The Tribo-Corrosion Behavior of Monel 400 Alloy in Marine Environment at Varied Rotational Velocities
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Tribo-Corrosion Measurement
2.3. Characterization
3. Results
3.1. Microstructure of Monel 400 Alloy
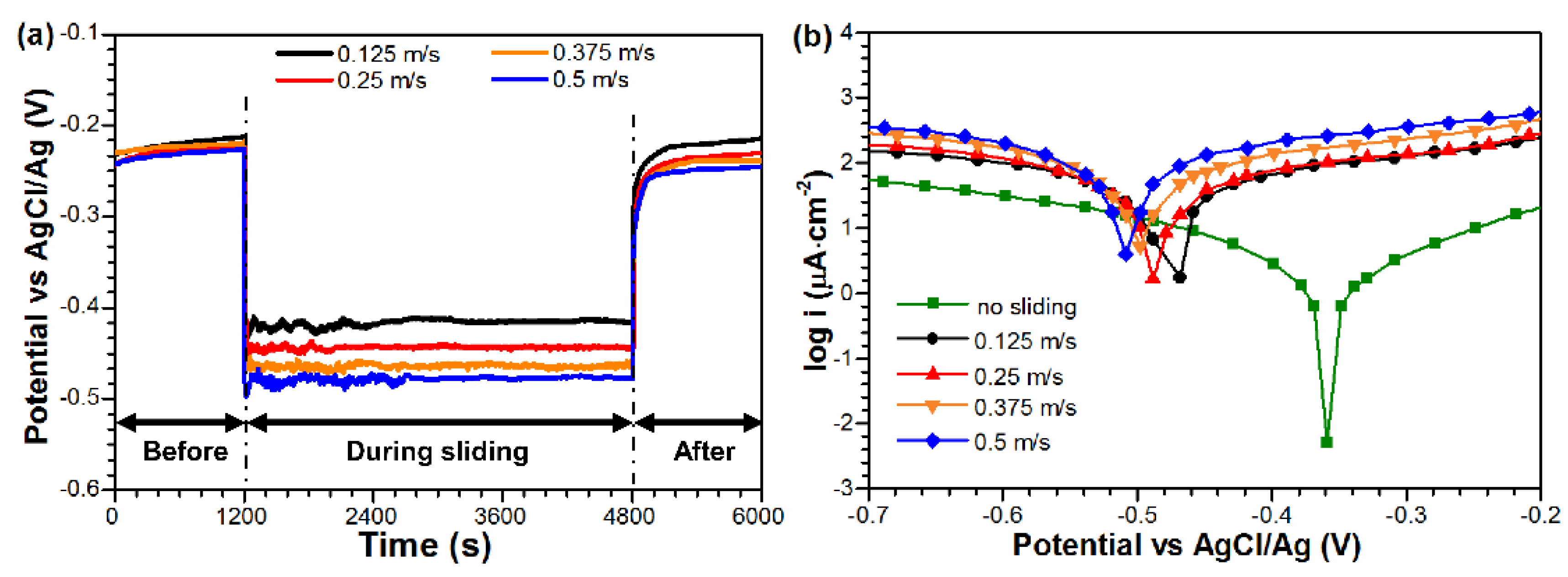
3.2. Electrochemical Corrosion Behavior
3.3. Tribo-Corrosion Behavior
3.4. Damage Morphologies
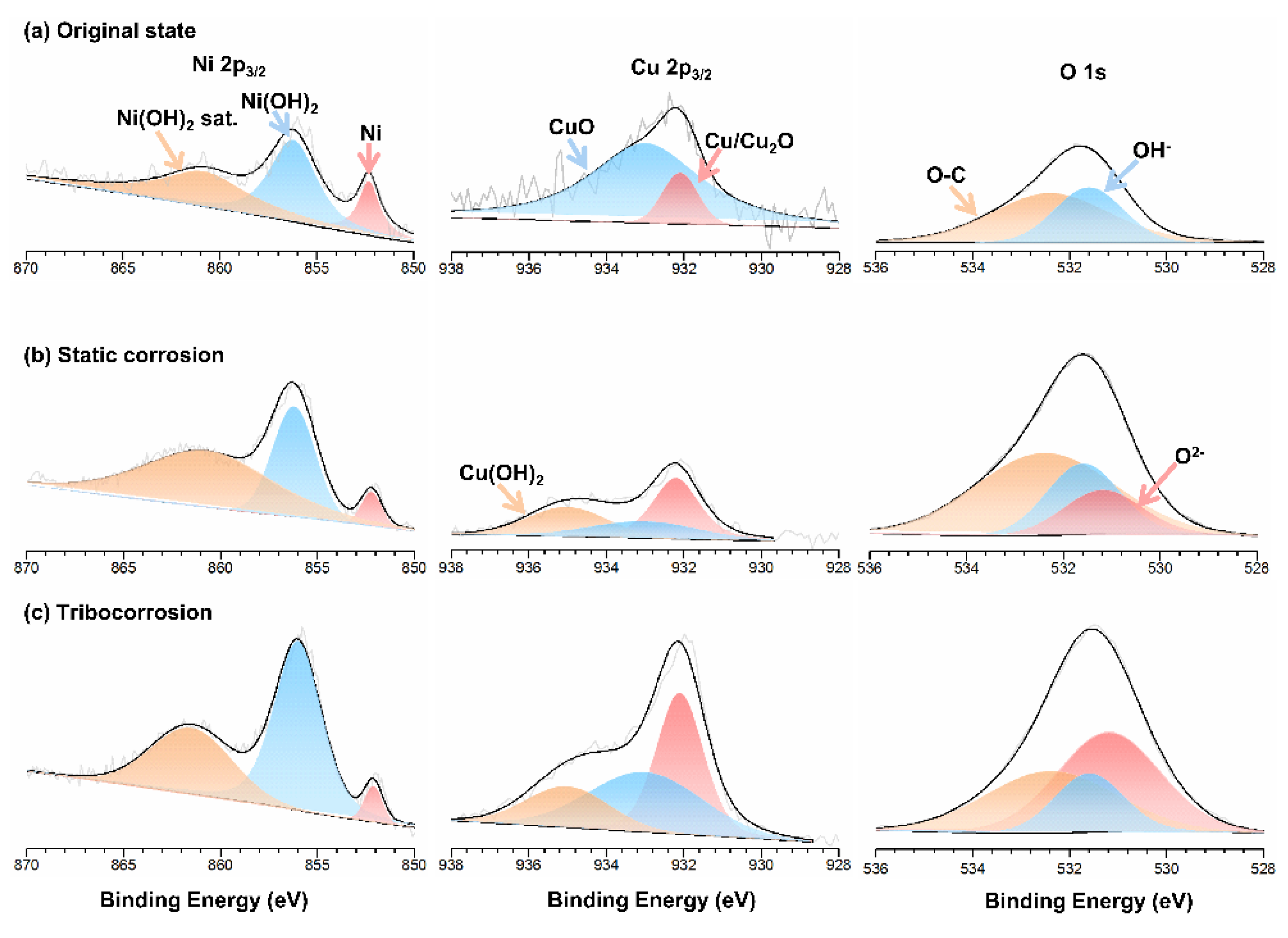
3.5. XPS Analysis of Corrosion Products
4. Discussion
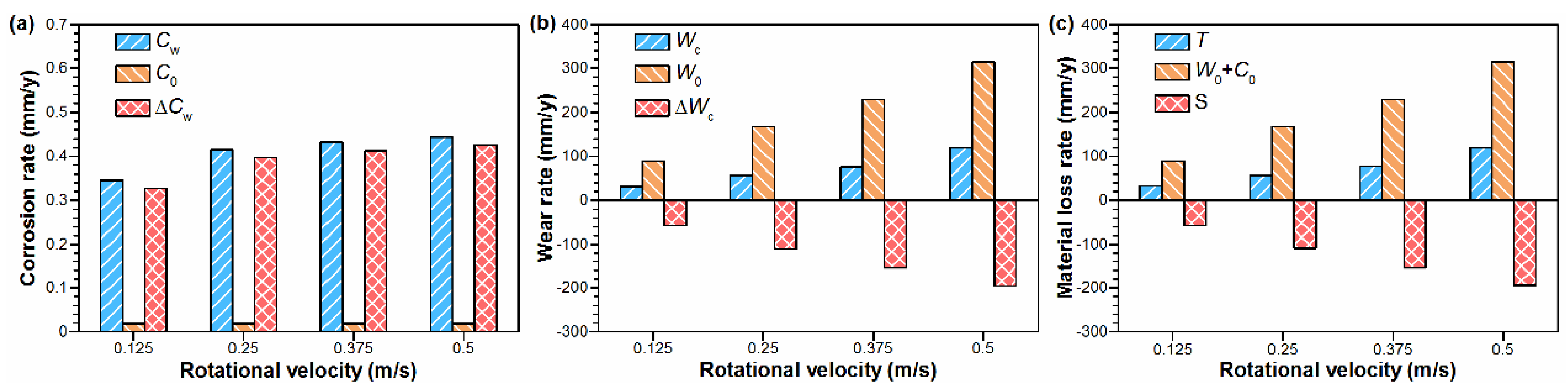
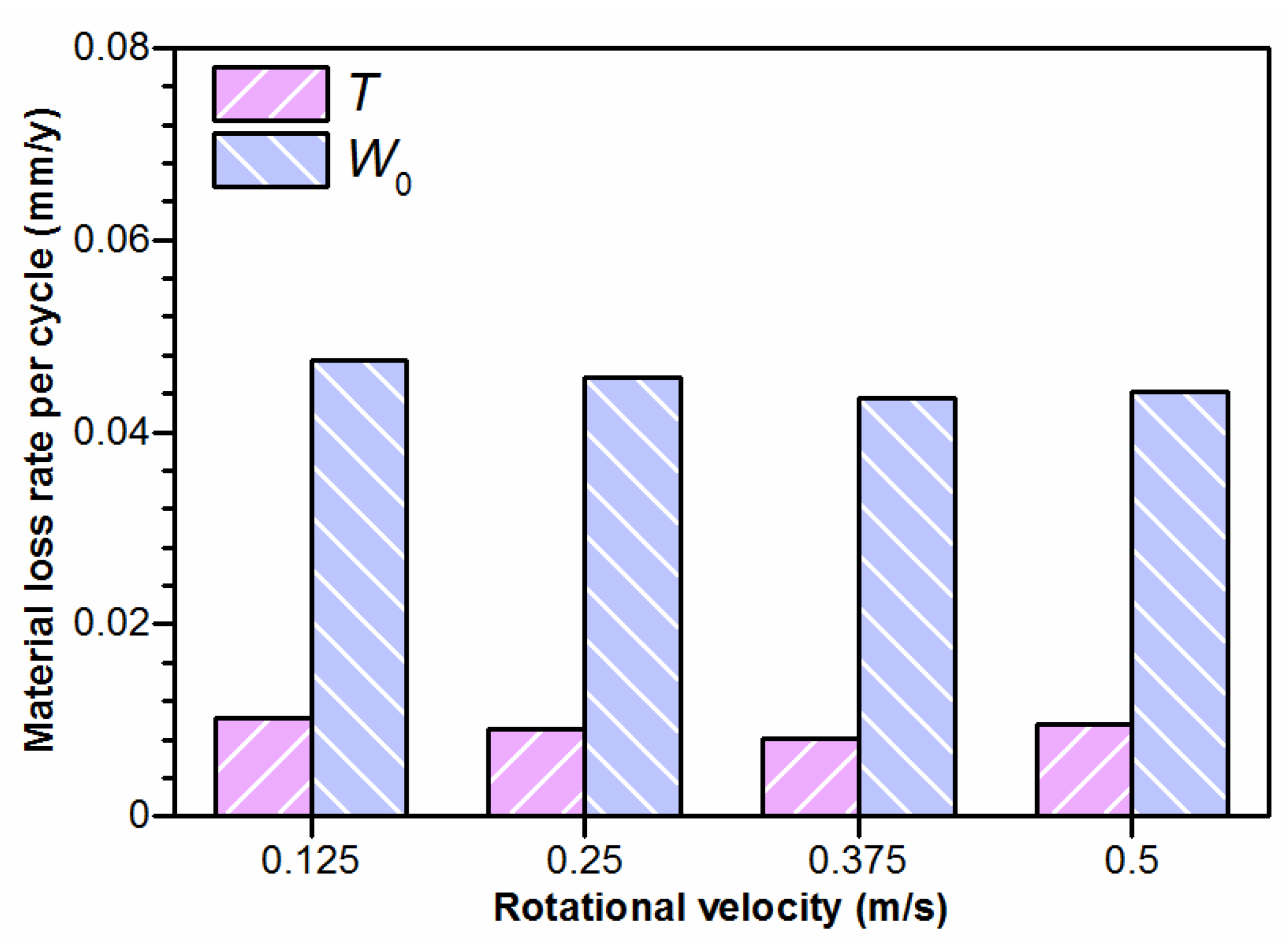
4.1. Effect of Rotational Velocity on Mechanical Wear
4.2. Effect of Rotational Velocity on Corrosion
5. Conclusions
- The total material loss and friction coefficient increased with the increasing velocity, presenting as delamination alongside the sliding direction at the surface and the crack nucleation and propagation at the subsurface.
- Compared with mechanical wear, the damage induced by tribo-corrosion was much lower due to the formation of the corrosion product’s layer in a thickness of several tens of nanometers, exhibiting excellent lubricating performance.
- After the mechanical wear tests, the increased material loss rate with the increasing velocity was mainly the result of the accumulation of the sliding distance.
- Since the thickness of the corrosion product’s layer decreases from 50 nm to 30 nm as the velocity increases from 0.125 m/s to 0.5 m/s, the material loss rate after the tribo-corrosion tests also increases with the velocities increasing, possibly due to the rebuilt balance between the passivation and slowed repassivation at higher velocities.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Wang, S.; Wang, H.; Ruan, G.; Xiao, Y.; Jiang, Z.; Li, X. Research status and prospects of tribological behaviors of key friction pairs of materials in marine equipment. Mater. Sci.-Medzg. 2021, 27, 148–154. [Google Scholar] [CrossRef]
