A Survey on the Oxidation Behavior of a Nickel-Based Alloy Used in Natural Gas Engine Exhaust Valve Seats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
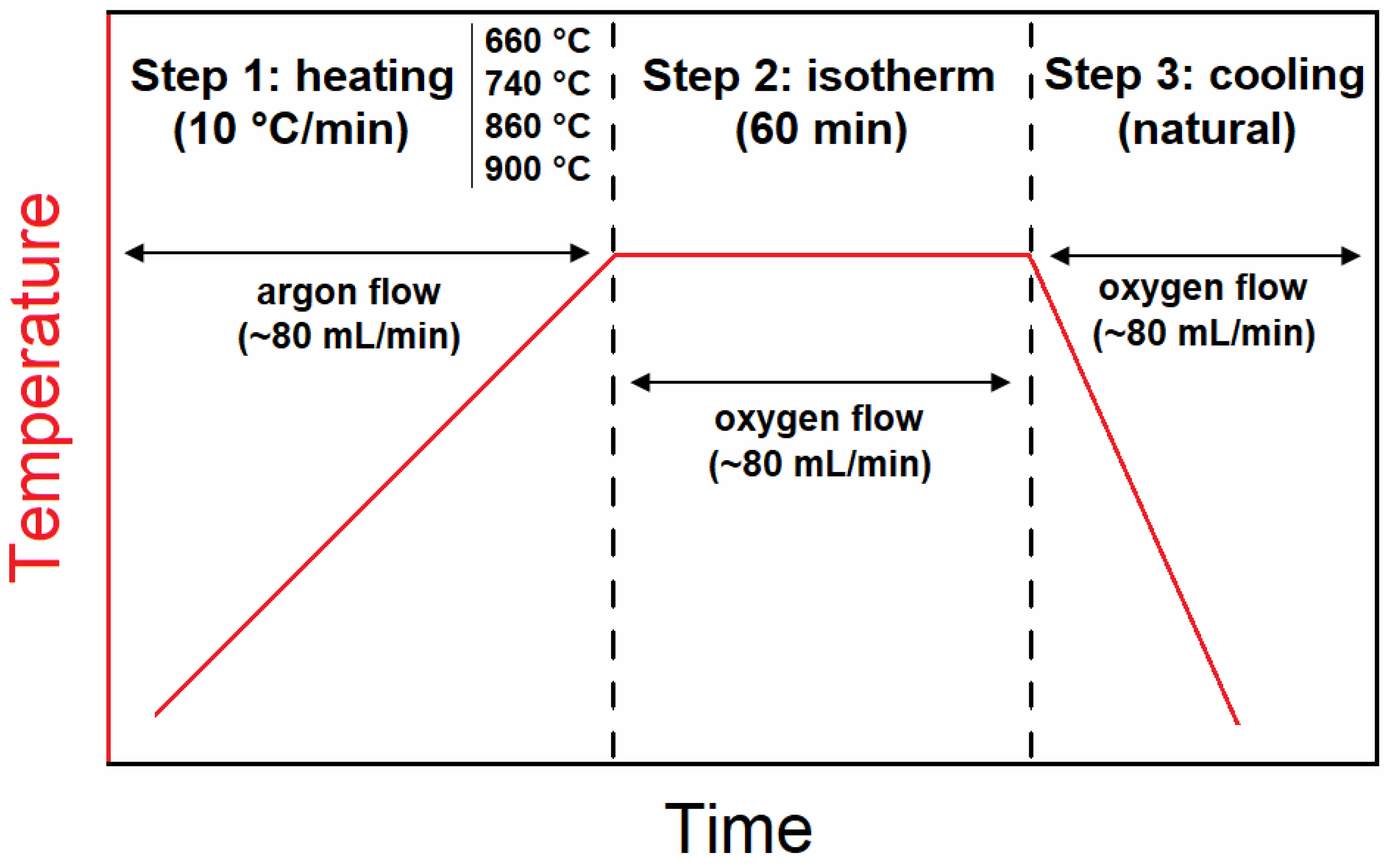
2.2. Isothermal Oxidation Tests
2.3. Materials Characterization
3. Results and Discussion
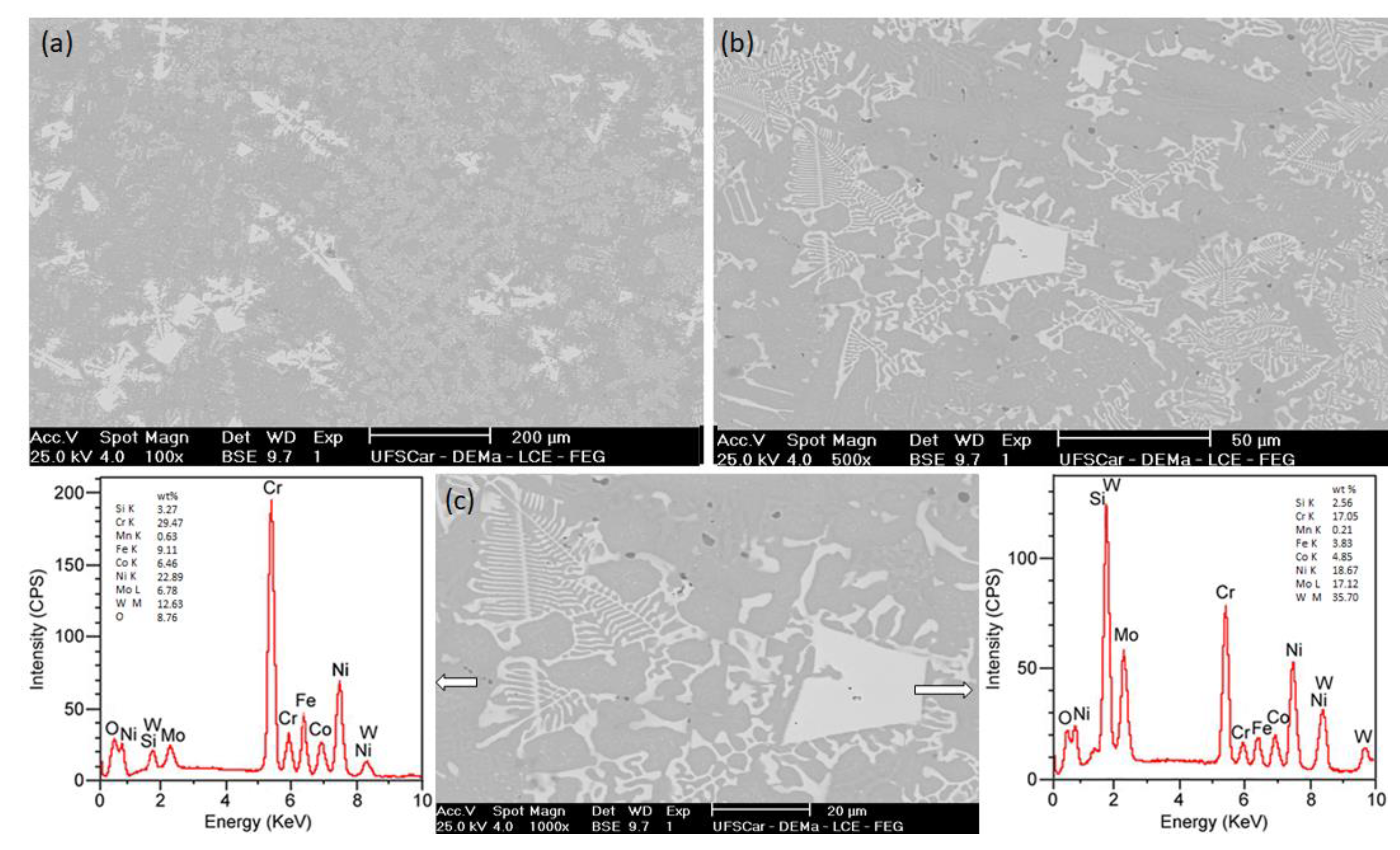
3.1. Nickel-Based Alloy Characterization
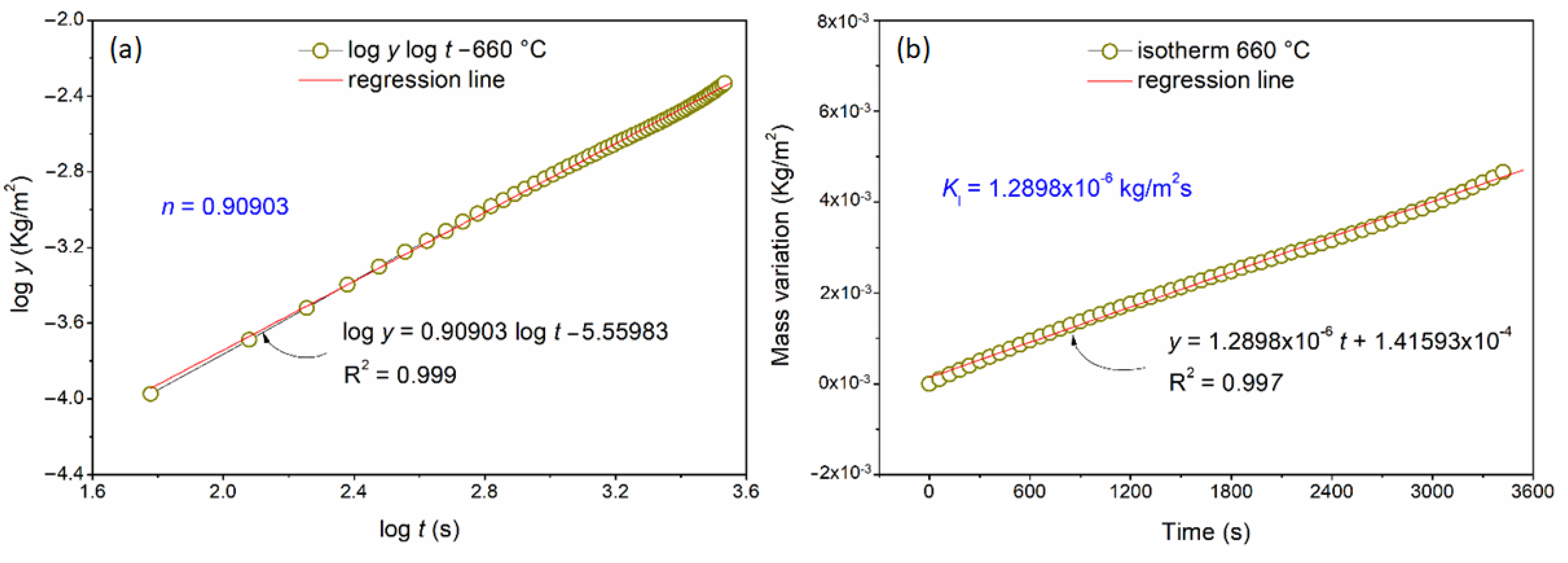
3.2. Thermogravimetric Analysis
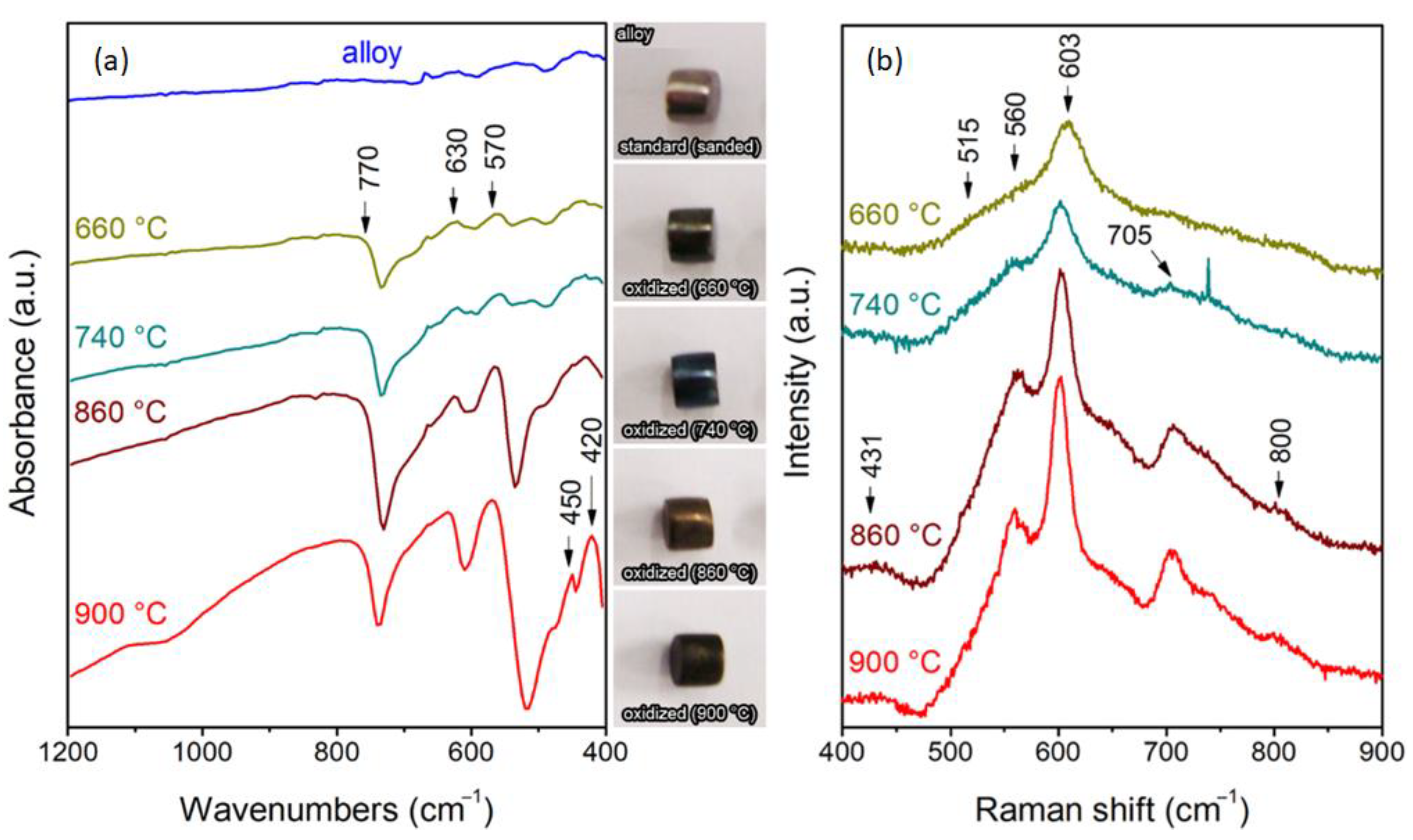
3.3. Characterization of the Formed Oxide Layer
4. Conclusions
- Based on the designed kinetic parameters, the oxidation rate increases as the temperature increases;
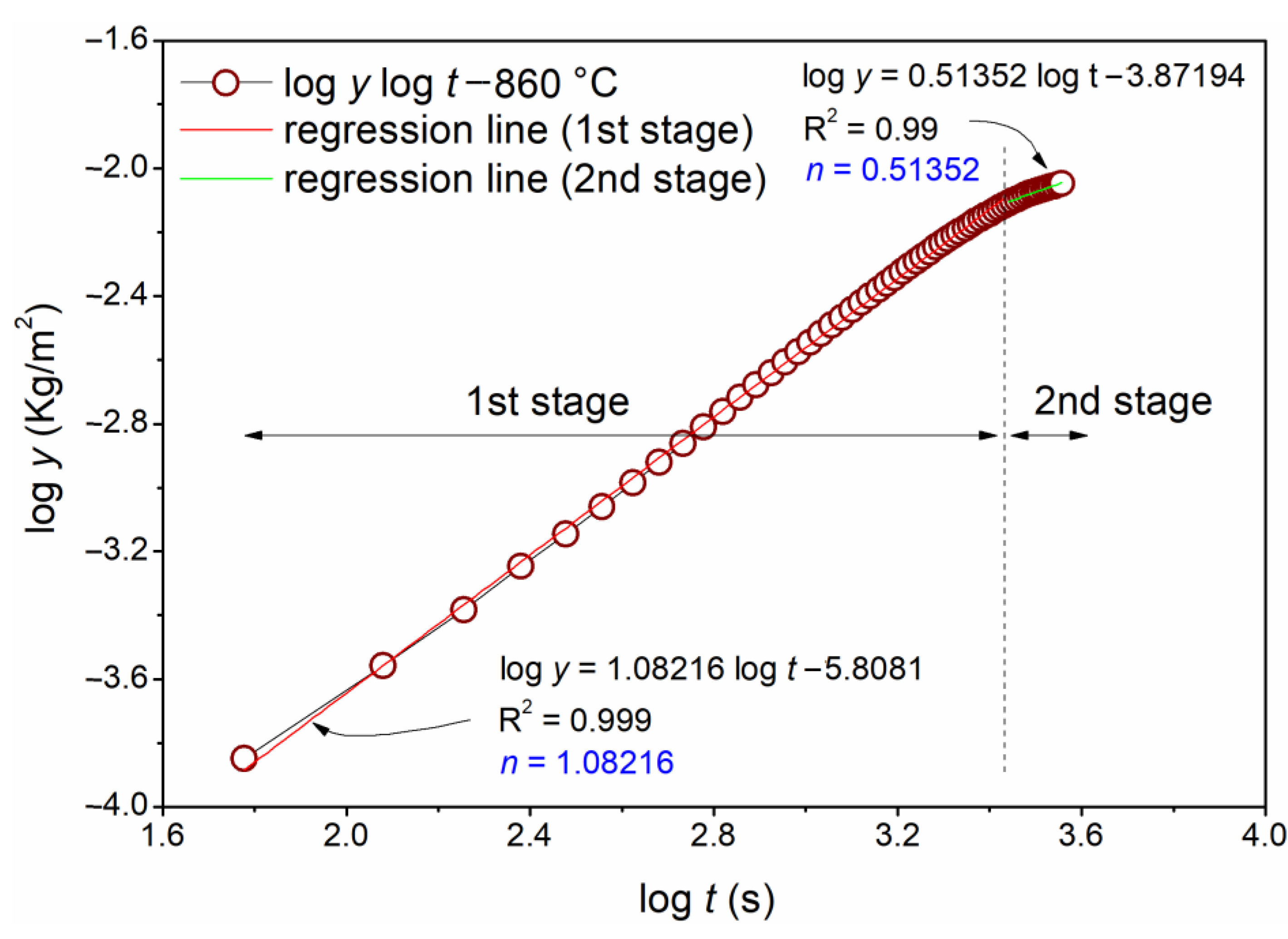
- The samples exposed at 860 and 900 °C exhibited two stages of oxidation, while at 660 and 740 °C, only one stage was observed;
- The first stage (linear behavior) was led by the oxygen reaction rate with the alloy surface. The second stage (parabolic behavior) was led mainly by Ni and Cr species diffusion through the formed oxide layer;
- The apparent activation energy for the first and second stages was 47 kJ/mol and 128 kJ/mol, respectively;
- The Cr2O3 layer that formed on the samples exposed at 900 °C presented a potentially protective character, with no noticeable cavities, pores, or microcracks.