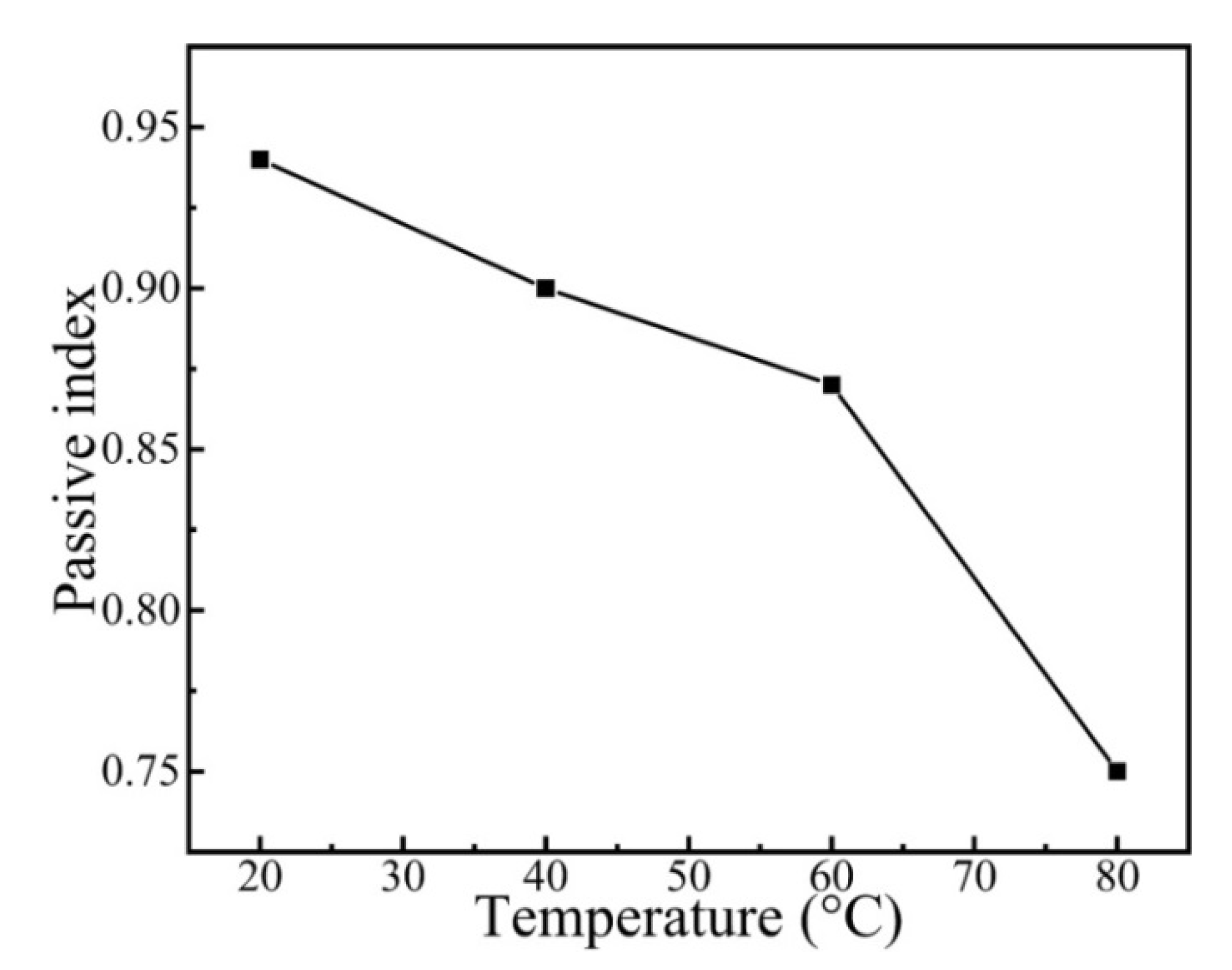
where i represent the anodic current density, A is a constant, t refers to the time, and n corresponds to the passivation index. The passivation index can be ascertained by fitting the curve of logi–logt, which could be used for explaining the formation rate of the passivation film. The fitting result of the passivation index is presented in Figure 9. As shown in Figure 9, the passivation index of the CrFeCoNi coating decreases with increasing solution temperature. This indicates that increasing solution temperature would decrease the formation rate of passivation film and corrosion resistance.
