Development of Recycled Expanded Polystyrene Nanofibers Modified by Chitosan for the Removal of Lead(II) from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EPS/CS Nanofibers
2.3. Characterization of EPS/CS Nanofibers
2.4. Batch Adsorption Experiments
2.5. Kinetic Models
2.6. Equilibrium and Thermodynamic Analysis
2.7. Parameters Estimation
2.8. Adsorption and Desorption Cycles: Reuse Experiments
3. Results and Discussion
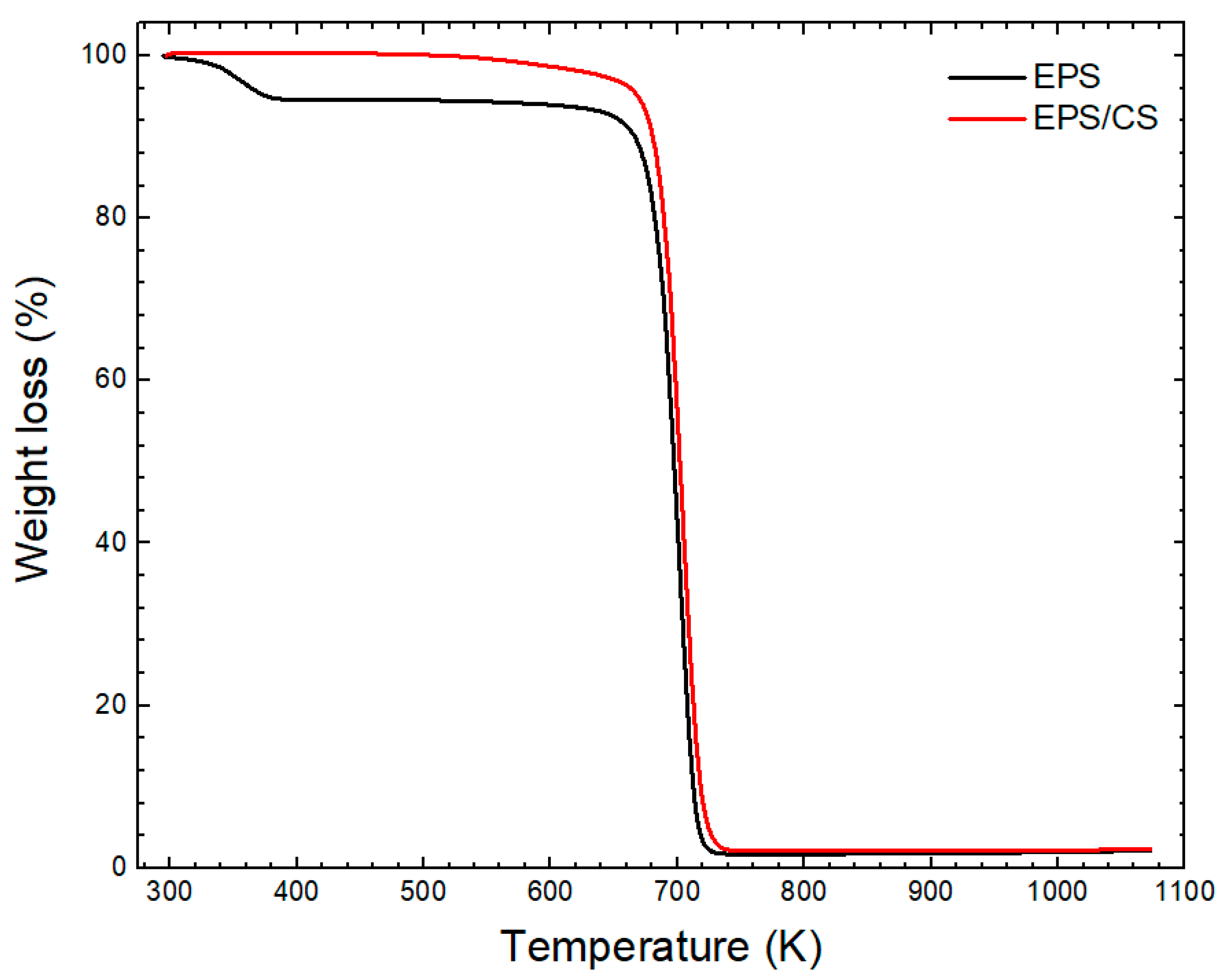
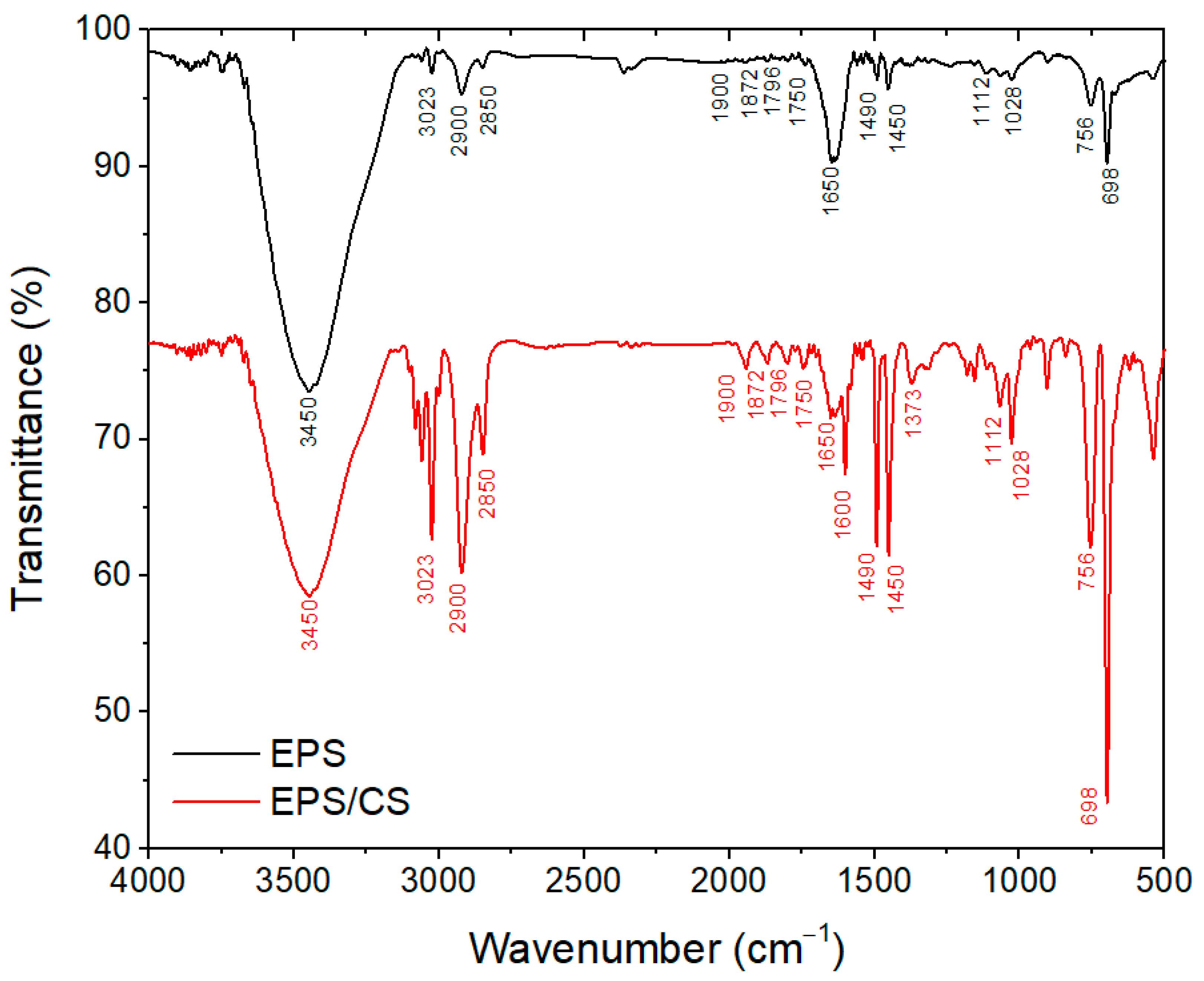
3.1. Characterization of EPS/CS Nanofibers
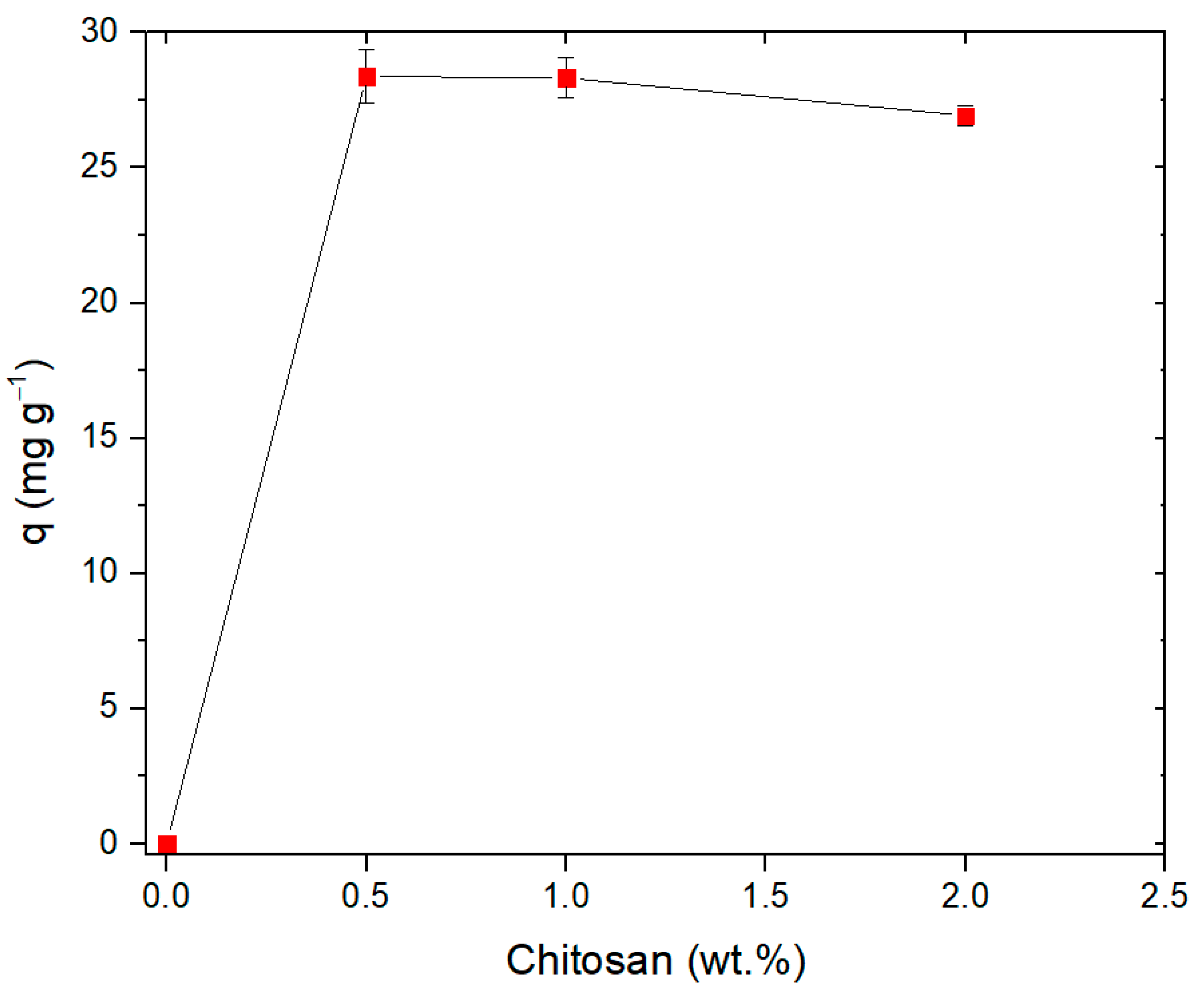
3.2. Effect of Chitosan Content on the Adsorption of Pb(II) by EPS/CS Nanofibers
3.3. Effect of pH on the Adsorption of Pb(II) by EPS/CS Nanofibers
3.4. Adsorption Kinetics
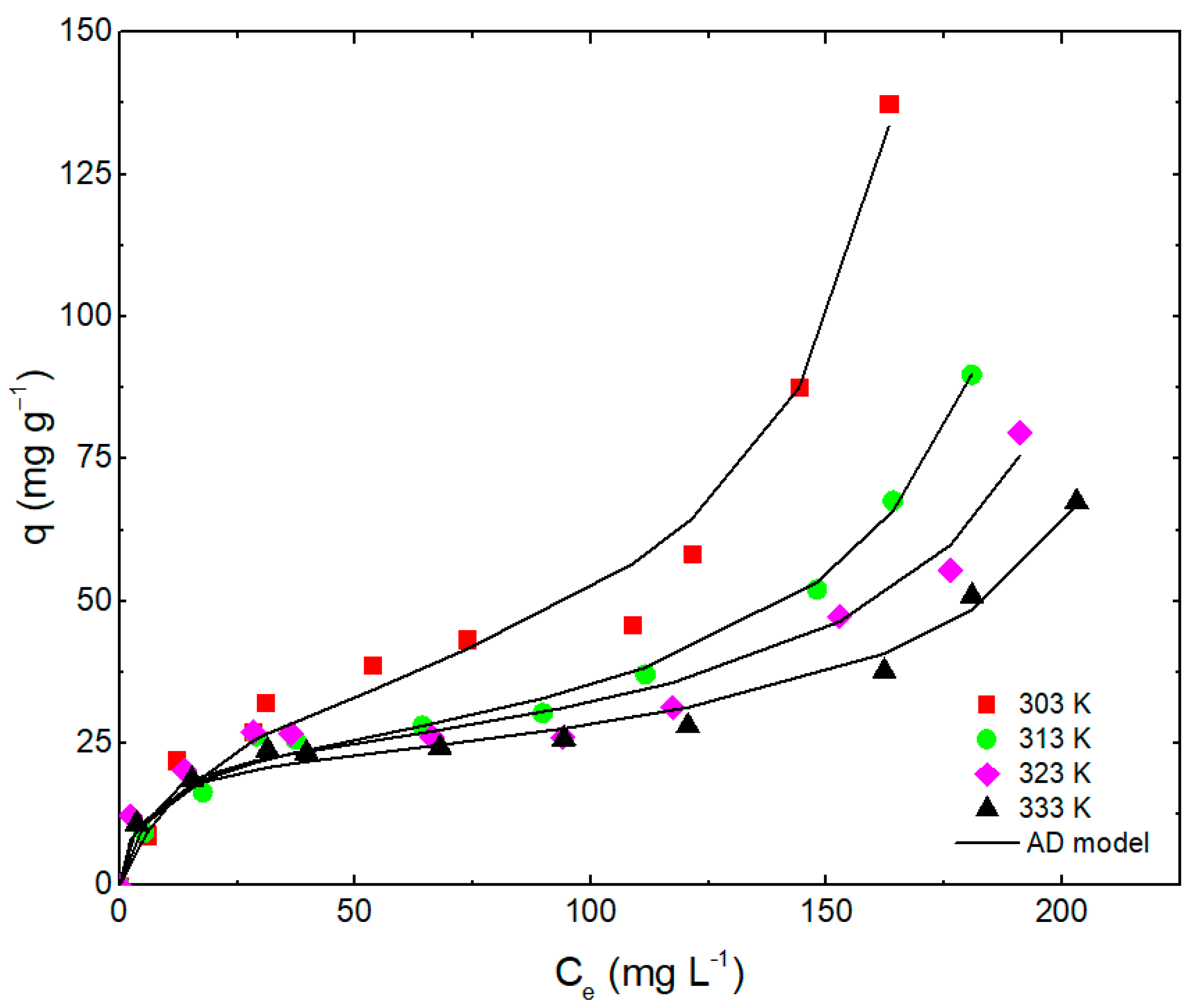
3.5. Adsorption Isotherms
| Adsorbent | Metal | pH | T (K) | qm (mg g−1) | References |
|---|---|---|---|---|---|
| EPS/CS nanofibers | Pb (II) | 6.0 | 303 | 137.35 | This work |
| CS nanofiber | Pb (II) | 7.0 | 298 | 110.20 | [60] |
| CS nanofiber | Pb (II) | 5.0 | 298 | 118.00 | [38] |
| Cellulose/CS nanofiber | Pb (II) | 6.0 | 298 | 57.30 | [25] |
| PEO/DTPA-CS nanofibers | Pb (II) | 5.0 | 298 | 142.00 | [61] |
| β-CD/CS/PVA | Pb (II) | 7.0 | 303 | 82.54 | [26] |
3.6. Adsorption Thermodynamic Results
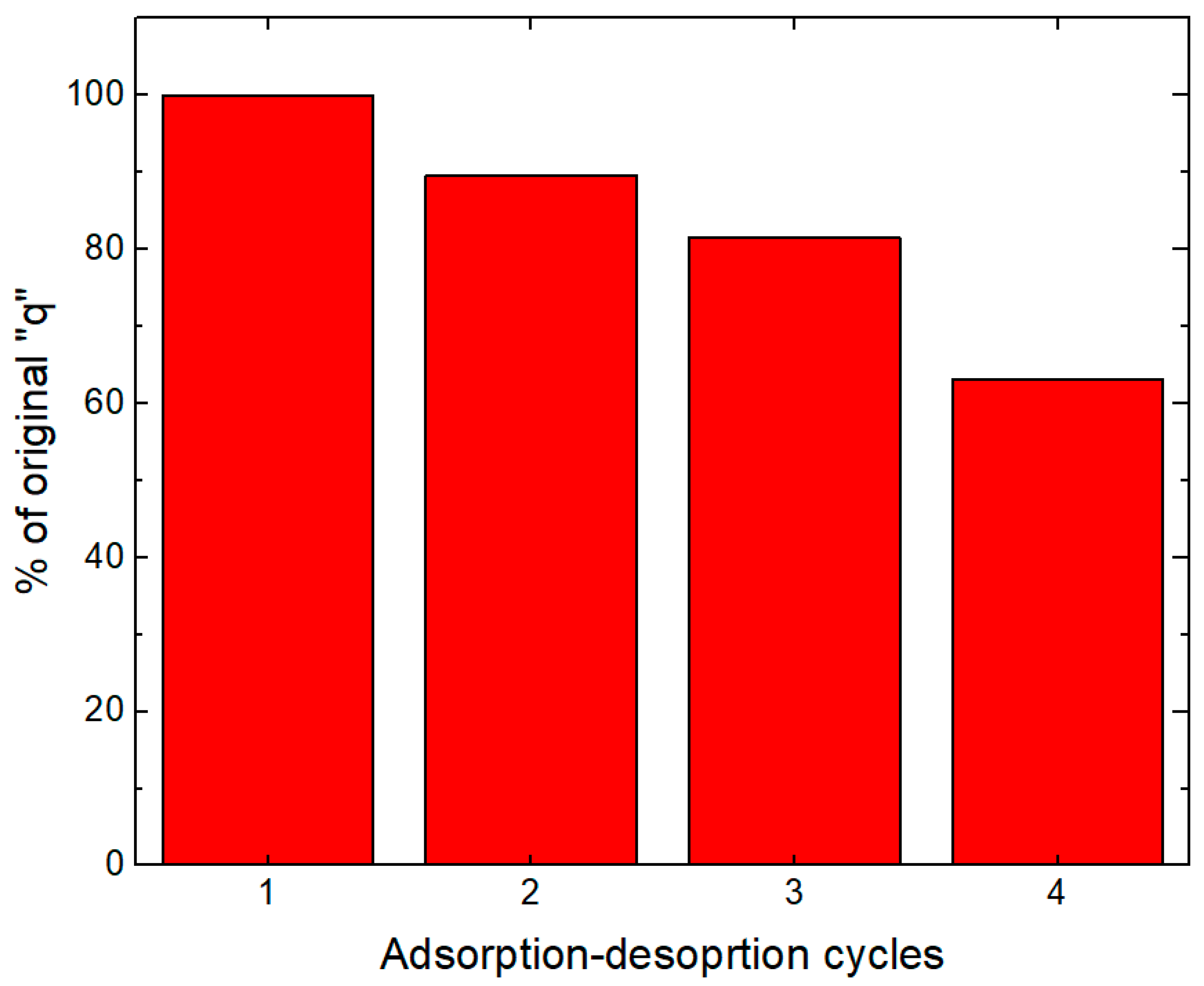
3.7. Adsorption and Desorption Cycles: Reuse Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perumal, S.; Atchudan, R.; Edison, T.; Babu, R.; Karpagavinayagam, P.; Vedhi, C. A Short Review on Recent Advances of Hydrogel-Based Adsorbents for Heavy Metal Ions. Metals 2021, 11, 864. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Abdulla, M. Lead. In Essential and Toxic Trace Elements and Vitamins in Human Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 181–191. