A Critical Review on Al-Co Alloys: Fabrication Routes, Microstructural Evolution and Properties
Abstract
:1. Introduction
2. Al-Co Phase Constitution
3. Microstructural Analysis
3.1. The Effect of the Fabrication Route on the Microstructure
3.2. The Effect of the Composition on the Microstructure
4. Mechanical Properties and Deformation Mechanisms
5. Corrosion Behavior
5.1. The Effect of the Fabrication Route on the Corrosion Behavior
5.2. The Effect of the Composition on the Corrosion Behavior
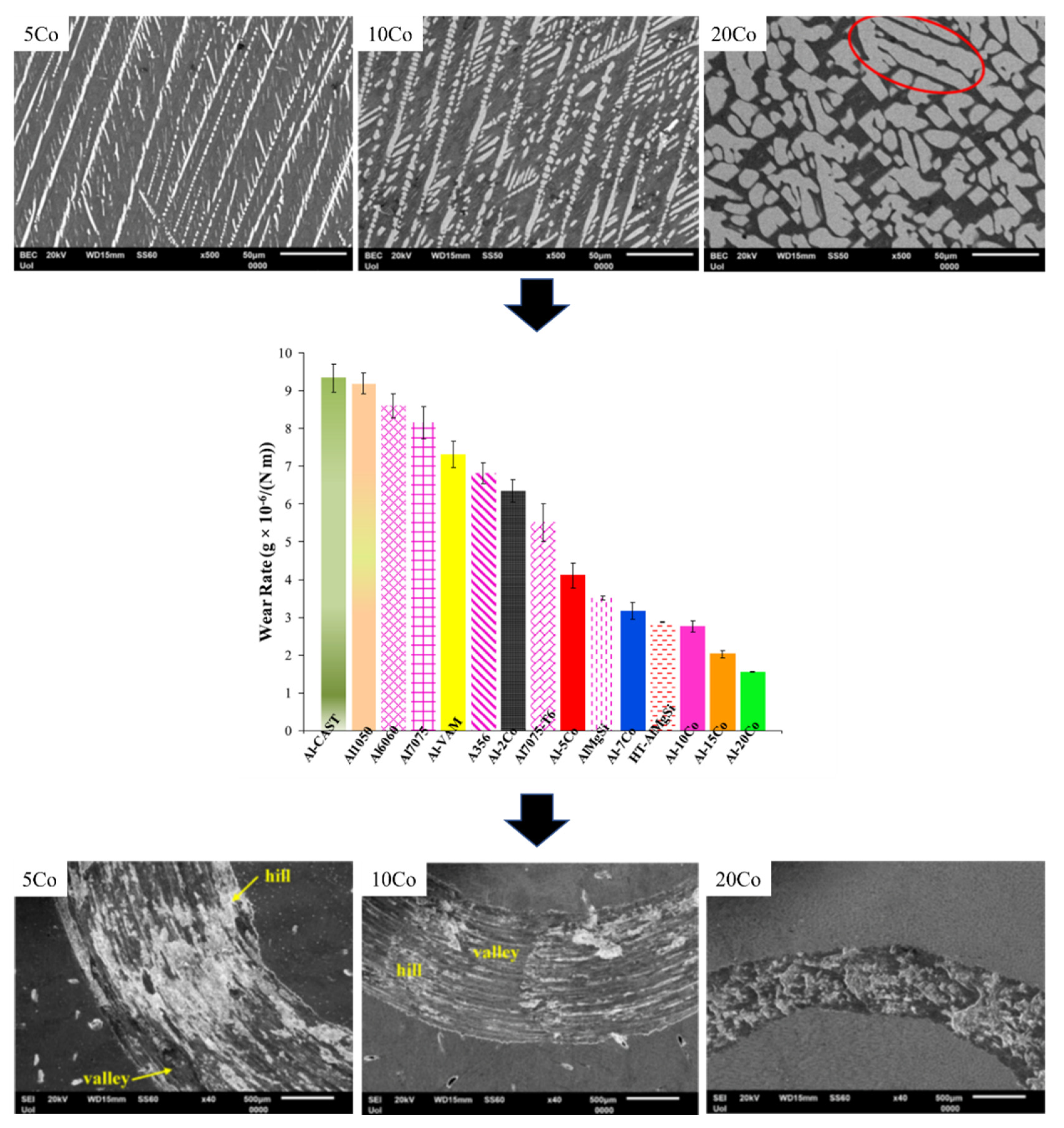
6. Wear Performance
6.1. The Effect of the Fabrication Route on the Wear Performance
6.2. The Effect of the Composition on the Wear Performance
7. Prospects and Potential Applications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.K.; Fabijanic, D.; Dorin, T.; Qiu, Y.; Wang, J.T.; Birbilis, N. Simultaneous improvement in the strength and corrosion resistance of Al via high-entropy ball milling and Cr alloying. Mater. Des. 2015, 84, 270–276. [Google Scholar] [CrossRef]
- Equivel, J.; Darling, K.A.; Murdoch, H.A.; Gupta, R.K. Corrosion and mechanical properties of Al-5 At. Pct Cr produced by cryomilling and subsequent consolidation at various temperatures. Metall. Mater. Trans. A 2018, 49, 3058–3065. [Google Scholar] [CrossRef]
- Esquivel, J.; Gupta, R.K. Influence of the V content on the microstructure and hardness of high-energy ball milled nanocrystalline Al-V alloys. J. Alloy. Compd. 2018, 760, 63–70. [Google Scholar] [CrossRef]
- Witharamage, C.S.; Christudasjustus, J.; Smith, J.; Gao, W.; Gupta, R.K. Corrosion behavior of an in situ consolidated nanocrystalline Al-V alloys. NPJ Mater. Degrad. 2022, 6, 15. [Google Scholar] [CrossRef]
- Witharamange, C.S.; Christudasjustus, J.; Gupta, R.K. The effect of milling time and speed on solid solubility, grain size, and hardness of Al-V alloys. J. Mater. Eng. Perform. 2021, 30, 3144–3158. [Google Scholar] [CrossRef]
- Esquivel, J.; Gupta, R.K. Corrosion behavior and hardness of Al-M (M: Mo, Si, Ti, Cr) alloys. Acta Metall. Sin. 2017, 30, 333–341. [Google Scholar] [CrossRef]
- Kakitani, R.; Reyes, R.V.; Garcia, A.; Spinelli, J.E.; Cheung, N. Relationship between spacing colonies and tensile properties of transient directionally solidified Al-Ni eutectic alloy. J. Alloy. Compd. 2018, 733, 59–68. [Google Scholar] [CrossRef]
- Esquivel, J.; Wachowiak, M.G.; O’Brien, S.O.; Gupta, R.K. Thermal stability of nanocrystalline Al-5at.% Ni and Al-5at.% V alloys produced by high-energy ball milling. J. Alloy. Compd. 2018, 744, 651–657. [Google Scholar] [CrossRef]
- Li, M.; Du, S.; Hou, Y.; Geng, H.; Jia, P.; Zhao, D. Study of liquid structure feature of Al100-XNiX with resistivity and rapid solidification method. J. Non-Cryst. Solids 2015, 411, 26–34. [Google Scholar] [CrossRef]
- Carraca, A.P.; Kakitani, R.; Garcia, A.; Cheung, N. Effect of cooling rate on microstructure and microhardness of hypereutectic Al-Ni alloy. Arch. Civ. Mech. Eng. 2021, 21, 1–9. [Google Scholar]
- Esquivel, J.; Murdoch, H.A.; Darling, K.A.; Gupta, R.K. Excellent corrosion resistance and hardness in Al alloys by extended solid solubility and nanocrystalline structure. Mater Res. Lett. 2018, 6, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Liu, Y.C.; Wei, C.; Zhang, Y.H.; Gao, Z.M. Influence of minor Co on the formation of intermetallic phases in the Al91Fe7Si2 alloy. J. Alloy. Compd. 2008, 466, 92–97. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Zhang, F.; Ma, Z.; Jia, L.; Yang, B.; Tao, T.; Zhang, H. Effect of cooling rate and Co content on the formation of Fe-rich intermetallics in hypereutectic Al7Si0.3Mg alloy with 0.5% Fe. Mater. Charact. 2018, 139, 116–124. [Google Scholar] [CrossRef]
- Gan, Z.; Wu, H.; Sun, Y.; Su, Y.; Wang, Y.; Wu, C.; Liu, L. Influence of Co content and super gravity-field on refinement of in-situ ultra-fined fibers in Al-2.5Ni eutectic alloys. J. Alloy. Compd. 2020, 822, 153607. [Google Scholar] [CrossRef]
- Daniels, S. Some sand-cast alloys of aluminium containing cobalt. Indust. Engin. Chem. 1926, 18, 686–691. [Google Scholar] [CrossRef]
