Phase Separation in a Novel Selective Lithium Extraction from Citrate Media with D2EHPA
Abstract
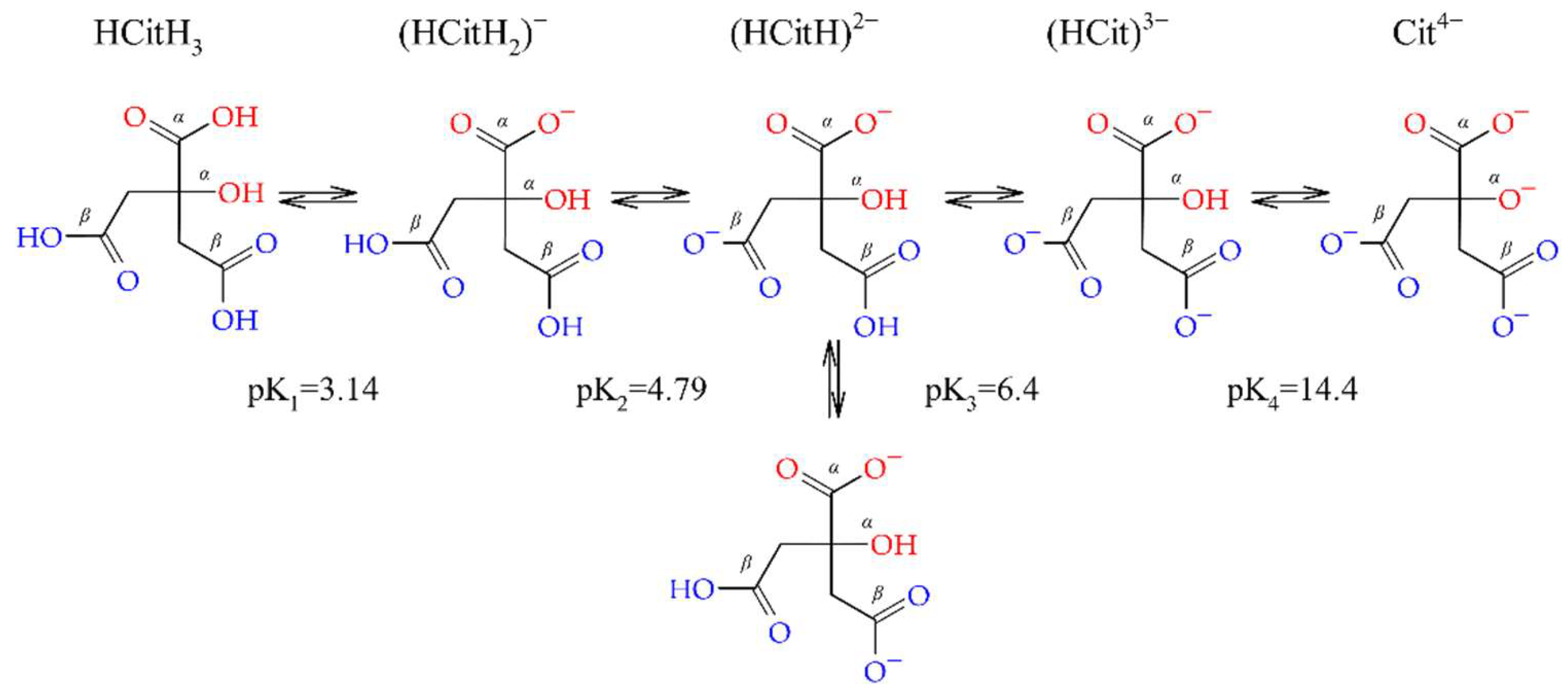
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Experimental Conditions
2.4. Experimental Procedure