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hekkert, M.P.; Hendriks, F.H.J.F.; Faaij, A.P.C.; Neelis, M.L. Natural gas as an alternative to crude oil in automotive fuel chains well-to-wheel analysis and transition strategy development. Energy Policy 2005, 33, 579–594. [Google Scholar] [CrossRef] [Green Version]
- Alkemade, U.G.; Shcumann, B. Engines and exhaust after treatment systems for future automotive applications. Solid State Ion. 2006, 177, 2291–2296. [Google Scholar] [CrossRef]
- Yamagata, H. The Science and Technology of Materials in Automotive Engines, 1st ed.; Woodhead Publishing and Maney Publishing on behalf of The Institute of Materials, Minerals & Mining: Cambridge, UK, 2005; ISBN 9781855739703. [Google Scholar]
- Jang, C.; Lee, D.; Kim, D. Oxidation behaviour of an Alloy 617 in very high-temperature air and helium environments. Int. J. Press. Vessel. Pip. 2008, 85, 368–377. [Google Scholar] [CrossRef]
- Seal, S.; Kuiry, S.C.; Bracho, L.A. Studies on the Surface Chemistry of Oxide Films Formed on IN-738LC Superalloy at Elevated Temperatures in Dry Air. Oxid. Met. 2001, 56, 583–603. [Google Scholar] [CrossRef]
- Huang, J.; Fang, H.; Fu, X.; Huang, F.; Wan, H.; Zhang, Q.; Deng, S.; Zu, J. High-temperature oxidation behavior and mechanism of a new type of wrought Ni-Fe-Cr-Al superalloy up to 1300 °C. Oxid. Met. 2000, 53, 273–287. [Google Scholar] [CrossRef]
