Nanoscale Tribological Properties of Nanostructure Fe3Al and (Fe,Ti)3Al Compounds Fabricated by Spark Plasma Sintering Method
Abstract
:1. Introduction
2. Materials and Experimental Procedures
3. Results and Discussion
4. Conclusions
- Fe3Al with nano-sized grains can be successfully synthesized by 30 h of mechanical alloying. In addition, a nanostructured (Fe,Ti)3Al intermetallic compound can also be formed during mechanical alloying, and it was found that Ti addition leads to the formation of a phase with a smaller grain size.
- Nanostructured Fe3Al and (Fe,Ti)3Al compounds with a nearly full relative density were consolidated by spark plasma sintering without any significant grain growth. Moreover, the nanoindentation test showed that (Fe,Ti)3Al has a higher hardness elasticity modulus than that of the Fe3Al sample, while a decrease in the plasticity index is observed by the addition of Ti to the Fe3Al phase. The increase in the hardness of (Fe,Ti)3Al compared to Fe3Al can be mainly attributed to grain refinement, solid-solution strengthening, and dispersion strengthening.
- The addition of Ti to Fe3Al led to a significant improvement in tribological behavior. This increase was due to more elastic recovery and also less plastic deformation, providing a better scratch resistance in the (Fe,Ti)3Al sample.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, S.-M.; Iwasaki, K. Characterization of mechanically alloyed ternary Fe–Ti–Al powders. Mater. Sci. Eng. A 1999, 270, 170–177. [Google Scholar] [CrossRef]
- Rafiei, M.; Enayati, M.H.; Karimzadeh, F. Thermodynamic analysis of solid solution formation in the nanocrystalline Fe–Ti–Al ternary system during mechanical alloying. J. Chem. Thermodyn. 2013, 59, 243–249. [Google Scholar] [CrossRef]
- Enayati, M.H.; Salehi, M. Formation mechanism of Fe3Al and FeAl intermetallic compounds during mechanical alloying. J. Mater. Sci. 2005, 40, 3933–3938. [Google Scholar] [CrossRef]
- Rafiei, M.; Enayati, M.H.; Karimzadeh, F. Characterization and formation mechanism of nanocrystalline (Fe,Ti)3Al intermetallic compound prepared by mechanical alloying. J. Alloy. Compd. 2009, 480, 392–396. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Stoloff, N.S. Iron aluminides: Present status and future prospects. Mater. Sci. Eng. A 1998, 258, 1–14. [Google Scholar] [CrossRef]
