Effect of High-Sintering-Temperature Reduction Behavior on Coke Solution Loss Reaction with Different Thermal Properties
Abstract
:1. Introduction
2. Experiment
2.1. Sample
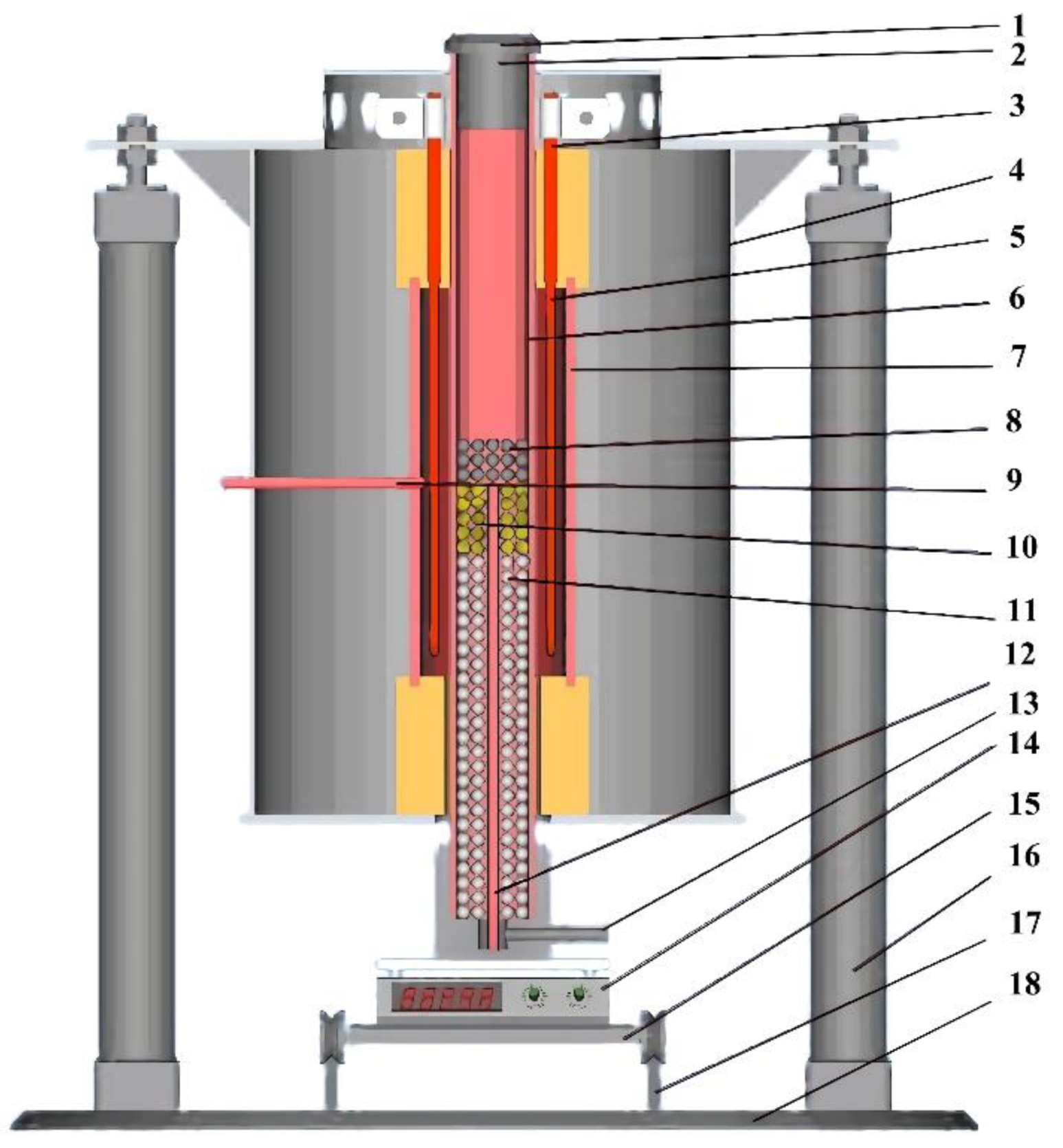
2.2. Experimental Device