3. Results
3.1. Influence of pH
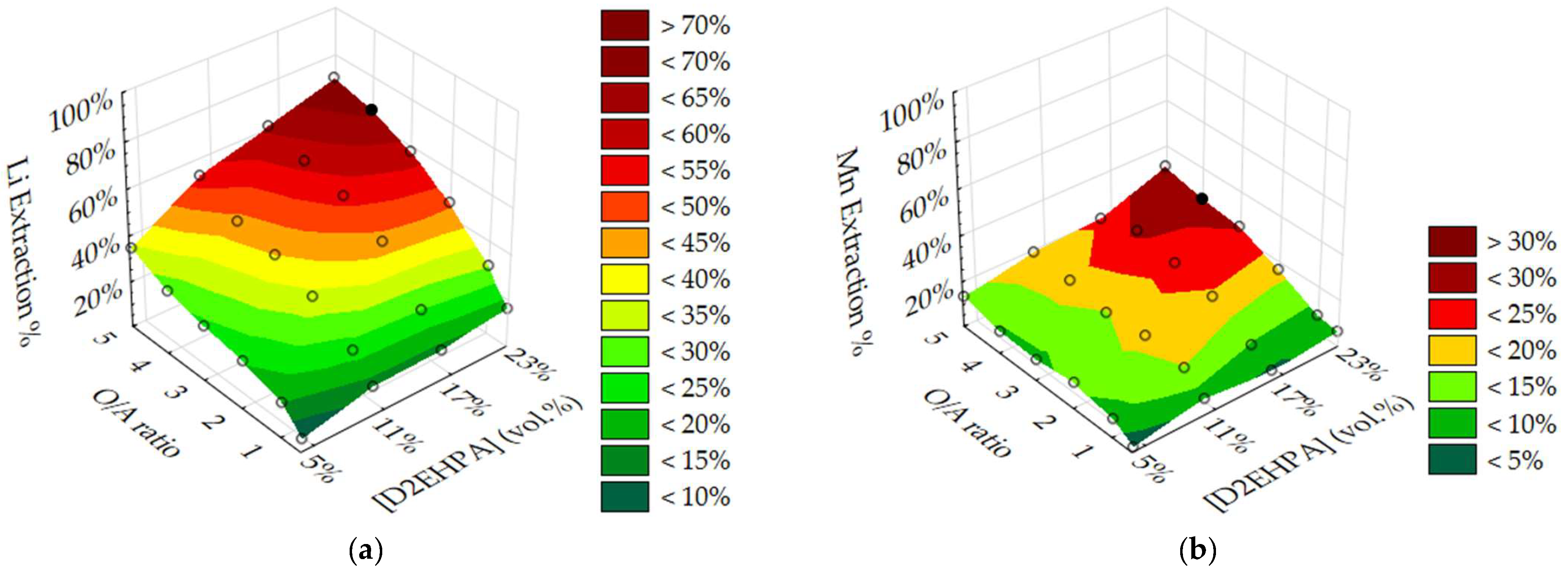
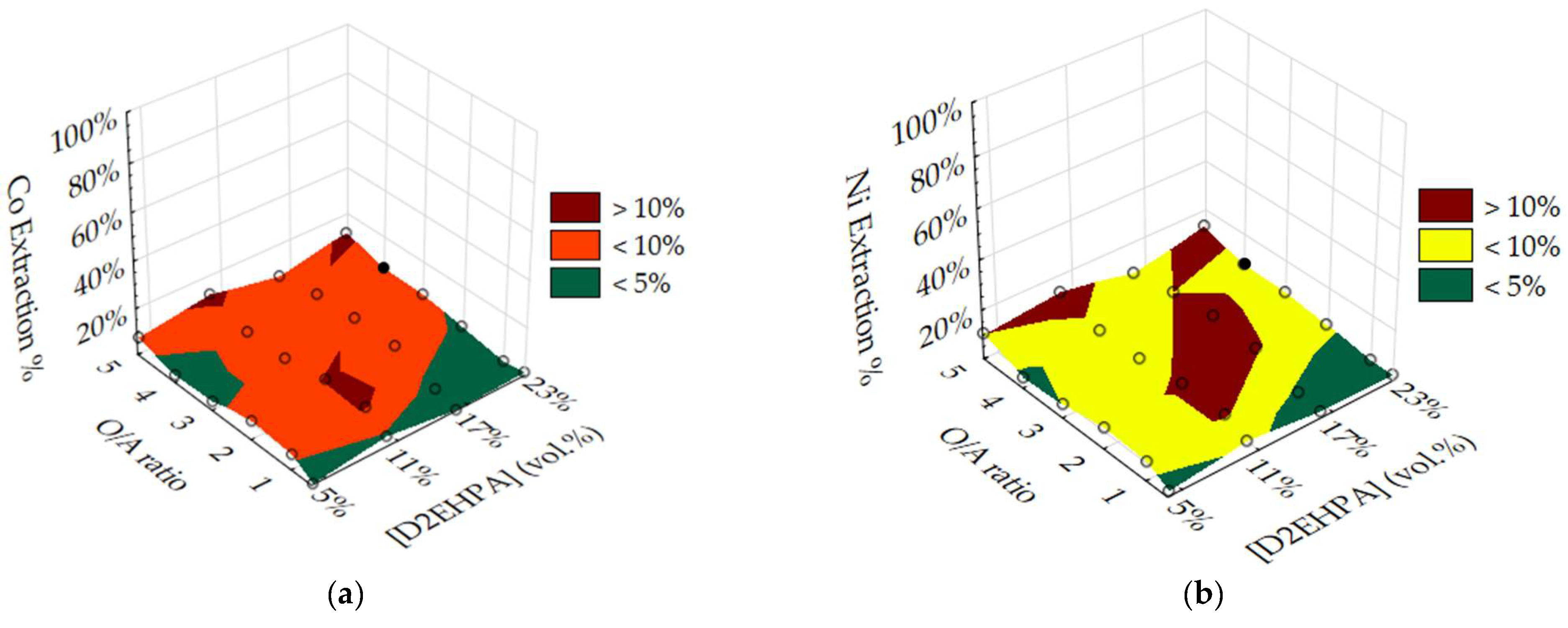
3.2. Optimisation of O/A Ratio and D2EHPA Concentration
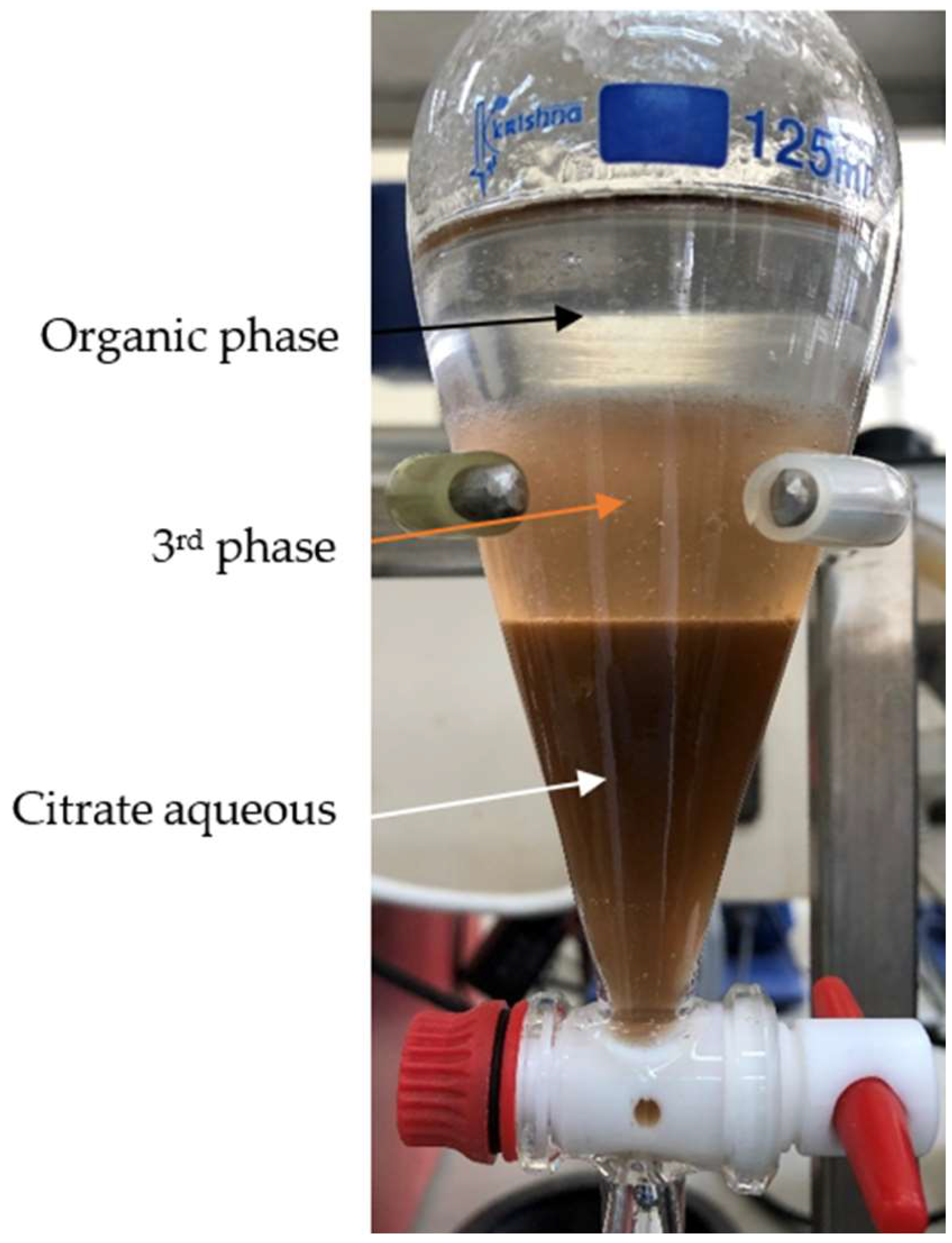
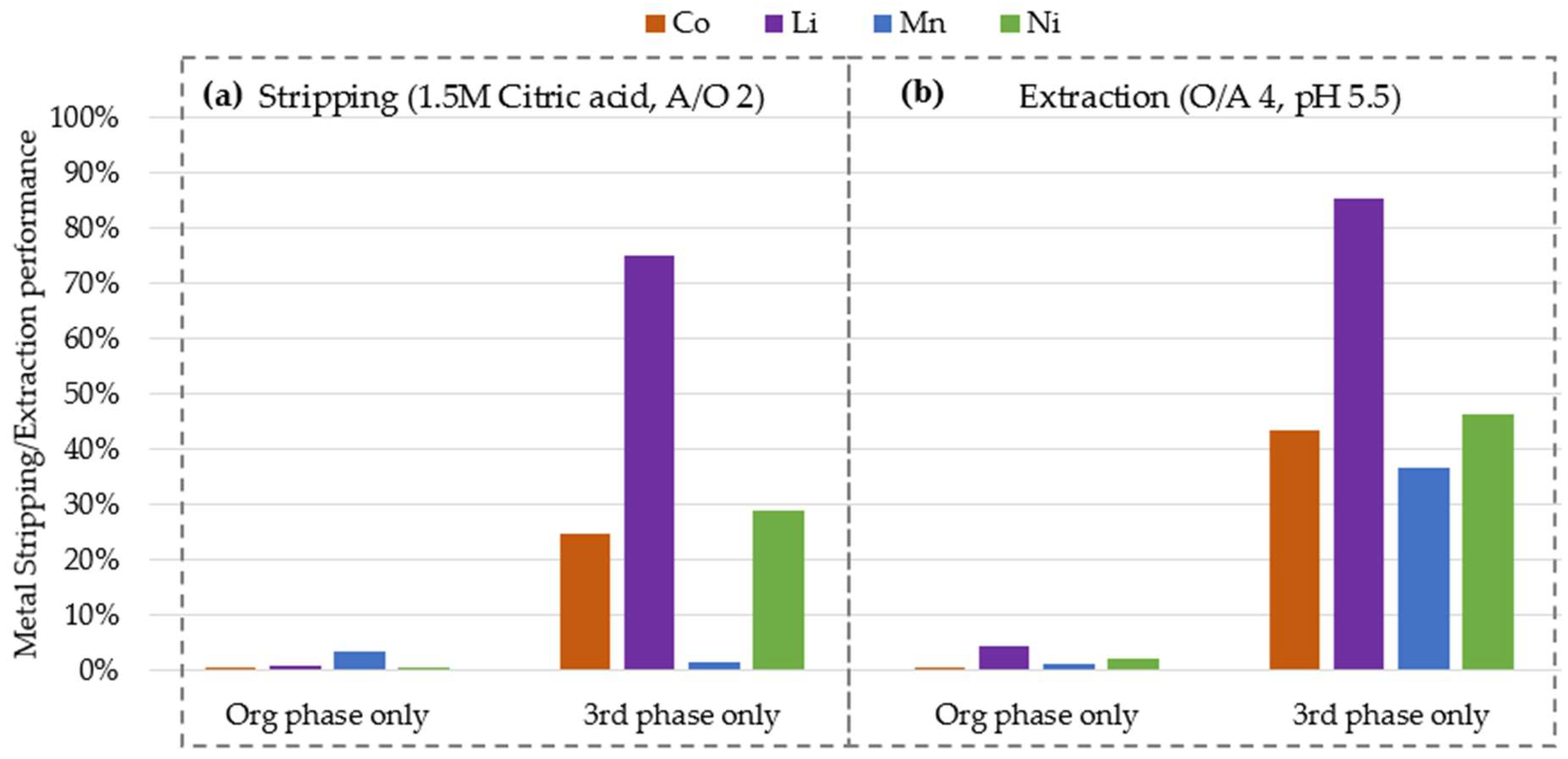
