Study of the Effect of Fluxing Ability of Flux Ores on Minimizing of Copper Losses with Slags during Copper Concentrate Smelting
Abstract
:1. Introduction
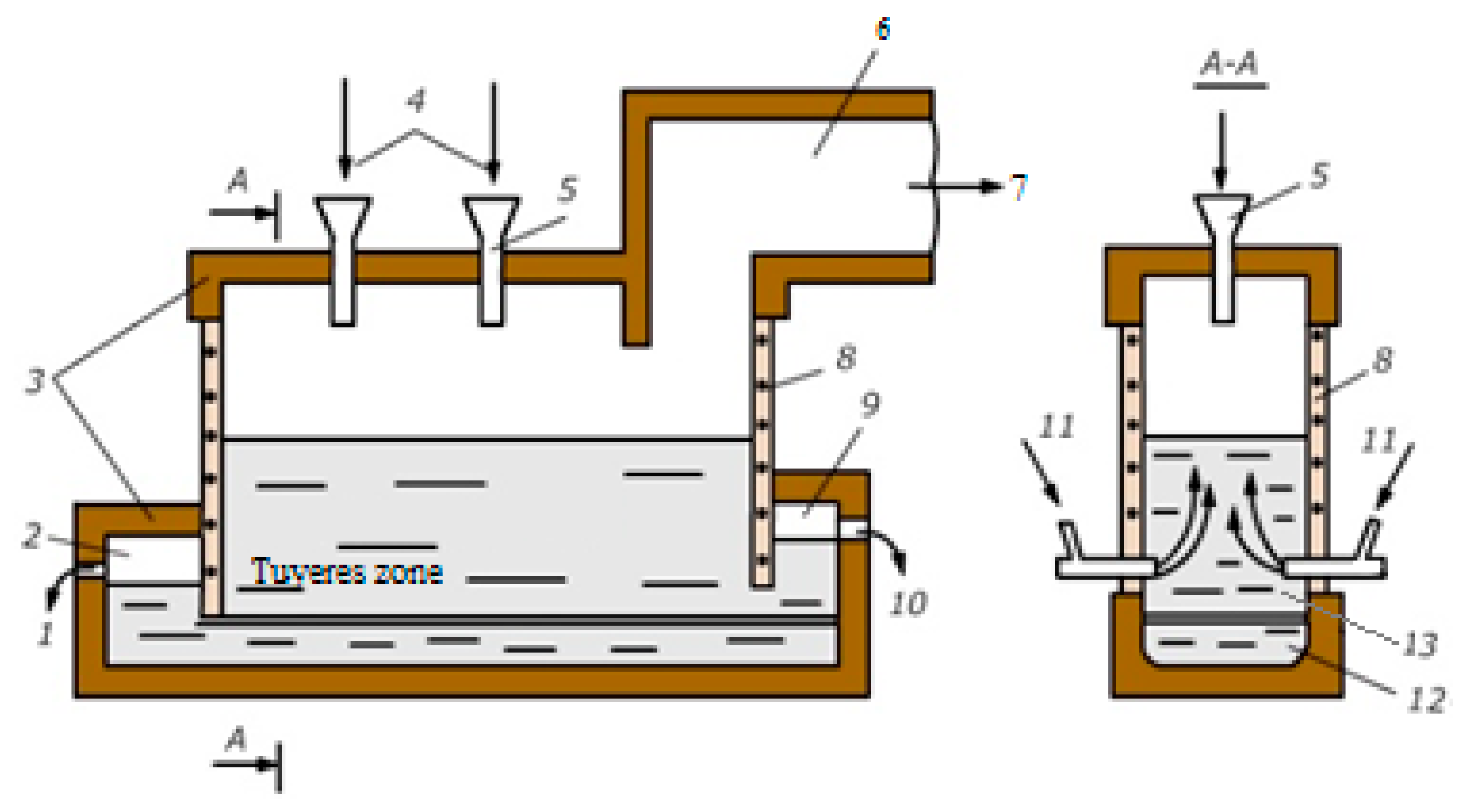
2. Materials and Methods
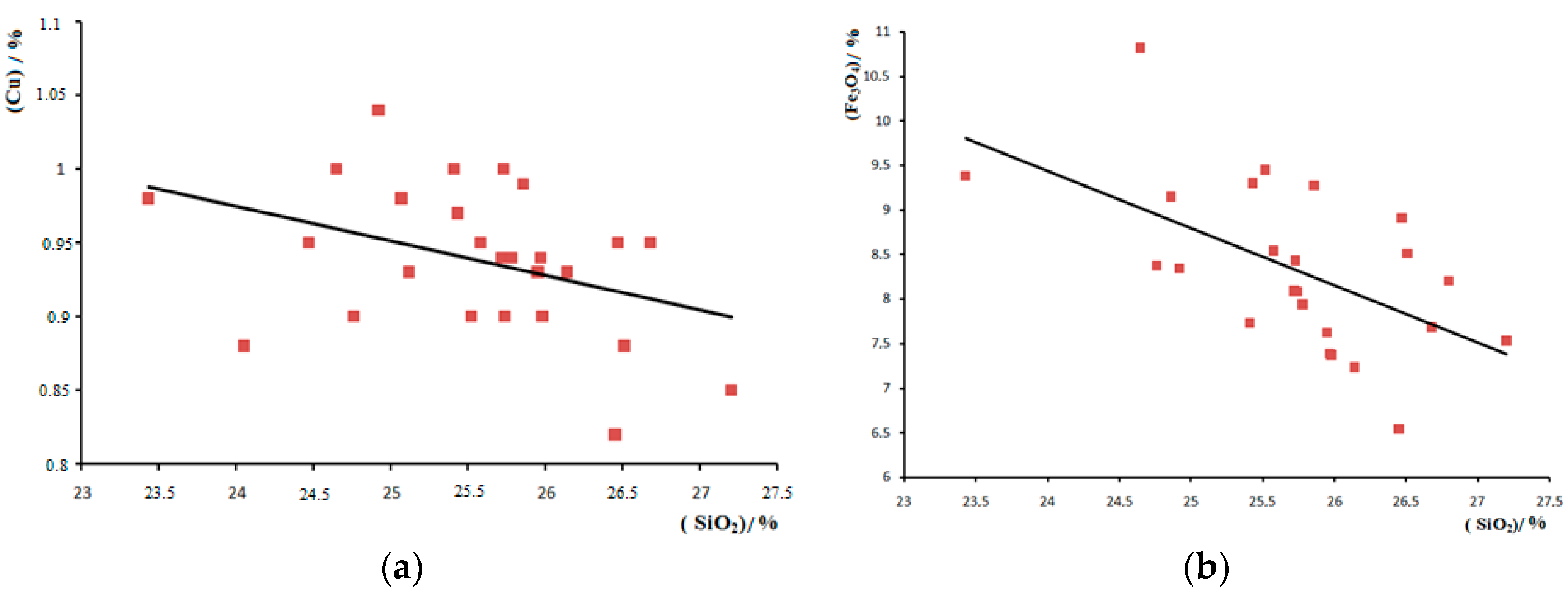
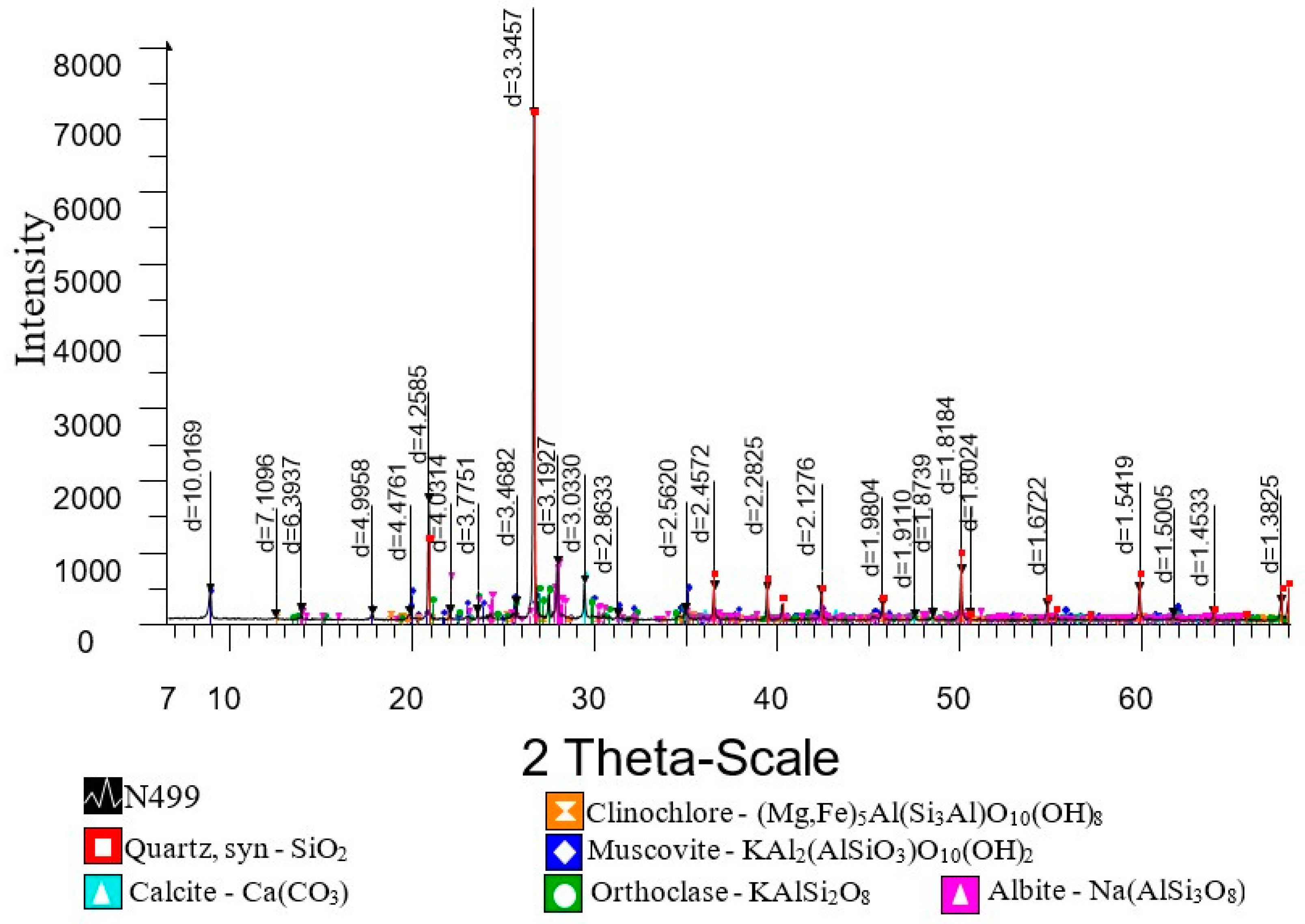
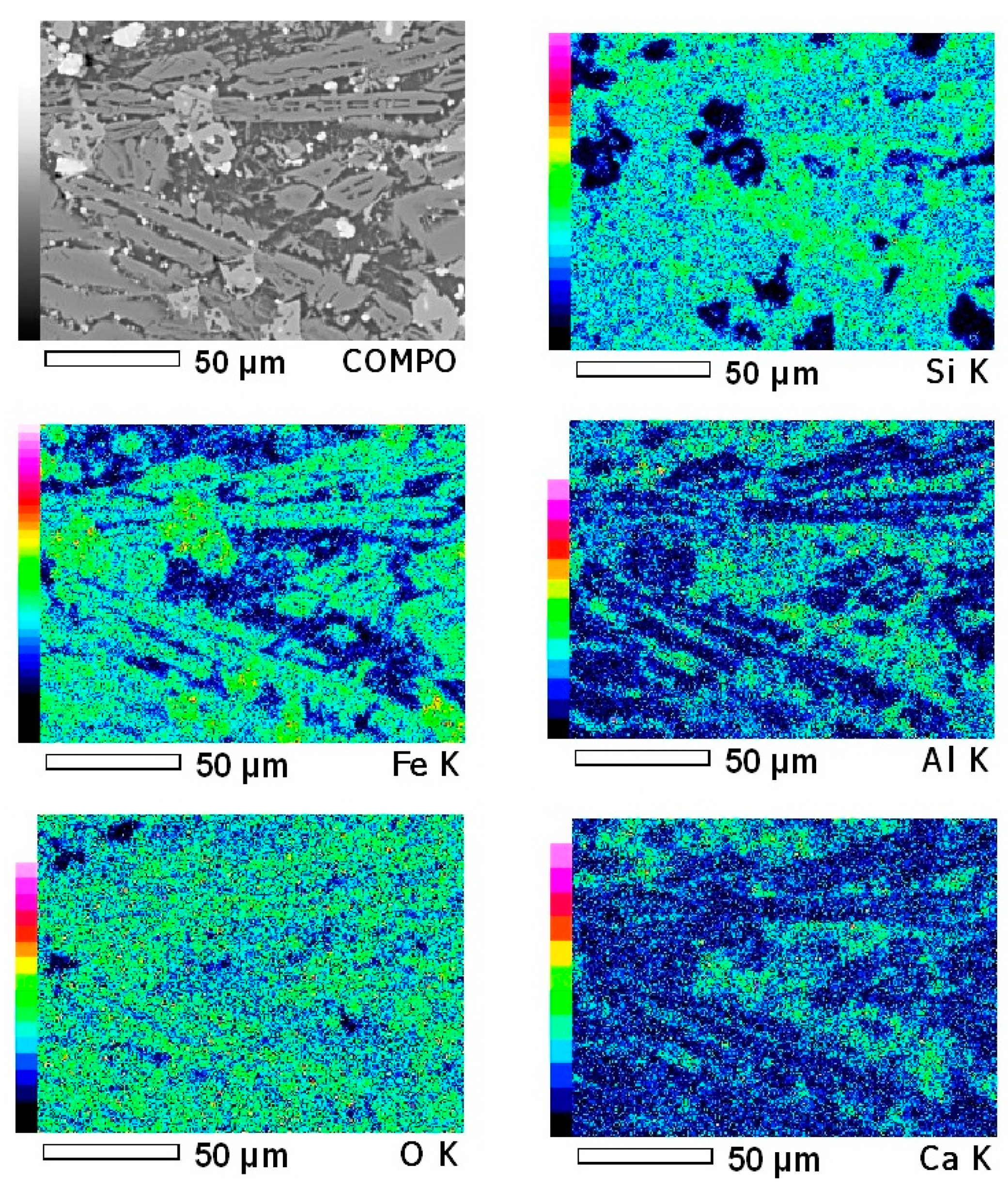
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gorai, B.; Jana, R. Characteristics and utilization of copper slag—A review. Resour. Conserv. Recycl. 2003, 39, 299–313. [Google Scholar] [CrossRef]
- Alp, I.; Deveci, H.; Sungun, H. Utilization of flotation wastes of copper slag as raw material in cement production. J. Hazard. Mater. 2008, 159, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, G.; Zhang, X.; Li, J. Characteristics and chemical speciation of waste copper slag. Environ. Sci. Pollut. Res. 2021, 28, 20012–20022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, G.; Zhang, L.; Zhou, C. Mineralogical and morphological factors affecting the separation of copper and arsenic in flash copper smelting slag flotation beneficiation process. J. Hazard. Mater. 2021, 401, 123293. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, A.; Takasaki, Y.; William, T.; Yamatodani, A.; Higuchi, Y.; Sunagawa, S.; Ono, E. Treatment of smelting residue for arsenic removal and recovery of copper using pyro–hydrometallurgical process. J. Hazard. Mater. 