- Geng, S.; Wang, F.; Zhu, S. High-temperature oxidation behavior of sputtered IN 738 nanocrystalline coating. Oxid. Met. 2002, 57, 231–243. [Google Scholar] [CrossRef]
- Berthod, P. Kinetics of high temperature oxidation and chromia volatilization for a binary Ni-Cr alloy. Oxid. Met. 2005, 64, 235–252. [Google Scholar] [CrossRef] [Green Version]
- Berthod, P.; Aranda, L.; Mathieu, S.; Vilasi, M. Influence of water vapour on the rate of oxidation of a Ni-25wt.%Cr alloy at high temperature. Oxid. Met. 2013, 79, 517–527. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, X.; Chen, G.; Lu, Z. Improvement of high-temperature oxidation resistance and strength in alumina-forming austenitic stainless steels. Mater. Lett. 2011, 65, 3285–3288. [Google Scholar] [CrossRef]
- Narasimhan, S.L.; Larson, J.M.; Whelan, E.P. Wear Characterization of New Nickel Base Alloys for Internal Combustion Engine Valve Seat Applications. In Proceedings of the Wear of Materials: International Conference on Wear of Materials, San Francisco, CA, USA, 30 March–1 April 1981; Volume 74, pp. 210–218. [Google Scholar]
- Pierce, D.; Haynes, A.; Hughes, J.; Graves, R.; Maziasz, P.; Muralidharan, G.; Shyam, A.; Wang, B.; England, R.; Daniel, C.; et al. High temperature materials for heavy duty diesel engines: Historical and future trends. Prog. Mater. Sci. 2019, 103, 109–179. [Google Scholar] [CrossRef]
- Roberge, P. Corrosion Engineering: Principles and Practice; McGraw-Hill Education: New York, NY, USA, 2008; ISBN 0071640878. [Google Scholar]
