Influence of Minor Additions of Be on the Eutectic Modification of an Al-33wt.%Cu Alloy Solidified under Transient Conditions
Abstract
1. Introduction
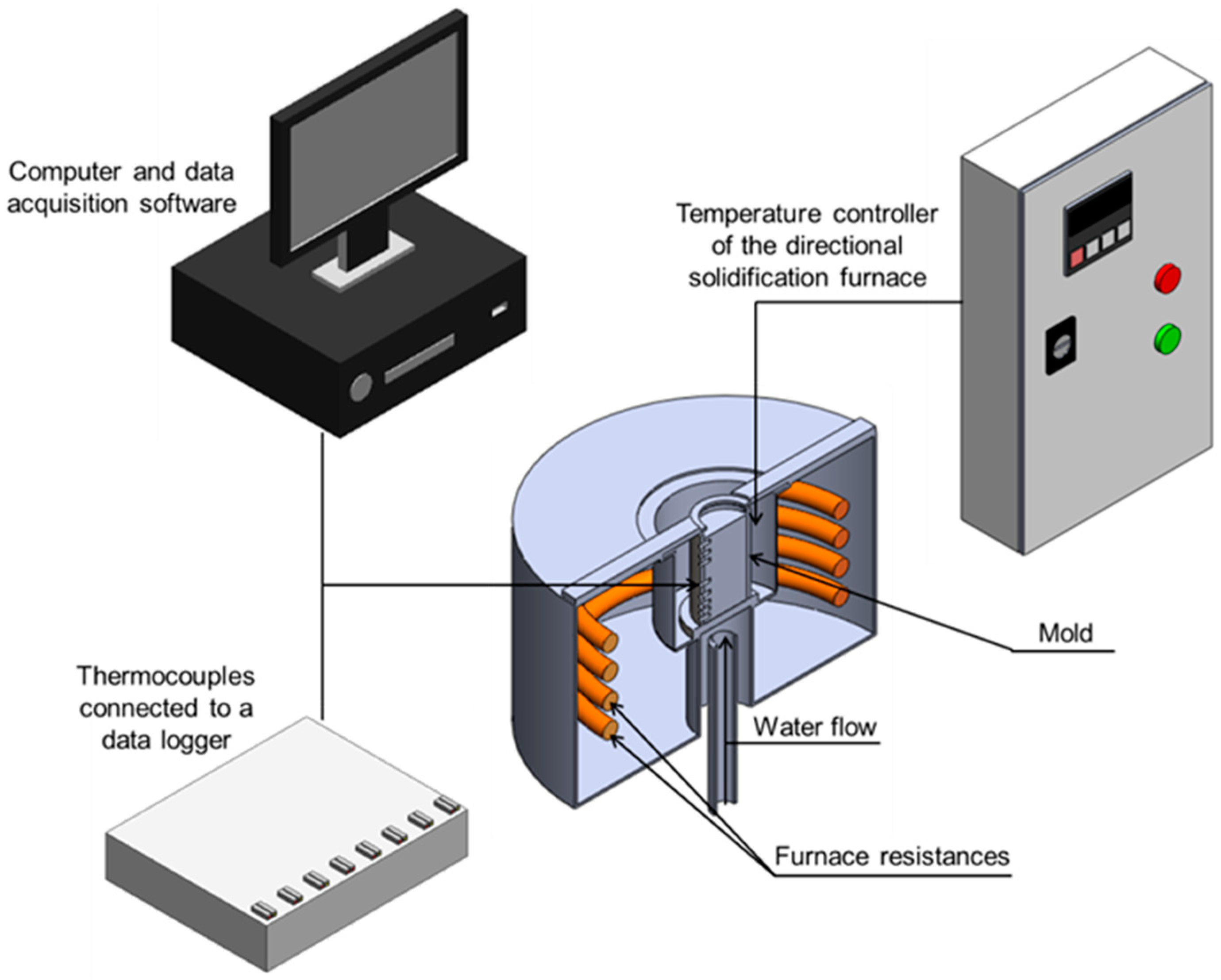