2010, 181, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, N.; Mao, F.; Zhang, J.; Huang, X.; Li, F.; Li, X.; Wu, P.; Dang, Z. A novel strategy for harmlessness and reduction of copper smelting slags by alkali disaggregation of fayalite (Fe2SiO4) coupling with acid leaching. J. Hazard. Mater. 2020, 402, 123791. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y.; Wan, J.; Gong, X.; Zhu, Y. Degradation of atrazine by a novel Fenton-like process and assessment the influence on the treated soil. J. Hazard. Mater. 2016, 312, 184–191. [Google Scholar] [CrossRef]
- Li, S.; Pan, J.; Zhu, D.; Guo, Z.; Xu, J.; Chou, J. A novel process to upgrade the copper slag by direct reduction-magnetic separation with the addition of Na2CO3 and CaO. Powder Technol. 2019, 347, 159–169. [Google Scholar] [CrossRef]
- Guo, Z.; Pan, J.; Zhu, D.; Zhang, F. Innovative methodology for comprehensive and harmless utilization of waste copper slag via selective reduction-magnetic separation process. J. Clean. Prod. 2018, 187, 910–922. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Dimitrijevic, M.D.; Stevanovic, Z.O.; Serbula, S.M.; Bogdanovic, G.D. Investigation of the possibility of copper recovery from the flotation tailings by acid leaching. J. Hazard. Mater. 2008, 158, 23–34. [Google Scholar] [CrossRef]
- Yang, Z.; Rui-lin, M.; Wang-dong, N.; Hui, W. Selective leaching of base metals from copper smelter slag. Hydrometallurgy 2010, 103, 25–29. [Google Scholar] [CrossRef]