i = A·t−n
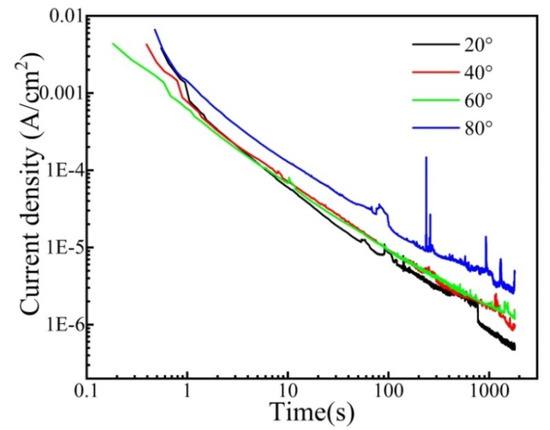
Figure 8.
Logi–Logt plots of the CrFeCoNi coating at 0 VRef at different temperatures in 0.5 mol/L H2SO4 solution.
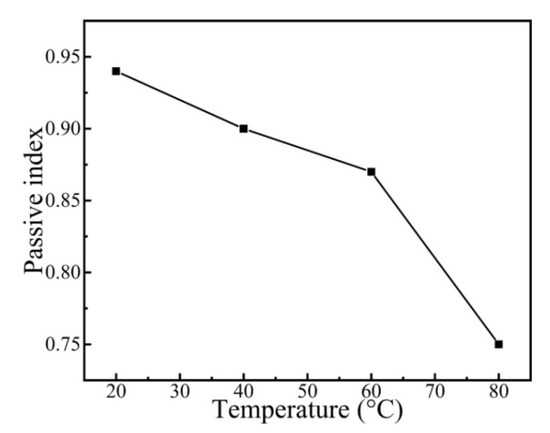
Figure 9.
Passive index of the CrFeCoNi coating at applied potential of 0 VRef at different temperatures in 0.5 mol/L H2SO4 solution.
Figure 10a shows the Nyquist diagram of the CrFeCoNi coating in 0.5 mol/L H2SO4 solution at an applied potential of 0 VRef at different temperatures. All the curves contain a semicircle and an upward sloping line. The semicircle is related to the charge transfer resistance, while the upward sloping line is related to the diffusion process. The diameter of the semicircle decreases with the increase in solution temperature. Figure 10b shows the Bode plot of the CrFeCoNi coating in 0.5 mol/L H2SO4 solution at an applied potential of 0 VRef at different temperatures. It is found that impedance modulus values of the coating gradually decrease in the low-frequency range with the increase in solution temperature.
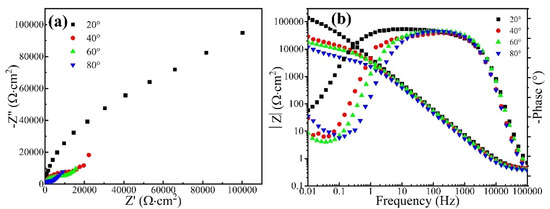
Figure 10.
Nyquist plot (a) and Bode plot (b) of the CrFeCoNi coating in 0.5 mol/L H2SO4 solution at applied potential of 0 VRef at different temperatures.