2. Materials and Methods
3. Results and Discussion
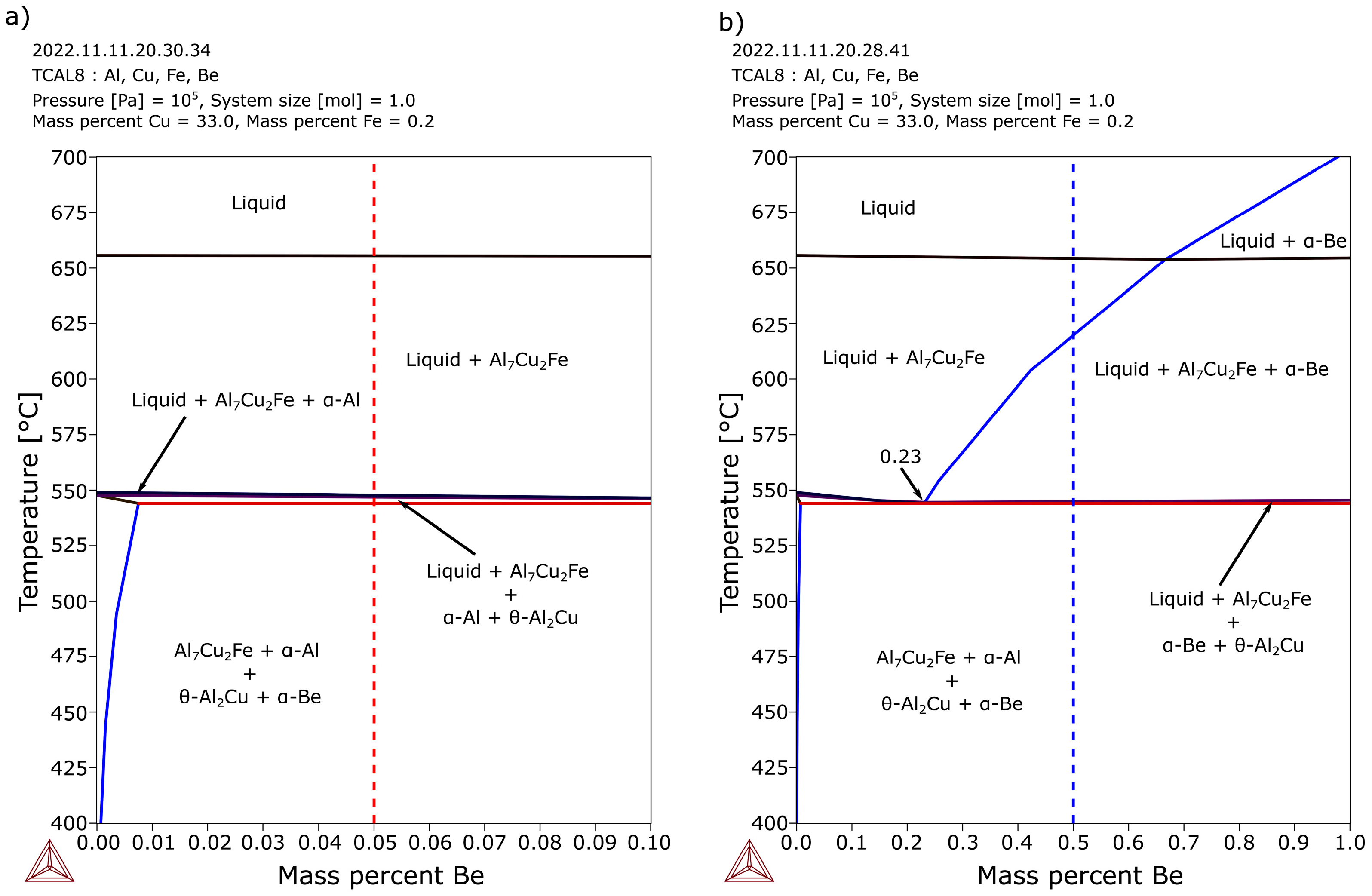
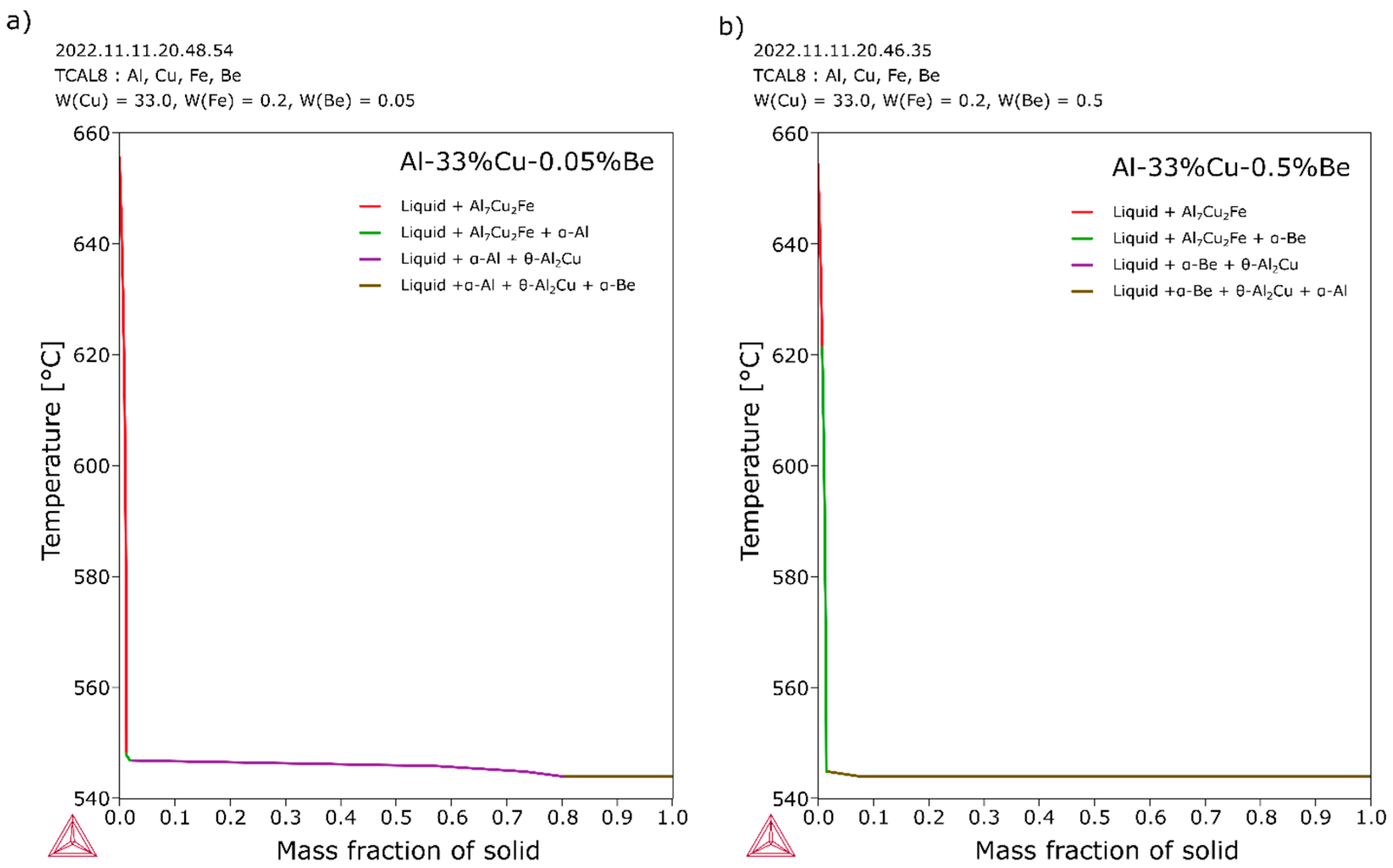
3.1. Thermo-Calc Simulations