- Garrett, R.K., Jr.; Sanders, T.H., Jr. The formation of coarse intermetallics in rapidly solidified Al-Co alloys. Mater. Sci. Eng. A 1983, 60, 269–274. [Google Scholar] [CrossRef]
- Menon, J.; Suryanarayana, C. Metallography of a melt quenched Aluminium-Cobalt alloy. Metallography 1988, 21, 179–197. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Menon, J. Electron microscopy of metastable phases in rapidly solidified Al-Co alloys. Bull. Mater. Sci. 1994, 17, 121–139. [Google Scholar] [CrossRef]
- Ma, X.L.; Kuo, K.H. Decagonal quasicrystal and related crystalline phases in slowly solidified Al-Co alloys. Metal. Trans. A 1992, 23A, 1121–1123. [Google Scholar] [CrossRef]
- Schroers, J.; Holland-Moritz, D.; Herlach, D.M.; Grushko, B.K. Urban, Undercooling and solidification behaviour of a metastable decagonal quasicrystalline phase and crystalline phases in Al-Co. Mater. Sci. Eng. 1997, A226–A228, 990–994. [Google Scholar] [CrossRef]
- Heggen, M.; Deng, D.; Feuerbacher, M. Plastic deformation properties of the orthorhombic complex metallic alloy phase Al13Co4. Intermetallics 2007, 15, 1425–1431. [Google Scholar] [CrossRef]
- Heggen, M.; Houben, L.; Feuerbacher, M. Metadislocations in the structurally complex orthorhombic alloy Al13Co4. Philos. Mag. 2008, 88, 2333–2338. [Google Scholar] [CrossRef]
- Heidelmann, M.; Heggen, M.; Dwyer, C.; Feuerbacher, M. Comprehensive model of metadislocation movement in Al13Co4. Scr. Mater. 2015, 98, 24–27. [Google Scholar] [CrossRef]
- Allarcon Villaseka, S.; Serkovic Loli, L.N.; Ledieu, J.; Fournee, V.; Gille, P.; Dubois, J.M.; Guadry, E. Oxygen adsorption on the Al9Co2(001) surface: First principles and STM study. J. Phys. Condens. Matter 2013, 25, 355003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allarcon Villaseka, S.; Ledieu, J.; Serkovic Loli, L.N.; de Weerd, M.C.; Gille, P.; Fournee, V.; Dubois, J.M.; Gaudry, E. Structural investigation of the (001) surface of the Al9Co2 complex metallic alloys. J. Phys. Chem. C 2011, 115, 14922–14932. [Google Scholar] [CrossRef]
- Addou, R.; Shukla, A.K.; Allarcon Villaseka, S.; Gaudry, E.; Deniozou, T.; Hegger, M.; Feuerbacher, M.; Widmen, R.; Groning, O.; Fournee, V.; et al. Lead adsorption on the Al13Co4 (100) surface: Heterogenous nucleation and pseudomorphic growth. J. Phys. 2011, 13, 103011. [Google Scholar] [CrossRef] [Green Version]
- Kandaskalov, D.; Fourne, V.; Ledieu, J.; Gaudry, E. Adsorption properties of the o- Al13Co4 (100) surface towards molecules involved in semihydrogeneration of acetylene. J. Phys. Chem. C 2014, 118, 23032–23041. [Google Scholar] [CrossRef]
- Kandaskalov, D.; Fourne, V.; Ledieu, J.; Gaudry, E. Catalytic semi-hydrogeneration f acetylene on the (100) surface of the o- Al13Co4 quasicrystalline approximant: A DFT study. J. Phys. Chem. C 2017, 121, 18738–18745. [Google Scholar] [CrossRef]
- Anand, K.; Fournee, V.; GPrevot, G.; Ledieu, J.; Gaudry, E. No-wetting behaviour of Al-Co quasicrystalline approximants owing to their unique electronic structures. Appl. Mater. Inter. 2020, 12–13, 15793–15801. [Google Scholar] [CrossRef]
- Allarcon Villaseka, S.; Dubois, J.M.; Gaudry, E. Lead adsorption on the pseudo-10-fold surface of the Al13Co4 complex metallic alloy: A first principle study. Int. J. Quantum Chem. 2012, 113, 1–7. [Google Scholar]
- Bobaru, S.; Gaudry, E.; de Weerd, M.C.; Ledieu, J.; Fournee, V. Competing allotropes of Bi deposited on the alloy Al13Co4 (100) surface. Phys. Rev. B 2012, 86, 214201. [Google Scholar] [CrossRef] [Green Version]
- Petucci, J.; Karimi, J.M.; Huang, Y.T.; Curtarolo, C. Ordering and growth of rare gas films (Xe, Kr, Ar, and Ne) on the pseudo-ten-fold quasicrystalline approximant Al13Co4 (100) surface. J. Phys Condens. Matter 2014, 26, 095003. [Google Scholar] [CrossRef]
- Fourne, V.; Gaudry, E.; Ledieu, J.; de Weerd, M.C.; Diehl, R.D. Quasi-ordered C60 molecular films grown on the pseudo-ten-fold (100) surface of the Al13Co4 quasicrystalline approximant. J. Phys. Condens. Matter 2016, 28, 355001. [Google Scholar] [CrossRef] [PubMed]
