Phase-Field Simulation of Spinodal Decomposition in Mn-Cu Alloys
Abstract
:1. Introduction
2. Numerical Formulation
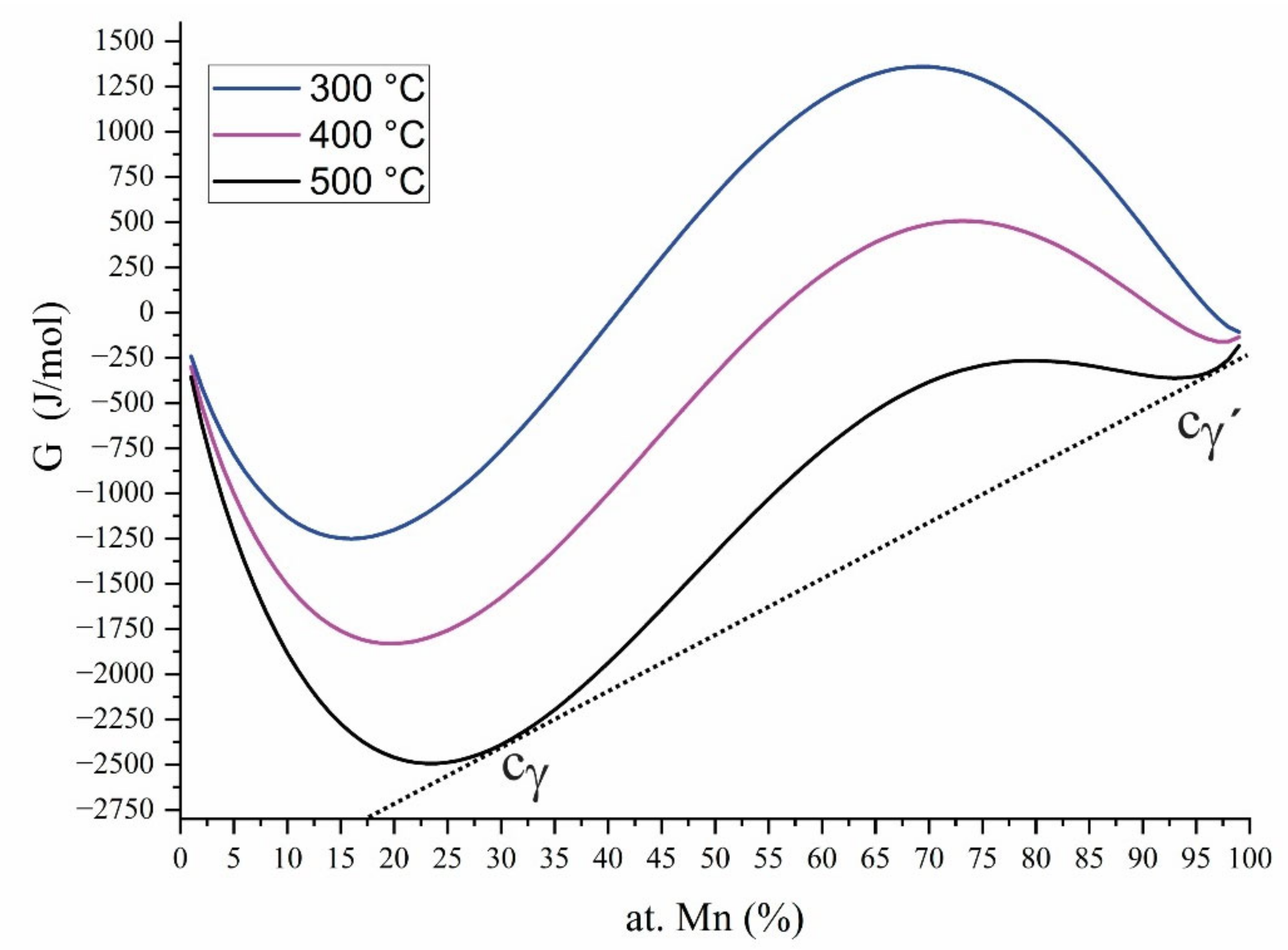
2.1. Thermodynamic Equilibrium
2.2. Elastic Strain Energy
2.3. Phase-Field Model
2.4. Simulation Conditions
3. Results
3.1. Evolution of Calculated Profiles