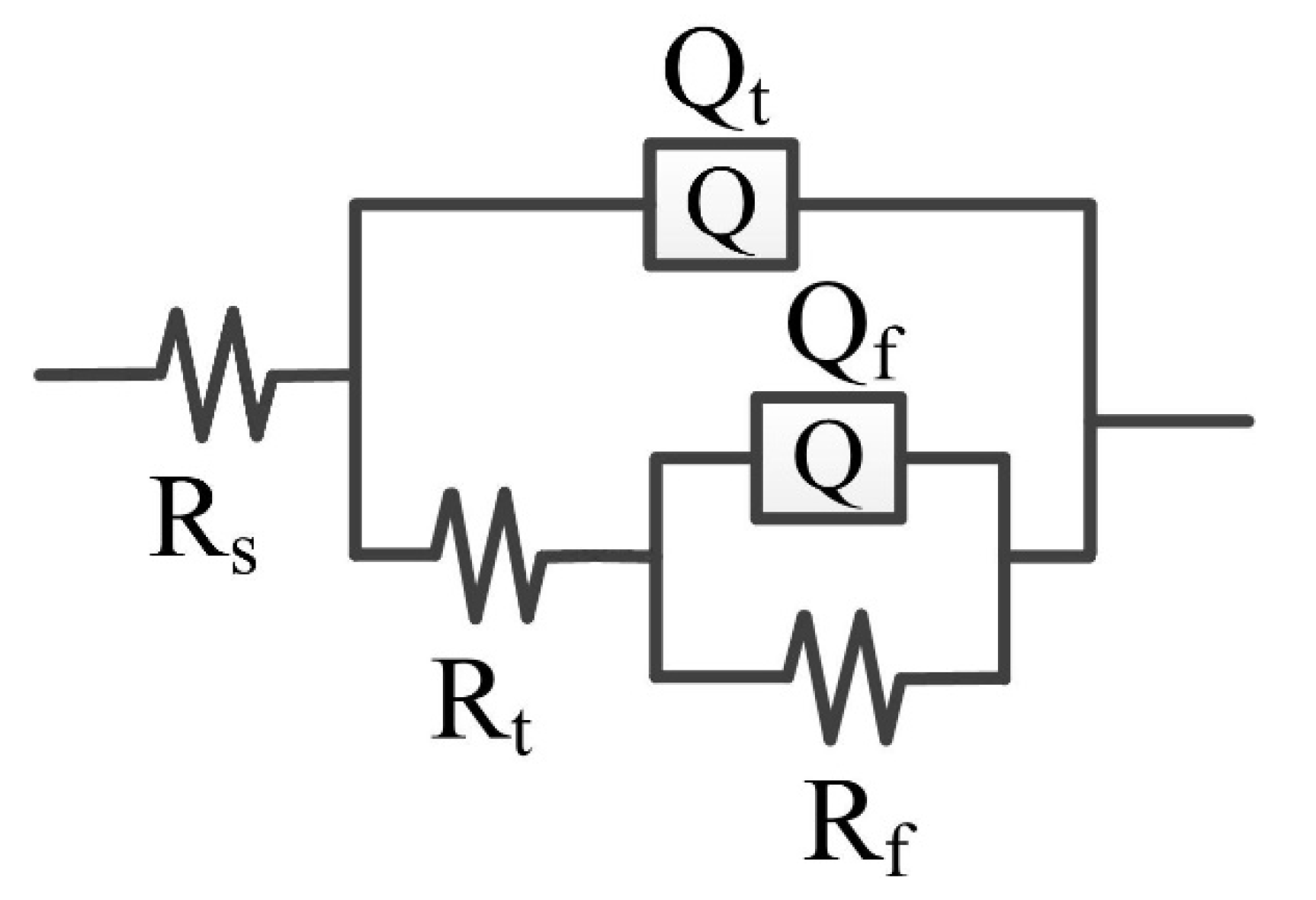
Figure 11 shows the EEC for fitting the EIS result of the CrFeCoNi coating in 0.5 mol/L H2SO4 solution at an applied potential of 0 VRef at different temperatures. Rs represents solution resistance, Rt and Qt represent charge transfer resistance and double layer capacitance, and Rf and Qf are passivation film resistance and capacitance [36]. The fitting results are shown in Table 5. At different temperatures, the solution resistance values are small. The passive film resistance decreases with the increase in solution temperature. This indicates that increasing solution temperature would result in decreasing corrosion resistance of the coating.
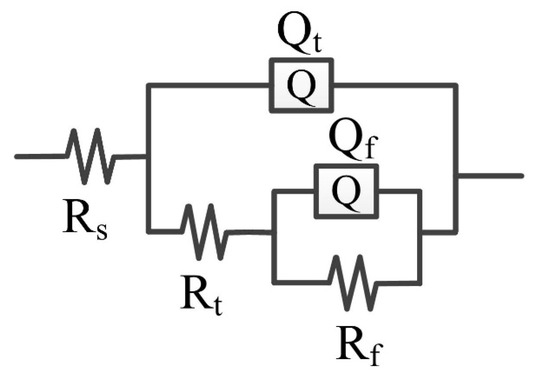
Figure 11.
EEC for fitting the EIS of the CrFeCoNi coating in 0.5 mol/L H2SO4 solution at applied potential of 0 VRef at different temperatures.

Table 5.