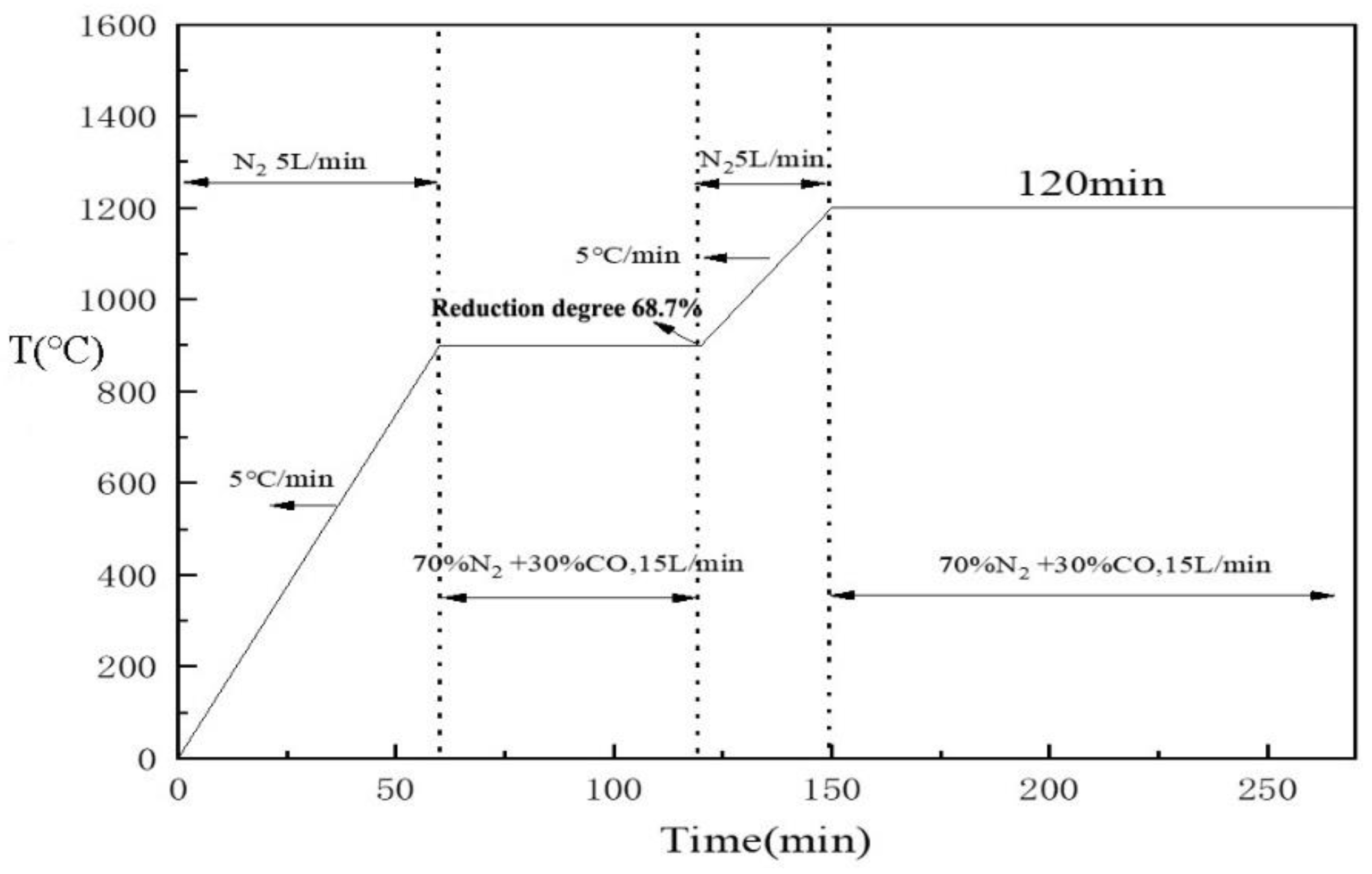
2.3. Experimental Methods
2.3.1. Sample
2.3.2. High-Temperature Reduction Experiment
2.3.3. Index
3. Experimental Results and Analysis
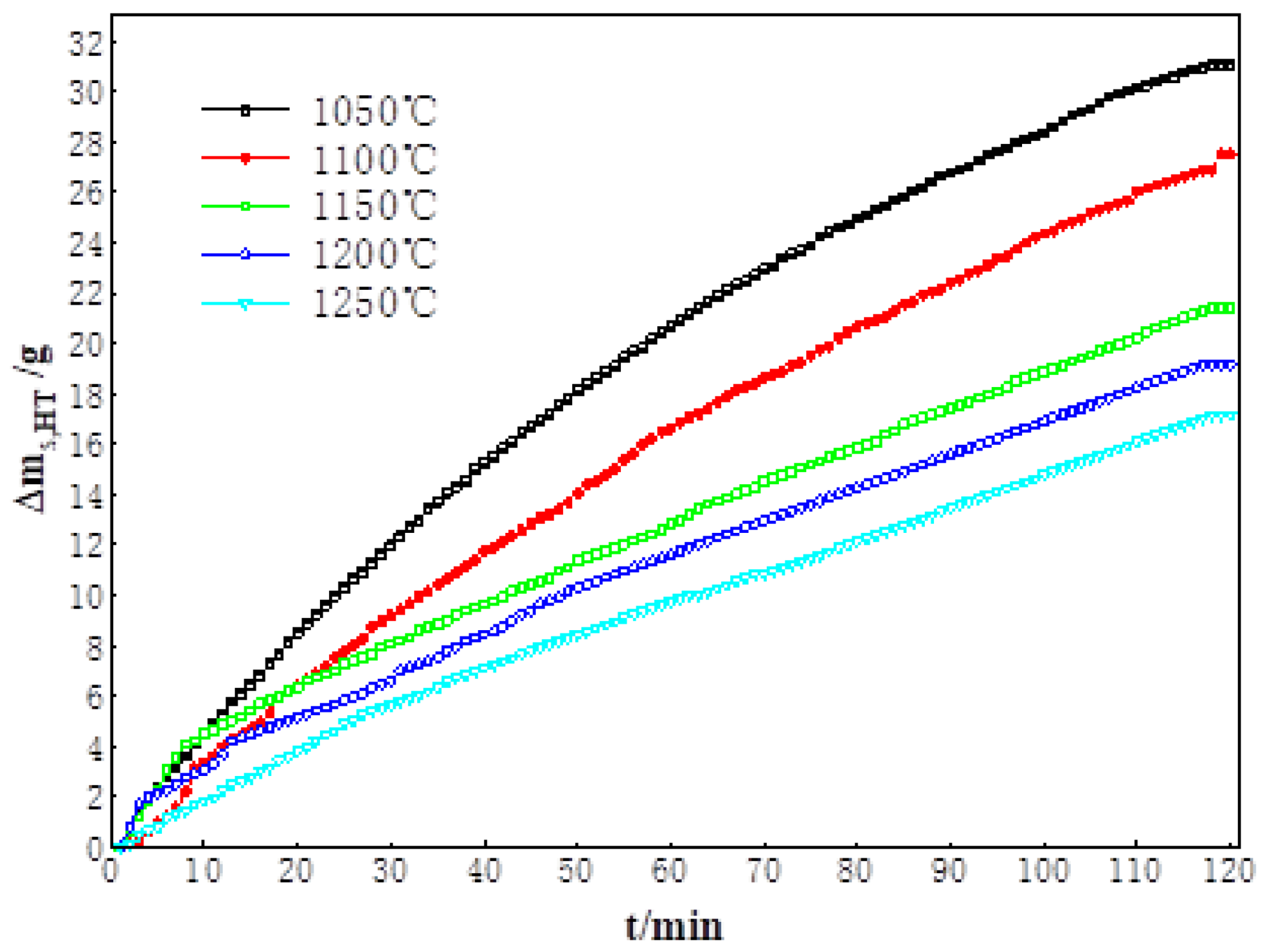