- Lekatou, A.G.; Sfikas, A.K.; Karantzalis, A.E. The influence of the fabrication route of the microstructure and surface degradation properties of Al reinforced by Al9Co2. Mater. Chem. Phys. 2017, 200, 33–49. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Sfikas, A.K.; Sioulas, D.; Kanterakis, D. Sliding wear performance of Al-Co alloys fabricated by vacuum arc melting and correlation with their microstructure. Mater. Chem. Phys. 2022, 276, 125411. [Google Scholar] [CrossRef]
- Bakoulis, G.; Lekatou, A.G.; Poulia, A.; Sfikas, A.K.; Lentzaris, K.; Karantzalis, A.E. Al-(Al9Co2-Al13Co4) powder metallurgy processed composite materials: Analysis of microstructure, sliding wear and aqueous corrosion. Mater. Sci. Eng. Adv. Res. 2017, Special Issue, 53–60. [Google Scholar]
- Geng, K.; Yang, Y.; Li, S.; Misra, R.D.K.; Zhu, Q. Enabling high-performance 3D printing by decorating with high laser absorbing Co phase. Addit. Manuf. 2020, 32, 101012. [Google Scholar] [CrossRef]
- McAllister, A.K. The Al-Co (Aluminium-Cobalt) system. Bull. Alloy Phase Diagr. 1989, 10, 646–650. [Google Scholar] [CrossRef]
- Elagin, V.I. Ways of developing high- strength and high- temperature structural aluminium alloys in the 21st century. Met. Sci. Heat Treat. 2007, 49, 427–434. [Google Scholar] [CrossRef]
- Grushko, B.; Wittenberg, R.; Bickmann, K.; Freiburg, C. The contritution of Al-Co alloys between Al9Co2 and Al5Co2. J. Alloy. Compd. 1996, 233, 279–287. [Google Scholar] [CrossRef]
- Simon, P.; Zelenina, I.; Ramlau, R.; Carillo-Cabrera, W.; Burkhardt, U.; Borrmann, H.; Cardoso Gil, R.; Feuerbacher, M.; Gille, P.; Grin, Y. Structural complexity of the intermerallic o-Al13Co4. J. Alloy. Compd. 2020, 820, 153363. [Google Scholar] [CrossRef]
- Fleischer, F.; Weber, T.; Jung, D.Y.; Steurer, W. o’-Al13Co4, a new quasicrystal approximant. J. Alloy. Compd. 2010, 500, 153–160. [Google Scholar] [CrossRef]
- Priputen, J.; Kusy, M.; Drienovsky, M.; Janickovic, D.; Cicka, R.; Cernickova, I.; Janovec, J. Experimental reinvestigation of Al-Co phase diagram in vinicity of Al13Co4 family of phases. J. Alloy. Compd. 2015, 647, 486–497. [Google Scholar] [CrossRef]
- Shevchenko, M.A.; Berezutskii, V.V.; Ivanov, M.I.; Kudin, V.G.; Sudavtsova, V.S. Thermodynamic properties of alloys of the Al-Co and Al-Co-Sc systems. Russ. J. Phys. Chem. A 2014, 88, 729–734. [Google Scholar] [CrossRef]
- Stein, F.; He, C.; Dupin, N. Melting behaviour and homogeneity range of B2 CoAl and updated thermodynamic description of the Al-Co system. Intermetallics 2013, 39, 58–68. [Google Scholar] [CrossRef]
- Okamoto, H. Supplemental literature review of binary phase diagrams: Ag-Yb, Al-Co, Al-I, Co-Cr, Cs-Te, In-Sr, Mg-Tl, Mn-Pd, Mo-O, Mo-Re, Ni-Os, V-Ze. J. Phase Equilib. Diffus. 2016, 37, 726–737. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.B.; Jiang, Y.; Huang, G.X.; Wang, X.; Jiang, Y.R.; Liang, J.S.; Zhang, L.G. Thermodynamic evaluation of phase equilibria and glass-forming ability of the Al-Co-Gd system. Calphad 2016, 52, 57–65. [Google Scholar] [CrossRef]
- Šulhánek, P.; Drienovský, M.; Černičková, I.; Ďuriška, L.; Skaudžius, R.; Gerhátová, Ž.; Palcut, M. Oxidation of Al-Co Alloys at High Temperatures. Materials 2020, 13, 3152. [Google Scholar] [CrossRef]
- Douglas, A.M.B. The structure of Co2Al9. Acta Cryst. 1950, 3, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Bostrom, M.; Prots, H.; Prots, Y.; Burkhardt, U.; Grin, Y. The Co2Al9 structure revised. Z. Anorg. Allg. Chem. 2005, 631, 534–541. [Google Scholar]
- Li, X.Z.; Ma, X.L.; Kuo, K.H. A structural model of orthorhombic Al3Co, derived from the monoclinic Al13Co4 by high-resoluton electron microscopy. Philos. Mag. Lett. 1994, 70, 221–229. [Google Scholar] [CrossRef]
- Grin, J.; Burkhardt, U.; Elner, M.; Peters, K. Crystal structure of orthorhombic Co4Al13. J. Alloy. Compd. 1994, 206, 243–247. [Google Scholar] [CrossRef]
- Hudd, R.C.; Taylor, W.H. The structure of Co4Al13. Acta Cryst. 1962, 15, 441–442. [Google Scholar] [CrossRef]