EEC fitting parameters for EIS of the CrFeCoNi coating in 0.5 mol/L H2SO4 solution at applied potential of 0 VRef at different temperatures.
Based on the above analysis, it can be concluded that the corrosion resistance of the CrFeCoNi coating decreases with increasing corrosion solution temperature in 3.5 wt% NaCl solution and 0.5 mol/L H2SO4 solution. These reasons could be attributed to two factors. On the one hand, the passivation film growth rate and passivation film decomposition rate increase with rising temperatures. The formation of the passivation film is due to the growth of passivation film dominating the reaction process. A high passivation film growth rate under high temperature would result in the generation of defects because the atoms lack enough time to spread out appropriate positions [37]. On the other hand, the formed defected passivation film at higher temperatures would further accelerate the corrosion, resulting in decreasing corrosion resistance.
4. Conclusions
The CrFeCoNi coating was fabricated by the laser remelting method. The corresponding microstructure was investigated. The corrosion behavior of the coating at different temperatures was explored. The main conclusion could be expressed as follows:
- (1)
- The CrFeCoNi coating is composed of numerous fine subgrains. The coating has higher Fe content than primary CrFeCoNi powder due to the dilution effect.
- (2)
- The corrosion current density and polarization resistance of the CrFeCoNi coating gradually decrease with the increase in solution temperature in 3.5 wt% NaCl solution, indicating that increasing solution temperature decrease the corrosion resistance of the CrFeCoNi coating.
- (3)
- As the temperature rises, the corrosion current density, passivation current density, and passivation film resistance decrease in 0.5 mol/L H2SO4 solution. This means that an increase in solution temperature results in a decrease in corrosion resistance of the CrFeCoNi coating.
Author Contributions
Conceptualization, C.W. and Y.Y.; methodology, C.W. and M.S.; validation, H.Z.; formal analysis, C.W.; investigation, C.W. and M.S.; resources, H.Z.; data curation, C.W. and Y.Y.; writing—original draft preparation, C.W.; writing—review and editing, C.W. and H.Z.; visualization, C.W.; supervision, Y.Y. and H.Z.; project administration, C.W. and H.Z.; funding acquisition, C.W. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Cross Scientific Research Exploration Project of Beijing Institute of Petrochemical Technology, grant number BIPTCFS-013 and by Award Cultivation Foundation from Beijing Institute of Petrochemical Technology, grant number BIPTACF-009 and by Beijing Science and Technology Plan Project, grant number KM202010017004.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, F.F.; Liaw, P.K.; Zhang, Y. Recent progress with BCC-structured high-entropy alloys. Metals 2022, 12, 501. [Google Scholar] [CrossRef]
- Wang, C.M.; Yu, J.X.; Yu, Y.; Zhang, Y. Phase evolution and solidification cracking sensibility in laser remelting treatment of the plasma-sprayed CrMnFeCoNi high entropy alloy coating. Mater. Des. 2019, 182, 108040. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Yang, B.; Liaw, P.K. Corrosion-resistance high entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.M.; Yu, Y.; Yu, J.X.; Zhang, Y.; Wang, F.C.; Li, H.D. Effect of the macro-segregation on corrosion behavior of CrMnFeCoNi coating prepared by arc cladding. J. Alloys Compd. 2020, 846, 156263. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Wu, C.J.; Liu, Y.; Peng, H.P.; Su, X.P. Eutectic reaction and microstructure stability in CoCrFeNiNbx high-entropy alloys. Metals 2022, 12, 756. [Google Scholar] [CrossRef]