- Li, R.; Wen, C.; Meng, X.; Xie, Y. Measurement of the friction force of sliding friction pairs in low-speed marine diesel engines and comparison with numerical simulation. Appl. Ocean. Res. 2022, 121, 103089. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, D.; Chen, B.; Zhao, J. Friction and wear study on friction pairs with a biomimetic non-smooth surface of 316L relative to CF/PEEK under a seawater lubricated condition. Chin. J. Mech. Eng. 2019, 32, 66. [Google Scholar] [CrossRef]
- Yuan, S.J.; Pehkonen, S.O. Surface characterization and corrosion behavior of 70/30 Cu-Ni alloy in pristine and sulfide-containing simulated seawater. Corros. Sci. 2007, 49, 1276–1304. [Google Scholar] [CrossRef]
- Marenych, O.; Kostryzhev, A. Strengthening Mechanisms in Nickel-Copper Alloys: A Review. Metals 2020, 10, 1358. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, S.; Wang, S. Effect of sliding speed and hardness on wear behavior and mechanism of AISI H13 steel. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2020, 234, 1822–1833. [Google Scholar] [CrossRef]
- Mahmud, D.N.F.; Bin Abdollah, M.F.; Bin Masripan, N.A.; Tamaldin, N.; Amiruddin, H. Influence of contact pressure and sliding speed dependence on the tribological characteristics of an activated carbon-epoxy composite derived from palm kernel under dry sliding conditions. Friction 2019, 7, 227–236. [Google Scholar] [CrossRef]
- Hedenqvist, P.; Olsson, M. Sliding Wear Testing of Coated Cutting-Tool Materials. Tribol. Int. 1991, 24, 143–150. [Google Scholar] [CrossRef]