- Khan, M.I.; Khan, M.A.; Shakoor, A. A failure analysis of the exhaust valve from a heavy duty natural gas engine. Eng. Fail. Anal. 2018, 85, 77–88. [Google Scholar] [CrossRef]
- Witek, L. Failure and thermo-mechanical stress analysis of the exhaust valve of diesel engine. Eng. Fail. Anal. 2016, 66, 154–165. [Google Scholar] [CrossRef]
- Ootani, T.; Yahata, N.; Fujiki, A.; Ehira, A. Impact Wear Characteristics of Engine Valve and Valve Seat Insert Materials at High Temperature: Impact Wear Tests of Martensitic Heat-Resistant Steel SUH3 Against Fe-Base Sintered Alloy Using Plane Specimens. Wear 1995, 188, 175–184. [Google Scholar] [CrossRef]
- Division, E.C.; Wang, Y.S.; Narasimhan, S.; Larson, J.M.E.; Larson, J.M.E.; Barber, G.C. The effect of operating conditions on heavy duty engine valve seat wear. Wear 1996, 201, 15–25. [Google Scholar]
- Chun, K.J.; Kim, J.H.; Hong, J.S. A study of exhaust valve and seat insert wear depending on cycle numbers. Wear 2007, 263, 1147–1157. [Google Scholar] [CrossRef]
- Khanna, A.S. Introduction to High Temperature Oxidation and Corrosion, 1st ed.; ASM International: Materials Park, OH, USA, 2002; ISBN 9780871707628. [Google Scholar]
- Kubaschewski, O.; Hopkins, B.E. Oxidation of Metals and Alloys, 2nd ed.; Butterworths: Oxford, UK, 1962; ISBN 9780608180144. [Google Scholar]