- Pocheć, E.; Jóźwiak, S.; Karczewski, K.; Bojar, Z. Fe–Al phase formation around SHS reactions under isothermal conditions. J. Alloy. Compd. 2011, 509, 1124–1128. [Google Scholar] [CrossRef]
- Sina, H.; Corneliusson, J.; Turba, K.; Iyengar, S. A study on the formation of iron aluminide (FeAl) from elemental powders. J. Alloy. Compd. 2015, 636, 261–269. [Google Scholar] [CrossRef]
- Krein, R.; Schneider, A.; Sauthoff, G.; Frommeyer, G. Microstructure and mechanical properties of Fe3Al-based alloys with strengthening boride precipitates. Intermetallics 2007, 15, 1172–1182. [Google Scholar] [CrossRef]
- Simas, P.; Juan, J.S.; Nó, M.L. High-temperature relaxation analysis in a fine-grain B2 FeAl intermetallic. Intermetallics 2010, 18, 1348–1352. [Google Scholar] [CrossRef]
- Dobeš, F.; Kratochvíl, P. The effect of Zr addition on creep of Fe-30 at.% Al alloys. Intermetallics 2013, 43, 142–146. [Google Scholar] [CrossRef]
- Senčekova, L.; Palm, M.; Pešička, J.; Veselý, J. Microstructures, mechanical properties and oxidation behaviour of single-phase Fe3Al (D03) and two-phase α-Fe,Al (A2) + Fe3Al (D03) FeAlV alloys. Intermetallics 2016, 73, 58–66. [Google Scholar] [CrossRef]
- Emdadi, A.; Sizova, I.; Bambach, M.; Hecht, U. Hot deformation behavior of a spark plasma sintered Fe-25Al-1.5Ta alloy with strengthening Laves phase. Intermetallics 2019, 109, 123–134. [Google Scholar] [CrossRef]
- Rafiei, M.; Enayati, M.H.; Karimzadeh, F. Mechanochemical synthesis of (Fe,Ti)3Al–Al2O3 nanocomposite. J. Alloy. Compd. 2009, 488, 144–147. [Google Scholar] [CrossRef]
- Wang, J.; Xing, J.; Tang, H.; Yang, B.; Li, Y. Microstructure and mechanical properties of Fe3Al alloys prepared by MA-PAS and MA-HP. Trans. Nonferrous Met. Soc. China 2011, 21, 2408–2414. [Google Scholar] [CrossRef]
- Wang, J.; Xing, J.; Cao, L.; Su, W.; Gao, Y. Dry sliding wear behavior of Fe3Al alloys prepared by mechanical alloying and plasma activated sintering. Wear 2010, 268, 473–480. [Google Scholar] [CrossRef]