3.3. Organic and Third Phase Composition
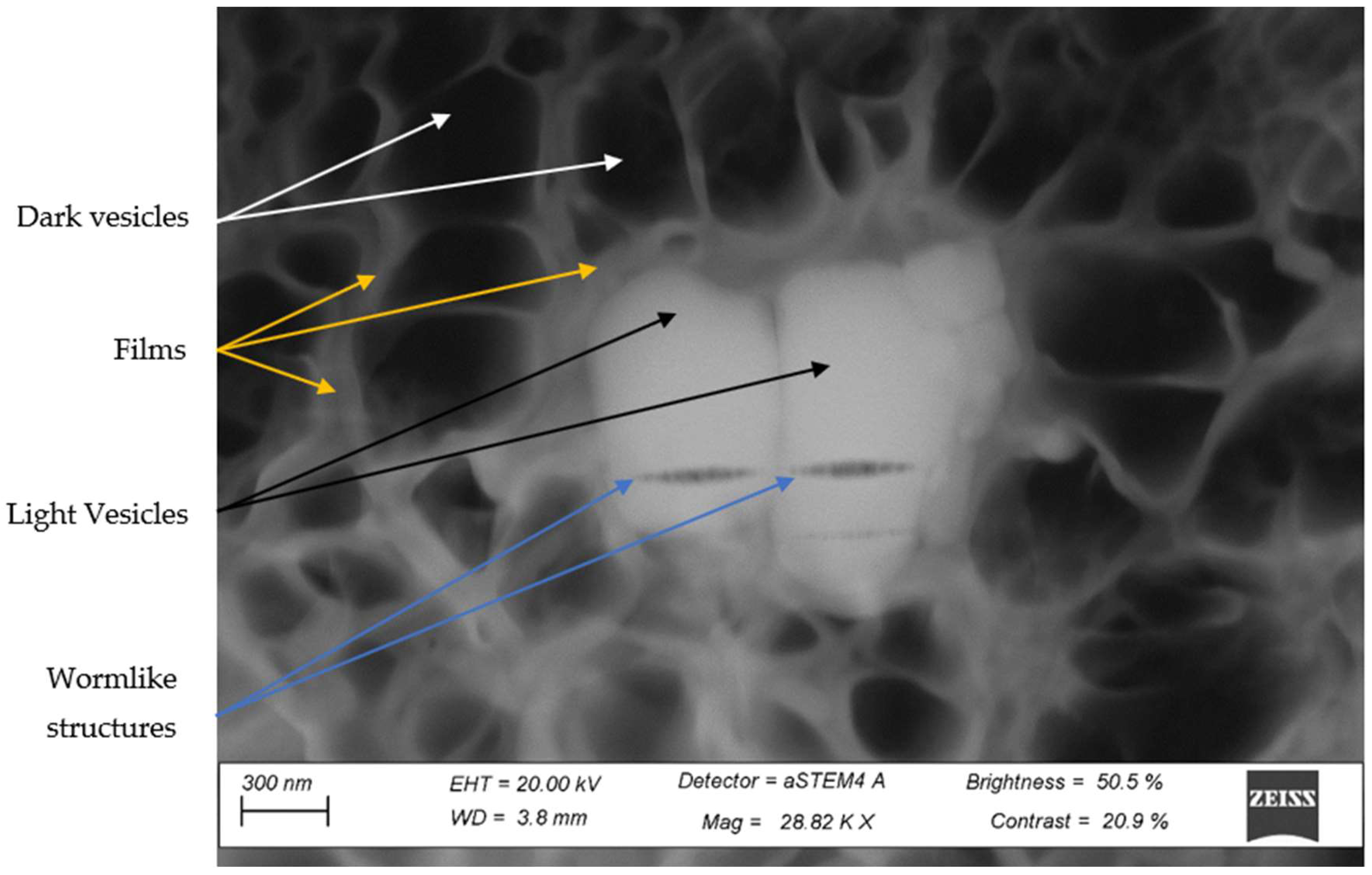
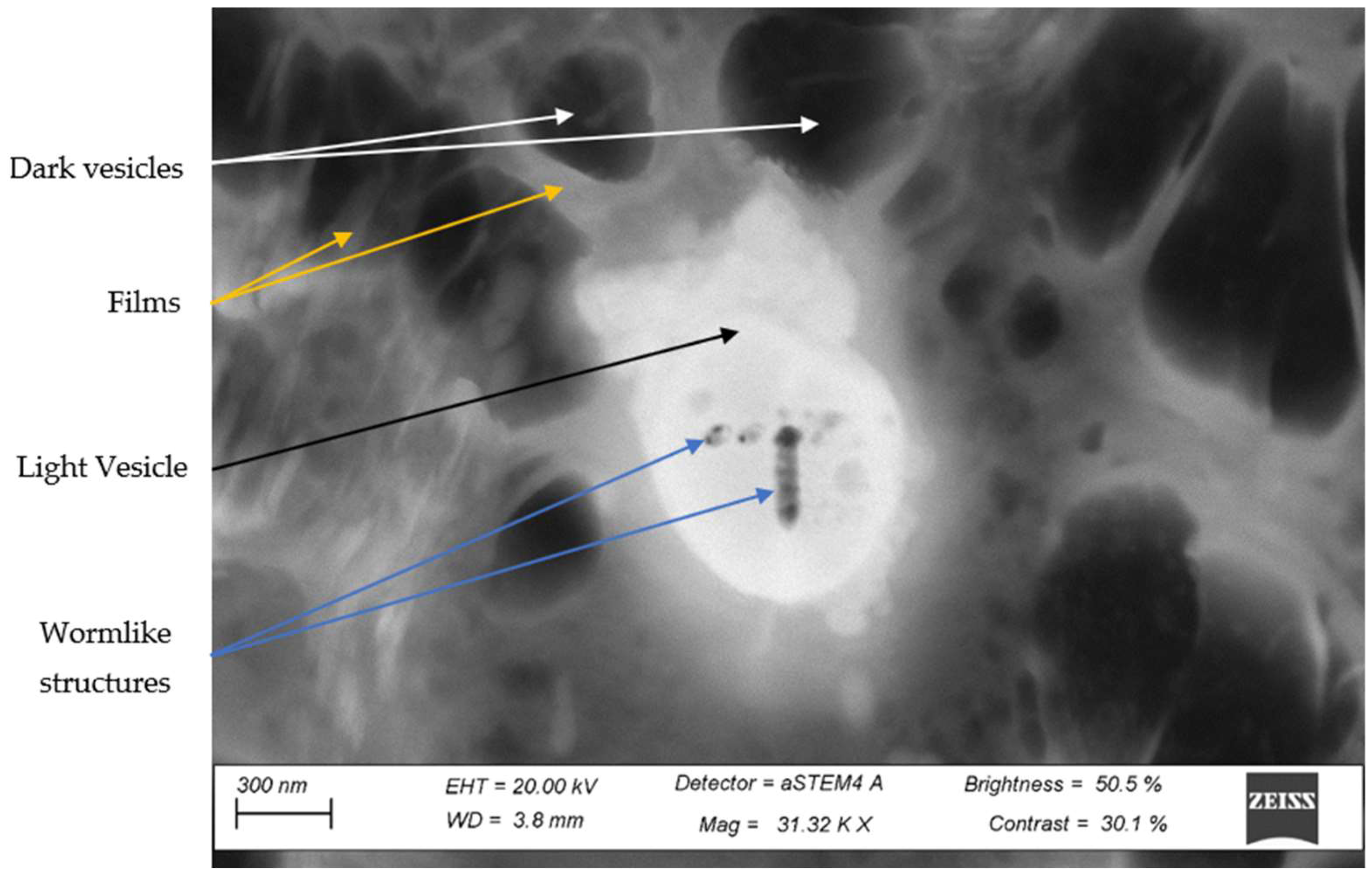
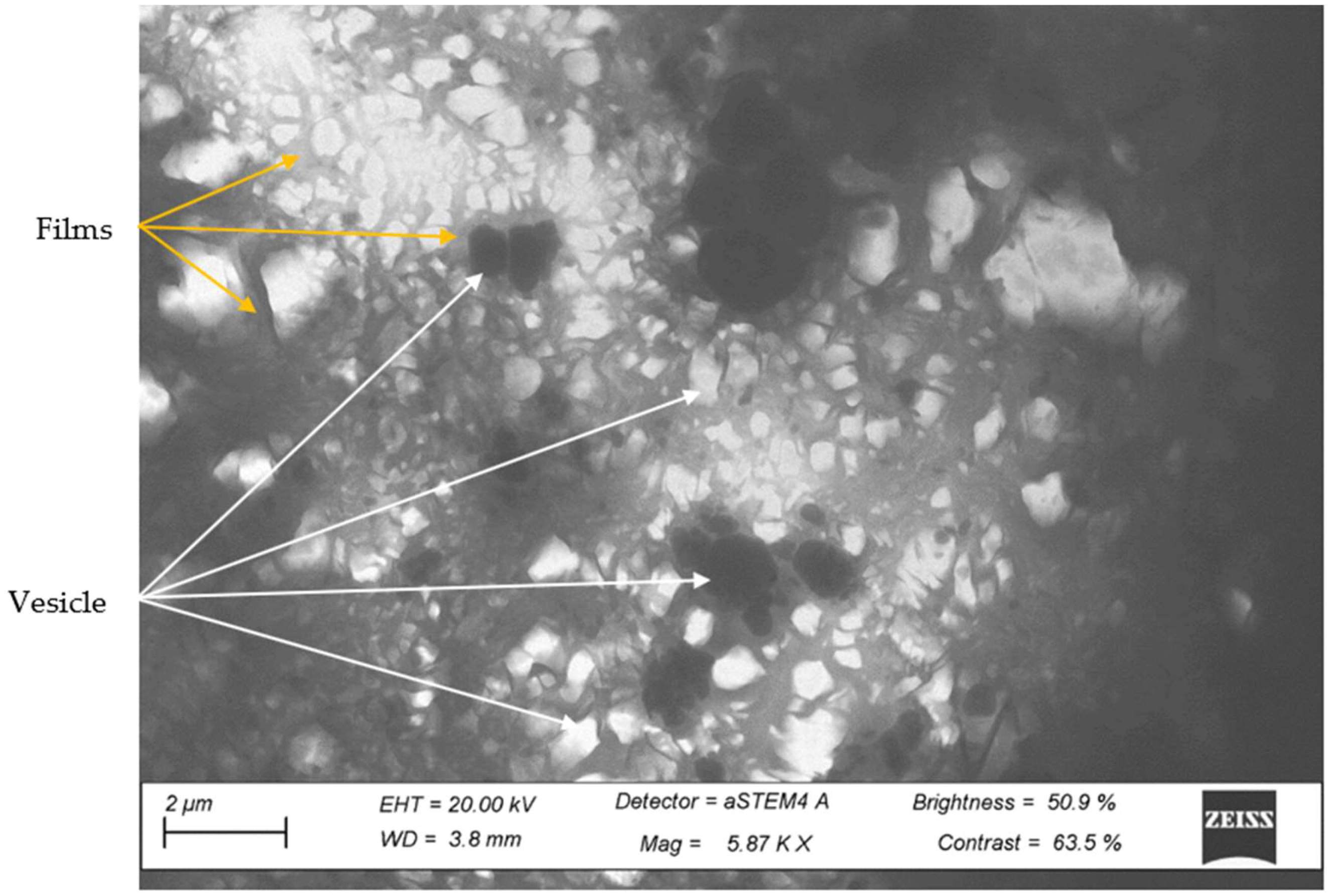
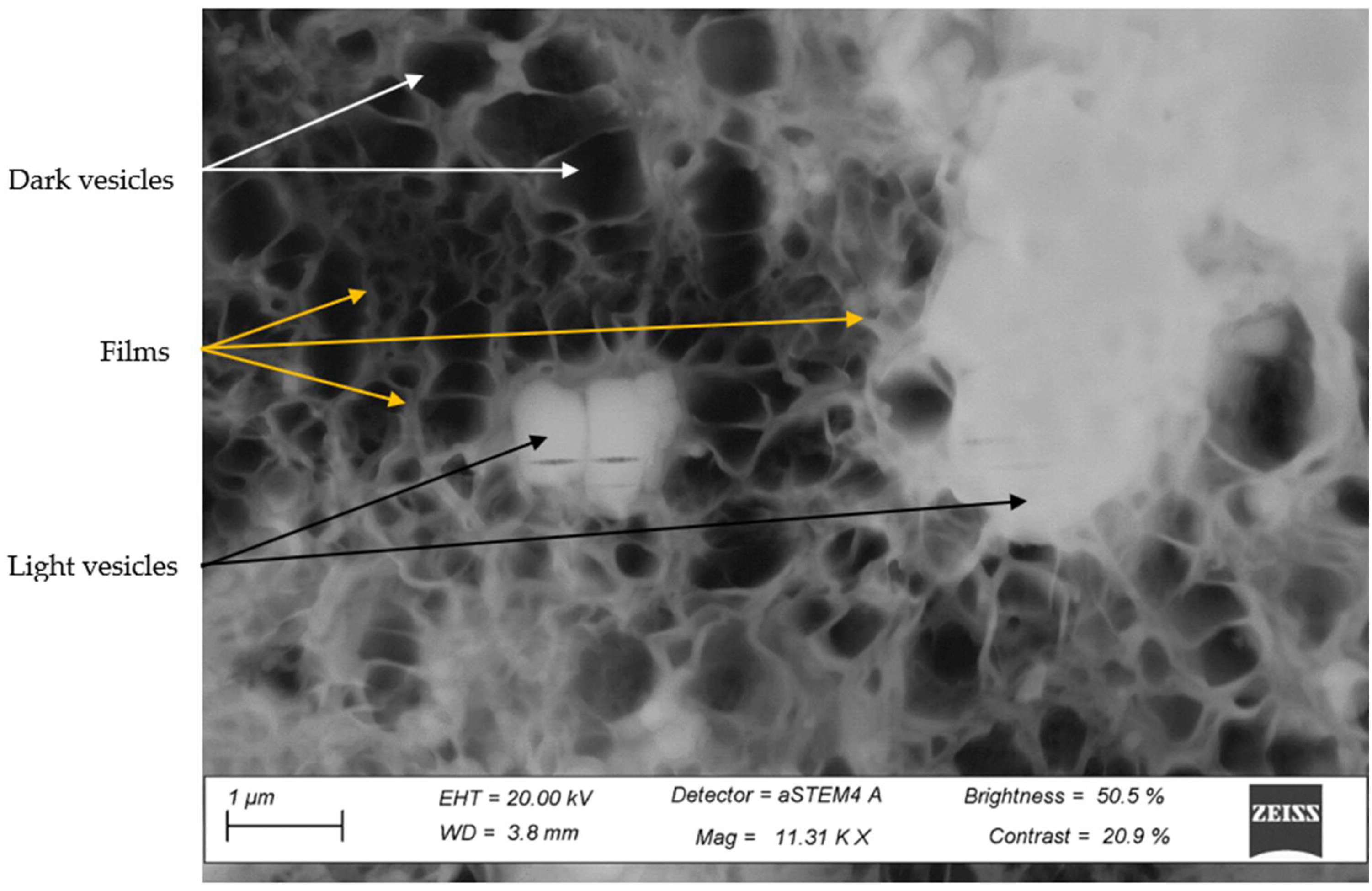
3.4. STEM Analysis of the Third Phase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- IEA The Role of Critical Minerals in Clean Energy Transitions. Available online: https://www.iea.org/reports/the-role-of-critical-minerals-in-clean-energy-transitions (accessed on 13 July 2021).