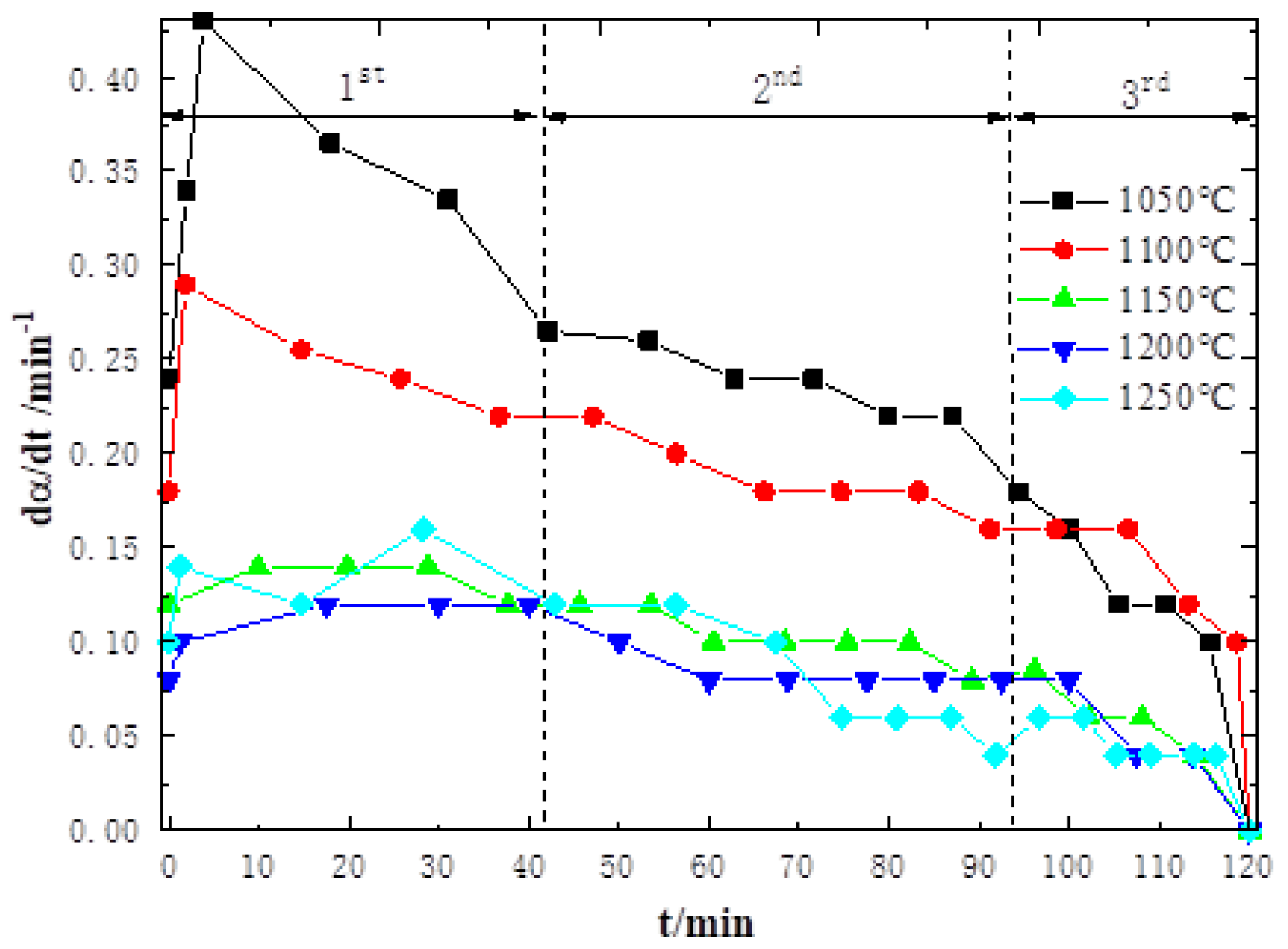
3.1. High-Temperature Reduction Behavior and Characteristics of Sinter
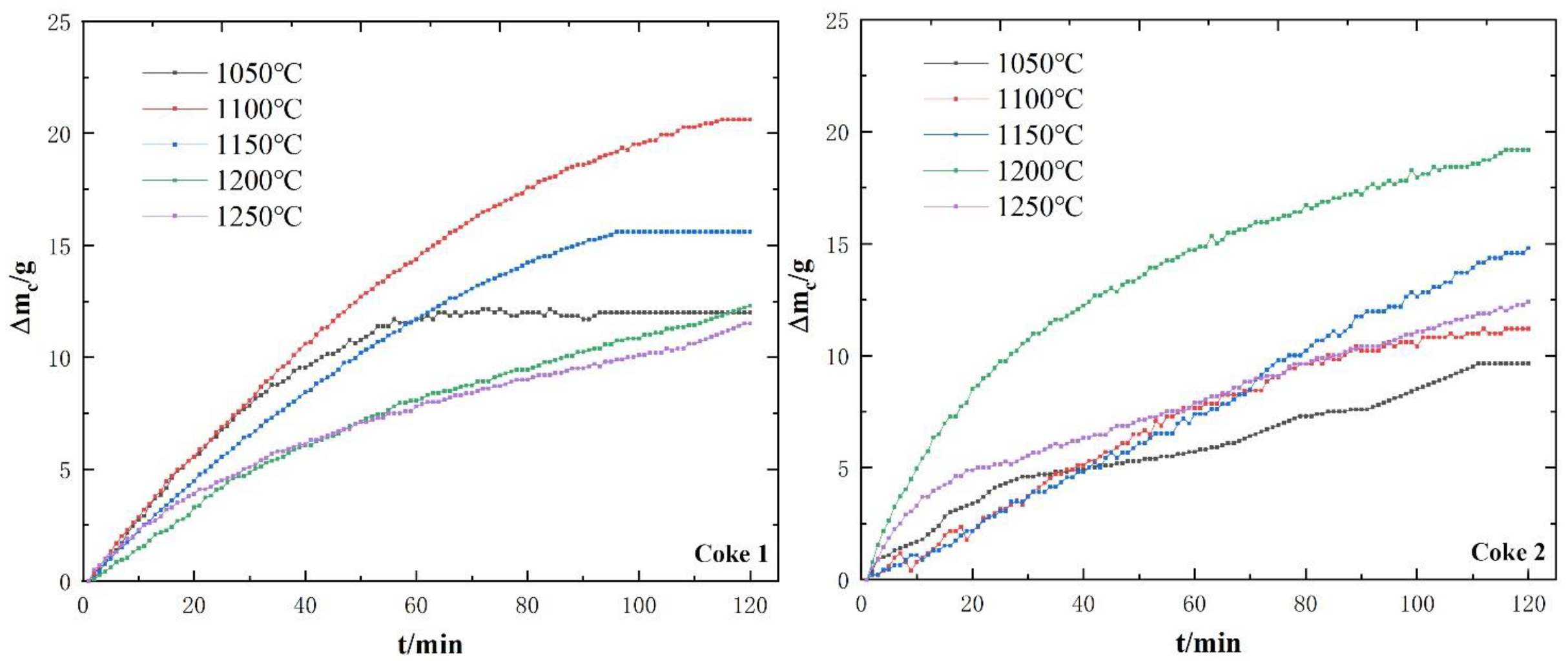
3.2. Behavior and Characteristics of Coke Dissolution Loss
4. Conclusions
- (1)
- From the mutual verification and analysis of non-isothermal equal conversion rate method and isothermal fitting model method, it is found that the diffusion step in the sinter reduction reaction is the limiting link in each stage of the high-temperature region. As a result, the reduction rate of sinter in the coupling reaction decreases with increasing temperature, which, in turn, affects the degradation behavior and characteristics of coke.
