Extraction of Rare-Earth Elements from Silicate-Based Ore through Hydrometallurgical Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.2.1. Direct Leaching
2.2.2. Dry Digestion and Acid Baking
3. Results and Discussion
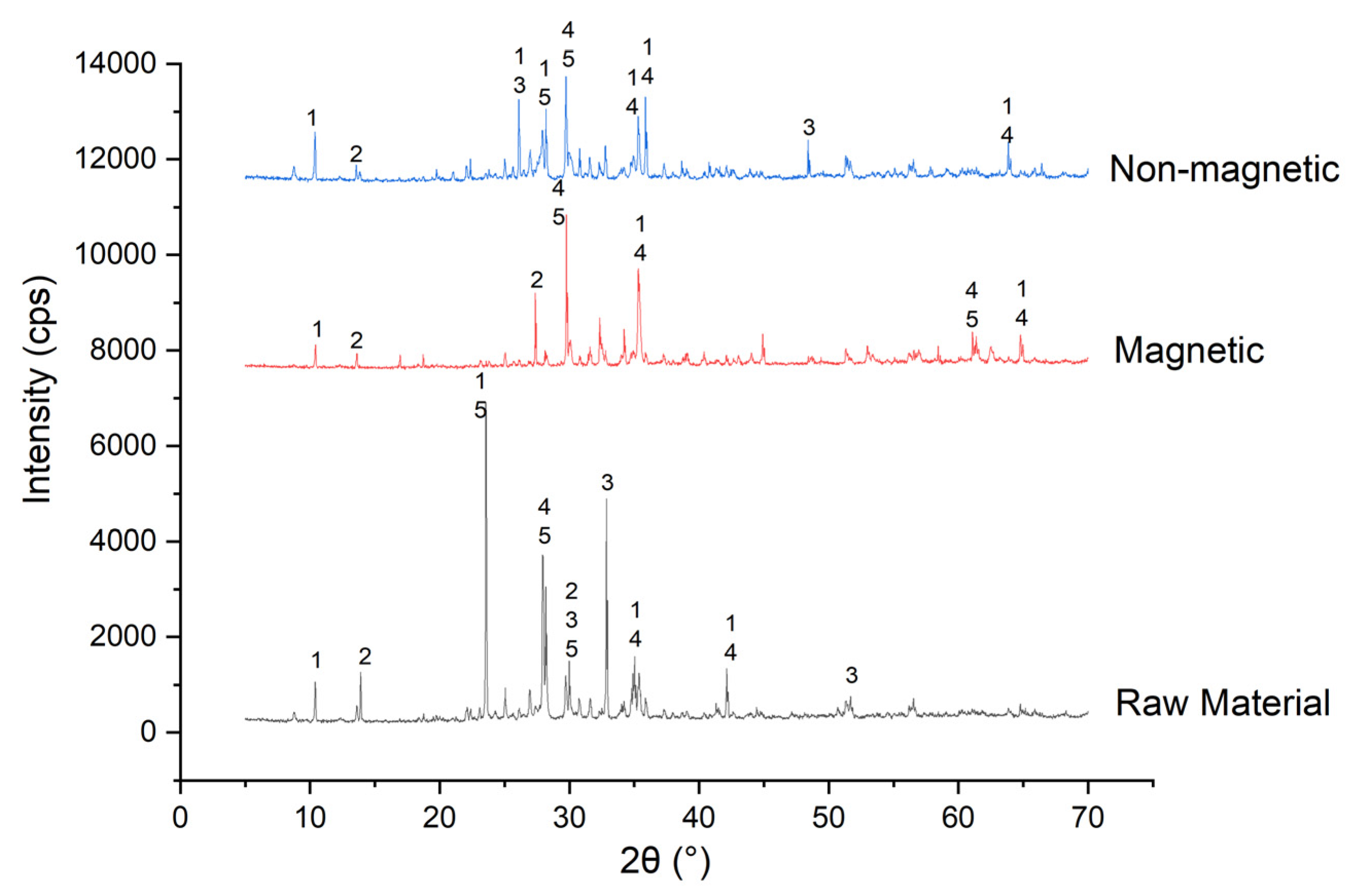
3.1. Characterization and Economic Analysis of Silicate-Based Ore
3.2. Direct Leaching
3.2.1. Effect of the Solid-Liquid Ratio and Acid Concentration