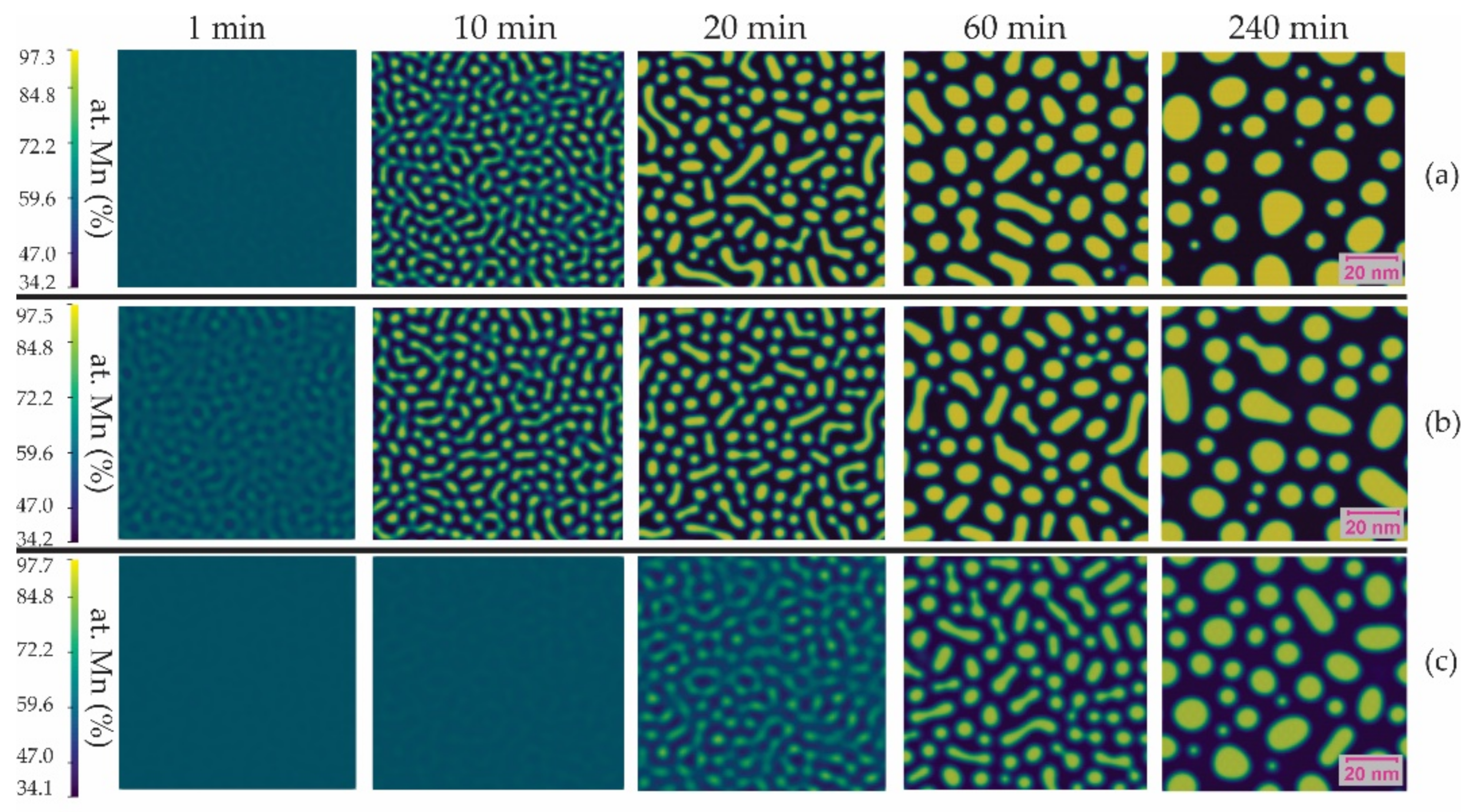
3.2. Microstructural Evolution
4. Discussion
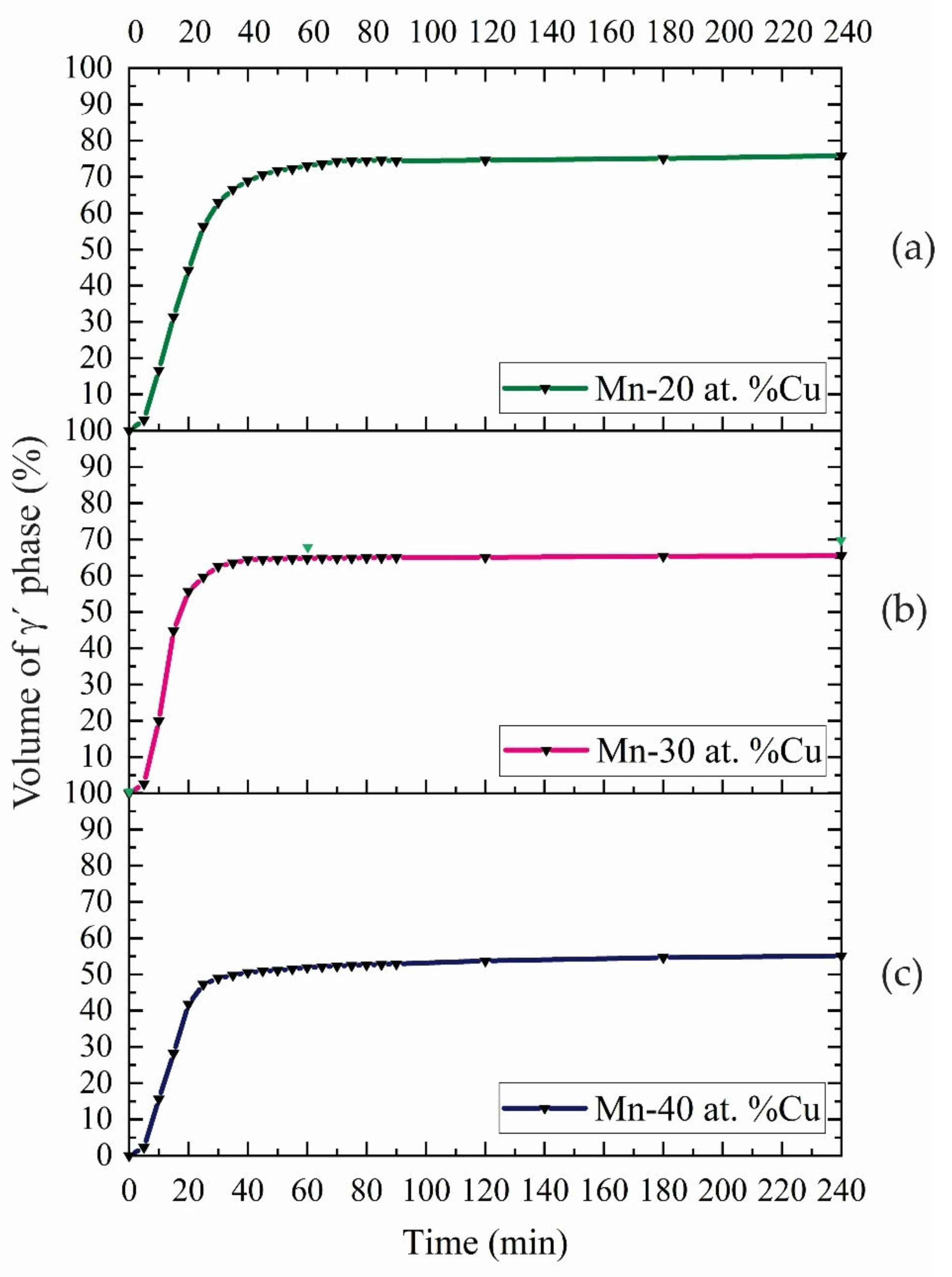
4.1. Growth Kinetics
4.2. Contribution of the Spinodal Decomposition to Mn-Cu Alloys
5. Conclusions
- The calculated Cu concentration profile confirmed the presence of spinodal decomposition at the early stages of aging because of the increase in the amplitude of modulation composition.
- The morphology of the decomposed phases was interconnected and of irregular shape, designated as percolated in the Cahn–Hilliard theory of spinodal decomposition. The growth kinetics of spinodal decomposition was slow at the early stages of aging.