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.S.; Pramanick, P.; Talukder, P.; Basak, A. Polyamines, metallothioneins, and phytochelatins—Natural defense of plants to mitigate heavy metals. Stud. Nat. Prod. Chem. 2021, 69, 227–261. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.; Oh, S. A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Process Eng. 2022, 45, 102518. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alcaraz, L.; García-Díaz, I.; López, F.A. Removal of Pb2+ in Wastewater via Adsorption onto an Activated Carbon Produced from Winemaking Waste. Metals 2018, 8, 697. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Zheng, Y.-M.; Zhang, B.-G.; Dai, Y.-R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef]
- Mehrotra, T.; Sinha, S.; Singh, R. Application of nanotechnology in the remediation of heavy metal toxicity. Nanotechnol. Food Agric. Environ. 2021, 359–373. [Google Scholar] [CrossRef]
- Rivero, P.J.; Redin, D.M.; Rodríguez, R.J. Electrospinning: A Powerful Tool to Improve the Corrosion Resistance of Metallic Surfaces Using Nanofibrous Coatings. Metals 2020, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, K.; Gomez, C.; Zambrano, S.; Ramirez, M.; de Hoyos, E.; Vasquez, H.; Lozano, K. Electrospinning to Forcespinning™. Mater. Today 2010, 13, 12–14. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Duan, Y.-S.; Xu, Q.; Zhang, B. A review on nanofiber fabrication with the effect of high-speed centrifugal force field. J. Eng. Fibers Fabr. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lu, Y. Centrifugal Spinning: An Alternative Approach to Fabricate Nanofibers at High Speed and Low Cost. Polym. Rev. 2014, 54, 677–701. [Google Scholar] [CrossRef]
- Segala, B.N.; Bertuol, D.A.; Tanabe, E.H. Production of polyacrylonitrile nanofibres modified with Cyanex 272 for recovery of gallium from solution. Environ. Technol. 2022, 43, 737–750. [Google Scholar] [CrossRef]