3.2. Solidification Thermal Parameters
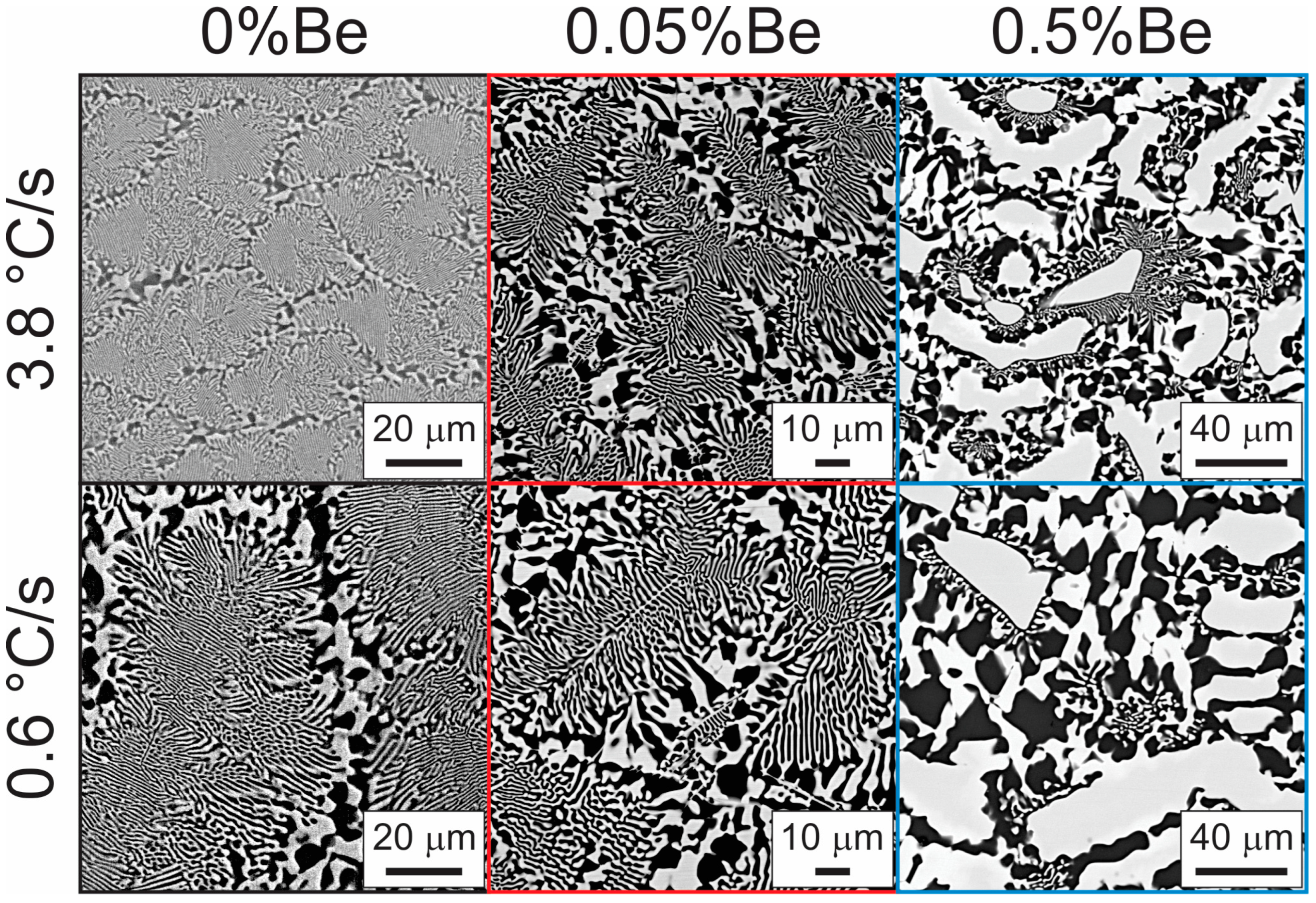