- Kofstad, V.P. High Temperature Corrosion, 1st ed.; Elsevier Applied Science: London, UK, 1988; ISBN 9781851661541. [Google Scholar]
- Brown, M.E. Introduction to Thermal Analysis: Techniques and Applications, 2nd ed.; Kluwer Academic Publisher: New York, NY, USA, 2001; ISBN 9781402004728. [Google Scholar]
- Wright, M.R. The kinetic analysis of experimental data. In An introduction to Chemical Kinetics, 1st ed.; John Wiley & Sons Ltd.: Chichester, UK, 2004; ISBN 9780470090589. [Google Scholar]
- Atkins, P.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry, 9th ed.; Oxford University Press: London, UUK, 2009; ISBN 9780198769866. [Google Scholar]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High-Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; ISBN 9781139163903. [Google Scholar]
- Ramanathan, L.V. Corrosão e Seu Controle, 1st ed.; Hemus: São Paulo, Brazil, 1997; ISBN 9788528900019. [Google Scholar]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Pearson: New Jersey, NJ, USA, 1995; ISBN 9780133599930. [Google Scholar]
- Gentil, V. Corrosão, 3rd ed.; LTC: Rio de Janeiro, Brazil, 1996; ISBN 9788521618041. [Google Scholar]
- Talbot, D.E.J.; Talbot, J.D.R. Corrosion Science and Technology, 3rd ed.; CRC Press: New York, NY, USA, 2017; ISBN 9780367735340. [Google Scholar]