- Deevi, S.C.; Zhang, W.J. Encyclopedia of Materials: Science and Technology; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2001; pp. 4165–4173. [Google Scholar]
- Liu, B.; Yang, Q.; Wang, Y. Intereaction and intermetallic phase formation between aluminum and stainless steel. Results Phys. 2019, 12, 514–524. [Google Scholar] [CrossRef]
- Alman, D.E.; Hawk, J.A.; Tylczak, J.H.; Doğan, C.P.; Wilson, R.D. Wear of iron–aluminide intermetallic-based alloys and composites by hard particles. Wear 2001, 251, 875–884. [Google Scholar] [CrossRef]
- Zhu, S.-M.; Tamura, M.; Sakamoto, K.; Iwasaki, K. Characterization of Fe3Al-based intermetallic alloys fabricated by mechanical alloying and HIP consolidation. Mater. Sci. Eng. A 2000, 292, 83–89. [Google Scholar] [CrossRef]
- Wang, J.; Xing, J.; Qiu, Z.; Zhi, X.; Cao, L. Effect of fabrication methods on microstructure and mechanical properties of Fe3Al-based alloys. J. Alloy. Compd. 2009, 488, 117–122. [Google Scholar] [CrossRef]
- Deevi, S.C.; Sikka, V.K.; Liu, C.T. Processing, properties, and applications of nickel and iron aluminides. Prog. Mater. Sci. 1997, 42, 177–192. [Google Scholar] [CrossRef]
- Zamanzade, M.; Barnoush, A.; Motz, C. A Review on the Properties of Iron Aluminide Intermetallics. Crystals 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Luu, W.C.; Wu, J.K. Moisture and hydrogen-induced embrittlement of Fe3Al alloys. Mater. Chem. Phys. 2001, 70, 236–241. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Liu, W.-X. Mechanical properties of Fe3Al-based alloys with addition of carbon, niobium and titanium. Mater. Sci. Eng. A 2006, 423, 343–349. [Google Scholar] [CrossRef]
- Khodaei, M.; Enayati, M.H.; Karimzadeh, F. The structure and mechanical properties of Fe3Al–30 vol.% Al2O3 nanocomposite. J. Alloy. Compd. 2009, 488, 134–137. [Google Scholar] [CrossRef]
- Khodaei, M.; Enayati, M.H.; Karimzadeh, F. Mechanochemically synthesized Fe3Al–Al2O3 nanocomposite. J. Alloy. Compd. 2009, 467, 159–162. [Google Scholar] [CrossRef]