- Henry, P.; Takadoum, J.; Bercot, P. Depassivation of some metals by sliding friction. Corros. Sci. 2011, 53, 320–328. [Google Scholar] [CrossRef]
- Chen, J.; Yan, F. Corrosive Wear Performance of Monel K500 Alloy in Artificial Seawater. Tribol. Trans. 2013, 56, 848–856. [Google Scholar] [CrossRef]
- Mischler, S.; Debaud, S.; Landolt, D. Wear-accelerated corrosion of passive metals in tribocorrosion systems. J. Electrochem. Soc. 1998, 145, 750–758. [Google Scholar] [CrossRef]
- Obadele, B.A.; Andrews, A.; Shongwe, M.B.; Olubambi, P.A. Tribocorrosion behaviours of AISI 310 and AISI 316 austenitic stainless steels in 3.5% NaCl solution. Mater. Chem. Phys. 2016, 171, 239–246. [Google Scholar] [CrossRef]
- Xu, Y.D.; Qi, J.H.; Nutter, J.; Sharp, J.; Bai, M.W.; Ma, L.; Rainforth, W.M. Correlation between the formation of tribofilm and repassivation in biomedical titanium alloys during tribocorrosion. Tribol. Int. 2021, 163, 107147. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Hao, X.H.; Huang, B.X.; Zhao, X.C.; Hu, C.C. Microstructure and tribocorrosion properties of NiTi/AlNi2Ti ternary intermetallic alloy. Vacuum 2021, 184, 109928. [Google Scholar] [CrossRef]
- Kossman, S.; Coelho, L.B.; Mejias, A.; Montagne, A.; Van Gorp, A.; Coorevits, T.; Touzin, M.; Poorteman, M.; Olivier, M.G.; Iost, A.; et al. Impact of industrially applied surface finishing processes on tribocorrosion performance of 316L stainless steel. Wear 2020, 456, 203341. [Google Scholar] [CrossRef]
- Ziomek-Moroz, M.; Miller, A.; Hawk, J.; Cadien, K.; Li, D.Y. An overview of corrosion-wear interaction for planarizing metallic thin films. Wear 2003, 255, 869–874. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.G.; Shi, C.B.; Wang, L.W.; Xue, X.D. Corrosion and wear behavior of Ti-30Zr alloy for dental implants. Mater. Res. Express 2019, 6, 0865c8. [Google Scholar] [CrossRef]