- Nijdam, T.J.; Sloof, W.G. Effect of y distribution on the oxidation kinetics of NiCoCrAlY bond coat alloys. Oxid. Met. 2008, 69, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Monceau, D.; Pieraggi, B. Determination of Parabolic Rate Constants from a Local Analysis of Mass-Gain Curves. Oxid. Met. 1998, 50, 477–493. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Jin, H.; Okamoto, T. A Fundamental Study of a New Kind of Medium Material for Chemical-Looping Combustion. Energy Fuels 1996, 10, 958–963. [Google Scholar] [CrossRef]
- Calvarin, G.; Molins, R.; Huntz, A.M. Oxidation Mechanism of Ni-20Cr Foils and Its Relation to the Oxide-Scale Microstructure. Oxid. Met. 2000, 53, 25–48. [Google Scholar] [CrossRef]
- Lopez-Navarrete, E.; Ocaña, M. Fine spherical particles of narrow size distribution in the Cr2O3-Al2O3 system. J. Mater. Sci. 2001, 36, 2383–2389. [Google Scholar] [CrossRef]
- Madi, C.; Tabbal, M.; Christidis, T.; Isber, S.; Nsouli, B.; Zahraman, K. Microstructural characterization of chromium oxide thin films grown by remote plasma assisted pulsed laser deposition. J. Phys. Conf. Ser. 2007, 59, 600–604. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Abbasi, S.M. Effect of vacuum degree in VIM furnace on mechanical properties of Ni–Fe–Cr based alloy. Trans. Nonferrous Met. Soc. China 2012, 22, 2124–2130. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Safyari, M. Effect of dwelling time in VIM furnace on chemical composition and mechanical properties of a Ni–Fe–Cr alloy. Vacuum 2019, 169, 108890. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Li, F.; Wang, Z.; Wang, X.; Wang, C.; Zhang, C.; Huang, J.; Mao, F.; Chen, C.; et al. High-Temperature Oxidation Behavior of Cr-Ni-Mo Hot-Work Die Steels. Mater 2022, 15, 5145. [Google Scholar] [CrossRef]
- Rabbani, F.; Ward, L.P.; Strafford, K.N. A Comparison of the Growth Kinetics and Scale Morphology for Three Superalloys at 930 °C in Air and Low PO2 Environments. Oxid. Met. 2000, 54, 139–153. [Google Scholar] [CrossRef]
- Koshelev, I.; Paulikas, A.P.; Veal, B.W. Refracted X-Ray Fluorescence (RXF) Applied to the Study of Thin Films and Thermally-Grown Oxide Scales. Oxid. Met. 1999, 51, 23–54. [Google Scholar] [CrossRef]



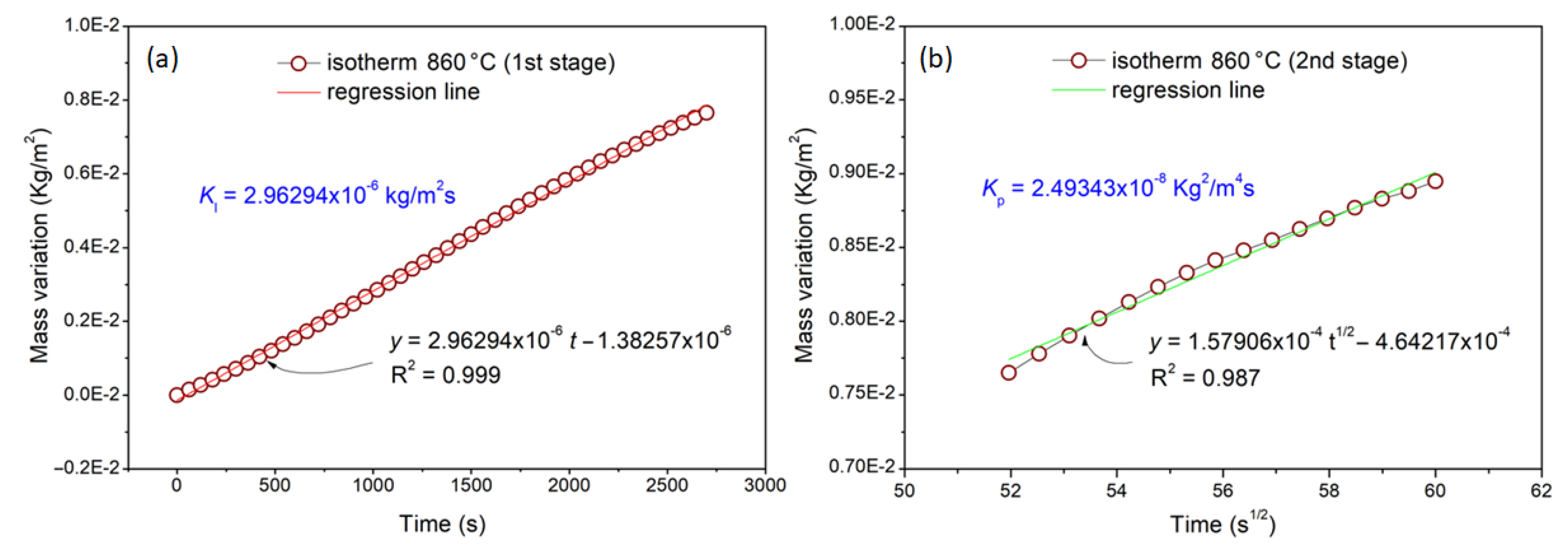
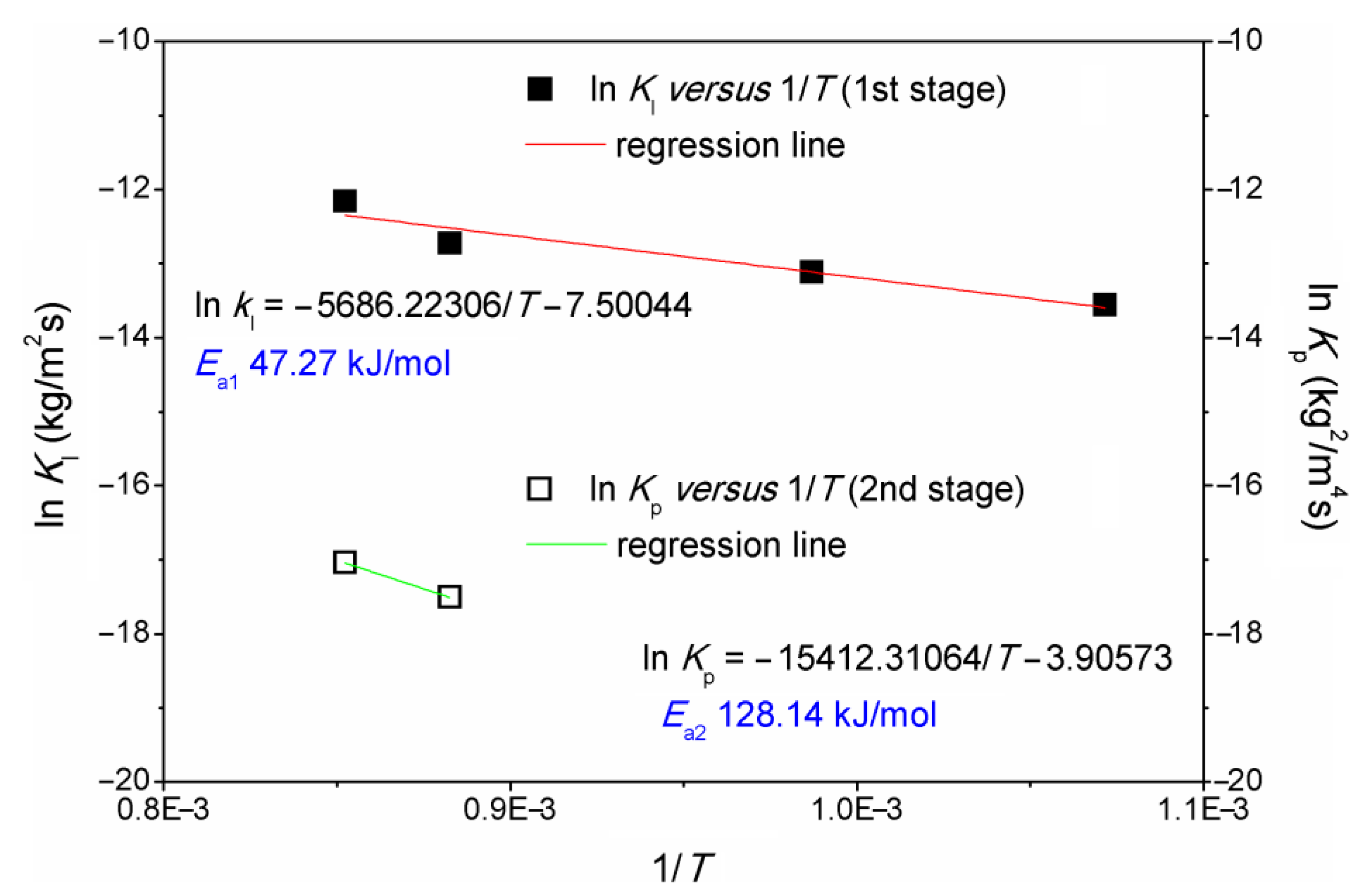



| Temperature (°C) | Oxidation Rate Index () | (kg/m2s) | (kg2/m4s) | |
|---|---|---|---|---|
| 1st Stage (Linear) | 2nd Stage (Parabolic) | |||
| 660 | 0.90903 (1) | - | 1.2898 × 10−6 | - |
| 740 | 0.91528 (1) | - | 2.0058 × 10−6 | - |
| 860 | 1.08216 (1) | 0.51352 (0.5) | 2.9629 × 10−6 | 2.49343 × 10−8 |
| 900 | 0.90381 (1) | 0.53096 (0.5) | 5.2203 × 10−6 | 3.96464 × 10−8 |
| (K−1) | lnKl (kg/m2s) | lnKp (kg2/m4s) | Ea1 (kJ/mol) 1st Stage (Linear) | Ea2 (kJ/mol) 2nd Stage (Parabolic) |
|---|---|---|---|---|