- Park, N.; Lee, S.-C.; Cha, P.-R. Effects of alloying elements on the stability and mechanical properties of Fe3Al from first-principles calculations. Comput. Mater. Sci. 2018, 146, 303–309. [Google Scholar] [CrossRef]
- McKamey, C.G.; DeVan, J.H.; Tortorelli, P.F.; Sikka, V.K. A review of recent developments in Fe3Al-based alloys. J. Mater. Res. 1991, 6, 1779–1805. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Al-Aqeeli, N. Mechanically alloyed nanocomposites. Prog. Mater. Sci. 2013, 58, 383–502. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D. Optimization of milling parameters for the synthesis of nano-structured duplex and ferritic stainless steel powders by high energy planetary milling. Powder Technol. 2015, 278, 34–45. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D. Development of nano-structured duplex and ferritic stainless steels by pulverisette planetary milling followed by pressureless sintering. Mater. Charact. 2015, 99, 220–229. [Google Scholar]
- Shashanka, R.; Chaira, D. Phase transformation and microstructure study of nano-structured austenitic and ferritic stainless steel powders prepared by planetary milling. Powder Technol. 2014, 259, 125–136. [Google Scholar]
- Shashanka, R.; Uzun, O.; Chaira, D. Synthesis of nano-structured duplex and ferritic stainless steel powders by dry milling and its comparision with wet milling. Arch. Metall. Mater. 2020, 65, 5–14. [Google Scholar]
- Fair, G.H.; Wood, J.V. Mechanical Alloying of Iron–Aluminium Intermetallics. Powder Metall. 1993, 36, 123–128. [Google Scholar] [CrossRef]
- Tang, W.M.; Zheng, Z.X.; Tang, H.J.; Ren, R.; Wu, Y.C. Structural evolution and grain growth kinetics of the Fe–28Al elemental powder during mechanical alloying and annealing. Intermetallics 2007, 15, 1020–1026. [Google Scholar] [CrossRef]
- Enzo, S.; Frattini, R.; Gupta, R.; Macrì, P.P.; Principi, G.; Schiffini, L.; Scipione, G. X-ray powder diffraction and Mössbauer study of nanocrystalline FeAl prepared by mechanical alloying. Acta Mater. 1996, 44, 3105–3113. [Google Scholar] [CrossRef]
- Morris, D.G.; Morris, M.A. Mechanical properties of FeAl-ZrB2 alloys prepared by rapid solidification. Acta Metall. Mater. 1991, 39, 1771–1779. [Google Scholar] [CrossRef]