- Melin, H.E.; Rajaeifar, M.A.; Ku, A.Y.; Kendall, A.; Harper, G.; Heidrich, O. Global Implications of the EU Battery Regulation. Science 2021, 373, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Usai, L.; Lamb, J.J.; Hertwich, E.; Burheim, O.S.; Strømman, A.H. Analysis of the Li-Ion Battery Industry in Light of the Global Transition to Electric Passenger Light Duty Vehicles until 2050. Environ. Res. Infrastruct. Sustain. 2022, 2, 011002. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. Retired Electric Vehicle (EV) Batteries: Integrated Waste Management and Research Needs. Environ. Sci. Technol. 2017, 51, 10927–10929. [Google Scholar] [CrossRef]
- Drabik, E.; Rizos, V. Prospects for Electric Vehicle Batteries in a Circular Economy. 2018. Available online: http://aei.pitt.edu/94326/1/RR_2018_05_Circular_Impacts_batteries.pdf (accessed on 24 July 2022).
- Choubey, P.K.; Chung, K.-S.; Kim, M.; Lee, J.; Srivastava, R.R. Advance Review on the Exploitation of the Prominent Energy-Storage Element Lithium. Part II: From Sea Water and Spent Lithium Ion Batteries (LIBs). Miner. Eng. 2017, 110, 104–121. [Google Scholar] [CrossRef]
- Swain, B. Recovery and Recycling of Lithium: A Review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Kang, D.H.P.; Chen, M.; Ogunseitan, O.A. Potential Environmental and Human Health Impacts of Rechargeable Lithium Batteries in Electronic Waste. Environ. Sci. Technol. 2013, 47, 5495–5503. [Google Scholar] [CrossRef]
- Punt, T.; Bradshaw, S.M.; van Wyk, P.; Akdogan, G. The Efficiency of Black Mass Preparation by Discharge and Alkaline Leaching for LIB Recycling. Minerals 2022, 12, 753. [Google Scholar] [CrossRef]
- Winslow, K.M.; Laux, S.J.; Townsend, T.G. A Review on the Growing Concern and Potential Management Strategies of Waste Lithium-Ion Batteries. Resour. Conserv. Recycl. 2018, 129, 263–277. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A Closed Loop Process for Recycling Spent Lithium Ion Batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Richa, K. Economies of Scale for Future Lithium-Ion Battery Recycling Infrastructure. Resour. Conserv. Recycl. 2014, 83, 53–62. [Google Scholar] [CrossRef]
- European Commission. Study on the EU’s List of Critical Raw Materials; EU Publications: Luxembourg City, Luxembourg, 2020. [Google Scholar]
- Ali, H.; Khan, H.A.; Pecht, M.G. Circular Economy of Li Batteries: Technologies and Trends. J. Energy Storage 2021, 40, 102690. [Google Scholar] [CrossRef]
- Vezzini, A. Manufacturers, Materials and Recycling Technologies. In Lithium-Ion Batteries: Advances and Applications; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 529–551. ISBN 9780444595133. [Google Scholar]
- Chagnes, A.; Pospiech, B. A Brief Review on Hydrometallurgical Technologies for Recycling Spent Lithium-Ion Batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of Lithium-Ion Batteries: Recent Advances and Perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Lie, J.; Liu, J.-C. Closed-Vessel Microwave Leaching of Valuable Metals from Spent Lithium-Ion Batteries (LIBs) Using Dual-Function Leaching Agent: Ascorbic Acid. Sep. Purif. Technol. 2021, 266, 118458. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of Cobalt and Lithium from Spent Lithium Ion Batteries Using Organic Citric Acid as Leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Hauschild, M.Z.; Huijbregts, M.; Jolliet, O.; Macleod, M.; Margni, M.; van de Meent, D.; Rosenbaum, R.K.; McKone, T.E. Building a Model Based on Scientific Consensus for Life Cycle Impact Assessment of Chemicals: The Search for Harmony and Parsimony. Environ. Sci. Technol. 2008, 42, 7032–7037. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, R.K.; Bachmann, T.M.; Gold, L.S.; Huijbregts, M.A.J.; Jolliet, O.; Juraske, R.; Koehler, A.; Larsen, H.F.; MacLeod, M.; Margni, M.; et al. USEtox—the UNEP-SETAC Toxicity Model: Recommended Characterisation Factors for Human Toxicity and Freshwater Ecotoxicity in Life Cycle Impact Assessment. Int. J. Life Cycle Assess. 2008, 13, 532–546. [Google Scholar] [CrossRef] [Green Version]
- OECD Citric Acid SIAR. Available online: https://hpvchemicals.oecd.org/UI/handler.axd?id=ff78c453-36c1-430d-9034-63e15899d24b (accessed on 8 July 2022).
- IPCS Citric Acid ICSC: 0855. Available online: https://www.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=0855&p_version=2 (accessed on 17 February 2021).
- ECHA Citric Acid Registration Dossier. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/15451 (accessed on 13 April 2021).