- (2)
- In the coupling reaction between high-reactive coke sample and sinter, the most severe dissolution rate, RCSL, is observed near 1100 °C, which is also the lowest point of the CSCSL value after the dissolution of high-reactive coke. However, in the coupling reaction between the low-reactivity coke sample and sinter, the most severe dissolution rate, RCSL, is observed near 1200 °C, which is also the lowest point of the CSCSL value after the dissolution of low-reactivity coke. Sinter reduction has different effects on the degradation of high- and low-reactivity coke.
- (3)
- In the coupling reaction between sinter and coke, the dissolution rate, RCSL, and the strength, CSCSL, of the two cokes have a good linear relationship at all temperature points and a good correlation with the coupling factor. Between sinter and coke, the temperature range with the best matching degree is the position where the coupling factor is closest to 1. The limiting link of the coupling reaction is obtained from the coupling factor, and the reason why high- and low-reaction coke can maintain their thermal performance in the high-temperature softening zone of a blast furnace due to the reduction behavior of sinter is revealed, so as to obtain a more accurate basis for evaluating and calculating the thermal performance of coke.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.-J. Relationship between essential characteristic of metallurgical coke and modern blast furnace smelting. Iron Steel 2016, 51, 78. [Google Scholar]
- Zhang, S.; Peng, L.; Rui, G.; Pengfei, L.; Yinhua, L. Preparation of high strength and highly reactive coke by the addition of steel slag. Coke Chem. 2014, 57, 391–397. [Google Scholar]
- Meng, F.; Shao, L.; Zou, Z. Investigation of the effects of coke reactivity and iron ore reducibility on the gas utilization efficiency of blast Furnace. Energies 2020, 13, 5062. [Google Scholar] [CrossRef]
- Rejdak, M.; Bigda, R.; Wojtaszek, M. Use of alternative raw materials in coke-making: New insights in the use of lignites for blast furnace coke production. Energies 2020, 13, 2832. [Google Scholar] [CrossRef]
- Naito, M.K.; Takeda, K.; Matsui, Y. Ironmaking technology for the last 100 years: Deployment to advanced technologies from introduction of technological know-how, and Evolution to Next-generation Process. ISIJ Int. 2015, 55, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.W.; Chu, M.S.; Liu, Z.G.; Han, D.; Cao, L.G.; Guo, J. Research progress on preparation and application of iron coke in blast furnace. Iron Steel 2020, 55, 38–48. [Google Scholar]
- Guo, D.Y.; Li, D.T.; Zhang, X.M.; Ma, C.; Dai, X.; Liu, Y. Optical structure and thermal and cold properties of different rank coal coke. Iron Steel 2020, 55, 20–26. [Google Scholar]
- Wang, Q. Effect of coke solution loss reaction on iron-making by blast furnace and discussion on high temperature properties of coke. Ansteel Technol. 2013, 1–8. [Google Scholar]
- Cheng, A. Coke quality requirements for blast furnaces, Part VIII. Ironmak. Steelmak. 2001, 28, 78–81. [Google Scholar]