- McKamey, C.G.; Liu, C.T. Chromium addition and environmental embrittlement in Fe3Al. Scr. Metall. Mater. 1990, 24, 2119–2122. [Google Scholar] [CrossRef]
- Yangshan, S.; Zhengjun, Y.; Zhonghua, Z.; Haibo, H. Mechanical properties of Fe3Al-based alloys with cerium addition. Scr. Metall. Mater. 1995, 33, 811–817. [Google Scholar] [CrossRef]
- Baligidad, R.G.; Prakash, U.; Radhakrishna, A.; Rao, V.R.; Rao, P.K.; Ballal, N.B. Effect of carbides on embrittlement of Fe3Al based intermetallic alloys. Scr. Mater. 1997, 36, 667–671. [Google Scholar] [CrossRef]
- Mostaan, H.; Karimzadeh, F.; Abbasi, M.H. Synthesis and formation mechanism of nanostructured NbAl3 intermetallic during mechanical alloying and a kinetic study on its formation. Thermochim. Acta 2012, 529, 36–44. [Google Scholar] [CrossRef]
- Mostaan, H.; Karimzadeh, F.; Abbasi, M.H. Thermodynamic analysis of nanocrystalline and amorphous phase formation in Nb–Al system during mechanical alloying. Powder Metall. 2012, 55, 142–147. [Google Scholar] [CrossRef]
- Xu, X.; Tao, T.; Zhang, X.; Cao, Z.; Huang, D.; Liang, H.; Hu, Y. Spark plasma sintering of ZrO2–Al2O3 nanocomposites at low temperatures aided by amorphous powders. Ceram. Int. 2019, 46, 4365–4370. [Google Scholar] [CrossRef]
- Pournajaf, R.; Hassanzadeh-Tabrizi, S.A.; Ebrahimi-Kahrizsangi, R.; Alhaji, A.; Nourbakhsh, A.A. Polycrystalline infrared-transparent MgO fabricated by spark plasma sintering. Ceram. Int. 2019, 45, 18943–18950. [Google Scholar] [CrossRef]
- Rominiyi, A.L.; Shongwe, M.B.; Ogunmuyiwa, E.N.; Babalola, B.J.; Lepele, P.F.; Olubambi, P.A. Effect of nickel addition on densification, microstructure and wear behaviour of spark plasma sintered CP-titanium. Mater. Chem. Phys. 2020, 240, 122130. [Google Scholar] [CrossRef]
- Rafiei, M.; Enayati, M.H.; Karimzadeh, F. The effect of Ti addition on alloying and formation of nanocrystalline structure in Fe–Al system. J. Mater. Sci. 2010, 45, 4058–4062. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M.I. Introduction, An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Liu, Y.; Chong, X.; Jiang, Y.; Zhou, R.; Feng, J. Mechanical properties and electronic structures of Fe-Al intermetallic. Phys. B Condens. Matter 2017, 506, 1–11. [Google Scholar] [CrossRef]