- Meng, F.; Liu, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective Recovery of Valuable Metals from Industrial Waste Lithium-Ion Batteries Using Citric Acid under Reductive Conditions: Leaching Optimization and Kinetic Analysis. Hydrometallurgy 2020, 191, 105160. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Z.; Xue, F.; Yang, B.; Guo, G.; Qiu, J. A More Simple and Efficient Process for Recovery of Cobalt and Lithium from Spent Lithium-Ion Batteries with Citric Acid. Sep. Purif. Technol. 2019, 215, 398–402. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of Lithium and Cobalt from Spent Lithium Ion Batteries (LIBs) Using Organic Acids as Leaching Reagents: A Review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Ultrasonic-Assisted Leaching Process. J. Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Apelblat, A. Citric Acid; Springer International Publishing: Cham, Switzerland, 2014; ISBN 978-3-319-11232-9. [Google Scholar]
- Heller, A.; Barkleit, A.; Foerstendorf, H.; Tsushima, S.; Heim, K.; Bernhard, G. Curium(Iii) Citrate Speciation in Biological Systems: A Europium(Iii) Assisted Spectroscopic and Quantum Chemical Study. Dalton Trans. 2012, 41, 13969. [Google Scholar] [CrossRef] [Green Version]
- Martell, A.E.; Sillen, L.G. Stability Constants of Metal-Ion Complexes, 2nd ed.; Chemistry Society: London, UK, 1964. [Google Scholar]
- Silva, A.M.N.; Kong, X.; Hider, R.C. Determination of the PKa Value of the Hydroxyl Group in the α-Hydroxycarboxylates Citrate, Malate and Lactate by 13C NMR: Implications for Metal Coordination in Biological Systems. BioMetals 2009, 22, 771–778. [Google Scholar] [CrossRef]
- Ma, L.; Nie, Z.; Xi, X.; Han, X. Cobalt Recovery from Cobalt-Bearing Waste in Sulphuric and Citric Acid Systems. Hydrometallurgy 2013, 136, 1–7. [Google Scholar] [CrossRef]
- Musariri, B. Development of an Environmentally Friendly Lithium-Ion Battery Recycling Process. Available online: http://scholar.sun.ac.za/handle/10019.1/106175 (accessed on 26 May 2021).
- Chen, X.; Zhou, T.; Kong, J.; Fang, H.; Chen, Y. Separation and Recovery of Metal Values from Leach Liquor of Waste Lithium Nickel Cobalt Manganese Oxide Based Cathodes. Sep. Purif. Technol. 2015, 141, 76–83. [Google Scholar] [CrossRef]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent Extraction: The Coordination Chemistry behind Extractive Metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhou, T. Hydrometallurgical Process for the Recovery of Metal Values from Spent Lithium-Ion Batteries in Citric Acid Media. Waste Manag. Res. J. A Sustain. Circ. Econ. 2014, 32, 1083–1093. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An Atom-Economic Process for the Recovery of High Value-Added Metals from Spent Lithium-Ion Batteries. J. Clean. Prod. 2016, 112, 3562–3570. [Google Scholar] [CrossRef]
- Punt, T.; Akdogan, G.; Bradshaw, S.; van Wyk, P. Development of a Novel Solvent Extraction Process Using Citric Acid for Lithium-Ion Battery Recycling. Miner. Eng. 2021, 173, 107204. [Google Scholar] [CrossRef]
- Joo, S.H.; Shin, D.J.; Oh, C.H.; Wang, J.P.; Senanayake, G.; Shin, S.M. Selective Extraction and Separation of Nickel from Cobalt, Manganese and Lithium in Pre-Treated Leach Liquors of Ternary Cathode Material of Spent Lithium-Ion Batteries Using Synergism Caused by Versatic 10 Acid and LIX 84-I. Hydrometallurgy 2016, 159, 65–74. [Google Scholar] [CrossRef]