- Namus, R.; Nutter, J.; Qi, J.; Rainforth, W.M. Sliding speed influence on the tribo-corrosion behaviour of Ti6Al4V alloy in simulated body fluid. Tribol. Int. 2021, 160, 107023. [Google Scholar] [CrossRef]
- Bi, Q.; Liu, W.; Ma, J.; Yang, J.; Pu, Y.; Xue, Q. Tribocorrosion behavior of Ni-17.5Si-29.3Cr alloy in sulfuric acid solution. Tribol. Int. 2009, 42, 1081–1087. [Google Scholar] [CrossRef]
- Ferreira, D.F.; Ale Almeida, S.M.; Soares, R.B.; Juliani, L.; Bracarense, A.Q.; Cunha Lins, V.d.F.; Rabelo Junqueira, R.M. Synergism between mechanical wear and corrosion on tribocorrosion of a titanium alloy in a Ringer solution. J. Mater. Res. Technol. 2019, 8, 1593–1600. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, H.; Zhang, D.; Wang, J.; Yan, F. Effect of polarization potentials on tribocorrosion behavior of Monel 400 alloy in seawater environment. Tribol. Int. 2022, 168, 107445. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Liu, H.; Wang, J.Z.; Yan, F.Y. Antagonistic effect of electrochemical corrosion on the mechanical wear of Monel 400 alloy in seawater. Corros. Sci. 2022, 198, 110120. [Google Scholar] [CrossRef]
- Ayiania, M.; Smith, M.; Hensley, A.J.R.; Scudiero, L.; McEwen, J.-S.; Garcia-Perez, M. Deconvoluting the XPS spectra for nitrogen-doped chars: An analysis from first principles. Carbon 2020, 162, 528–544. [Google Scholar] [CrossRef]
- Lee, E.; Lee, C.E.; Han, J.H. Effects of proton irradiation on single-stranded DNA studied by using X-ray photoelectron spectroscopy. J. Korean Phys. Soc. 2016, 69, 578–583. [Google Scholar] [CrossRef]