- Friák, M.; Deges, J.; Krein, R.; Frommeyer, G.; Neugebauer, J. Combined ab initio and experimental study of structural and elastic properties of Fe3Al-based ternaries. Intermetallics 2010, 18, 1310–1315. [Google Scholar] [CrossRef]
- Deevil, S.C.; Maziasz, P.J.; Sikka, V.K.; Cahn, R.W. International Symposium on Nickel and Iron Aluminides: Processing, Properties, and Applications. Mater. Technol. 1997, 12, 165–170. [Google Scholar] [CrossRef]
- Zhu, S.-M.; Sakamoto, K.; Tamura, M.; Iwasaki, K. Effects of Titanium Addition on the Microstructure and Mechanical Behavior of Iron Aluminide Fe3Al. Mater. Trans. 2001, 42, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.A.; Morris, D.G. Dispersoid additions and their effect on high temperature deformation of Fe-Al. Acta Metall. Mater. 1990, 38, 551–559. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Mai, Y.-W. Nanoscratching of nylon 66-based ternary nanocomposites. Acta Mater. 2007, 55, 635–646. [Google Scholar] [CrossRef]
- Popov, V. Several Aspects of Application of Nanodiamonds as Reinforcements for Metal Matrix Composites. Appl. Sci. 2021, 11, 4695. [Google Scholar] [CrossRef]
- Bai, Y.; Li, M.; Cheng, C.; Li, J.; Guo, Y.; Yang, Z. Study on Microstructure and In Situ Tensile Deformation Behavior of Fe-25Mn-xAl-8Ni-C Alloy Prepared by Vacuum Arc Melting. Metals 2021, 11, 814. [Google Scholar] [CrossRef]
- Salman, K.D.; Al-Maliki, W.A.K.; Alobaid, F.; Epple, B. Microstructural Analysis and Mechanical Properties of a Hybrid Al/Fe2O3/Ag Nano-Composite. Appl. Sci. 2022, 12, 4730. [Google Scholar] [CrossRef]
- Voiculescu, I.; Geanta, V.; Stefanescu, E.V.; Simion, G.; Scutelnicu, E. Effect of Diffusion on Dissimilar Welded Joint between Al0.8CoCrFeNi High-Entropy Alloy and S235JR Structural Steel. Metals 2022, 12, 548. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Li, Q.; Wang, S.; Wu, M.; Yang, S.; Xiang, D. Effects of Al or Mo Addition on Microstructure and Mechanical Properties of Fe-Rich Nonequiatomic FeCrCoMnNi High-Entropy Alloy. Metals 2022, 12, 191. [Google Scholar] [CrossRef]