| 1.07164 × 10−3 | −13.56102 | - | 47.27 | - |
| 9.87021 × 10−4 | −13.11947 | - | ||
| 8.82496 × 10−4 | −12.72934 | −17.50703 | 128.14 | |
| 8.52046 × 10−4 | −12.16296 | −17.04328 |
| Sample | Mass (mg) | Thickness (µm) | Height (mm) | Diameter (mm) | Volume (mm3) | Density (g/cm3) |
|---|---|---|---|---|---|---|
| Standard (sanded) | 79.80000 | - | 2.0000 | 2.5000 | 9.8125 | 8.13 |
| Oxidized (900 °C) | 80.06957 | - | 2.0040 | 2.5040 | 9.8636 | - |
| Oxide layer | 0.26957 | ~2 | - | - | 0.0511 | 5.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alano, J.H.; Siqueira, R.L.; Martins Júnior, C.B.; Silva, R.; Vacchi, G.d.S.; Rovere, C.A.D. A Survey on the Oxidation Behavior of a Nickel-Based Alloy Used in Natural Gas Engine Exhaust Valve Seats. Metals 2023, 13, 49. https://doi.org/10.3390/met13010049
Alano JH, Siqueira RL, Martins Júnior CB, Silva R, Vacchi GdS, Rovere CAD. A Survey on the Oxidation Behavior of a Nickel-Based Alloy Used in Natural Gas Engine Exhaust Valve Seats. Metals. 2023; 13(1):49. https://doi.org/10.3390/met13010049
Chicago/Turabian StyleAlano, José Henrique, Renato Luiz Siqueira, Claudio Beserra Martins Júnior, Rodrigo Silva, Guilherme dos Santos Vacchi, and Carlos Alberto Della Rovere. 2023. "A Survey on the Oxidation Behavior of a Nickel-Based Alloy Used in Natural Gas Engine Exhaust Valve Seats" Metals 13, no. 1: 49. https://doi.org/10.3390/met13010049