- Nataraj, S.; Yang, K.; Aminabhavi, T. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Shin, C.; Chase, G.; Reneker, D. Recycled expanded polystyrene nanofibers applied in filter media. Colloids Surf. A Physicochem. Eng. Asp. 2005, 262, 211–215. [Google Scholar] [CrossRef]
- Rajak, A.; Hapidin, D.A.; Iskandar, F.; Munir, M.M.; Khairurrijal, K. Electrospun nanofiber from various source of expanded polystyrene (EPS) waste and their characterization as potential air filter media. Waste Manag. 2020, 103, 76–86. [Google Scholar] [CrossRef]
- Chinchillas-Chinchillas, M.J.; Orozco-Carmona, V.M.; Alvarado-Beltrán, C.G.; Almaral-Sánchez, J.L.; Sepulveda-Guzman, S.; Jasso-Ramos, L.E.; Castro-Beltrán, A. Synthesis of Recycled Poly(ethylene terephthalate)/Polyacrylonitrile/Styrene Composite Nanofibers by Electrospinning and Their Mechanical Properties Evaluation. J. Polym. Environ. 2019, 27, 659–669. [Google Scholar] [CrossRef]
- Bonfim, D.; Cruz, F.; Guerra, V.; Aguiar, M. Development of Filter Media by Electrospinning for Air Filtration of Nanoparticles from PET Bottles. Membranes 2021, 11, 293. [Google Scholar] [CrossRef]
- de Oliveira, C.T.; Luna, M.M.; Campos, L.M. Understanding the Brazilian expanded polystyrene supply chain and its reverse logistics towards circular economy. J. Clean. Prod. 2019, 235, 562–573. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Haddad, M.Y.; Aijaz, M.O.; Assaifan, A.K.; Karim, M.R. Electrospun Bilayer PAN/Chitosan Nanofiber Membranes Incorporated with Metal Oxide Nanoparticles for Heavy Metal Ion Adsorption. Coatings 2020, 10, 285. [Google Scholar] [CrossRef] [Green Version]
- Keshtkar, A.R.; Tabatabaeefar, A.; Vaneghi, A.S.; Moosavian, M.A. Electrospun polyvinylpyrrolidone/silica/3-aminopropyltriethoxysilane composite nanofiber adsorbent: Preparation, characterization and its application for heavy metal ions removal from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1248–1258. [Google Scholar] [CrossRef]