- Nomura, S.; Ayukawa, H.; Kitaguchi, H.; Tahara, T.; Matsuzaki, S.; Naito, M.; Koizumi, S.; Ogata, Y.; Nakayama, T.; Abe, T. Improvement in blast furnace reaction efficiency through the use of highly reactive calcium rich coke. ISIJ Int. 2005, 45, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Nomura, S.; Naito, M.; Yamaguchi, K. Post-reaction strength of catalyst-added highly reactive coke. ISIJ Int. 2007, 47, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Guo, R.; Zhao, X.F.; Sun, J.F.; Zhang, S.; Liu, W.Z. A new testing and evaluation method of cokes with greatly varied CRI and CSR. Fuel 2016, 182, 879. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Q.; Zhang, S. Kinetics and mechanisms of coke and sinter on the coupling reaction to evaluate the integrated effects of coke solution loss reaction on blast furnace processes. Metall. Res. Technol. 2021, 118, 506. [Google Scholar] [CrossRef]
- Zamalloa, M.; Utigard, T.A. Characterization of industrial coke structures. ISIJ Int. 1995, 35, 449–457. [Google Scholar] [CrossRef]
- Hilding, T.; Gupta, S.; Sahajwalla, V.; Bjoerkman, B.; Wikstroem, J.O. Degradation behaviour of a high CSR coke in an experimental blast furnace: Effect of carbon structure and alkali reactions. ISIJ Int. 2005, 45, 1041–1050. [Google Scholar] [CrossRef] [Green Version]
- Rao, Y.K. The kinetics of reduction of hematite by carbon. Metall. Trans. 1971, 2, 1439–1447. [Google Scholar] [CrossRef]
- Fruehan, R.J. The rate of reduction of iron oxides by carbon. Metall. Trans. B 1977, 8, 279–286. [Google Scholar] [CrossRef]
- Freeman, E.S.; Carroll, B. The application of thermoanalytical techniques to reaction kinetics: The thermogravimetric evaluation of the kinetics of the decomposition of calcium oxalate monohydrate. J. Phys. Chem. 1958, 62, 394–397. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2. Thermochim. Acta 2007, 456, 75–88. [Google Scholar] [CrossRef]
- Huang, B.H.; Lu, W.K. Kinetics and mechanisms of reactions in iron ore/coal composites. ISIJ Int. 2007, 33, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Patisson, F.; François, M.G.; Ablitzer†, D. A non-isothermal, non-equimolar transient kinetic model for gas-solid reactions. Chem. Eng. Sci. 2014, 53, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Qie, Y.; Lyu, Q.; Jianpeng, L.I.; Lan, C.; Liu, X. Effect of hydrogen addition on reduction kinetics of iron oxides in gas-injection BF. ISIJ Int. 2017, 57, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Fott, P.; Straka, P. Effect of pore diffusion on reactivity of lump coke. Fuel 1987, 66, 1287–1288. [Google Scholar] [CrossRef]
- Homma, S.; Ogata, S.; Koga, J.; Matsumoto, S. Gas–solid reaction model for a shrinking spherical particle with unreacted shrinking core. Chem. Eng. Sci. 2005, 60, 4971–4980. [Google Scholar] [CrossRef]
- The British Carbonization Resarch Association (BCRA). The Evaluation of the Nippon Steel Corporation Reactivity and Postreaction Strength Test for Coke; Carbonization Research Report 91; British Carbonization Research Association: Chesterfield, UK, 1980. [Google Scholar]
- Sohn, H.Y.; Szekely, J. A structural model for gas—Solid reactions with a moving boundary—IV. Langmuir—Hinshelwood kinetics. Chem. Eng. Sci. 1972, 27, 763–778. [Google Scholar] [CrossRef]
- Maalmi, M.; Strieder, W.C.; Varma, A. Spatiotemporal evolution of conversion and selectivity for simultaneous noncatalytic gas−solid reactions in a compact of particles. Ind. Eng. Chem. Res. 1997, 36, 1470–1479. [Google Scholar] [CrossRef]