- Vaidya, M.; Pradeep, K.G.; Murty, B.S.; Wilde, G.; Divinski, S.V. Bulk tracer diffusion in CoCrFeNi and CoCrFeMnNi high entropy alloys. Acta Mater. 2018, 146, 211–224. [Google Scholar] [CrossRef]
- Zhang, W.R.; Li, Y.S.; Liaw, P.K.; Zhang, Y. A strategic design route to find a depleted uranium high-entropy alloy with great strength. Metals 2022, 12, 699. [Google Scholar] [CrossRef]
- Ye, Q.F.; Feng, K.; Li, Z.G.; Lu, F.G.; Li, R.F.; Huang, J.; Wu, Y.X. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Sha, M.H.; Jia, C.T.; Qiao, J.; Feng, W.Q.; Ai, X.G.; Jing, Y.A.; Shen, M.G.; Li, S.L. Microstructure and properties of high-entropy AlxCoCrFe2.7MoNi alloy coatings prepared by laser cladding. Metals 2019, 9, 1243. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Cui, X.F.; Jin, G.; Liu, Y.F.; Zhang, Y.; Zhang, X.R.; Liu, E.B.; Tian, H.L.; Fang, Y.C. Corrosion and tribo-corrosion behaviors of nano-lamellar Ni1.5CrCoFe0.5Mo0.1Nbx eutectic high-entropy alloy coatings: The role of dual-phase microstructure. Corros. Sci. 2022, 201, 110305. [Google Scholar] [CrossRef]
- Li, C.W.; Ma, A.B.; Jiang, J.H. Mechanical properties and corrosion behavior of novel Al-Mg-Zn-Cu-Si lightweight high entropy alloys. J. Alloys Compd. 2022, 900, 163508. [Google Scholar]
- Wang, C.M.; Yu, Y.; Zhang, H.; Xu, L.X.; Ma, X.Y.; Wang, F.F.; Song, B.Y. Microstructure and corrosion properties of laser remelted CrFeCoNi and CrMnFeCoNi high entropy alloys coatings. J. Mater. Res. Technol. 2021, 15, 5187–5196. [Google Scholar] [CrossRef]
- Qzturk, S.; Alptekin, F.; Qnal, S.; Sunbul, S.E.; Sahin, O.; Icin, K. Effect of titanium addition on the corrosion behavior of CoCuFeNiMn high entropy alloy. J. Alloys Compd. 2022, 903, 163867. [Google Scholar] [CrossRef]
- Shuang, S.; Yu, Q.; Gao, X.; He, Q.F.; Zhang, J.Y.; Shi, S.Q.; Yang, Y. Tuning the microstructure for superb corrosion resistance in eutectic high entropy alloy. J. Mater. Sci. Technol. 2022, 109, 197–208. [Google Scholar] [CrossRef]
- Thota, H.; Jeyaraam, R.; Bairi, L.R.; Tirunilai, A.S.; Kauffmann, A.; Freudenberger, J.; Heilmaier, M.; Mandal, S.; Vadlamani, S.S. Grain boundary engineering and its implications on corrosion behavior of equiatomic CoCrFeMnNi high entropy alloy. J. Alloys Compd. 2021, 25, 161500. [Google Scholar] [CrossRef]
- Parakh, A.; Vaidya, M.; Kumar, N.; Chetty, R.; Murty, B.S. Effect of crystal structure and grain size on corrosion properties of AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2021, 863, 158056. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Z.W.; Liu, H.M.; Zhang, Y.B.; Li, H.X.; Shi, C.W.; Liu, P.; Yan, D.F. Recent progress on the microstructure and properties of high entropy alloy coatings prepared by laser processing technology: A review. J. Manuf. Process. 2022, 76, 397–411. [Google Scholar] [CrossRef]
- Jiao, W.N.; Li, T.X.; Chang, X.X.; Lu, Y.P.; Yin, G.M.; Cao, Z.Q. A novel Co-free Al0.75CrFeNi eutectic high entropy alloy with superior mechanical properties. J. Alloys Compd. 2022, 902, 163814. [Google Scholar] [CrossRef]
- Osintsev, K.A.; Konovalov, S.V.; Gromov, V.E.; Ivanov, Y.F.; Panchenko, I.A. Microstructure and mechanical properties of non-equiatomic Co25.4Cr15Fe37.9Mn3.5Ni16.8Si1.4 high-entropy alloy produced by wire-arc additive manufacturing. Mater. Lett. 2022, 312, 131675. [Google Scholar] [CrossRef]