- Sakib, M.N.; Mallik, A.K.; Rahman, M.M. Update on chitosan-based electrospun nanofibers for wastewater treatment: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100064. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Phan, D.-N.; Lee, H.; Huang, B.; Mukai, Y.; Kim, I.-S. Fabrication of electrospun chitosan/cellulose nanofibers having adsorption property with enhanced mechanical property. Cellulose 2019, 26, 1781–1793. [Google Scholar] [CrossRef]
- Fan, J.-P.; Luo, J.-J.; Zhang, X.-H.; Zhen, B.; Dong, C.-Y.; Li, Y.-C.; Shen, J.; Cheng, Y.-T.; Chen, H.-P. A novel electrospun β-CD/CS/PVA nanofiber membrane for simultaneous and rapid removal of organic micropollutants and heavy metal ions from water. Chem. Eng. J. 2019, 378, 122232. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Zhu, Y.; Li, Z.; Kumar, R. Recycled chitosan nanofibril as an effective Cu(II), Pb(II) and Cd(II) ionic chelating agent: Adsorption and desorption performance. Carbohydr. Polym. 2014, 111, 469–476. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafarirad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef]
- Kummer, G.; Schonhart, C.; Fernandes, M.G.; Dotto, G.L.; Missio, A.L.; Bertuol, D.A.; Tanabe, E.H. Development of Nanofibers Composed of Chitosan/Nylon 6 and Tannin/Nylon 6 for Effective Adsorption of Cr(VI). J. Polym. Environ. 2018, 26, 4073–4084. [Google Scholar] [CrossRef]
- Lou, T.; Yan, X.; Wang, X. Chitosan coated polyacrylonitrile nanofibrous mat for dye adsorption. Int. J. Biol. Macromol. 2019, 135, 919–925. [Google Scholar] [CrossRef]
- Thamer, B.M.; Aldalbahi, A.; Moydeen, A.M.; Al-Enizi, A.M.; El-Hamshary, H.; El-Newehy, M.H. Fabrication of functionalized electrospun carbon nanofibers for enhancing lead-ion adsorption from aqueous solutions. Sci. Rep. 2019, 9, 19467. [Google Scholar] [CrossRef]
- Ho, Y.; Mckay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Freundlich, H. Over the Adsorption in Solution. J. Phys. Chem. 1907, 57, 385–470. [Google Scholar]