- The rate of growth of spinodal decomposition was faster for the Mn-20 and 30 at. %Cu alloys than for Mn-40 at. %Cu during aging at 500 °C because of its higher driving force.
- The growth kinetics of phase decomposition increased with the decrease in aging temperature because of a reduction in driving force.
- The size of the decomposed phases increased with the aging temperature. The coarsening of the decomposed phases was a diffusion-controlled process despite the nanometric size.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, D. High Damping Metals for Engineering Applications. Mater. Sci. Eng. 1969, 4, 1–8. [Google Scholar] [CrossRef]
- Zuo, S.; Xiao, F.; Fukuda, T. Orientation dependence of damping behavior in a Mn-Cu shape memory alloy. Scr. Mater. 2019, 170, 95–98. [Google Scholar] [CrossRef]
- Sun, L.; Vasin, R.; Islamov, A.; Bobrikov, I.; Cifre, J.; Golovin, I.; Balagurov, A. Influence of spinodal decomposition on structure and thermoelastic martensitic transition in MnCuAlNi alloy. Mater. Lett. 2020, 275, 128069. [Google Scholar] [CrossRef]
- Lewin, K.; Sichen, D. Thermodynamic Study of the Cu-Mn system. Scand. J. Metall. 1993, 22, 310–316. [Google Scholar]
- Wang, C.; Liu, X.; Ohnuma, I.; Kainuma, R.; Ishida, K. Thermodynamic assessments of the Cu-Mn-X (X:Fe, Co) systems. J. Alloy. Compd. 2007, 438, 129–141. [Google Scholar] [CrossRef]
- Vitek, J.M.; Warlimont, H. On a metastable miscibility gap in γ-Mn-Cu alloys and the origin of their high damping capacity. Mater. Sci. 1976, 10, 7–13. [Google Scholar] [CrossRef]
- Smith, J.H.; Vance, E.R. Decomposition of Gamma-Phase Manganese Copper Alloys. J. Appl. Phys. 1969, 40, 4853–4858. [Google Scholar] [CrossRef]
- Gokcen, N.A. The Cu-Mn (Copper-Manganese) System. J. Phase Equilibria 1993, 14, 76–83. [Google Scholar] [CrossRef]
- Cowlam, N. Structural aspect of decomposition of metastable γ-Mn-Cu alloys. Met. Sci. 1978, 12, 483–489. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Peng, L.-M.; Zeng, X.-Q.; Ding, W.-J. Effects of Cu and Mn on mechanical properties and damping capacity of Mg-Cu-Mn alloy. Trans. Nonferrous Met. Soc. China 2008, 18, s55–s58. [Google Scholar] [CrossRef]
- Findik, F. Improvements in spinodal alloys from past to present. Mater. Des. 2012, 42, 131–146. [Google Scholar] [CrossRef]
- Shi, S.; Liu, C.; Wan, J.; Rong, Y.; Zhang, J. Thermodynamics of fcc-fct martensitic transformation in Mn-X(X=Cu,Fe) alloys. Mater. Des. 2016, 92, 960–970. [Google Scholar] [CrossRef]
- Turchanin, M.; Nikolaenko, I.V. Entalpies of Formation of Liquid (Copper+Manganese) Alloys. Metall. Mater. Trans. B 1977, 28, 470–473. [Google Scholar] [CrossRef]
- Velazquez, D.; Romero, R. Spinodal decomposition and martensitic transformation in Cu-Al-Mn Shape memory alloy. J. Therm. Anal. Calorim. 2017, 130, 2007–2013. [Google Scholar] [CrossRef]
- Jiazhen, Y.; Ning, L.; Xu, F.; Ying, Z. The strengthening effect of spinodal decomposition and twinning structure in MnCu-based alloy. Mater. Sci. Eng. A 2014, 618, 205–209. [Google Scholar] [CrossRef]
- Sato, K.; Stobbs, W.M. Quantification of the spinodal wave in Cu2.5Mn0.5Al by dark-field image analysis. Philos. Mag. A 1994, 69, 349–377. [Google Scholar] [CrossRef]
- Saito, Y.; Suwa, Y.; Ochi, K.; Aoki, T.; Goto, K.; Abe, K. Kinetics of Phase Separation in Ternary Alloys. J. Phys. Soc. Jpn. 2022, 71, 808–812. [Google Scholar] [CrossRef]