- Freiburg, C.; Grushko, B.; Wittenberg, R.; Reichert, W. Once more about monoclinic Al13Co4. Mater. Sci. Forum 1996, 228–132, 583–586. [Google Scholar] [CrossRef]
- Ma, X.L.; Li, X.Z.; Kuo, K.H. A family of τ-inflated monoclinic Al13Co4 phases. Acta Cryst. 1995, B-51, 36–43. [Google Scholar] [CrossRef]
- Li, X.Z.; Hiraga, K. High-resolution electron microscopy of the ε-Al3Co, a monoclinic approximant of the Al-Co decagonal quasicrystal. J. Alloy. Compd. 1998, 269, L13–L16. [Google Scholar] [CrossRef]
- Mo, Z.M.; Sui, H.X.; Ma, X.L.; Kuo, K.H. Structural models of τ2-inflated monoclinic and orthorhombic Al-Co phases. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1998, 29, L13–L16. [Google Scholar] [CrossRef]
- Christensen, J.; Oleynikov, P.; Hovmuller, S.; Zou, X.D. Solving approximant structures using a “strong reflections” approach. Ferroelectrics 2004, 305, 273–277. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.J.; Wang, S.C.; Li, Z.C.; Hovmuller, S.; Zou, X.D. A structural model for τ2- Al13Co4 deducted by the strong relations approach. J. Comput. Theor. Nanosci. 2008, 5, 1735–1737. [Google Scholar] [CrossRef]
- Sugiyama, K.; Yasuhara, A.; Hiraga, K. Structure of τ2- Al3Co a monoclinic approximant of the Al-Co decagonal quasicrystals. In Aperiodic Crystals; Smidt, S., Withers, R.K., Lifshitz, R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 237–242. [Google Scholar]
- Bradley, A.J. Crustal structure of Co2Al5. Z. Krist. 1938, 99, 480–487. [Google Scholar]
- Newkirt, J.B.; Black, P.J.; Damjanovic, A. The refinement of Co2Al5 structures. Acta Cryst. 1961, 14, 532–533. [Google Scholar] [CrossRef]
- Burkhardt, U.; Ellner, M.; Grin, Y.; Baumgartner, M. Powder diffraction refinement of Co2Al5 structure. Powder Diffr. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Cooper, M.J. The electron distribution in phases CoAl and NiAl. Philos. Mag. 1963, 8, 811–821. [Google Scholar] [CrossRef]
- Dutchak, Y.I.; Chekh, V.G. High temperature X-ray diffraction study of the lattice dynamics of the compounds Al-Co and Al-Ni. Russ. J. Phys. Chem. 1981, 55, 1326–1328. [Google Scholar]
- Adam, A.M. Dendrite refinement of Al9Co2 compound by a continuous increase of the cooling rate during solidification. UPB Sc. Bull. Ser. B 2012, 74, 289–300. [Google Scholar]
- Adam, A.M. Influence of rapid solidification on the microstructure on aluminium rich hypereutectuc Al-Co alloys. UPB Sci. Bull. Ser. B 2011, 73, 217–228. [Google Scholar]
- Hung, C.J.; Nayak, S.K.; Sun, Y.; Fennessy, C.; Vedula, V.K.; Tulyani, S.; Lee, S.W.; Alpay, S.P.; Hebert, R.J. Novel Al-X alloys with improved hardness. Mater. Des. 2020, 192, 108699. [Google Scholar] [CrossRef]
- Silva, C.A.P.; Kakitani, R.; Cante, M.V.; Bryto, C.; Garcia, A.; Spinelli, J.E.; Cheung, N. Microstructure, phase morphology, eutectic coupled zone and hardness of Al-Co alloys. Mater. Charact. 2020, 169, 110617. [Google Scholar] [CrossRef]
- Sun, Y.; Hung, C.; Hebert, R.J.; Fennessy, C.; Tulyani, S.; Aindow, M. Eutectic microstructures in dilute Al-Ce and Al-Co alloys. Mater. Charact. 2019, 154, 269–276. [Google Scholar] [CrossRef]
- Osorio, W.R.; Spinelli, J.E.; Ferreira, I.L.; Garcia, A. The roles of macrosegregation and of dendritic array spacings of the electrochemical behavior of an Al-4.5 wt.% Cu alloy. Electrochim. Acta 2007, 52, 3265–3273. [Google Scholar] [CrossRef]
- Lekatou, A.; Sfikas, A.K.; Karantzalis, A.E.; Sioulas, D. Microstructure and corrosion performance of Al-32%Co alloys. Corros. Sci. 2012, 63, 193–209. [Google Scholar] [CrossRef]
- Brodova, I.G.; Men`Shikova, S.G.; Lad`yanov, V.I.; Yablonskikh, T.I.; Bel’tyukov, A.L.; Astaf`ev, V.V. Crystallization of eutectic alloys Al88.8Co1.2 under equilibrium and nonequilibrium conditions. Met. Sci. Heat Treat. 2015, 57, 344–349. [Google Scholar] [CrossRef]
- Men`Shikova, S.G.; Shirinkina, I.G.; Brodova, I.G.; Lad`yanov, V.I.; Suslov, A.A. Structures of thin ribbons from an Al-Co alloy under rapid cooling. Met. Sci. Heat Treat. 2016, 58, 393–399. [Google Scholar] [CrossRef]
- Froes, F.H. Rapid solidification of lightweight metals alloys. Mater. Sci. Eng. 1989, A117, 19–32. [Google Scholar] [CrossRef]