- Wang, J.; Huang, C.; Allen, H.E.; Cha, D.K.; Kim, D.W. Adsorption Characteristics of Dye onto Sludge Particulates. J. Colloid Interface Sci. 1998, 208, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Aranovich, G.L.; Donohue, M.D. A New Approach to Analysis of Multilayer Adsorption. J. Colloid Interface Sci. 1995, 173, 515–520. [Google Scholar] [CrossRef]
- Brião, G.D.V.; da Silva, M.G.C.; Vieira, M.G.A.; Chu, K.H. Correlation of type II adsorption isotherms of water contaminants using modified BET equations. Colloids Interface Sci. Commun. 2022, 46, 100557. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Eldin, M.M.; Abu-Saied, M.; Tamer, T.; Youssef, M.; Hashem, A.; Sabet, M. Development of polystyrene based nanoparticles ions exchange resin for water purification applications. Desalin. Water Treat. 2016, 57, 14810–14823. [Google Scholar] [CrossRef]
- Dotto, G.; Rodrigues, F.; Tanabe, E.; Fröhlich, R.; Bertuol, D.; Martins, T.; Foletto, E. Development of chitosan/bentonite hybrid composite to remove hazardous anionic and cationic dyes from colored effluents. J. Environ. Chem. Eng. 2016, 4, 3230–3239. [Google Scholar] [CrossRef]
- Lou, T.; Cui, G.; Xun, J.; Wang, X.; Feng, N.; Zhang, J. Synthesis of a terpolymer based on chitosan and lignin as an effective flocculant for dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 149–154. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, T.; Zhao, C. Preparation of chitosan–nylon-6 blended membranes containing silver ions as antibacterial materials. Carbohydr. Res. 2008, 343, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Young, C.W.; DuVall, R.B.; Wright, N. Characterization of Benzene Ring Substitution by Infrared Spectra. Anal. Chem. 1951, 23, 709–714. [Google Scholar] [CrossRef]