- Okada, K.; Kotani, A. Oxygen is core-level photoemission as a tool to investigate the unoccupied electronic states of cuprates. J. Phys. Soc. Jpn. 2006, 75, 123703. [Google Scholar] [CrossRef]
- Chen, W.; Chu, M.; Gao, L.; Mao, L.; Yuan, J.; Shangguan, W. Ni(OH)(2) loaded on TaON for enhancing photocatalytic water splitting activity under visible light irradiation. Appl. Surf. Sci. 2015, 324, 432–437. [Google Scholar] [CrossRef]
- Jeong, D.J.; Kim, W.S.; Choi, Y.K.; Sung, Y.E. Intercalation/deinterealation characteristics of electrodeposited and anodized nickel thin film on ITO electrode in aqueous and nonaqueous electrolytes. J. Electroanal. Chem. 2001, 511, 79–87. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Wang, M.; Guo, X. Facile In Situ Synthesis of Hierarchical Porous Ni/Ni(OH)(2) Hybrid Sponges with Excellent Electrochemical Energy-Storage Performances for Supercapacitors. Chem. Asian J. 2014, 9, 2590–2596. [Google Scholar] [CrossRef]
- Tao, Z.; Gu, J.; Fang, H.; Chen, L.; Chen, J. Cu doped epsilon-Fe2-3N films with manipulable magnetic and transport properties. Mater. Lett. 2019, 250, 131–134. [Google Scholar] [CrossRef]
- Arun, L.; Karthikeyan, C.; Philip, D.; Sasikumar, M.; Elanthamilan, E.; Merlin, J.P.; Unni, C. Effect of Ni2+ doping on chemocatalytic and supercapacitor performance of biosynthesized nanostructured CuO. J. Mater. Sci.-Mater. Electron. 2018, 29, 21180–21193. [Google Scholar] [CrossRef]
- Pinon-Espitia, M.; Lardizabal-Gutierrez, D.; Camacho-Rios, M.L.; Herrera-Perez, G.; Ochoa-Lara, M.T. Electronic structure comparison of Cu 2p and O 1s X-Ray photoelectron spectra for CuxO nanofibers (x = 1, 2, i). Mater. Chem. Phys. 2021, 272, 124981. [Google Scholar] [CrossRef]