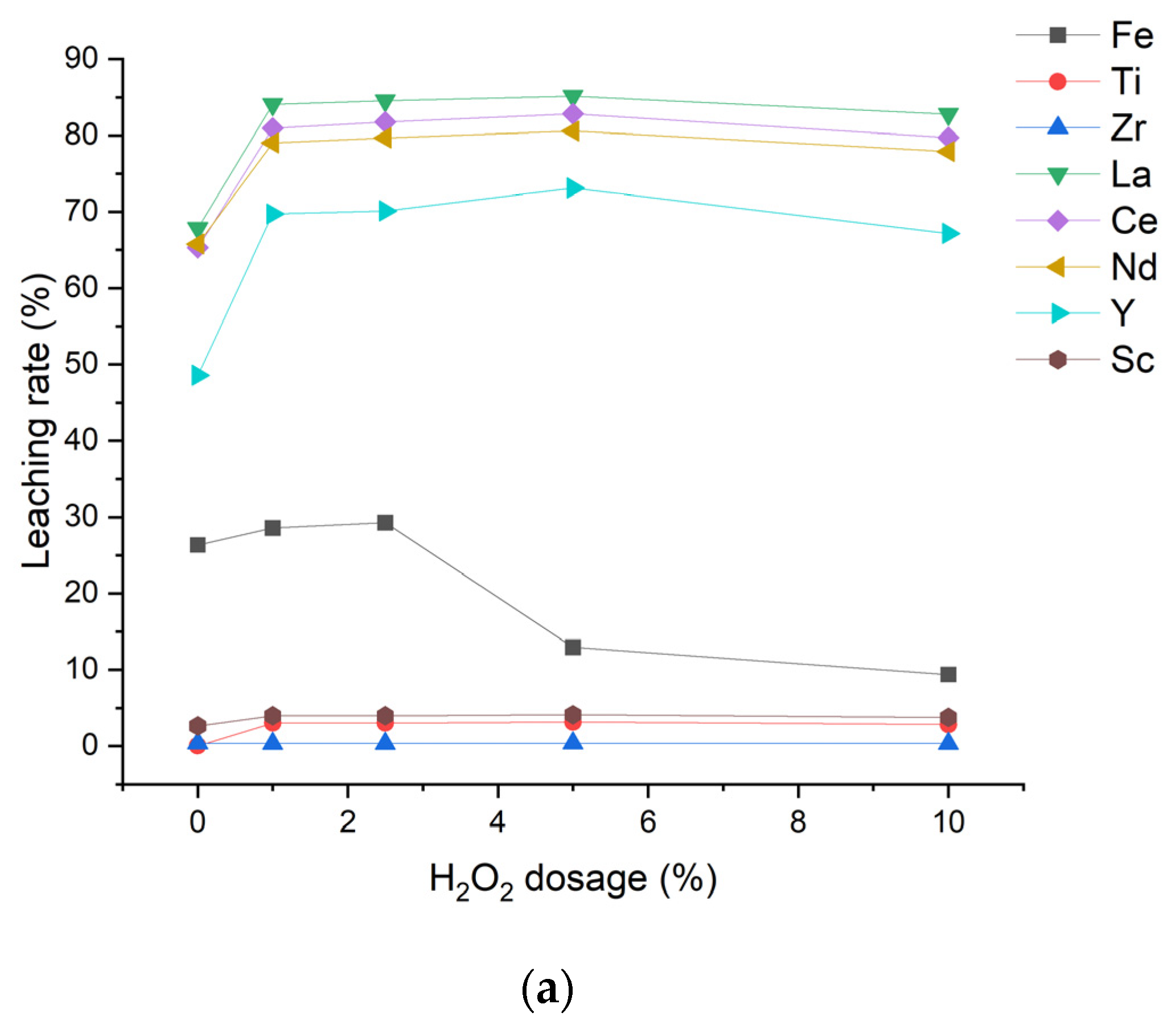
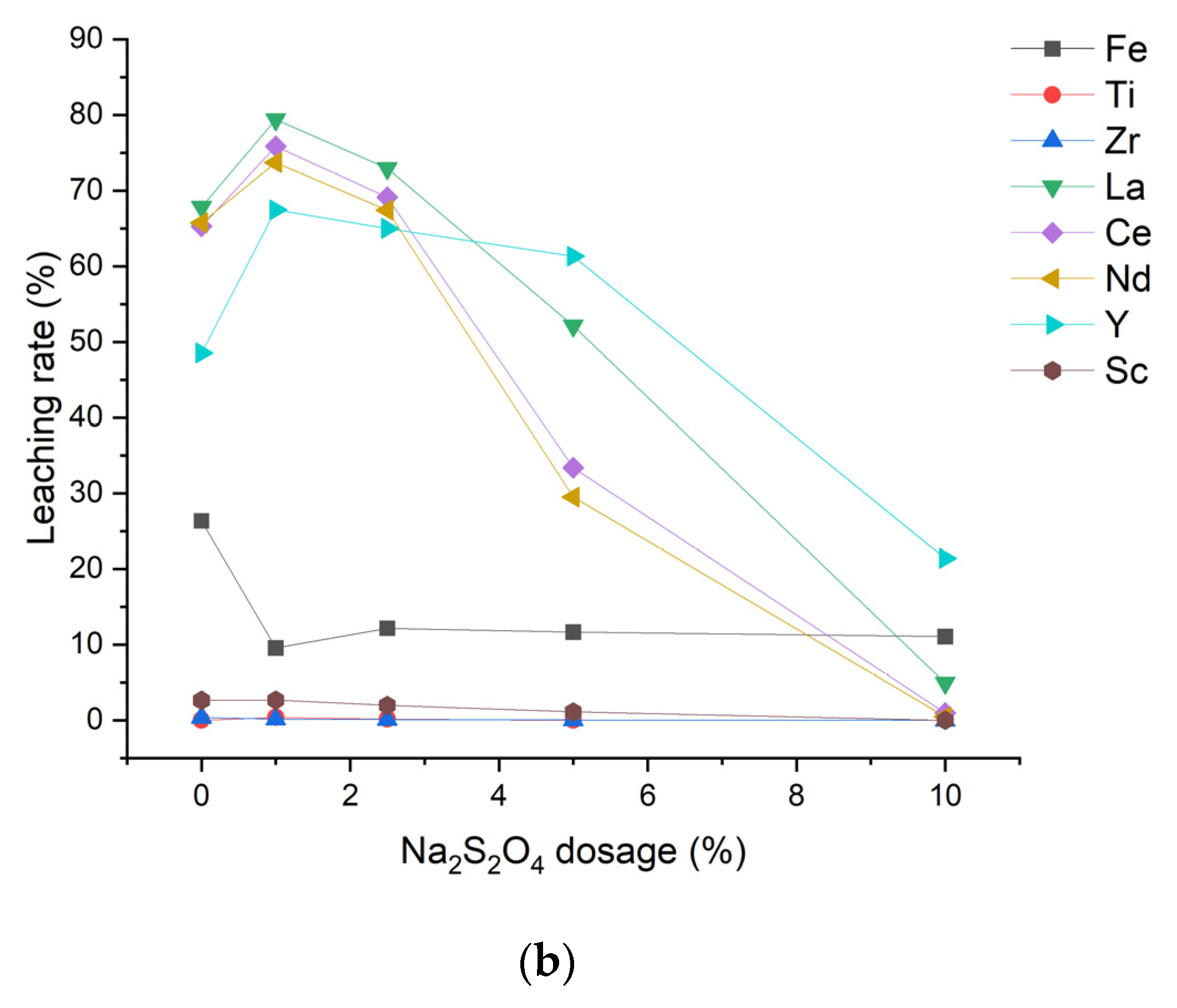
3.2.2. Oxidizing and Reducing Leaching Experiments
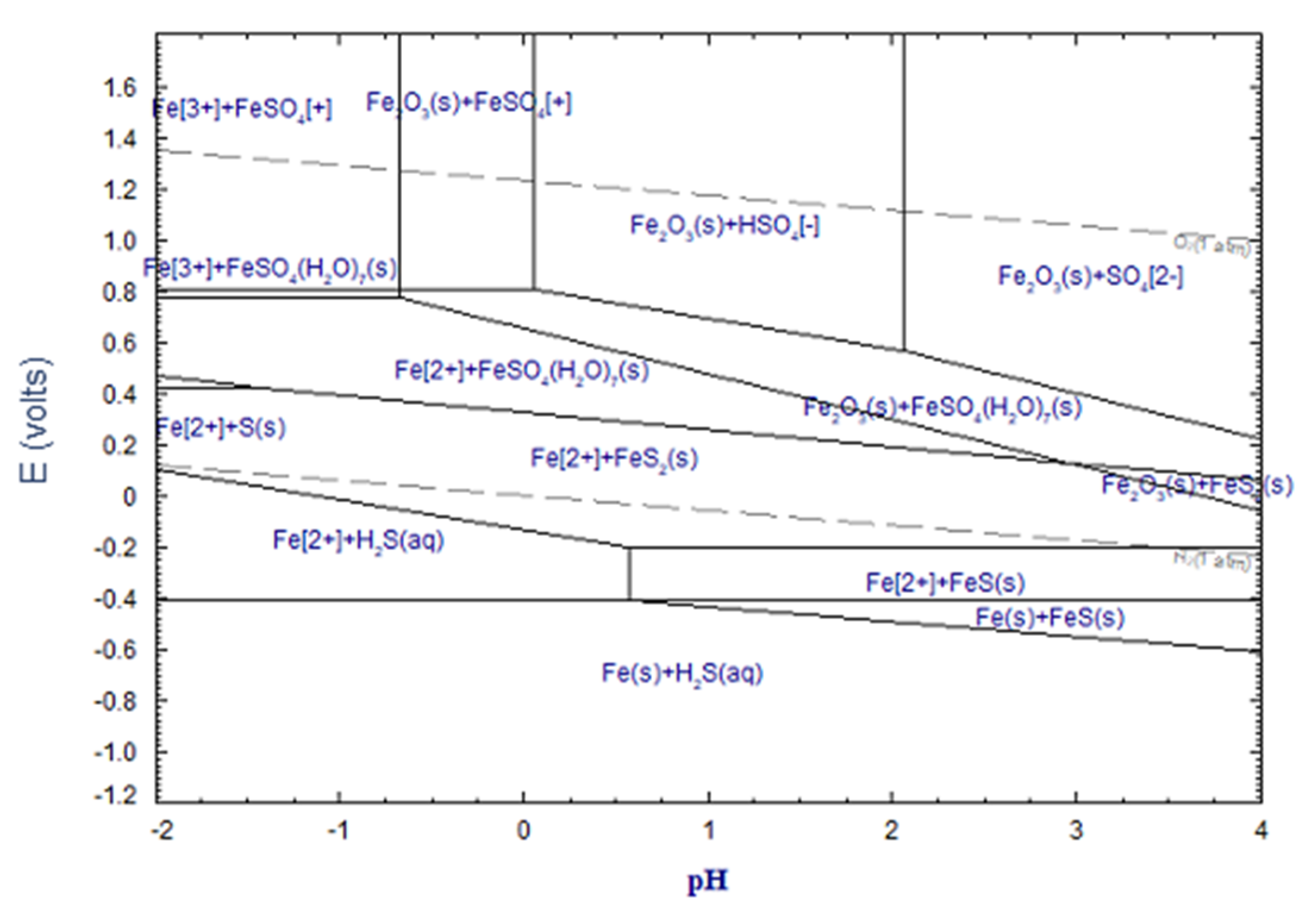
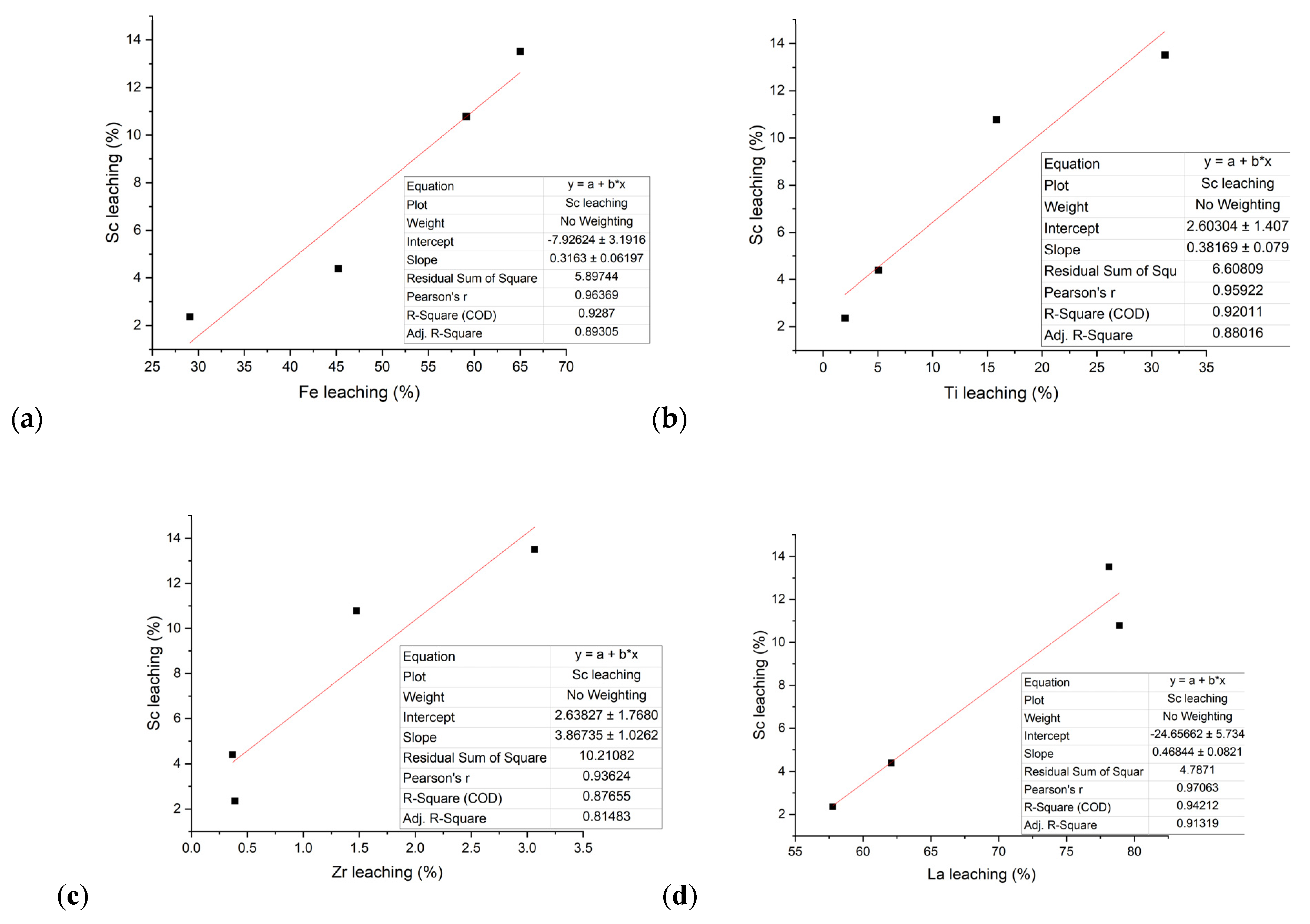