- Lekatou, A.; Sfikas, A.K.; Petsa, C.; Karantzalis, A.E. Al-Co Alloys Prepared by Vacuum Arc Melting: Correlating Microstructure Evolution and Aqueous Corrosion Behavior with Co Content. Metals 2016, 6, 46. [Google Scholar] [CrossRef] [Green Version]
- Van Tendeloo, G.; Menon, J.; Suryanarayana, C. An electron microscopic study of rapidly solidified Al-5 wt% Co alloy. J. Mater. Res. 1987, 2, 547–556. [Google Scholar] [CrossRef]
- Sfikas, A.K.; Lekatou, A.G. Electrochemical behavior of Al-Al9Co2 in sulfuric acid. Corros. Mater. Degrad. 2020, 1, 249–272. [Google Scholar] [CrossRef]
- Palcut, M.; Priputen, P.; Kusy, M.; Janovec, J. Corrosion behaviour of Al-29at%Co in aqueous NaCl. Corros. Sci. 2013, 75, 461–466. [Google Scholar] [CrossRef]
- Palcut, M.; Priputen, P.; Salgo, K.; Janovec, J. Phase constitution and corrosion resistance of Al-Co alloys. Mater. Chem. Phys. 2015, 166, 95–104. [Google Scholar] [CrossRef]
- Priputen, P.; Palcut, M.; Babinec, M.; Misik, J.; Cernickova, I.; Janovec, J. Correlation between microstructure and corrosion behaviour of near equilibrium Al-Co alloys in various environments. J. Mater. Eng. Perform. 2017, 26, 3970–3976. [Google Scholar] [CrossRef]
- Browmik, A.; Dolbnya, I.P.; Britton, T.B.; Jones, N.G.; Sernicola, G.; Walter, C.; Gille, P.; Dye, D.; Clegg, W.J.; Giuliani, F. Using coupled micropillar compression ad micro-Laue diffraction do investigate deformation mechanisms in a complex metallic alloy Al13Co4. Appl. Phys. Lett. 2016, 108, 1111902. [Google Scholar]
- Korte-Kerzel, S.; Schnabel, V.; Clegg, W.J.; Heggen, M. Room temperature plasticity in m-Al13Co4 studied my microcompression and high-resolution scanning transmission electron microscopy. Scr. Mater. 2018, 148, 327–330. [Google Scholar] [CrossRef]
- Walter, C.; Wheeler, J.M.; Barnard, J.S.; Raghavan, R.; Korte- Kerzel, S.; Gille, P.; Michler, J.; Clegg, W.J. Anomaloys yielding in the complex metallic alloy Al13Co4. Acta Mater. 2013, 61, 7189–7196. [Google Scholar] [CrossRef] [Green Version]
- Xue, S.; Li, Q.; Xie, D.Y.; Zhang, Y.F.; Wang, H.; Wang, H.; Wang, J.; Zhang, X. High strength, deformable nanotwinned Al-Co alloys. Mater. Res. Lett. 2019, 7, 33–39. [Google Scholar] [CrossRef]
- do Nascimento, A.M.; Ielardi, M.C.F.; Kina, A.Y.; Tavares, S.S.M. Pitting corrosion resistance of cast duplex stainless steels in 3.5% NaCl solution. Mater. Charact. 2008, 59, 1736–1740. [Google Scholar] [CrossRef]
- Lifka, B. Corrosion of aluminum and aluminium alloys. In Corrosion Engineering Handbook; Schweitzer, P.A., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 99–155. [Google Scholar]
- Esquivel, J.; Gupta, R.K. Review—Corrosion resistant metastable Al alloys: An overview of corrosion mechanisms. J. Electrochem. Soc. 2020, 167, 081504. [Google Scholar] [CrossRef]
- Davis, J.R. Corrosion of Aluminum and Aluminum Alloys; ASM International: Materials Park, OH, USA, 1999. [Google Scholar]
- Gousia, V.; Tsioukis, A.; Lekatou, A.; Karantzalis, A.E. Al-MoSi2 composite materials: Analysis of microstructure, sliding wear, solid particle erosion, and aqueous corrosion. J. Mater. Eng. Perform. 2016, 25, 3107–3120. [Google Scholar] [CrossRef]
- Lekatou, A.; Karantzalis, A.E.; Evangelou, A.; Gousia, V.; Kaptay, G.; Gacsi, Z.; Baumli, P.; Simon, A. Aluminium reinforced by WC and TiC nanoparticles (ex-situ) and aluminide particles (in-situ): Microstructure, wear and corrosion behaviour. Mater. Des. 2015, 65, 1121–1135. [Google Scholar] [CrossRef]
- Wu, J.M.; Li, Z.Z. Contributions of the particulate reinforcement to dry sliding wear resistance of rapidly solidified Al-Ti alloys. Wear 2000, 244, 147–153. [Google Scholar] [CrossRef]
- Lentzaris, K.; Lekatou, A.G.; Ntoumazios, A.; Karantzalis, A.E.; Sfikas, A.K. Solid particle erosion of aluminum in-situ reinforced with a cobalt aluminide. Mater. Sci. Eng. Adv. Res. 2017, 19–25. Available online: http://verizonaonlinepublishing.com/MSCPDF/MaterialScienceandEngineeringwithAdvancedResearch3S.pdf (accessed on 10 May 2022).