- Luo, Q.; Zeng, S.; Shu, Y.; Fu, Z.; Zhao, H.; Su, S. A novel green process for tannic acid hydrolysis using an internally sulfonated hollow polystyrene sphere as catalyst. RSC Adv. 2018, 8, 17151–17158. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Chen, S.; Huang, H.; Chen, B.; Xiao, X.; Lin, J.; Chen, Q. Novel Anisotropic Janus Composite Particles Based on Urushiol-erbium Chelate Polymer/Polystyrene. Soft Mater. 2015, 13, 237–242. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless Melt-Electrospinning of Polypropylene Nanofibres. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Adibzadeh, S.; Bazgir, S.; Katbab, A.A. Fabrication and characterization of chitosan/poly(vinyl alcohol) electrospun nanofibrous membranes containing silver nanoparticles for antibacterial water filtration. Iran. Polym. J. 2014, 23, 645–654. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Wang, W.; Li, Y.; Liu, Y.; Lu, C.; Zhang, Z. Rheological transitions and in-situ IR characterizations of cellulose/LiCl·DMAc solution as a function of temperature. Cellulose 2018, 25, 4955–4968. [Google Scholar] [CrossRef]
- Margoshes, M.; Fassel, V. The infrared spectra of aromatic compounds: I. The out-of-plane C-H bending vibrations in the region 625–900 cm−1. Spectrochim. Acta 1955, 7, 14–24. [Google Scholar] [CrossRef]
- Karim, M.R.; Aijaz, M.O.; Alharth, N.H.; Alharbi, H.F.; Al-Mubaddel, F.S.; Awual, M.R. Composite nanofibers membranes of poly(vinyl alcohol)/chitosan for selective lead(II) and cadmium(II) ions removal from wastewater. Ecotoxicol. Environ. Saf. 2019, 169, 479–486. [Google Scholar] [CrossRef]
- Dewage, N.B.; Fowler, R.E.; Pittman, C.U.; Mohan, D.; Mlsna, T. Lead (Pb2+) sorptive removal using chitosan-modified biochar: Batch and fixed-bed studies. RSC Adv. 2018, 8, 25368–25377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci. Rep. 2020, 10, 3285. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.-L.; Qiu, W.; Wang, P.-P.; Liu, Y.-L.; Zou, J.; Wang, L.; Ma, J. Mechanism study about the adsorption of Pb(II) and Cd(II) with iron-trimesic metal-organic frameworks. Chem. Eng. J. 2020, 385, 123507. [Google Scholar] [CrossRef]