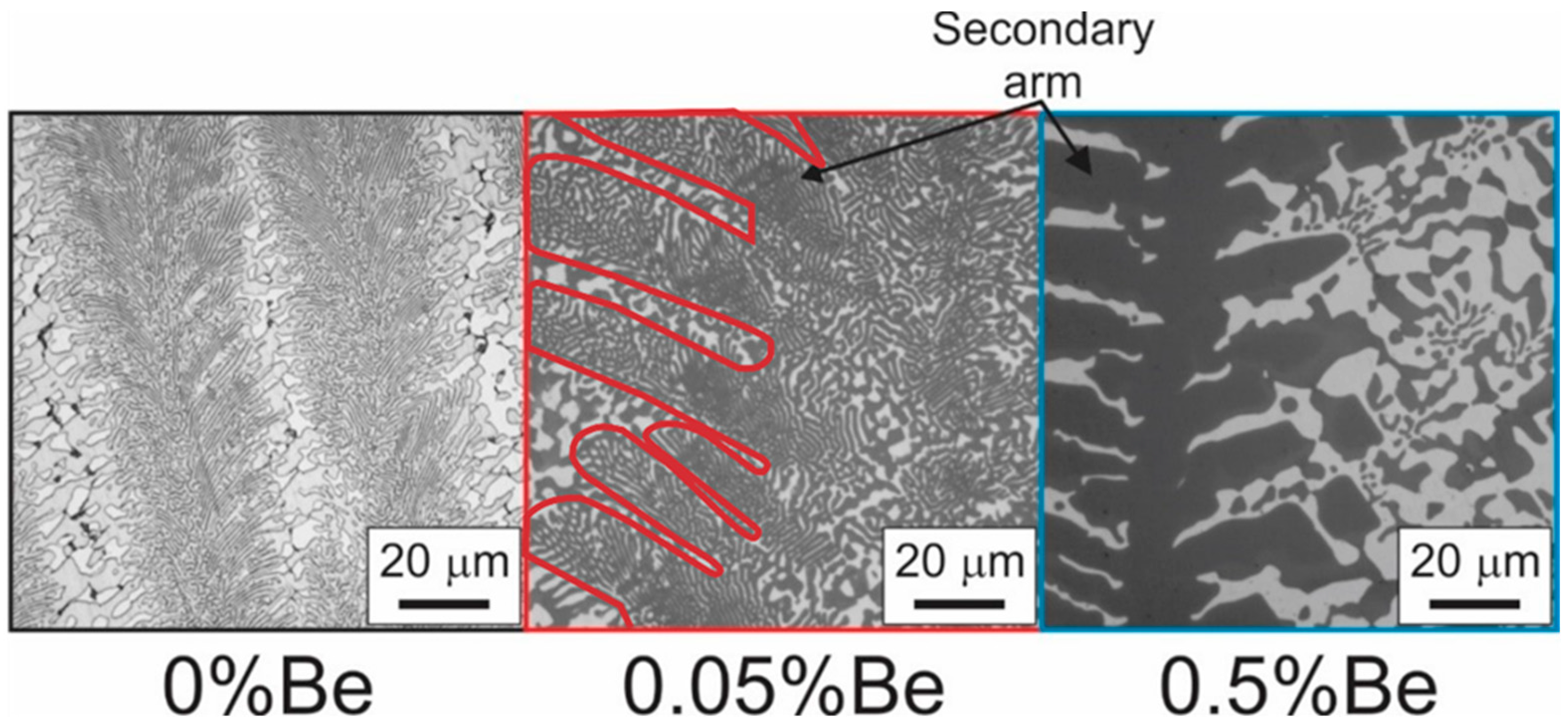
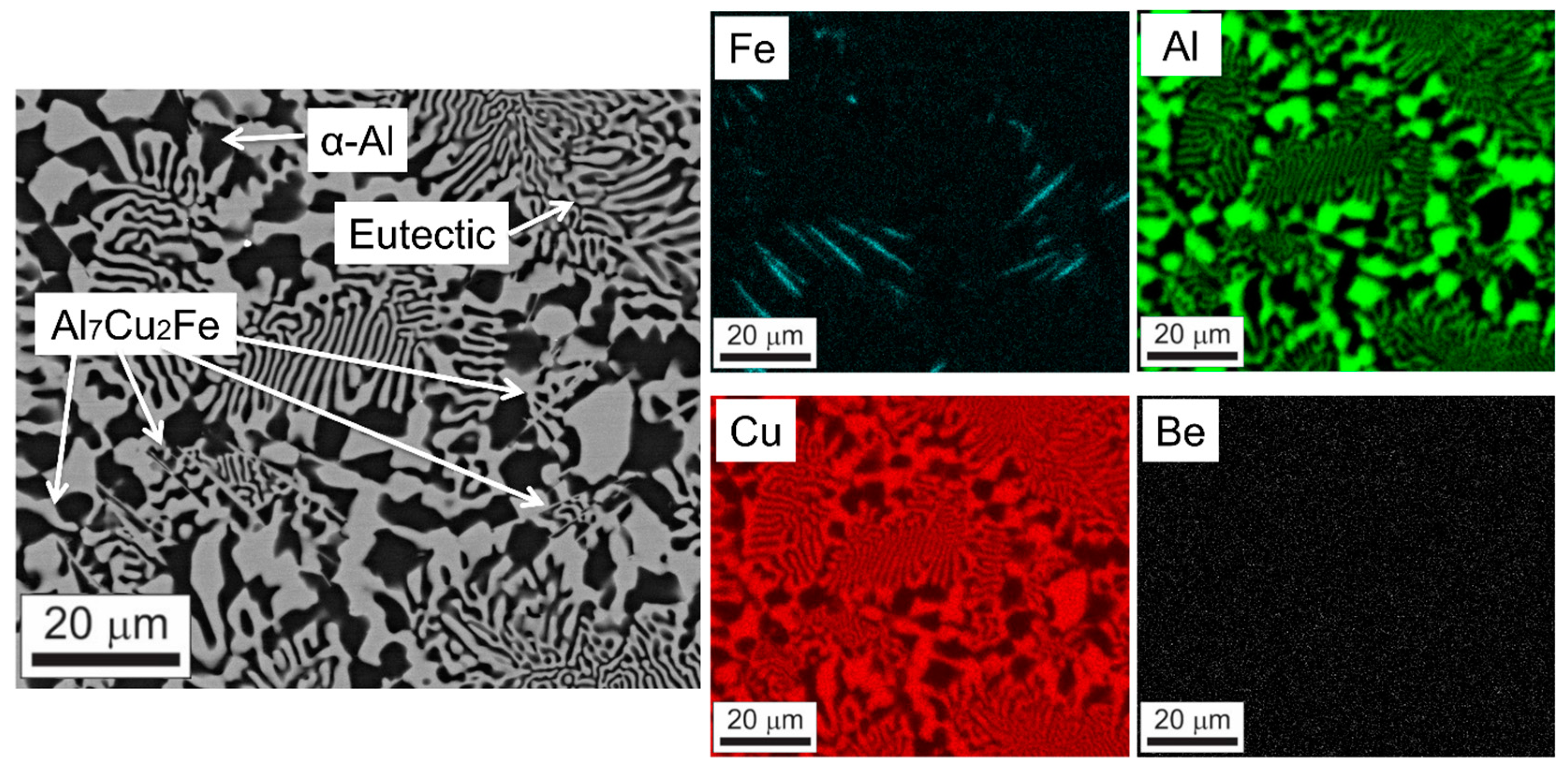