- Li, Y.; Perederiy, I.; Papangelakis, V.G. Cleaning of waste smelter slags and recovery of valuable metals by pressure oxidative leaching. J. Hazard. Mater. 2008, 152, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Kenzhaliev, B.K.; Kvyatkovsky, S.A.; Kozhakhmetov, S.M.; Sokolovskaya, L.V.; Semenova, A.S. Depletion of waste slag of Bakkhash copper smelter. Kompleks. Ispolz. Miner. Syra 2018, 306, 45–53. [Google Scholar] [CrossRef]
- Coursol, P.; Valencia, N.C.; Macrey, P.; Bell, S.; Davis, B. Minimization of Copper losses in Copper Smelting Slag During Electric Furnace Treatment. JOM 2012, 64, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Guo, Z.; Pan, J.; Zhu, D.; Yang, C.; Xue, Y.; Li, S.; Wang, D. Comprehensive review on metallurgical recycling and cleaning of copper slag (Review). Resour. Conserv. Recycl. 2021, 168, 105366. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, S.; Rao, D.S.; Pradhan, N.; Mohapatra, U.; Angadi, S.; Mishra, B.K. Extraction of copper from copper slag: Mineralogical insights, physical beneficiation and bioleaching studies. Korean J. Chem. Eng. 2015, 32, 667–676. [Google Scholar] [CrossRef]
- Heo, J.H.; Kim, B.S.; Park, J.H. Erratum to: Effect of CaO addition on iron recovery from copper smelting slags by solid carbon. Metall. Mater. Trans. B 2013, 44, 1352–1363. [Google Scholar] [CrossRef]