- Jnr, M.H.; Spiff, A.I. Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium bicolor (Wild Cocoyam) biomass. Electron. J. Biotechnol. 2005, 8, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Yan, Y.; Sun, X.; Li, J.; Han, W.; Shen, J.; Liu, X.; Wang, L.; Zheng, F. Recycling Phosphated Residue for Pb2+ Removal from Wastewater. Environ. Eng. Sci. 2016, 33, 207–214. [Google Scholar] [CrossRef]
- Perez, J.P.H.; Folens, K.; Leus, K.; Vanhaecke, F.; Van Der Voort, P.; Du Laing, G. Progress in hydrometallurgical technologies to recover critical raw materials and precious metals from low-concentrated streams. Resour. Conserv. Recycl. 2019, 142, 177–188. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Xu, C.; Zhou, Y. Crosslinked chitosan nanofiber mats fabricated by one-step electrospinning and ion-imprinting methods for metal ions adsorption. Sci. China Ser. B Chem. 2016, 59, 95–105. [Google Scholar] [CrossRef]
- Surgutskaia, N.S.; Di Martino, A.; Zednik, J.; Ozaltin, K.; Lovecká, L.; Bergerová, E.D.; Kimmer, D.; Svoboda, J.; Sedlarik, V. Efficient Cu2+, Pb2+ and Ni2+ ion removal from wastewater using electrospun DTPA-modified chitosan/polyethylene oxide nanofibers. Sep. Purif. Technol. 2020, 247, 116914. [Google Scholar] [CrossRef]
- Singh, R.; Bhateria, R. Experimental and Modeling Process Optimization of Lead Adsorption on Magnetite Nanoparticles via Isothermal, Kinetics, and Thermodynamic Studies. ACS Omega 2020, 5, 10826–10837. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ouyang, X.-K.; Yang, L.-Y. Adsorption of lead ions from aqueous solutions by porous cellulose nanofiber–sodium alginate hydrogel beads. J. Mol. Liq. 2021, 324, 115122. [Google Scholar] [CrossRef]




| Pseudo First-Order Model (PFO) | Pseudo Second Order Model (PSO) | ||
|---|---|---|---|
| q1 (mg g−1) | 26.87 | q2 (mg g−1) | 27.33 |
| k1 (min−1) | 0.7925 | k2 (g mg−1 min−1) | 0.1001 |
| R2 (%) | 99.02 | R2 (%) | 99.49 |
| ARE (%) | 2.73 | ARE (%) | 1.89 |