- Soriano-Vargas, O.; Avila-Davila, E.O.; Lopez-Hirata, V.M.; Cayetano-Castro, N.; Gonzalez-Velazquez, J.L. Effect of Spinodal decomposition on the mechanical behavior of Fe-Cr alloys. Mater. Sci. Eng. A 2010, 527, 2910–2914. [Google Scholar] [CrossRef]
- Avila-Davila, E.O.; Lopez-Hirata, V.; Saucedo-Muñoz, M.L.; Gonzalez-Velazquez, J.L. Microstructural simulation of phase decomposition in Cu–Ni alloys. J. Alloy. Compd. 2008, 460, 206–212. [Google Scholar] [CrossRef]
- Zhang, S.; Kumar, R. Effects of the local order parameter dependent transport coefficient in deblock copolymers under applied electric fields. J. Chem. Phys. 2022, 156, 174903. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, W.; Tonks, M.R. Assessment of four strain energy decomposition methods for phase field fracture models using quasi-static and dynamic benchmark cases. Mater. Theory 2022, 6, 6. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, D.-U.; Jiang, W.; Tonk, M.R. A phase field model of crack propagation in anisotropic brittle materials with preferred fracture planes. Comput. Mater. Sci. 2021, 193, 1104000. [Google Scholar] [CrossRef]
- Rezaei, Y.; Jafari, M.; Jamshidian, M. Phase-field modeling of magnetic field-induced grain growth in polycrystalline metals. Comput. Mater. Sci. 2021, 200, 110786. [Google Scholar] [CrossRef]
- Miyoshi, E.; Takaki, T.; Sakane, S.; Ohno, M.; Shibuta, Y.; Aoki, T. Large-scale phase-field study of anisotropic grain growth: Effects of misorientation-dependent grain boundary energy and mobility. Comput. Mater. Sci. 2021, 186, 109992. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, D.C.; Pi, Z.P.; Lin, J.G.; Wen, C. Phase field simulation of spinodal decomposition in Zr–Nb alloys for implant materials. J. Appl. Phys. 2019, 126, 085102. [Google Scholar] [CrossRef]
- Avila-Davila, E.O.; Lopez-Hirata, V.M.; Saucedo-Muñoz, M.L. Application of Phase-Field Method to the Analysis of Phase Decomposition of Alloys. In Modeling and Simulation in Engineering Sciences; InTechOpen: Vienna, Austria, 2015; pp. 221–242. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-Q. Computer Simulation of Spinodal Decomposition in Ternary Systems. Acta Met. Mater. 1994, 42, 3503–3513. [Google Scholar] [CrossRef]
- Koyama, T.; Hashimoto, K.; Onodera, H. Phase-Field Simulation of Phase Transformation in Fe-Cu-Mn-Ni Quaternary Alloy. Mater. Trans. 2006, 47, 2765–2772. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Jing, T.; Jie, W.; Liu, B. Phase-field simulation of solidification in multicomponent alloys coupled with thermodynamic and diffusion mobility. Acta Mater. 2006, 54, 2235–2239. [Google Scholar] [CrossRef]
- Kim, D.-C.; Ogura, T.; Hamada, R.; Yamashita, S.; Saida, K. Establishment of a theoretical model on the phase-field method for predicting the γ phase precipitation in Fe-Cr-Ni ternary alloys. Mater. Today Commun. 2021, 26, 101932. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Enomoto, M.; Lee, J.K. Mechanisms of Diffusional Phase Transformations in Metals and Alloys; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–47. [Google Scholar]
- Kostorz, G. Phase Transformations in Materials; Wiley-VCH: Weinheim, Germany, 2001; pp. 409–480. [Google Scholar]
- Dinsdale, A. SGTE Data for pure elements. CALPHAD 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic Representation of Thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 1948, 40, 345–348. [Google Scholar] [CrossRef]