- Guo, Z.; Pan, J.; Zhu, D.; Congcong, Y. Mechanism of composite additive in promoting reduction of copper slag to produce direct reduction iron for weathering resistant steel. Powder Technol. 2018, 329, 55–64. [Google Scholar] [CrossRef]
- Sarfo, P.; Wyss, G.; Ma, G.; Das, A.; Young, C. Carbothermal reduction of copper smelter slag for recycling into pig iron and glass. Miner. Eng. 2017, 107, 8–19. [Google Scholar] [CrossRef]
- Bellemans, I.; De Wilde, E.; Moelans, N.; Verbeken, K. Metal losses in pyrometallurgical operations—A review. Adv. Colloid Interface Sci. 2018, 255, 47–63. [Google Scholar] [CrossRef]
- Kenzhaliev, B.K.; Kvyatkovskii, S.A.; Kozhakhmetov, S.M.; Kenzhaliev, É.B.; Semenova, A.S. Determination of Optimum Production Parameters for Depletion of Balkhash Copper-Smelting Plant Dump Slags. Metallurgist 2019, 63, 759–765. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E. An overview of recovery of metals from slag. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Yannopoulos, J. Control of copper losses in reverberatory slags—A literature review. Can. Metall. Q. 1970, 10, 291–307. [Google Scholar] [CrossRef]
- Dyussebekova, M.A.; Kenzhaliyev, B.K.; Kvyatkovskiy, S.A.; Sit’ko, E.A.; Nurhadiyanto, D. The main reasons for increased copper losses with slags from vanyukov furnace. Metalurgija 2021, 60, 309–312. [Google Scholar]
| № of Flux | Content of Components (mas. %) * | |||||
|---|---|---|---|---|---|---|
| SiO2 | Fe | CaO | Al2O3 | MgO | Rest | |