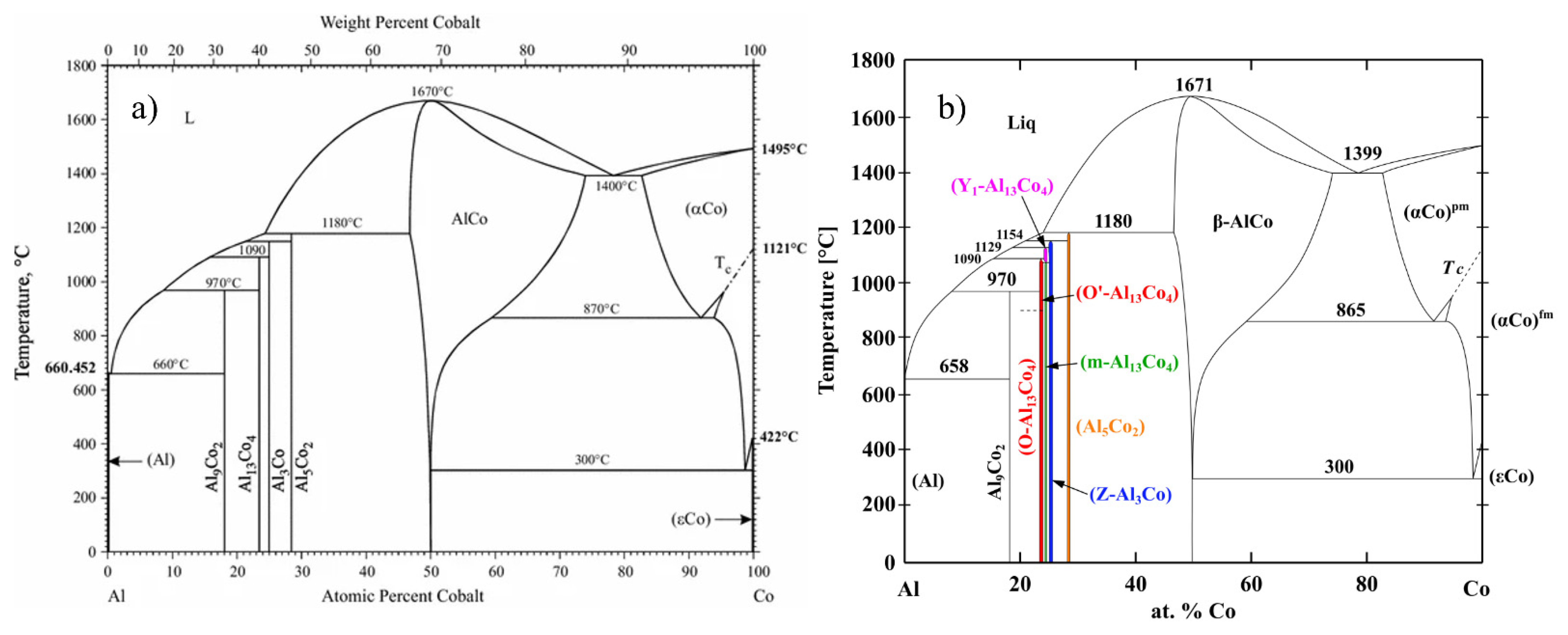
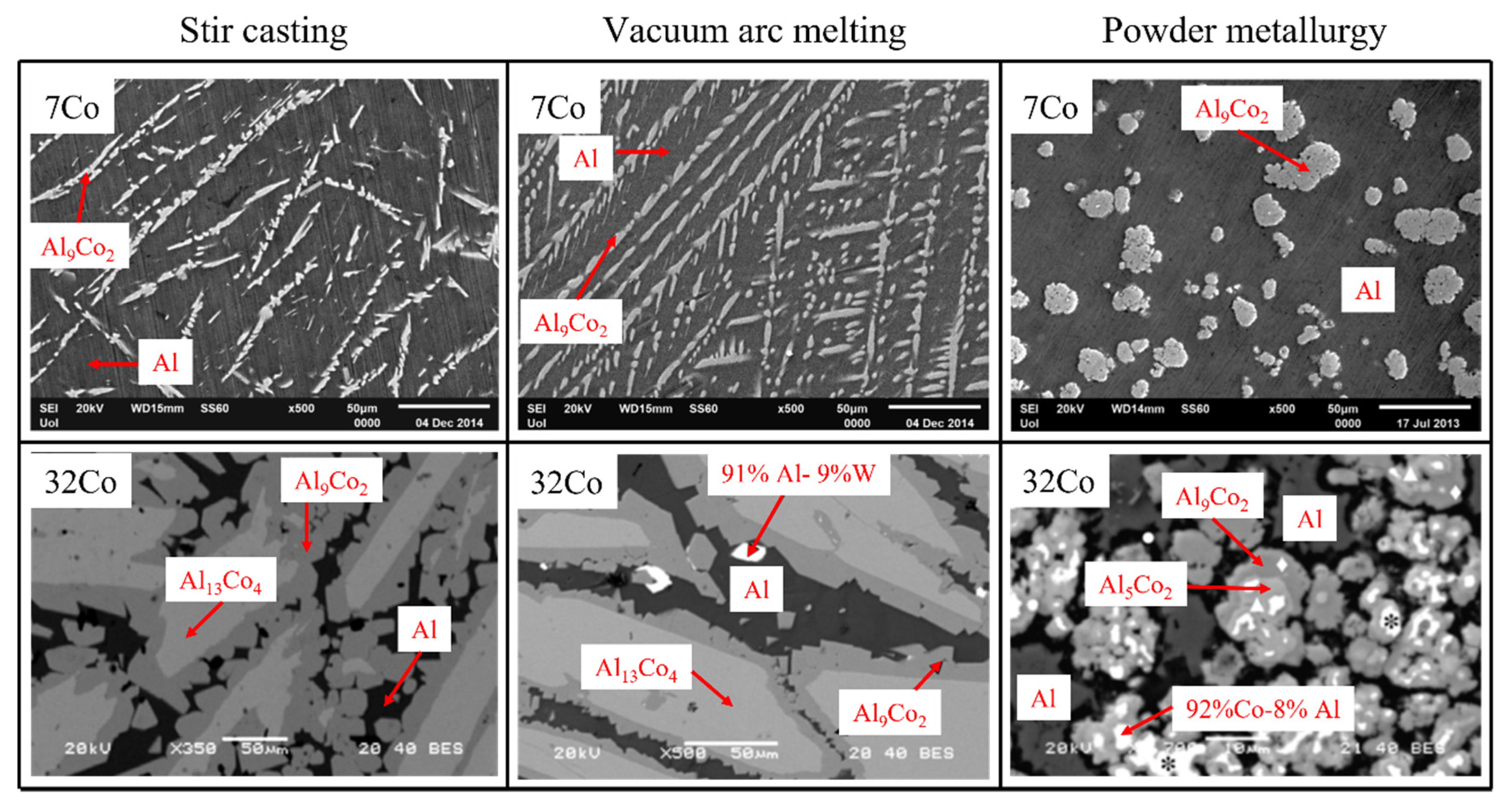
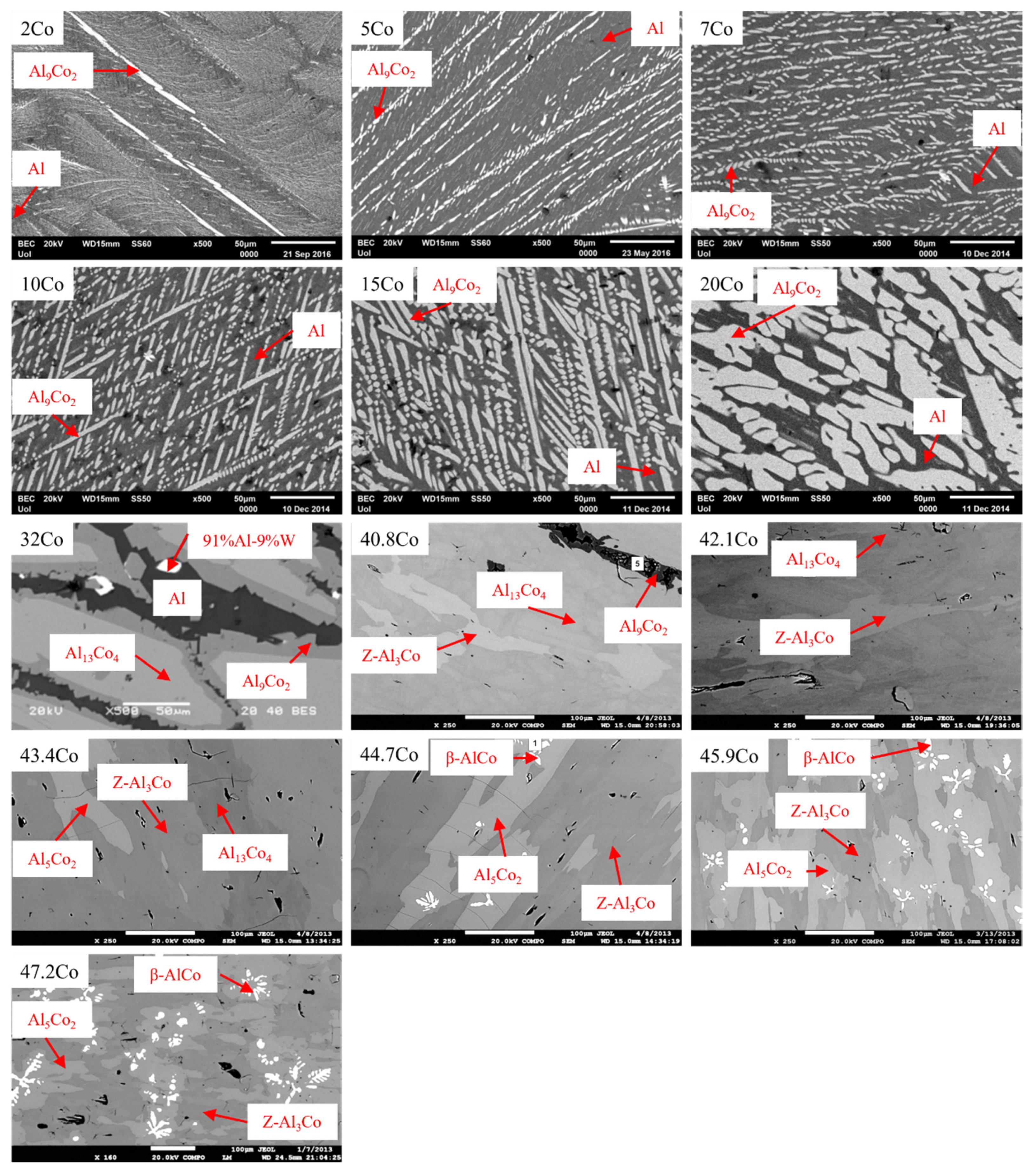

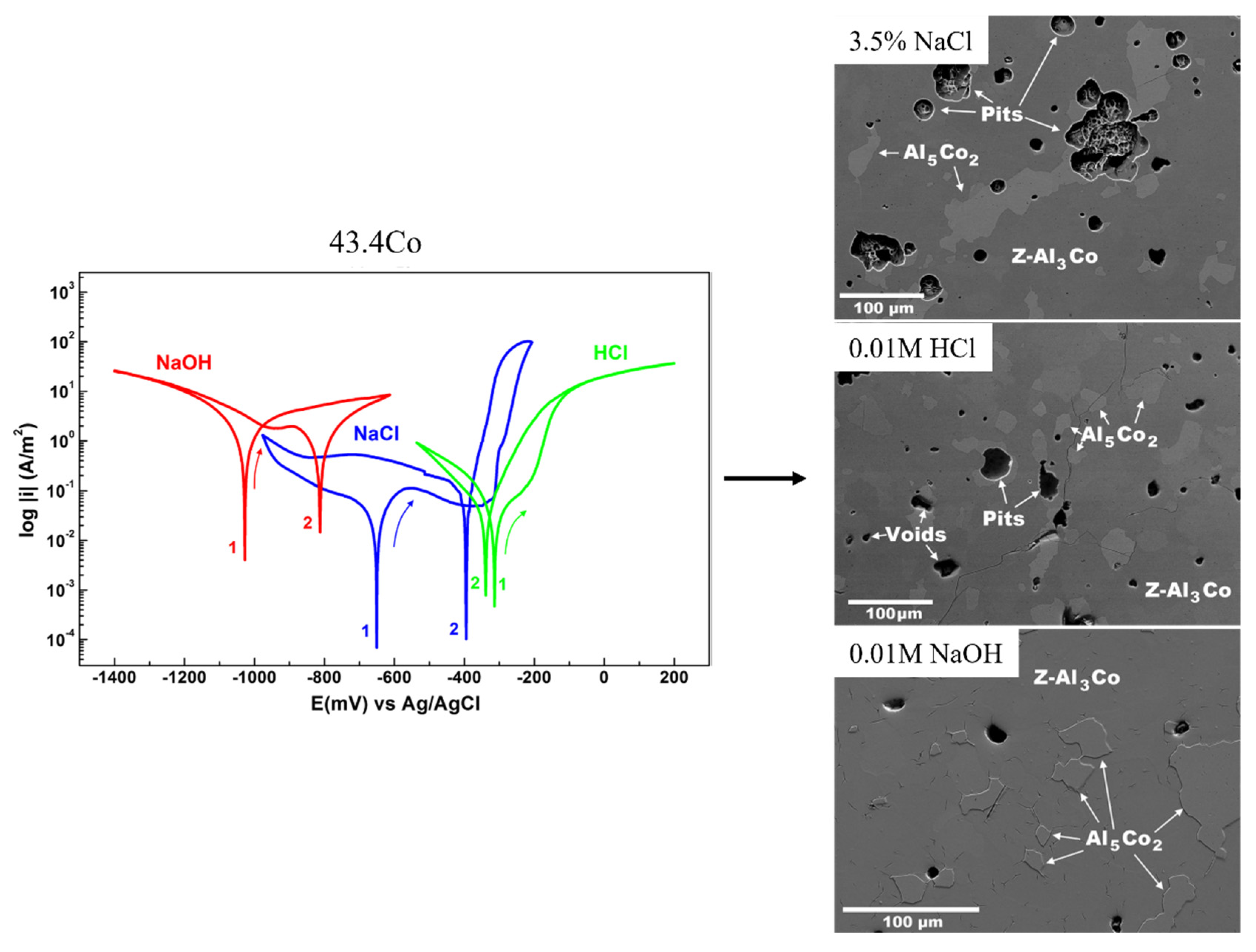
| Phase | Space Groups | a (Å) | b (Å) | c (Å) | β (°) | References |
|---|---|---|---|---|---|---|
| Al9Co2 | P21/C | 6.21 | 6.28 | 8.55 | 94.77 | [49,50] |
| Y1-Al13Co4 | C2/m (12) | 17.06 | 4.10 | 7.50 | 116.02 | [43] |
| Y2-Al13Co4 | Immm (71) | 12.03 | 7.58 | 15.35 | - | [43] |
| O-Al13Co4 | Pmn21 | 8.15 | 12.34 | 14.45 | - | [41,51,52] |
| O’-Al13Co4 | Pnma | 28.89 | 8.13 | 12.34 | - | [42] |
| Μ-Al13Co4 | C2/m | 15.17 | 8.10 | 12.34 | 107.84 | [53,54] |