3.3. Microstructural Analysis
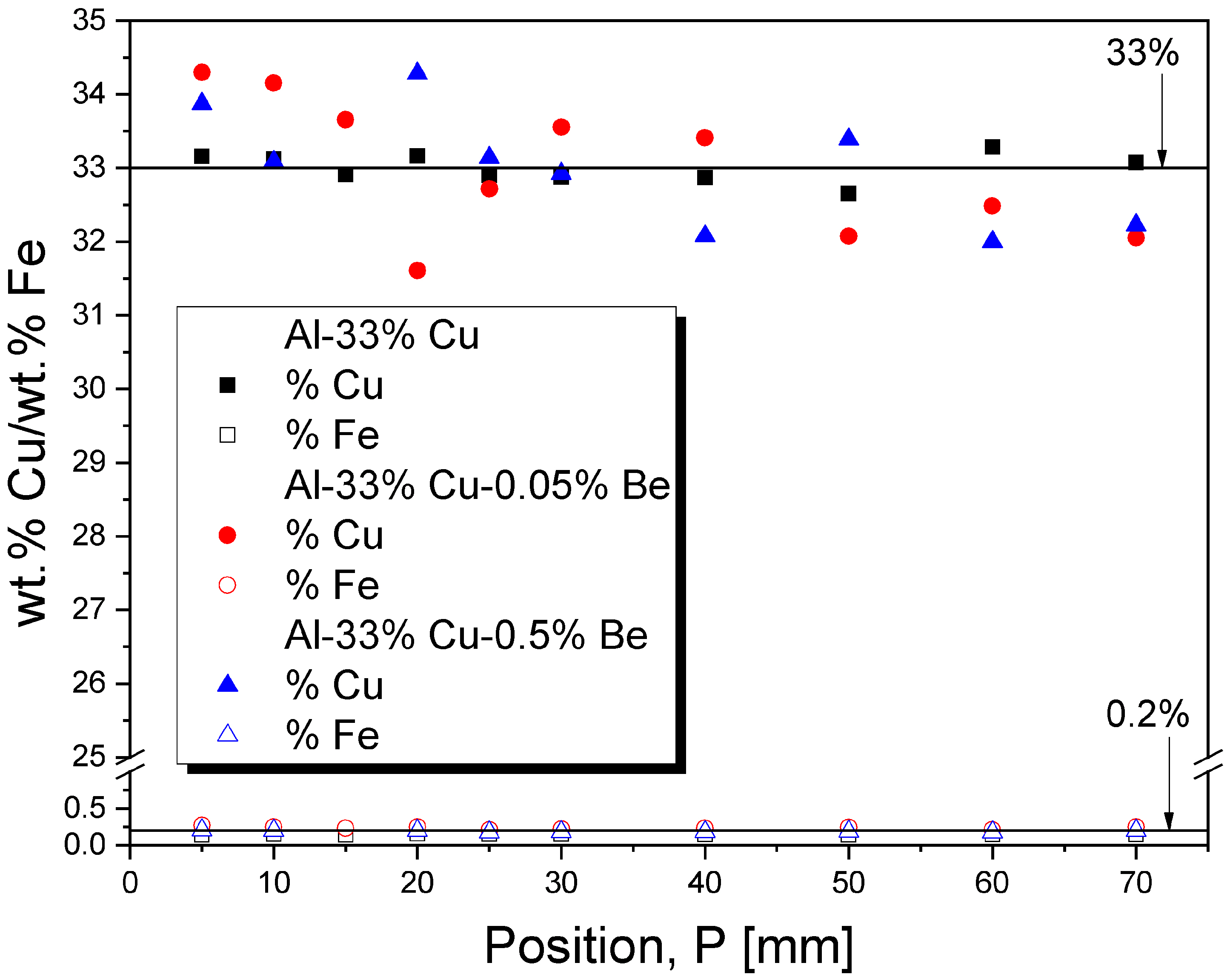
3.4. Chemical Composition