- Hillert, M.; Jarl, M. A model for alloying effects in ferromagnetic metals. CALPHAD 1978, 2, 227–238. [Google Scholar] [CrossRef]
- Lukas, H.; Fries, S.; Sundman, B. Computational Thermodynamics: The Calphad Method; Cambridge University Press: Cambridge, UK, 2007; pp. 79–160. [Google Scholar]
- He, C.; Du, Y.; Chen, H.-L.; Liu, S.; Xu, H.; Ouyang, Y.; Liu, Z.-K. Thermodynamic modeling of the Cu-Mn system supported by key experiments. J. Alloy. Compd. 2008, 457, 233–238. [Google Scholar] [CrossRef]
- Kim, S.; Sanders, T.H. Thermodynamic Modeling of the Miscibility Gaps and the Metastable Liquid in the Zn–Bi, Zn–Pb, and Zn–Tl systems. Z. Met. 2003, 94, 390–395. [Google Scholar] [CrossRef]
- Neumann, G. Self-Difussion and Impurity Difussion in Pure Metals: Handbook of Experimental Data, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 37–91. [Google Scholar]
- Pearson, W. A Handbook of Lattice Spacings and Structures of Metals and Alloys, 2nd ed.; Pergamon Press: Oxford, UK, 1958; Volume 4, pp. 3–113. [Google Scholar]
- Simmons, G.; Wang, H. Single Crystal Elastic Constants and Calculated Aggregate Properties: A Handbook, 1st ed.; The Massachusetts Institute of Technology: Cambridge, MA, USA, 1971. [Google Scholar]
- Cahn, J.W.; Hilliard, J.E. Free Energy of a Nonuniform System. I. Interfacial Free Energy. J. Chem. Phys. 1958, 28, 258–267. [Google Scholar] [CrossRef]
- Lifshitz, I.; Slyozov, V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids J. Phys. Chem. Solids. 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Dieter, G. Mechanical Metallurgy, 3rd ed.; McGraw-Hill: New York, NY, USA, 1986; pp. 212–219. [Google Scholar]
- Corti, C. The evaluation of the Interparticle Spacing in Dispersion Alloys. Int. Metall. Rev. 1974, 19, 77–88. [Google Scholar] [CrossRef]







| Parameter | FCC_A1 γ-Phase | Reference |
|---|---|---|
J/mol | −7770.458 + 130.485235 T − 24.112392 T ln(T) − 2.65684 × 10−3 T2 + 0.129223 × 106 T3 + 52,478 T−1 | [33] |
J/mol | −3439.3 + 131.884 T − 24.5177 T ln (T) − 6 × 10−3 T2 + 69,600 T−1 + | |
| 540/(−3) | ||
| 0.62/(−3) | ||
| 20,235.508 − 13.2437 T | [37] | |
| −12,154.853 + 2.9399 T | ||
| Diffusion coefficient m2s−1 | [28,39] | |
| Q J/mol | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigala-García, D.A.; López-Hirata, V.M.; Saucedo-Muñoz, M.L.; Dorantes-Rosales, H.J.; Villegas-Cárdenas, J.D. Phase-Field Simulation of Spinodal Decomposition in Mn-Cu Alloys. Metals 2022, 12, 1220. https://doi.org/10.3390/met12071220
Sigala-García DA, López-Hirata VM, Saucedo-Muñoz ML, Dorantes-Rosales HJ, Villegas-Cárdenas JD. Phase-Field Simulation of Spinodal Decomposition in Mn-Cu Alloys. Metals. 2022; 12(7):1220. https://doi.org/10.3390/met12071220
Chicago/Turabian StyleSigala-García, Darío A., Víctor M. López-Hirata, Maribel L. Saucedo-Muñoz, Héctor J. Dorantes-Rosales, and José D. Villegas-Cárdenas. 2022. "Phase-Field Simulation of Spinodal Decomposition in Mn-Cu Alloys" Metals 12, no. 7: 1220. https://doi.org/10.3390/met12071220
APA StyleSigala-García, D. A., López-Hirata, V. M., Saucedo-Muñoz, M. L., Dorantes-Rosales, H. J., & Villegas-Cárdenas, J. D. (2022). Phase-Field Simulation of Spinodal Decomposition in Mn-Cu Alloys. Metals, 12(7), 1220. https://doi.org/10.3390/met12071220