| Isotherm Model | Temperature (K) | |||
|---|---|---|---|---|
| 303 | 313 | 323 | 333 | |
| Freundlich | ||||
| KF ((mg g−1) (mg L−1)−1/nF) | 0.5004 | 0.8577 | 2.459 | 2.56 |
| 1/nF | 1.0534 | 0.8555 | 0.6072 | 0.5674 |
| R2 | 82.82 | 87.69 | 78.66 | 81.91 |
| ARE (%) | 35.61 | 27.74 | 30.41 | 25.51 |
| BET | ||||
| qBET (mg g−1) | 25.04 | 20.54 | 18.86 | 17.78 |
| KBET1 (L mg−1) | 0.2365 | 0.2262 | 1.1246 | 0.6508 |
| KBET2 (L mg−1) × 103 | 4.998 | 4.255 | 3.927 | 3.592 |
| R2 | 98.25 | 99.01 | 96.87 | 97.76 |
| ARE (%) | 16.25 | 8.86 | 10.86 | 9.37 |
| Aranovich-Donohue | ||||
| qAD (mg g−1) | 37.33 | 24.05 | 23.12 | 21.72 |
| KL-AD (L mg−1) | 0.0557 | 0.1491 | 0.2277 | 0.2401 |
| KAD (L mg−1) × 103 | 5.424 | 4.742 | 4.448 | 4.337 |
| n | 0.6361 | 0.6949 | 0.6364 | 0.5378 |
| RL-AD | 0.0565 | 0.0219 | 0.0144 | 0.0137 |
| R2 | 99.05 | 99.68 | 98.47 | 99.37 |
| ARE (%) | 11.3585 | 7.17205 | 12.801 | 6.42509 |
| Temperature (K) | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (kJ mol−1 K−1) |
|---|---|---|---|
| 303 | −27.75 | 38.75 | 0.22 |
| 313 | −31.07 | ||
| 323 | −33.16 | ||
| 333 | −34.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, T.R.; Costa, P.S.; Bertuol, D.A.; Aguiar, M.L.; Tanabe, E.H. Development of Recycled Expanded Polystyrene Nanofibers Modified by Chitosan for the Removal of Lead(II) from Water. Metals 2022, 12, 1334. https://doi.org/10.3390/met12081334
Martins TR, Costa PS, Bertuol DA, Aguiar ML, Tanabe EH. Development of Recycled Expanded Polystyrene Nanofibers Modified by Chitosan for the Removal of Lead(II) from Water. Metals. 2022; 12(8):1334. https://doi.org/10.3390/met12081334
Chicago/Turabian StyleMartins, Thiago R., Patrick S. Costa, Daniel A. Bertuol, Mônica L. Aguiar, and Eduardo H. Tanabe. 2022. "Development of Recycled Expanded Polystyrene Nanofibers Modified by Chitosan for the Removal of Lead(II) from Water" Metals 12, no. 8: 1334. https://doi.org/10.3390/met12081334