3.2.3. Thermodynamic Experiments
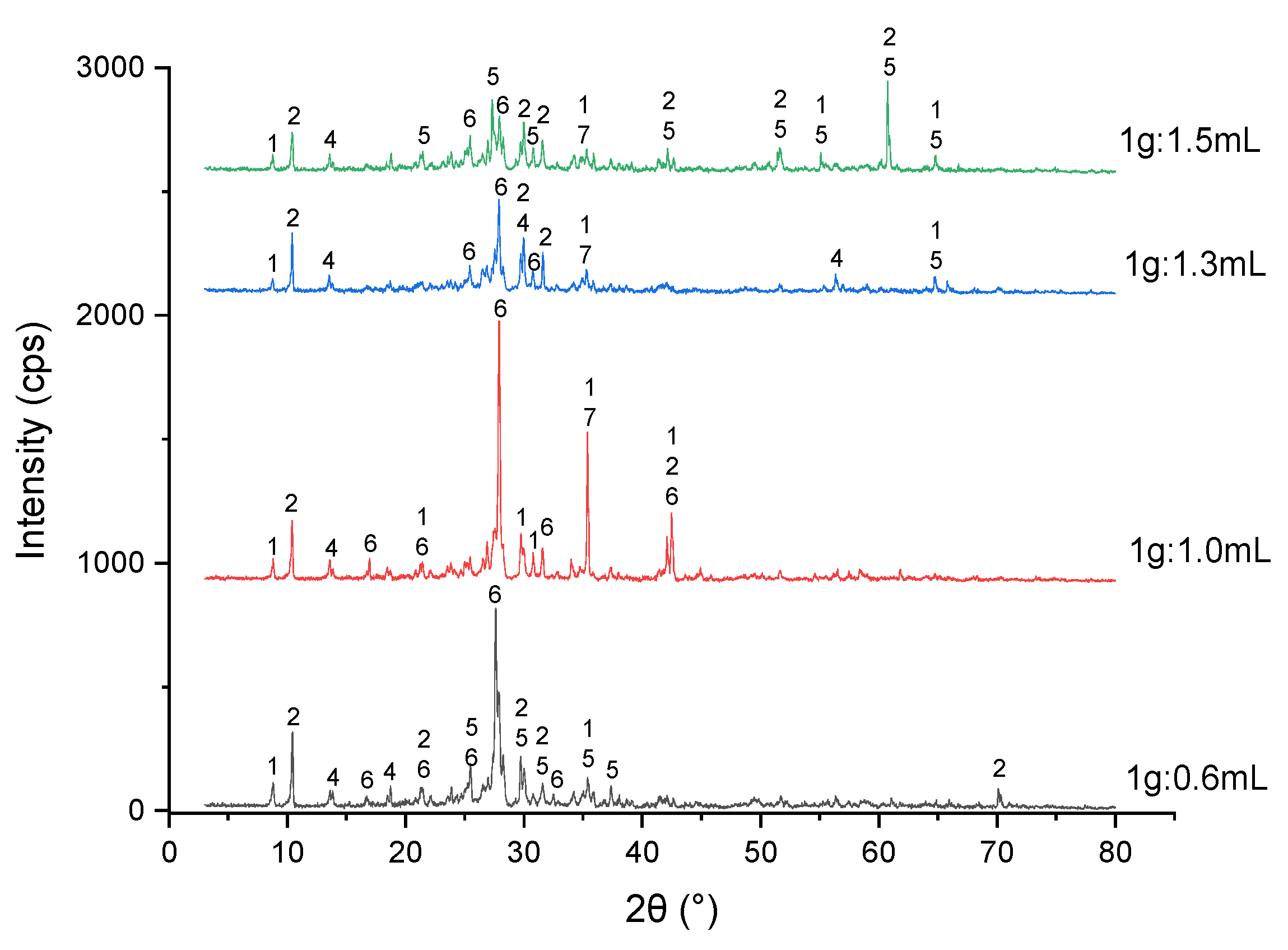
3.3. Dry Digestion and Acid Baking Experiments
- (a)
- Complete destruction of the silicate structure and cation release, generating silica gel;
- (b)
- Partial dissolution of the silicate structure and cation release, which leads the silica compound in the solid phase; and
- (c)
- The acid does not react with the silicate.