- Cui, C.; Nie, J.; Li, Y.; Guan, Q.; Cai, J.; Zhang, P.; Wu, J. Wear Resistance of FeCrAlNbNi Alloyed Zone via Laser Surface Alloying on 304 Stainless Steel. Metals 2022, 12, 467. [Google Scholar] [CrossRef]
- Romero-Orozco, A.J.; Taha-Tijerina, J.J.; de Luna-Alanís, R.; López-Morelos, V.H.; Ramírez-López, M.; Salazar-Martínez, M.; Curiel-López, F.F. Evaluation of Microstructural and Mechanical Behavior of AHSS CP780 Steel Welded by GMAW-Pulsed and GMAW-Pulsed-Brazing Processes. Metals 2022, 12, 530. [Google Scholar] [CrossRef]
- He, Z.; Hu, X.; Han, W.; Mao, X.; Chou, K.-C. Erosion Layer Growth between Solid 316L Stainless Steel and Al–Li Alloy Melt. Metals 2022, 12, 350. [Google Scholar] [CrossRef]
- Ananiadis, E.; Argyris, K.T.; Matikas, T.E.; Sfikas, A.K.; Karantzalis, A.E. Microstructure and Corrosion Performance of Aluminium Matrix Composites Reinforced with Refractory High-Entropy Alloy Particulates. Appl. Sci. 2021, 11, 1300. [Google Scholar] [CrossRef]
- Feng, K.; Yang, M.; Long, S.-L.; Li, B. The Effect of a Composite Nanostructure on the Mechanical Properties of a Novel Al-Cu-Mn Alloy through Multipass Cold Rolling and Aging. Appl. Sci. 2020, 10, 8109. [Google Scholar] [CrossRef]
- Mudang, M.; Hamzah, E.; Bakhsheshi-Rad, H.R.; Berto, F. Effect of heat treatment on microstructure and creep behavior of Fe-40Ni-24Cr alloy. Appl. Sci. 2021, 11, 7951. [Google Scholar] [CrossRef]
- Fang, C.; Souissi, M.; Que, Z.; Fan, Z. Crystal Chemistry and Electronic Properties of the Al-Rich Compounds, Al2Cu, ω-Al7Cu2Fe and θ-Al13Fe4 with Cu Solution. Metals 2022, 12, 329. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. Graphene family nanomaterial reinforced magnesium-based matrix composites for biomedical application: A comprehensive review. Metals 2020, 10, 1002. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. The Effect of Co-Encapsulated GO-Cu Nanofillers on Mechanical Properties, Cell Response, and Antibacterial Activities of Mg-Zn Composite. Metals 2022, 12, 207. [Google Scholar] [CrossRef]