| Z-Al3Co | P2/m | 39.83 | 8.12 | 32.18 | 108.03 | [55,56,57,58,59,60] |
| Al5Co2 | P63/mmc | 7.67 | - | 7.60 | - | [61,62,63] |
| B2-AlCo | Pm-3m | 2.86 | - | - | - | [64,65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfikas, A.K.; Gonzalez, S.; Lekatou, A.G.; Kamnis, S.; Karantzalis, A.E. A Critical Review on Al-Co Alloys: Fabrication Routes, Microstructural Evolution and Properties. Metals 2022, 12, 1092. https://doi.org/10.3390/met12071092
Sfikas AK, Gonzalez S, Lekatou AG, Kamnis S, Karantzalis AE. A Critical Review on Al-Co Alloys: Fabrication Routes, Microstructural Evolution and Properties. Metals. 2022; 12(7):1092. https://doi.org/10.3390/met12071092
Chicago/Turabian StyleSfikas, Athanasios K., Sergio Gonzalez, Angeliki G. Lekatou, Spyros Kamnis, and Alexandros E. Karantzalis. 2022. "A Critical Review on Al-Co Alloys: Fabrication Routes, Microstructural Evolution and Properties" Metals 12, no. 7: 1092. https://doi.org/10.3390/met12071092
APA StyleSfikas, A. K., Gonzalez, S., Lekatou, A. G., Kamnis, S., & Karantzalis, A. E. (2022). A Critical Review on Al-Co Alloys: Fabrication Routes, Microstructural Evolution and Properties. Metals, 12(7), 1092. https://doi.org/10.3390/met12071092