3.3.1. Effect of Acid Dosage
3.3.2. Effect of Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4665-7638-4. [Google Scholar]
- Qi, D. Extraction of Rare Earths From RE Concentrates. In Hydrometallurgy of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–185. ISBN 9780128139202. [Google Scholar]
- European Commission 2020 List of Critical Raw Materials for the EU. Available online: https://ec.europa.eu/growth/tools-databases/regional-innovation-monitor/base-profile/berlin (accessed on 3 August 2021).
- Ni’am, A.C.; Wang, Y.F.; Chen, S.W.; You, S.J. Recovery of Rare Earth Elements from Waste Permanent Magnet (WPMs) via Selective Leaching Using the Taguchi Method. J. Taiwan Inst. Chem. Eng. 2019, 97, 137–145. [Google Scholar] [CrossRef]
- Surampally, R.; Batchu, N.K.; Mannepalli, L.K.; Bontha, R.R. Studies on Solvent Extraction of Dy(III) and Separation Possibilities of Rare Earths Using PC-88A from Phosphoric Acid Solutions. J. Taiwan Inst. Chem. Eng. 2012, 43, 839–844. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. The Use of Computational Thermodynamic for Yttrium Recovery from Rare Earth Elements-Bearing Residue. J. Rare Earths 2021, 39, 201–207. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Selective Separation of Sc(III) and Zr(IV) from the Leaching of Bauxite Residue Using Trialkylphosphine Acids, Tertiary Amine, Tri-Butyl Phosphate and Their Mixtures. Sep. Purif. Technol. 2021, 279, 119798. [Google Scholar] [CrossRef]
- Binnemans, K.; McGuiness, P.; Jones, P.T. Rare-Earth Recycling Needs Market Intervention. Nat. Rev. Mater. 2021, 6, 459–461. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.Y.H.; Liu, Z.R.R.; Zhou, M.F. Scandium: Ore Deposits, the Pivotal Role of Magmatic Enrichment and Future Exploration. Ore Geol. Rev. 2021, 128, 103906. [Google Scholar] [CrossRef]
- Levin, L.A.; Amon, D.J.; Lily, H. Challenges to the Sustainability of Deep-Seabed Mining. Nat. Sustain. 2020, 3, 784–794. [Google Scholar] [CrossRef]
- Jyothi, R.K.; Thenepalli, T.; Ahn, J.W.; Parhi, P.K.; Chung, K.W.; Lee, J.Y. Review of Rare Earth Elements Recovery from Secondary Resources for Clean Energy Technologies: Grand Opportunities to Create Wealth from Waste. J. Clean. Prod. 2020, 267, 122048. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Ali, S.H.; Bazilian, M.; Radley, B.; Nemery, B.; Okatz, J.; Mulvaney, D. Sustainable Minerals and Metals for a Low-Carbon Future. Science 2020, 367, 30–33. [Google Scholar] [CrossRef]
- Demol, J.; Ho, E.; Soldenhoff, K.; Senanayake, G. The Sulfuric Acid Bake and Leach Route for Processing of Rare Earth Ores and Concentrates: A Review. Hydrometallurgy 2019, 188, 123–139. [Google Scholar] [CrossRef]
- Gambogi, J. Rare Earths. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-rare-earths.pdf (accessed on 21 March 2021).