- Badkoobeh, F.; Mostaan, H.; Rafiei, M.; Bakhsheshi-Rad, H.R.; Berto, F. Microstructural Characteristics and Strengthening Mechanisms of Ferritic–Martensitic Dual-Phase Steels: A Review. Metals 2022, 12, 101. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. Carbon nanotubes (CNTs)-reinforced magnesium-based matrix composites: A comprehensive review. Materials 2020, 13, 4421. [Google Scholar] [CrossRef]
- Badkoobeh, F.; Mostaan, H.; Rafiei, M.; Bakhsheshi-Rad, H.R.; Berto, F. Friction Stir Welding/Processing of Mg-Based Alloys: A Critical Review on Advancements and Challenges. Materials 2021, 14, 6726. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yun, Y.; Rui, J. Enhanced Tensile Plasticity in Ultrafine Lamellar Eutectic Al-Cu Based Composites with α-Al Dendrites Prepared by Progressive Solidification. Appl. Sci. 2019, 9, 3922. [Google Scholar] [CrossRef] [Green Version]
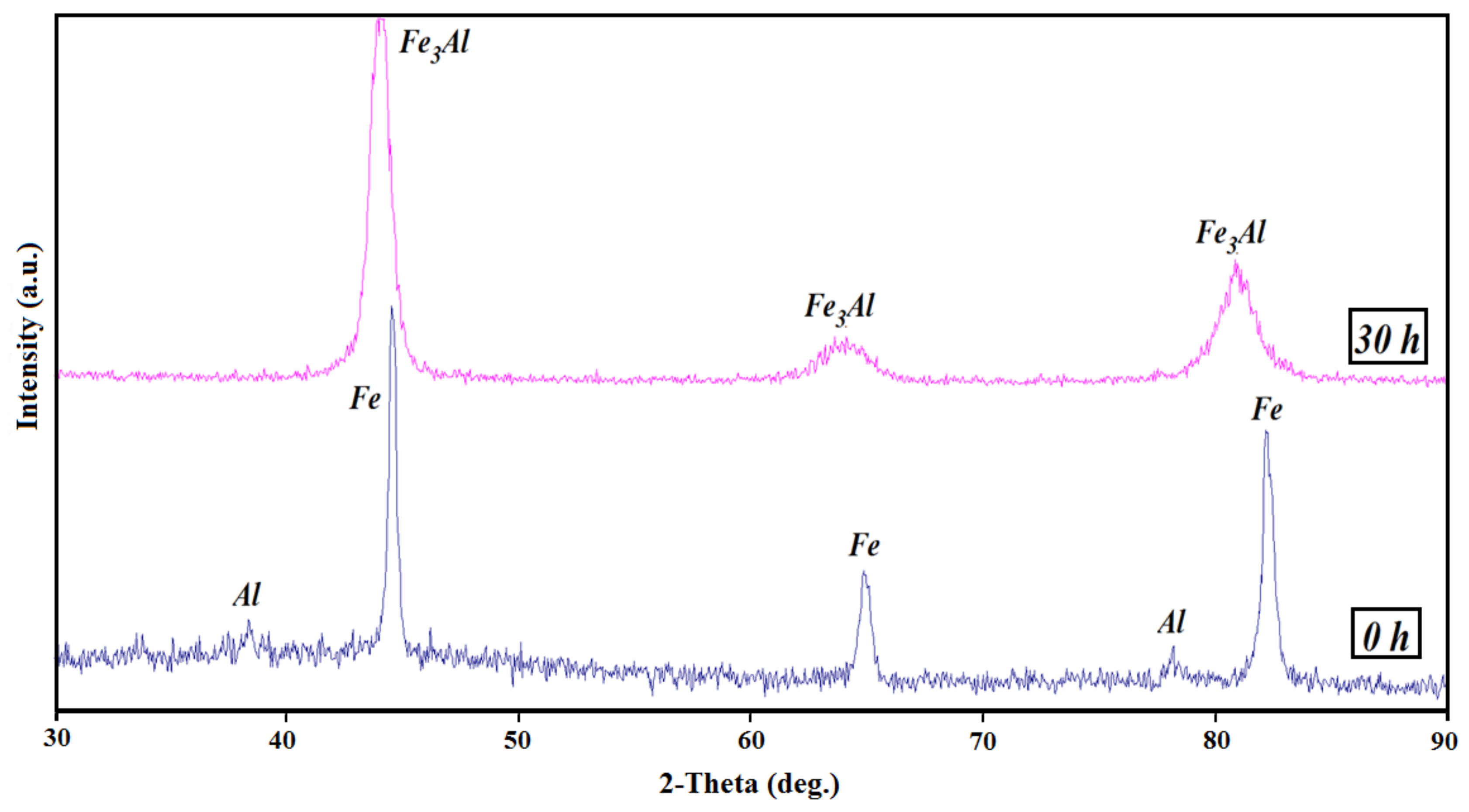
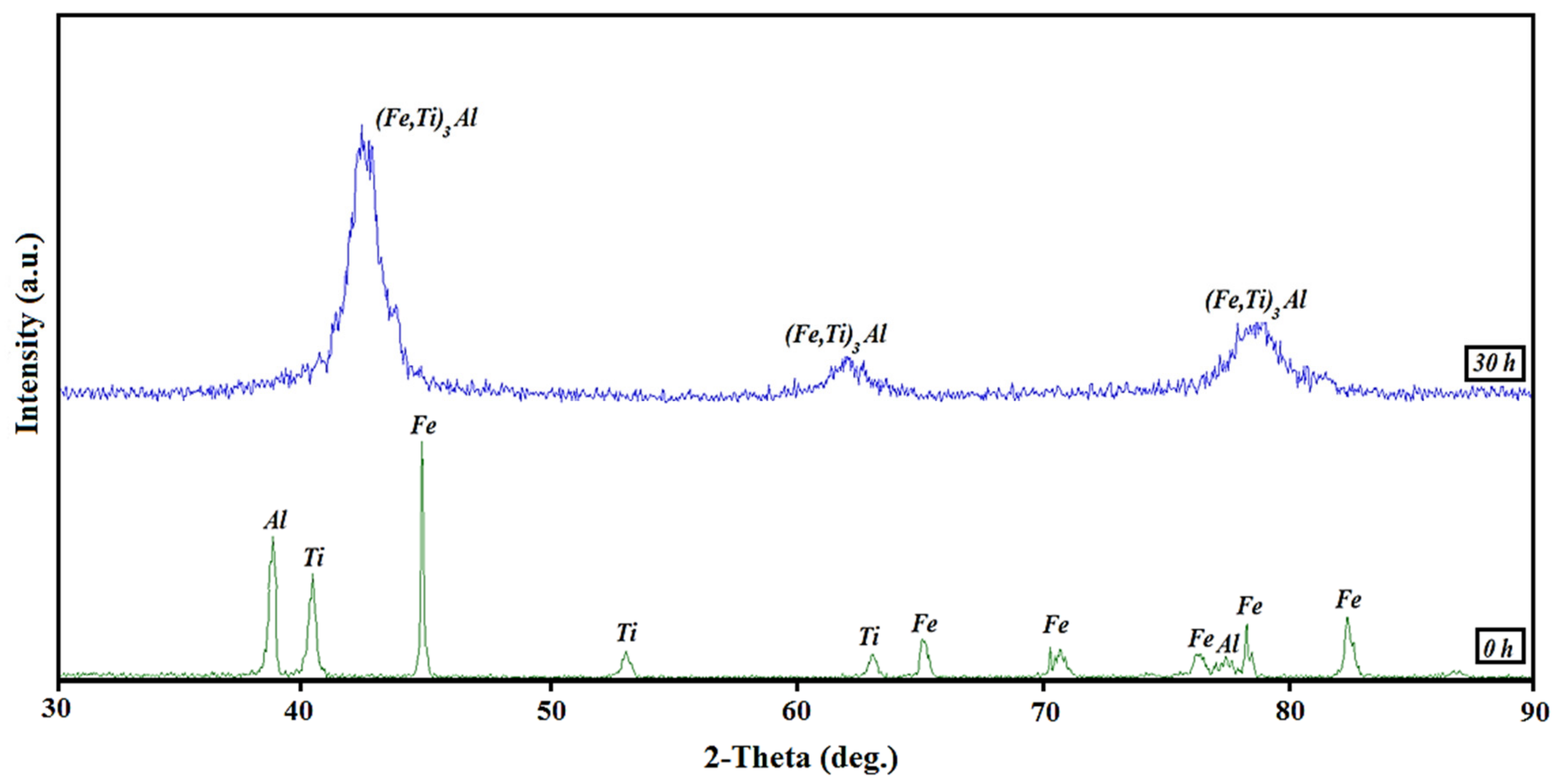

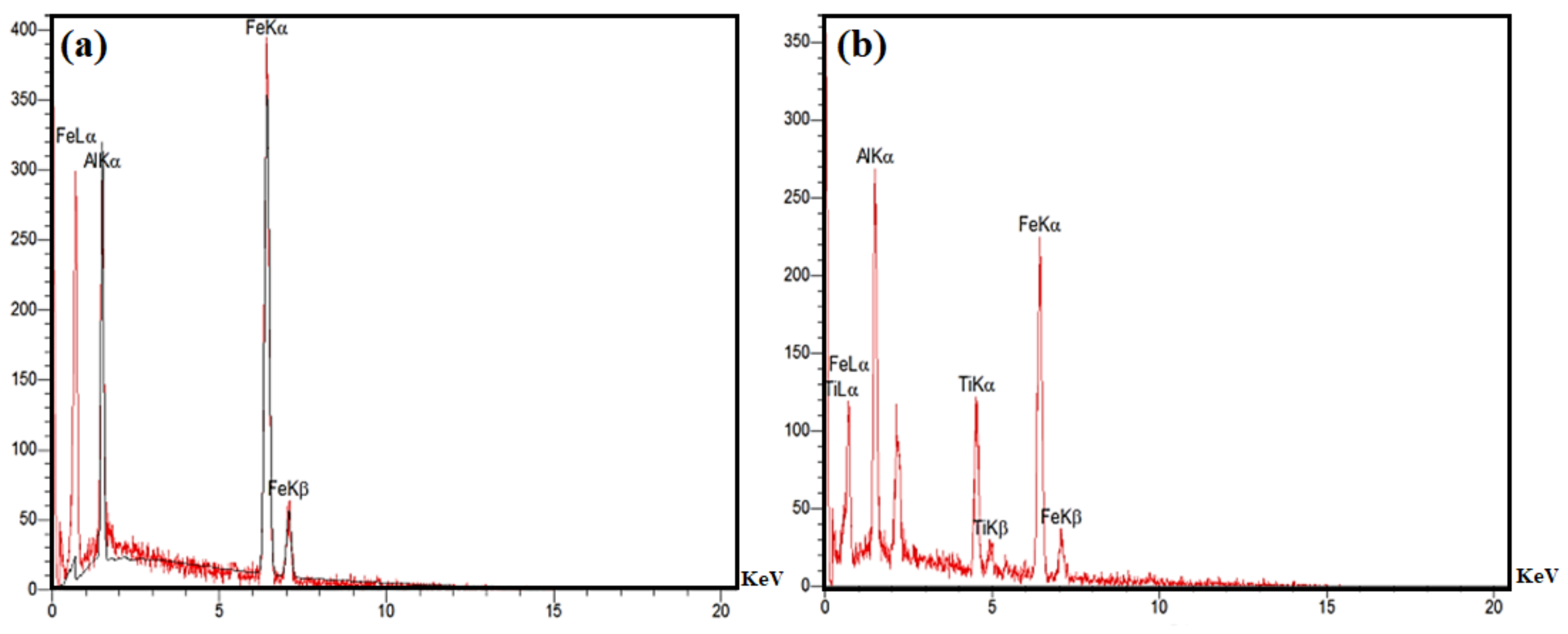
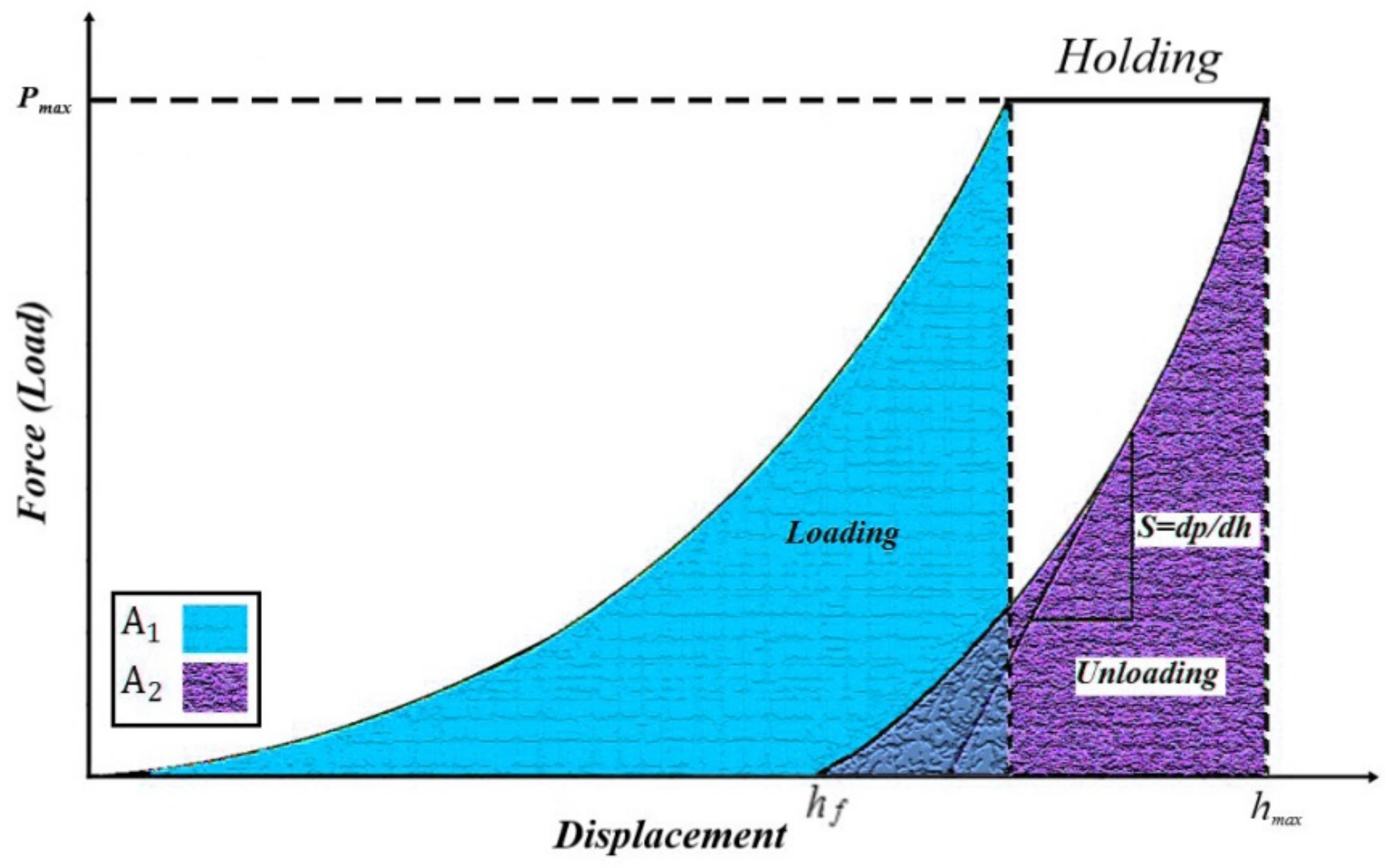

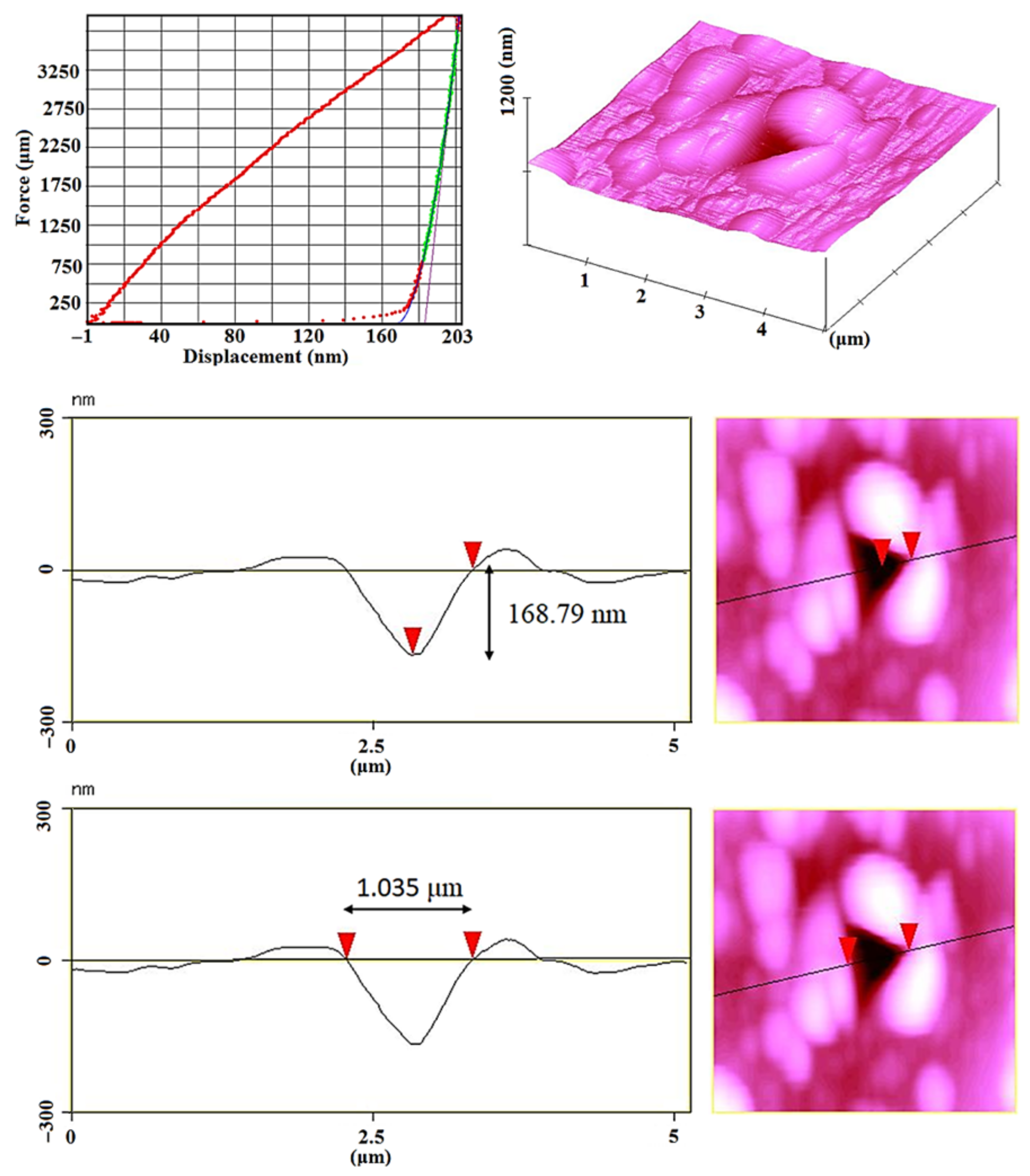
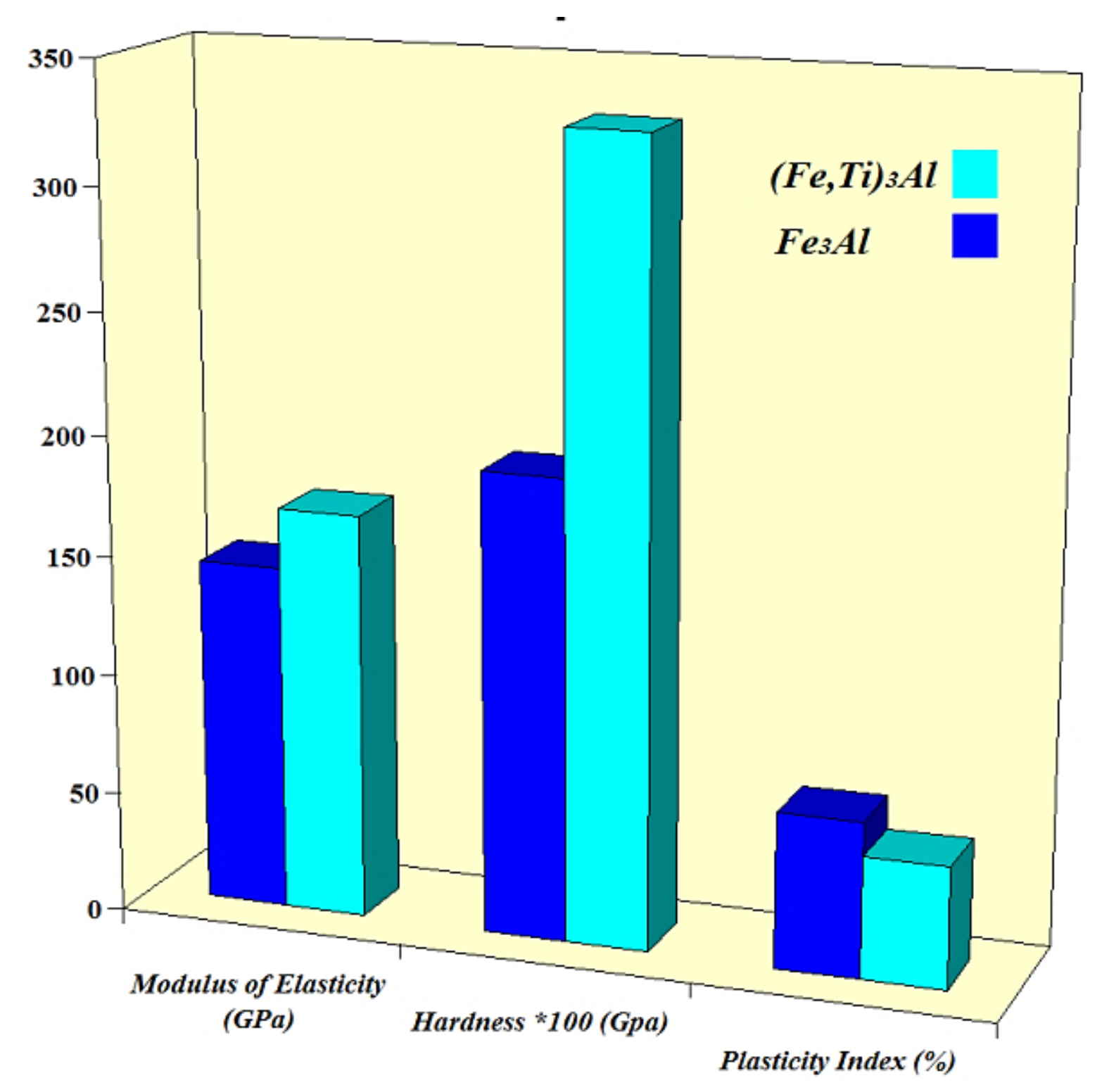
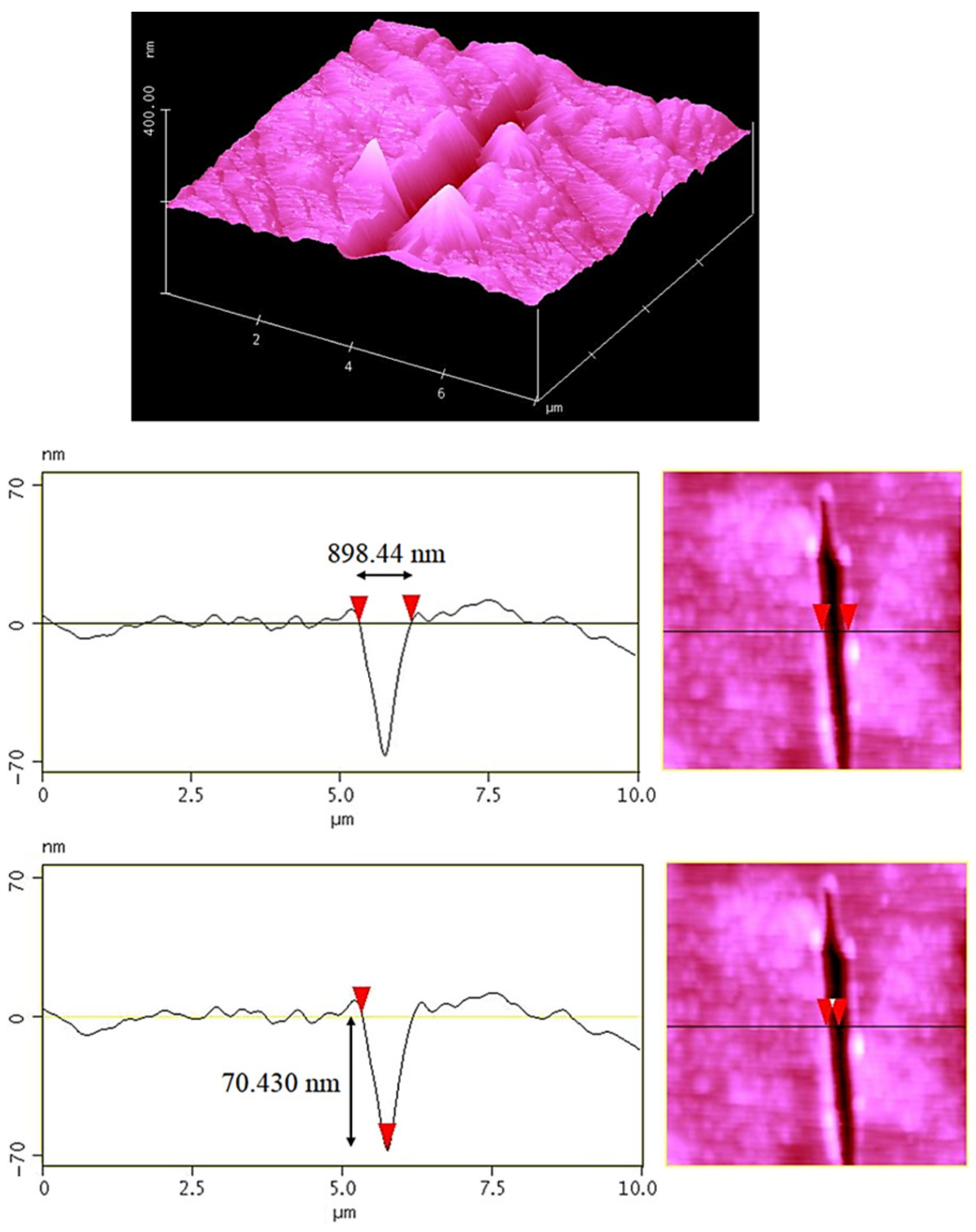


| Sample | Elastic Modulus (GPa) | Hardness × 100 | Plasticity Index (%) |
|---|---|---|---|
| Fe3Al | 144 ± 4 | 1.92 ± 0.15 | 0.65 ± 0.02 |
| (Fe,Ti)3Al | 169 ± 3 | 3.29 ± 0.2 | 0.51 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghvaei, M.M.; Mostaan, H.; Rafiei, M.; Bakhsheshi-Rad, H.R.; Berto, F. Nanoscale Tribological Properties of Nanostructure Fe3Al and (Fe,Ti)3Al Compounds Fabricated by Spark Plasma Sintering Method. Metals 2022, 12, 1077. https://doi.org/10.3390/met12071077
Taghvaei MM, Mostaan H, Rafiei M, Bakhsheshi-Rad HR, Berto F. Nanoscale Tribological Properties of Nanostructure Fe3Al and (Fe,Ti)3Al Compounds Fabricated by Spark Plasma Sintering Method. Metals. 2022; 12(7):1077. https://doi.org/10.3390/met12071077
Chicago/Turabian StyleTaghvaei, Mohammad Mahdi, Hossein Mostaan, Mahdi Rafiei, Hamid Reza Bakhsheshi-Rad, and Filippo Berto. 2022. "Nanoscale Tribological Properties of Nanostructure Fe3Al and (Fe,Ti)3Al Compounds Fabricated by Spark Plasma Sintering Method" Metals 12, no. 7: 1077. https://doi.org/10.3390/met12071077