- Hansen, N.; Jensen, D.J. Development of microstructure in FCC metals during cold work. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 1999, 357, 1447–1469. [Google Scholar] [CrossRef]
- Ebrahimi, G.R.; Momeni, A.; Ezatpour, H.R.; Jahazi, M.; Bocher, P. Dynamic recrystallization in Mone1400 Ni-Cu alloy: Mechanism and role of twinning. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2019, 744, 376–385. [Google Scholar] [CrossRef]
- Singh, J.B.; Cai, W.; Bellon, P. Dry sliding of Cu-15 wt%Ni-8 wt%Sn bronze: Wear behaviour and micro structures. Wear 2007, 263, 830–841. [Google Scholar] [CrossRef]
- Roncery, L.M.; Jacome, L.A.; Aghajani, A.; Theisen, W.; Weber, S. Subsurface characterization of high-strength high-interstitial austenitic steels after impact wear. Wear 2018, 402, 137–147. [Google Scholar] [CrossRef]
- Stemp, M.; Mischler, S.; Landolt, D. The effect of mechanical and electrochemical parameters on the tribocorrosion rate of stainless steel in sulphuric acid. Wear 2003, 255, 466–475. [Google Scholar] [CrossRef]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Benea, L.; Ponthiaux, P.; Wenger, F.; Galland, J.; Hertz, D.; Malo, J.Y. Tribocorrosion of stellite 6 in sulphuric acid medium: Electrochemical behaviour and wear. Wear 2004, 256, 948–953. [Google Scholar] [CrossRef]






| Element | Cu | Fe | Mn | Si | C | S | P | Ni |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 29.65 | 1.85 | 1.09 | 0.22 | 0.08 | 0.003 | 0.003 | Bal. |
| Components | Concentration (g/L) |
|---|---|
| NaCl | 24.53 |
| MgCl2 | 5.20 |
| Na2SO4 | 4.09 |
| CaCl2 | 1.16 |
| KCl | 0.695 |
| NaHCO3 | 0.201 |
| KBr | 0.101 |
| SrCl2 | 0.025 |
| H3BO3 | 0.027 |
| NaF | 0.003 |