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of Rare Earths from Bauxite Residue (Red Mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Kaya, Ş.; Dittrich, C.; Stopic, S.; Friedrich, B. Concentration and Separation of Scandium from Ni Laterite Ore Processing Streams. Metals 2017, 7, 557. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical Processes for Scandium Recovery from Various Resources: A Review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Vaughan, J.; Tenório, J.A.S. Recovery of Scandium from Various Sources: A Critical Review of the State of the Art and Future Prospects. Miner. Eng. 2021, 172, 107148. [Google Scholar] [CrossRef]
- Martins, L.S.; Guimarães, L.F.; Botelho Junior, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Electric Car Battery: An Overview on Global Demand, Recycling and Future Approaches towards Sustainability. J. Environ. Manag. 2021, 295, 113091. [Google Scholar] [CrossRef] [PubMed]
- Talan, D.; Huang, Q. A Review of Environmental Aspect of Rare Earth Element Extraction Processes and Solution Purification Techniques. Miner. Eng. 2022, 179, 107430. [Google Scholar] [CrossRef]
- Chaikin, L.; Shoppert, A.; Valeev, D.; Loginova, I.; Napol’skikh, J. Concentration of Rare Earth Elements (Sc, Y, La, Ce, Nd, Sm) in Bauxite Residue (Red Mud) Obtained by Water and Alkali Leaching of Bauxite Sintering Dust. Minerals 2020, 10, 500. [Google Scholar] [CrossRef]
- Anawati, J.; Azimi, G. Recovery of Scandium from Canadian Bauxite Residue Utilizing Acid Baking Followed by Water Leaching. Waste Manag. 2019, 95, 549–559. [Google Scholar] [CrossRef]
- Kim, J.; Azimi, G. Recovery of Scandium and Neodymium from Blast Furnace Slag Using Acid Baking–Water Leaching. RSC Adv. 2020, 10, 31936–31946. [Google Scholar] [CrossRef]
- Zou, D.; Li, H.; Deng, Y.; Chen, J.; Bai, Y. Recovery of Lanthanum and Cerium from Rare Earth Polishing Powder Wastes Utilizing Acid Baking-Water Leaching-Precipitation Process. Sep. Purif. Technol. 2021, 261, 118244. [Google Scholar] [CrossRef]
- Pan, J.; Hassas, B.V.; Rezaee, M.; Zhou, C.; Pisupati, S.V. Recovery of Rare Earth Elements from Coal Fly Ash through Sequential Chemical Roasting, Water Leaching, and Acid Leaching Processes. J. Clean. Prod. 2021, 284, 124725. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Pinheiro, É.F.; Espinosa, D.C.R.; Tenório, J.A.S.; Baltazar, M.D.P.G. Adsorption of Lanthanum and Cerium on Chelating Ion Exchange Resins: Kinetic and Thermodynamic Studies. Sep. Sci. Technol. 2022, 57, 60–69. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Characterization of Bauxite Residue from a Press Filter System: Comparative Study and Challenges for Scandium Extraction. Min. Metall. Explor. 2021, 38, 161–176. [Google Scholar] [CrossRef]
- Reid, S.; Tam, J.; Yang, M.; Azimi, G. Technospheric Mining of Rare Earth Elements from Bauxite Residue (Red Mud): Process Optimization, Kinetic Investigation, and Microwave Pretreatment. Sci. Rep. 2017, 7, 15252. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, H.; Sun, M.; Zhang, Y.; Meng, X.; Zhang, L.; Lv, X.; Davaasambuu, S.; Qiu, G. Scandium Extraction from Silicates by Hydrometallurgical Process at Normal Pressure and Temperature. J. Mater. Res. Technol. 2020, 9, 709–717. [Google Scholar] [CrossRef]
- Luo, J.; Li, G.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Atmospheric Leaching Characteristics of Nickel and Iron in Limonitic Laterite with Sulfuric Acid in the Presence of Sodium Sulfite. Miner. Eng. 2015, 78, 38–44. [Google Scholar] [CrossRef]
- Han, K.N.; Rubcumintara, T.; Fuerstenau, M.C. Leaching Behavior of Ilmenite with Sulfuric Acid. Metall. Trans. B 1987, 18, 325–330. [Google Scholar] [CrossRef]
- Haverkamp, R.G.; Kruger, D.; Rajashekar, R. The Digestion of New Zealand Ilmenite by Hydrochloric Acid. Hydrometallurgy 2016, 163, 198–203. [Google Scholar] [CrossRef]
- Azimi, G.; Papangelakis, V.G. The Solubility of Gypsum and Anhydrite in Simulated Laterite Pressure Acid Leach Solutions up to 250 °C. Hydrometallurgy 2010, 102, 1–13. [Google Scholar] [CrossRef]
- Han, K.N. Characteristics of Precipitation of Rare Earth Elements with Various Precipitants. Minerals 2020, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel Approach for Enhanced Scandium and Titanium Leaching Efficiency from Bauxite Residue with Suppressed Silica Gel Formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef] [PubMed]