| 1 (Zhezkazgan) | 65.2 | 3.3 | 3.45 | 11.30 | 0.43 | 16.32 |
| 2 (Taskara) | 65.5 | 3.3 | 1.70 | 13.95 | 1.30 | 14.25 |
| 3 (Konyrat) | 65.7 | 3.4 | 0.70 | 17.50 | 0.18 | 12.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyussebekova, M.; Kenzhaliyev, B.; Kvyatkovskiy, S.; Kozhakhmetov, S.; Semenova, A.; Sukurov, B. Study of the Effect of Fluxing Ability of Flux Ores on Minimizing of Copper Losses with Slags during Copper Concentrate Smelting. Metals 2022, 12, 1240. https://doi.org/10.3390/met12081240
Dyussebekova M, Kenzhaliyev B, Kvyatkovskiy S, Kozhakhmetov S, Semenova A, Sukurov B. Study of the Effect of Fluxing Ability of Flux Ores on Minimizing of Copper Losses with Slags during Copper Concentrate Smelting. Metals. 2022; 12(8):1240. https://doi.org/10.3390/met12081240
Chicago/Turabian StyleDyussebekova, Maral, Bagdaulet Kenzhaliyev, Sergey Kvyatkovskiy, Sultanbek Kozhakhmetov, Anastasiya Semenova, and Bulat Sukurov. 2022. "Study of the Effect of Fluxing Ability of Flux Ores on Minimizing of Copper Losses with Slags during Copper Concentrate Smelting" Metals 12, no. 8: 1240. https://doi.org/10.3390/met12081240
APA StyleDyussebekova, M., Kenzhaliyev, B., Kvyatkovskiy, S., Kozhakhmetov, S., Semenova, A., & Sukurov, B. (2022). Study of the Effect of Fluxing Ability of Flux Ores on Minimizing of Copper Losses with Slags during Copper Concentrate Smelting. Metals, 12(8), 1240. https://doi.org/10.3390/met12081240