| Samples | Ni 2p3/2/Binding Energy/Amount | Cu 2p3/2/Binding Energy/Amount | O 1s/Binding Energy/Amount | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Original State | Ni metal Ni(OH)2 | 852.3 | 15.5% | Cu/Cu2O | 932.1 | 12.9% | O2− | / | / | |
| Main | 856.2 | 43.8% | CuO | 933.0 | 87.1% | OH− | 531.6 | 36.9% | ||
| Sat. | 860.8 | 40.7% | Cu(OH)2 | / | / | O-C | 532.4 | 63.1% | ||
| Static Corrosion | Ni metal Ni(OH)2 | 852.3 | 5.2% | Cu/Cu2O | 932.1 | 45.5% | O2− | 531.2 | 17.1% | |
| Main | 856.2 | 54.2% | CuO | 933.0 | 23.7% | OH− | 531.6 | 25.8% | ||
| Sat. | 860.8 | 40.6% | Cu(OH)2 | 935.0 | 30.8% | O-C | 532.4 | 57.1% | ||
| Tribo-Corrosion | Ni metal | 852.3 | 4.1% | Cu/Cu2O | 932.1 | 41.7% | O2− | 531.2 | 46.1% | |
| Ni(OH)2 | Main | 856.2 | 61.1% | CuO | 933.0 | 38.7% | OH− | 531.6 | 18.2% | |
| Sat. | 860.8 | 34.8% | Cu(OH)2 | 935.0 | 19.6% | O–C | 532.4 | 35.7% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wang, J.; Liu, H.; Yan, F. The Tribo-Corrosion Behavior of Monel 400 Alloy in Marine Environment at Varied Rotational Velocities. Metals 2022, 12, 1503. https://doi.org/10.3390/met12091503
Zhu Y, Wang J, Liu H, Yan F. The Tribo-Corrosion Behavior of Monel 400 Alloy in Marine Environment at Varied Rotational Velocities. Metals. 2022; 12(9):1503. https://doi.org/10.3390/met12091503
Chicago/Turabian StyleZhu, Yuhua, Jianzhang Wang, Hao Liu, and Fengyuan Yan. 2022. "The Tribo-Corrosion Behavior of Monel 400 Alloy in Marine Environment at Varied Rotational Velocities" Metals 12, no. 9: 1503. https://doi.org/10.3390/met12091503
APA StyleZhu, Y., Wang, J., Liu, H., & Yan, F. (2022). The Tribo-Corrosion Behavior of Monel 400 Alloy in Marine Environment at Varied Rotational Velocities. Metals, 12(9), 1503. https://doi.org/10.3390/met12091503