- Čermák, V.; Smutek, M.; Cermak, V.; Smutek, M.; Čermák, V.; Smutek, M. Mechanism of Decomposition of Dithionite in Aqueous Solutions. Collect. Czechoslov. Chem. Commun. 2012, 40, 3241–3264. [Google Scholar] [CrossRef]
- Lister, M.W.; Garvie, R.C. Sodium Dithionite Decomposition in Aqueous Solution and in the Solid State. Can. J. Chem. 1959, 37, 1567–1574. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, S.; Fowler, S.; Raghavan, S.; Fowler, S. Use of Dithionite in the Removal of Nickel from Ammoniacal Solutions. Hydrometallurgy 1983, 11, 125–129. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Jiménez Correa, M.M.; Espinosa, D.C.R.; Tenório, J.A.S. Study of the Reduction Process of Iron in Leachate from Nickel Mining Waste. Braz. J. Chem. Eng. 2018, 35, 1241–1248. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Wen, Y.; Liu, W.; Chu, Y.; Wang, R.; Xu, Z. Leaching Kinetics and Mechanism of Laterite with NH4Cl-HCl Solution. Minerals 2020, 10, 754. [Google Scholar] [CrossRef]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Van Gerven, T.; Marin, R.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Gerven, T. Van Extraction of Rare Earths from Bauxite Residue (Red Mud) by Dry Digestion Followed by Water Leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Terry, B. The Acid Decomposition of Silicate Minerals Part I. Reactivities and Modes of Dissolution of Silicates. Hydrometallurgy 1983, 10, 135–150. [Google Scholar] [CrossRef]








| Variables | Conditions |
|---|---|
| Solid-liquid ratio (S/L) | 1/5–1/50 |
| Concentration | 0.5–4.0 mol/L |
| Na2S2O4 | 1–10% |
| H2O2 | 1–10% |
| Compounds | wt% |
|---|---|
| SiO2 | 39.4 |
| Fe2O3 | 36.3 |
| CaO | 8.79 |
| Al2O3 | 4.61 |
| TiO2 | 2.32 |
| Na2O | 1.64 |
| MgO | 1.44 |
| K2O | 1.42 |
| MnO | 1.09 |
| P2O5 | 0.43 |
| Elements | Concentration (mg/kg) | Economic Value (in US$) |
|---|---|---|
| Zr | 8090 | 6.0% |
| Ce | 5420 | 0.8% |
| La | 2330 | 0.3% |
| Nd | 2140 | 7.3% |
| Y | 1100 | 0.2% |
| Pr | 626 | 3.2% |
| Th | 375 | 1.7% |
| Sm | 358 | 0.1% |
| Gd | 268 | 0.4% |
| Dy | 238 | 2.8% |
| Sc | 191 | 76.8% |
| Magnetic Fraction | Non-Magnetic Fraction | ||
|---|---|---|---|
| Hedenbergite | 14.28% | Phlogopite | 9.14% |
| Hilairite | 1.49% | Hedenbergite | 15.20% |
| Ilmenite | 14.91% | Zirconia | 4.73% |
| Zirconia | 1.45% | Fayalite | 11.81% |
| Fayalite | 10.87% | Diopside | 21.20% |
| Diopside | 31.46% | Hastingsite | 1.65% |
| Ferrohornblende | 10.94% | Albite | 22.02% |
| Magnetite | 14.60% | Ferrohornblende | 14.25% |
| Compounds | Magnetic Fraction | Non-Magnetic Fraction | Elements | Magnetic Fraction | Non-Magnetic Fraction |
|---|---|---|---|---|---|
| SiO2 | 25.6 | 42.3 | Zr | N.D. | 8210 |
| Fe total | 36.54 | 23.14 | Ce | N.D. | 4960 |
| CaO | 7.21 | 9.05 | La | N.D. | 2210 |
| Al2O3 | 1.79 | 4.97 | Nd | N.D. | 2060 |
| TiO2 | 7.36 | 1.48 | Y | N.D. | 995 |
| Pr | N.D. | 565 | |||
| Th | N.D. | 369 | |||
| Sm | N.D. | 339 | |||
| Gd | N.D. | 247 | |||
| Dy | N.D. | 226 | |||
| Sc | 164 | 214 |
| Fe | Ti | Zr | La | Ce | Nd | Y | Sc | |
|---|---|---|---|---|---|---|---|---|
| Activation energy (J/mol) | 11,217.87 | 39,404.73 | 31,617.41 | 4661.09 | 5387.19 | 6830.66 | 4541.61 | 25,448.91 |
| Frequency factor (×10−2) | 3.38 | 5.90 × 10−4 | 9.73 × 10−2 | 26.19 | 19.83 | 12.73 | 29.48 | 0.14 |
| Linear correlation (r2) | 0.8911 | 0.9639 | 0.8492 | 0.788 | 0.7051 | 0.8401 | 0.8663 | 0.9098 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botelho Junior, A.B.; Espinosa, D.C.R.; Vaughan, J.; Tenório, J.A.S. Extraction of Rare-Earth Elements from Silicate-Based Ore through Hydrometallurgical Route. Metals 2022, 12, 1133. https://doi.org/10.3390/met12071133
Botelho Junior AB, Espinosa DCR, Vaughan J, Tenório JAS. Extraction of Rare-Earth Elements from Silicate-Based Ore through Hydrometallurgical Route. Metals. 2022; 12(7):1133. https://doi.org/10.3390/met12071133
Chicago/Turabian StyleBotelho Junior, Amilton Barbosa, Denise Crocce Romano Espinosa, James Vaughan, and Jorge Alberto Soares Tenório. 2022. "Extraction of Rare-Earth Elements from Silicate-Based Ore through Hydrometallurgical Route" Metals 12, no. 7: 1133. https://doi.org/10.3390/met12071133