- Sohn, I.; Fruehan, R.J. The reduction of iron oxides by volatiles in a rotary hearth furnace process: Part II. The reduction of iron oxide/carbon composites. Metall. Mater. Trans. B 2006, 37, 223–229. [Google Scholar] [CrossRef]
- Ma, Z.; Bai, J.; Wen, X.; Li, X.; Shi, Y.; Bai, Z.; Kong, L.; Guo, Z.; Yan, J.; Li, W. Mineral transformation in char and its effect on coal char gasification reactivity at high temperatures part 3: Carbon thermal reaction. Energy Fuels 2014, 28, 3066–3073. [Google Scholar] [CrossRef]





| TFe | FeO | SiO2 | CaO | MgO | R2 |
|---|---|---|---|---|---|
| 56.00 | 8.40 | 5.28 | 10.10 | 1.79 | 1.91 |
| Fixed C | Ash | Volatiles | S | M10 | M40 | Mt | P | |
|---|---|---|---|---|---|---|---|---|
| coke1 | 87.4 | 12.85 | 0.98 | 0.88 | 5.7 | 81.5 | 0.29 | 0.033 |
| coke2 | 87.12 | 13.98 | 1.29 | 0.87 | 6.2 | 81.9 | 0.31 | 0.035 |
| Temperature | 1050 °C | 1100 °C | 1150 °C | 1200 °C | 1250 °C |
|---|---|---|---|---|---|
| Rs,HT | 27.7 | 23.5 | 18.3 | 14.0 | 11.4 |
| Reaction Model | Symbol | f(α) | g(α) |
|---|---|---|---|
| Mampel power law | M1 | 4α3/4 | α1/4 |
| Mampel power law | M2 | 3α2/3 | α1/3 |
| Mampel power law | M3 | 2α1/2 | α1/2 |
| Avrami–Erofeev | A1 | 4 (1 − α)[−ln(1 − α)]3/4 | [−ln(1 − α)]1/4 |
| Avrami–Erofeev | A2 | 3 (1 − α)[−ln(1 − α)]2/3 | [−ln(1 − α)]1/3 |
| Avrami–Erofeev | A3 | 2 (1 − α)[−ln(1 − α)]1/2 | [−ln(1 − α)]1/2 |
| Reaction order | R1 | 4 (1 − α)3/4 | 1 −(1 − α)1/4 |
| Reaction order | R2 | 3 (1 − α)2/3 | 1 −(1 − α)1/3 |
| Reaction order | R3 | 2 (1 − α)1/2 | 1 −(1 − α)1/2 |
| One-dimensional diffusion | D1 | 1/2α−1 | α2 |
| Ginstling–Brounshtein diffusion | D2 | 3/2 [(1 − α)1/3 −1]−1 | 1 − 2/3α − (1 − α)2/3 |
| Jander diffusion | D3 | 3/2 (1 − α)2/3 [1 − (1 − α)1/3]−1 | [1 −(1 − α)1/3]2 |
| g(Rs,HT) | E/kJ mol−1 | A/s−1 | R2 | |
|---|---|---|---|---|
| 1st Stage | [1 − (1 − α)1/3]2 | 45.79 | 1.09 × 106 | 0.9858 |
| 2nd Stage | 1 − 2α/3 − (1 − α)2/3 | 68.67 | 9.3 × 106 | 0.9871 |
| 3rd Stage | α2 | 63.45 | 6.9 × 106 | 0.9998 |
| T | 1050 °C | 1100 °C | 1150 °C | 1200 °C | 1250 °C | ||
|---|---|---|---|---|---|---|---|
| RCSL | Coke 1 | experiment | 15 | 25 | 19.5 | 15.6 | 14.3 |
| sample | 17.5 | 25.4 | 20 | 16.5 | 15 | ||
| Coke 2 | experiment | 11.8 | 14 | 18.5 | 24 | 15.5 | |
| sample | 12 | 16 | 20 | 25 | 18 |
| T | 1050 °C | 1100 °C | 1150 °C | 1200 °C | 1250 °C |
| Coke 1 | 0.494 | 0.998 | 1.025 | 0.985 | 0.995 |
| Coke 2 | 0.488 | 0.538 | 0.899 | 1.091 | 1.098 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Wang, Q.; Zhang, S. Effect of High-Sintering-Temperature Reduction Behavior on Coke Solution Loss Reaction with Different Thermal Properties. Metals 2023, 13, 117. https://doi.org/10.3390/met13010117
Jin J, Wang Q, Zhang S. Effect of High-Sintering-Temperature Reduction Behavior on Coke Solution Loss Reaction with Different Thermal Properties. Metals. 2023; 13(1):117. https://doi.org/10.3390/met13010117
Chicago/Turabian StyleJin, Jin, Qi Wang, and Song Zhang. 2023. "Effect of High-Sintering-Temperature Reduction Behavior on Coke Solution Loss Reaction with Different Thermal Properties" Metals 13, no. 1: 117. https://doi.org/10.3390/met13010117