- Zou, H.; Gratz, E.; Apelian, D.; Wang, Y. A Novel Method to Recycle Mixed Cathode Materials for Lithium Ion Batteries. Green Chem. 2013, 15, 1183–1191. [Google Scholar] [CrossRef]
- Wang, R.-C.; Lin, Y.-C.; Wu, S.-H. A Novel Recovery Process of Metal Values from the Cathode Active Materials of the Lithium-Ion Secondary Batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Mayyas, A.; Steward, D.; Mann, M. The Case for Recycling: Overview and Challenges in the Material Supply Chain for Automotive Li-Ion Batteries. Sustain. Mater. Technol. 2019, 19, e00087. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, G.; Ma, J.S. Versatile Framework Solids Constructed from Divalent Transition Metals and Citric Acid: Syntheses, Crystal Structures, and Thermal Behaviors. Cryst. Growth Des. 2006, 6, 375–381. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Chmurzyński, L. Thermodynamics of Citrate Complexation with Mn2+, Co2+, Ni2+ and Zn2+ Ions. J. Therm. Anal. Calorim. 2010, 102, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.-H.; Deng, Y.-F.; Wan, H.-L. Structural Diversities of Cobalt(II) Coordination Polymers with Citric Acid. Cryst. Growth Des. 2005, 5, 1109–1117. [Google Scholar] [CrossRef]
- Hedwig, G.R.; Liddle, J.R.; Reeves, R.D. Complex Formation of Nickel(II) Ions with Citric Acid in Aqueous Solution: A Potentiometric and Spectroscopic Study. Aust. J. Chem. 1980, 33, 1685–1693. [Google Scholar] [CrossRef]
- Li, N.C.; Lindenbaum, A.; White, J.M. Some Metal Complexes of Citric and Tricarballylic Acids. J. Inorg. Nucl. Chem. 1959, 12, 122–128. [Google Scholar] [CrossRef]
- Zelenin, O.Y. Interaction of the Ni2+ Ion with Citric Acid in an Aqueous Solution. Russ. J. Coord. Chem. 2007, 33, 346–350. [Google Scholar] [CrossRef]
- Campi, E.; Ostacoli, G.; Meirone, M.; Saini, G. Stability of the Complexes of Tricarballylic and Citric Acids with Bivalent Metal Ions in Aqueous Solution. J. Inorg. Nucl. Chem. 1964, 26, 553–564. [Google Scholar] [CrossRef]
- Francis, A.J.; Dodge, C.J.; Gillow, J.B. Biodegradation of Metal Citrate Complexes and Implications for Toxic-Metal Mobility. Nature 1992, 356, 140–142. [Google Scholar] [CrossRef]
- Deng, Y.-F.; Zhou, Z.-H.; Wan, H.-L.; Ng, S.W. Δ-Aqua- S -Citrato(2–)Manganese(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, m310–m312. [Google Scholar] [CrossRef]
- Glusker, J.P.; van der Helm, D.; Love, W.E.; Dornberg, M.; Minkin, J.A.; Johnson, C.K.; Patterson, A.L. X-Ray Crystal Analysis of the Substrates of Aconitase. VI. The Structures of Sodium and Lithium Dihydrogen Citrates. Acta Crystallogr. 1965, 19, 561–572. [Google Scholar] [CrossRef]
- Oruch, R.; Elderbi, M.A.; Khattab, H.A.; Pryme, I.F.; Lund, A. Lithium: A Review of Pharmacology, Clinical Uses, and Toxicity. Eur. J. Pharmacol. 2014, 740, 464–473. [Google Scholar] [CrossRef]
- Rammohan, A.; Kaduk, J.A. Crystal Structures of Alkali Metal (Group 1) Citrate Salts. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2018, 74, 239–252. [Google Scholar] [CrossRef]