- Yin, S.; Li, W.Y.; Song, B.; Yan, X.C.; Kuang, M.; Xu, Y.X.; Wen, K.; Lupoi, R. Deposition of FeCoNiCrMn high entropy alloy (HEA) coating via cold spraying. J. Mater. Sci. Technol. 2019, 35, 1003–1007. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Zhang, X.M.; Quan, H.; Chen, Y.J.; Wang, S.; Zhang, S. Effect of Mo addition on structures and properties of FeCoNiCrMn high entropy alloy film by direct current magnetron sputtering. J. Alloys Compd. 2020, 895, 162709. [Google Scholar] [CrossRef]
- Lin, X.; Gao, Y.Q.; Wang, Z.T.; Gao, J.; Wang, L.L.; Huang, W.D. Regular eutectic and anomalous eutectic growth behavior in laser remelting of Ni-30wt% Sn alloy. Acta Mater. 2017, 126, 210–220. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.K.; Guo, P.S.; Gao, X.; Yang, L.J.; Song, Z.L. Effects of laser surface remelting on microstructure and properties of biodegradable Zn-Zr alloy. Mater. Lett. 2018, 226, 52–54. [Google Scholar] [CrossRef]
- Torbati-Sarraf, H.; Shabani, M.; Jablonski, P.D.; Pataky, G.J.; Poursaee, A. The effect of incorporation of Mn on the pitting corrosion performance of CrFeCoNi High Entropy Alloy at different temperatures. Mater. Des. 2019, 184, 108170. [Google Scholar] [CrossRef]
- Vogt, J.B.; Serre, I.P. A review of the surface modifications for corrosion mitigation of steels in lead and LBE. Coatings 2021, 11, 53. [Google Scholar] [CrossRef]
- Li, B.S.; Liao, Q.; Zhang, H.P.; Shen, T.L.; Ge, F.F.; Daghbouj, N. The effects of stress on corrosion behaviour of SIMP martensitic steel in static liquid lead-bismuth eutectic. Corros. Sci. 2021, 187, 109477. [Google Scholar] [CrossRef]
- Wang, P.; Karsten, N.; Gary, S.W. Reproducing shadow corrosion on Zircaloy-2 using in-situ proton irradiation. J. Nucl. Mater. 2022, 558, 153406. [Google Scholar] [CrossRef]
- Daghbouj, N.; Sen, H.S.; Callisti, M.; Vronka, M.; Karlik, M.; Duchoň, J.; Čech, J.; Havánek, V.; Polcar, T. Revealling nanoscale strain mechanisms in ion-irradiated multilayers. Acta Mater. 2022, 229, 117807. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.M.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Della Rovere, C.A.; Alano, J.H.; Silva, R.; Nascente, P.A.P.; Otubo, J.; Kuri, S.E. Characterization of passive films on shape memory stainless steels. Corros. Sci. 2021, 57, 154–161. [Google Scholar] [CrossRef]
- Wang, C.M.; Yu, Y.; Yu, J.X.; Zhang, Y.; Zhao, Y.; Yuan, Q.W. Microstructure evolution and corrosion behavior of dissimilar 304/430 stainless steel welded joints. J. Manuf. Process. 2020, 50, 183–191. [Google Scholar] [CrossRef]
- Kocijan, A.; Merl, D.K.; Jenko, M. The corrosion behaviour of austenitic stainless steels in srtificial saliva with the addition of fluoride. Corros. Sci. 2011, 53, 776–783. [Google Scholar] [CrossRef]
- Escriva-Cerdan, C.; Blasco-Tamarit, E.; Garcia-Garcia, D.M.; Garcia-Anton, J.; Guenbour, A. Passivation behavior of Alloy 31 (UNS N08031) in polluted phosphoric acid at different temperatures. Corros. Sci. 2012, 56, 114–122. [Google Scholar] [CrossRef]
- Wang, C.M.; Yu, J.X.; Yu, Y.; Zhao, Y.; Zhang, Y.; Han, X.X. Comparison of the corrosion and passivity behavior between CrMnFeCoNi and CrFeCoNi coatings prepared by argon arc cladding. J. Mater. Res. Technol. 2020, 9, 8482–8496. [Google Scholar] [CrossRef]
- Leon, A.; Levy, G.K.; Ron, T.; Shirizly, A.; Aghion, E. The effect of strain rate on stress corrosion performance of Ti6Al4V alloy produced by additive manufacturing process. J. Mater. Res. Technol. 2020, 9, 4097–4105. [Google Scholar] [CrossRef]
- Cui, Z.Y.; Wang, L.W.; Ni, H.T.; Hao, W.K.; Man, C.; Chen, S.S.; Wang, X.; Liu, Z.Y.; Li, X.G. Influence of temperature on the electrochemical and passivation behavior of 2507 super duplex stainless steel in simulated desulfurized flue gas condensates. Corros. Sci. 2017, 18, 31–48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).