- Rossi, M.; Rickles, L.F.; Glusker, J.P. Trilithium Citrate Pentahydrate, C6H5O73−. 3Li+.5H2O. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1983, 39, 987–990. [Google Scholar] [CrossRef] [Green Version]
- Haussühl, S.; Jiyang, W. Elastic Properties of Citric Acid, Citric Acid Hydrate, Trilithium Citrate Tetrahydrate, Trisodium Citrate Pentahydrate, and Tripotassium Citrate Hydrate. Z. Für Krist. Cryst. Mater. 1999, 214, 85–89. [Google Scholar] [CrossRef]
- Schreuer, J.; Haussühl, S. The Pseudo-Orthorhombic Crystal Structure of Monoclinic Trilithium Citrate Tetrahydrate, Li3HOC(COO)(CH2COO)2 4H2O. Z. Für Krist. New Cryst. Struct. 2000, 215, 369–370. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, L.; Li, H.; Guo, R.; Zhao, M.; Yang, L. Highly Selective Lithium Ion Adsorbents: Polymeric Porous Microsphere with Crown Ether Groups. Trans. Tianjin Univ. 2019, 25, 101–109. [Google Scholar] [CrossRef]
- Kobiro, K. New Class of Lithium Ion Selective Crown Ethers with Bulky Decalin Subunits. Coord. Chem. Rev. 1996, 148, 135–149. [Google Scholar] [CrossRef]
- Torrejos, R.E.C.; Nisola, G.M.; Song, H.S.; Limjuco, L.A.; Lawagon, C.P.; Parohinog, K.J.; Koo, S.; Han, J.W.; Chung, W.-J. Design of Lithium Selective Crown Ethers: Synthesis, Extraction and Theoretical Binding Studies. Chem. Eng. J. 2017, 326, 921–933. [Google Scholar] [CrossRef]
- Dutta, D.; Kumari, A.; Panda, R.; Jha, S.; Gupta, D.; Goel, S.; Jha, M.K. Close Loop Separation Process for the Recovery of Co, Cu, Mn, Fe and Li from Spent Lithium-Ion Batteries. Sep. Purif. Technol. 2018, 200, 327–334. [Google Scholar] [CrossRef]
- Pranolo, Y.; Zhang, W.; Cheng, C.Y. Recovery of Metals from Spent Lithium-Ion Battery Leach Solutions with a Mixed Solvent Extractant System. Hydrometallurgy 2010, 102, 37–42. [Google Scholar] [CrossRef]
- Hamley, I. Neutral Block Copolymers in Dilute Solution. In Block Copolymers in Solution: Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2005; pp. 7–104. ISBN 9780470016985. [Google Scholar]
- Hamley, I. Applications. In Block Copolymers in Solution: Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2005; pp. 241–283. ISBN 9780470016985. [Google Scholar]
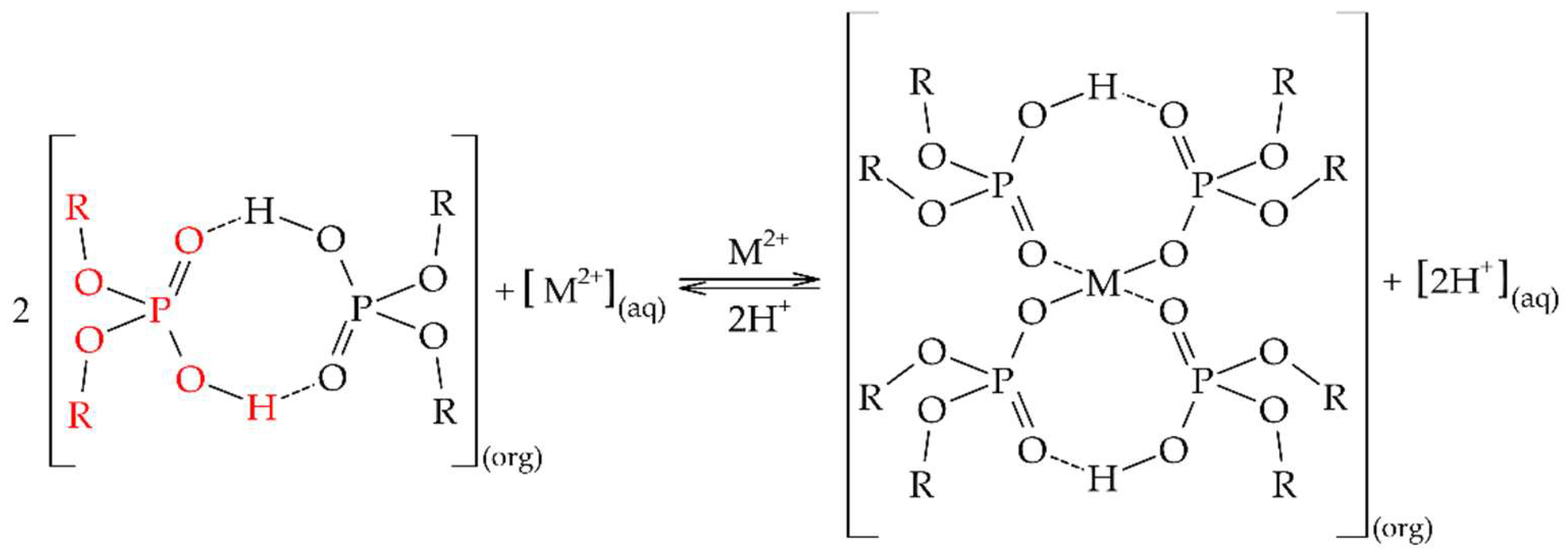
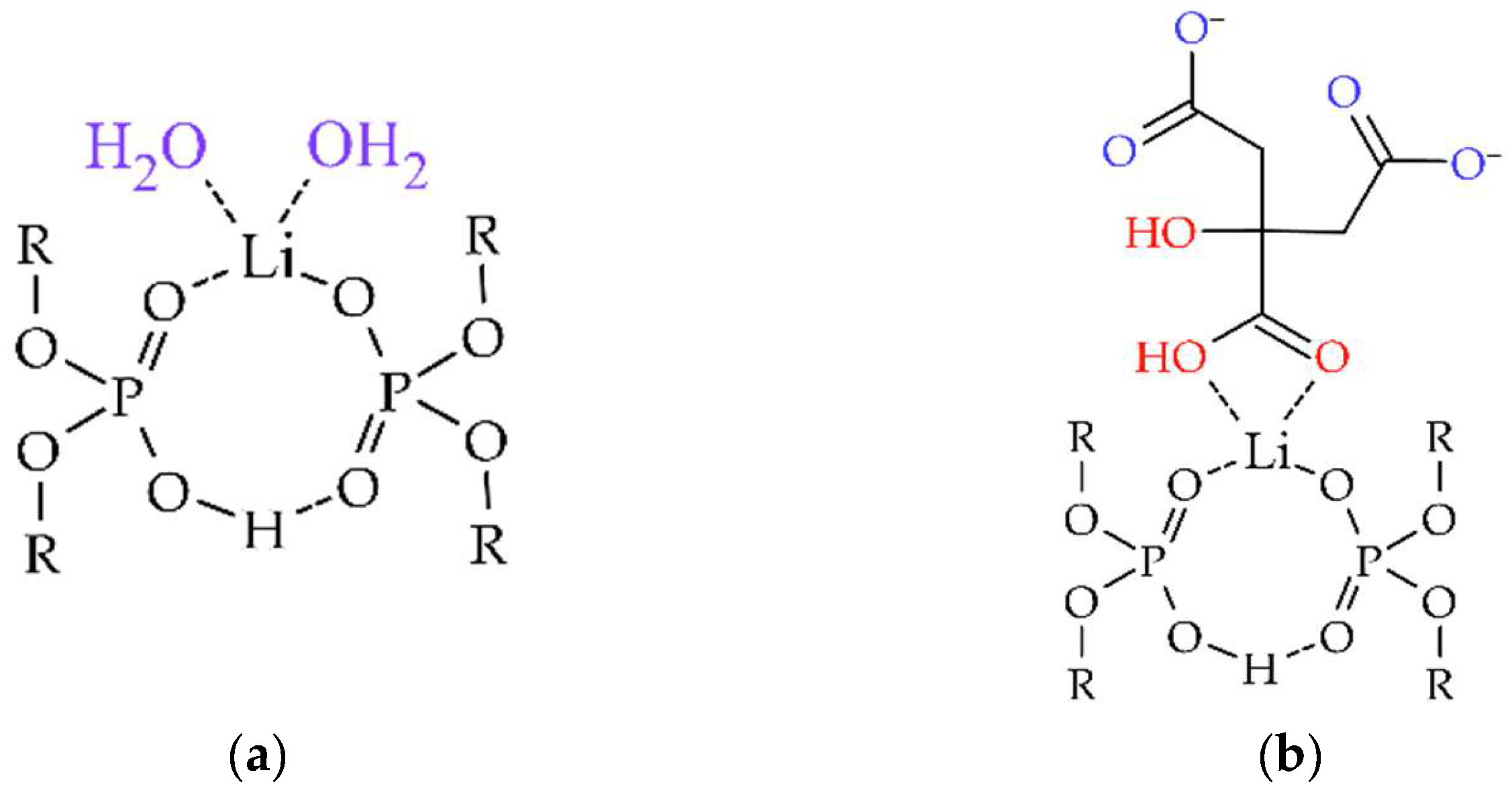
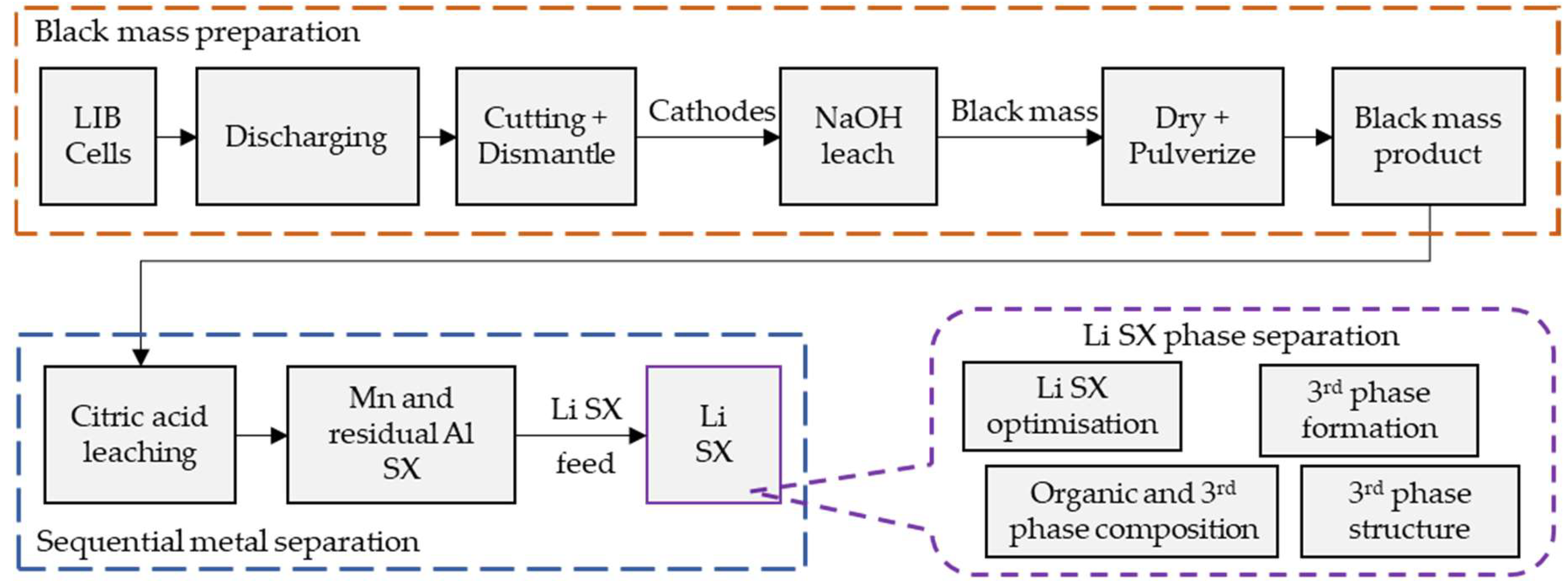

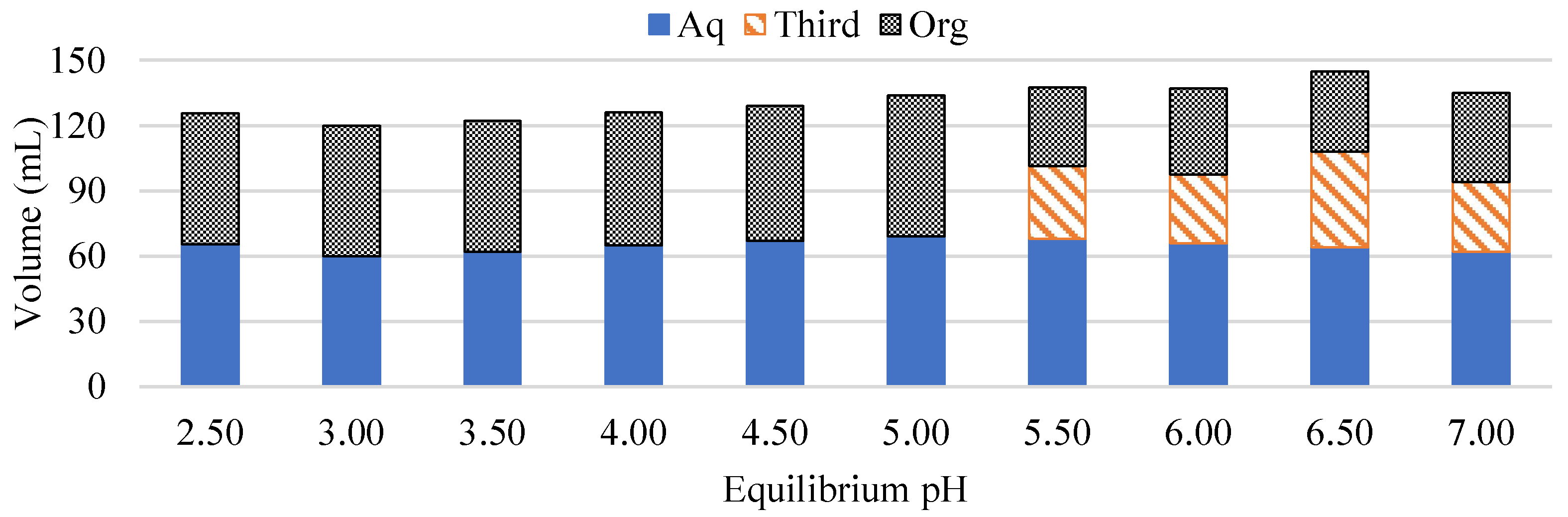
| Al | Co | Li | Mn | Ni | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weight % | 1.2% | ±0.0% | 35.4% | ±1.4% | 10.2% | ±0.4% | 23.4% | ±0.9% | 29.7% | ±1.2% |
| Metal | Co | Li | Mn | Ni | ||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mg/L) | 4026 | ±22 | 1165 | ±9 | 273.8 | ±6.1 | 3951 | ±36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punt, T.; Bradshaw, S.M.; Van Wyk, P.; Akdogan, G. Phase Separation in a Novel Selective Lithium Extraction from Citrate Media with D2EHPA. Metals 2022, 12, 1400. https://doi.org/10.3390/met12091400
Punt T, Bradshaw SM, Van Wyk P, Akdogan G. Phase Separation in a Novel Selective Lithium Extraction from Citrate Media with D2EHPA. Metals. 2022; 12(9):1400. https://doi.org/10.3390/met12091400
Chicago/Turabian StylePunt, Tiaan, Steven M. Bradshaw, Petrie Van Wyk, and Guven Akdogan. 2022. "Phase Separation in a Novel Selective Lithium Extraction from Citrate Media with D2EHPA" Metals 12, no. 9: 1400. https://doi.org/10.3390/met12091400