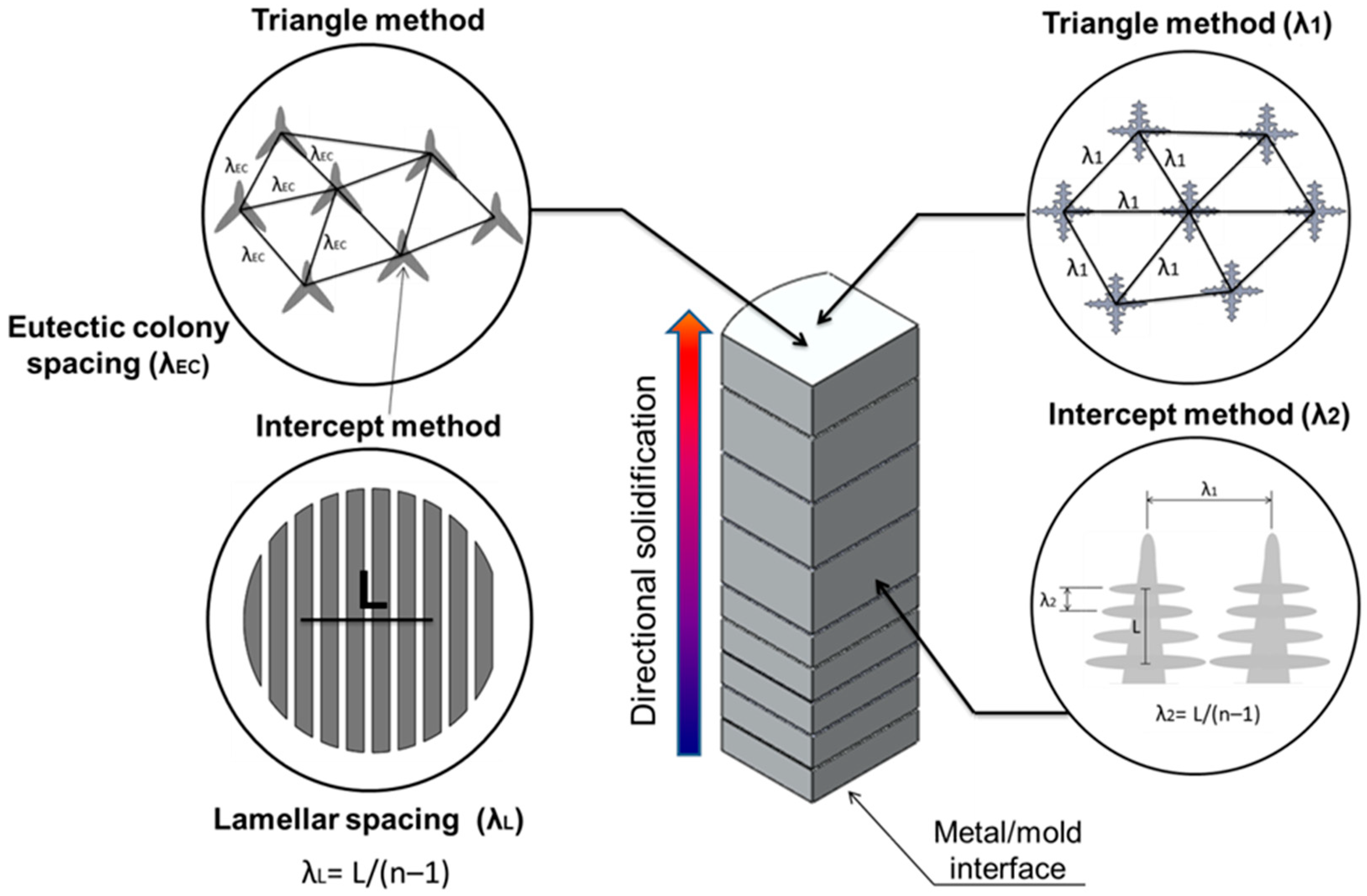
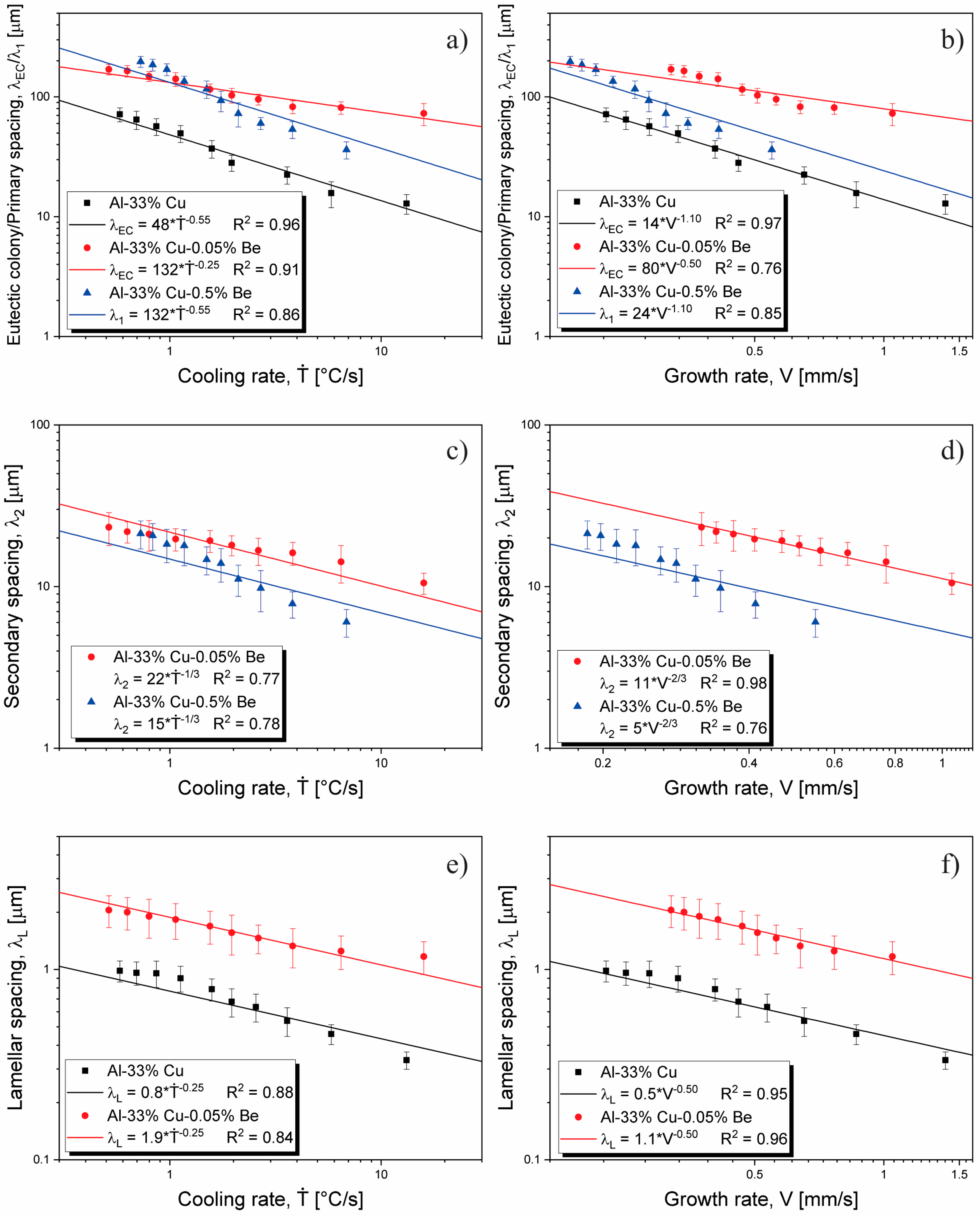
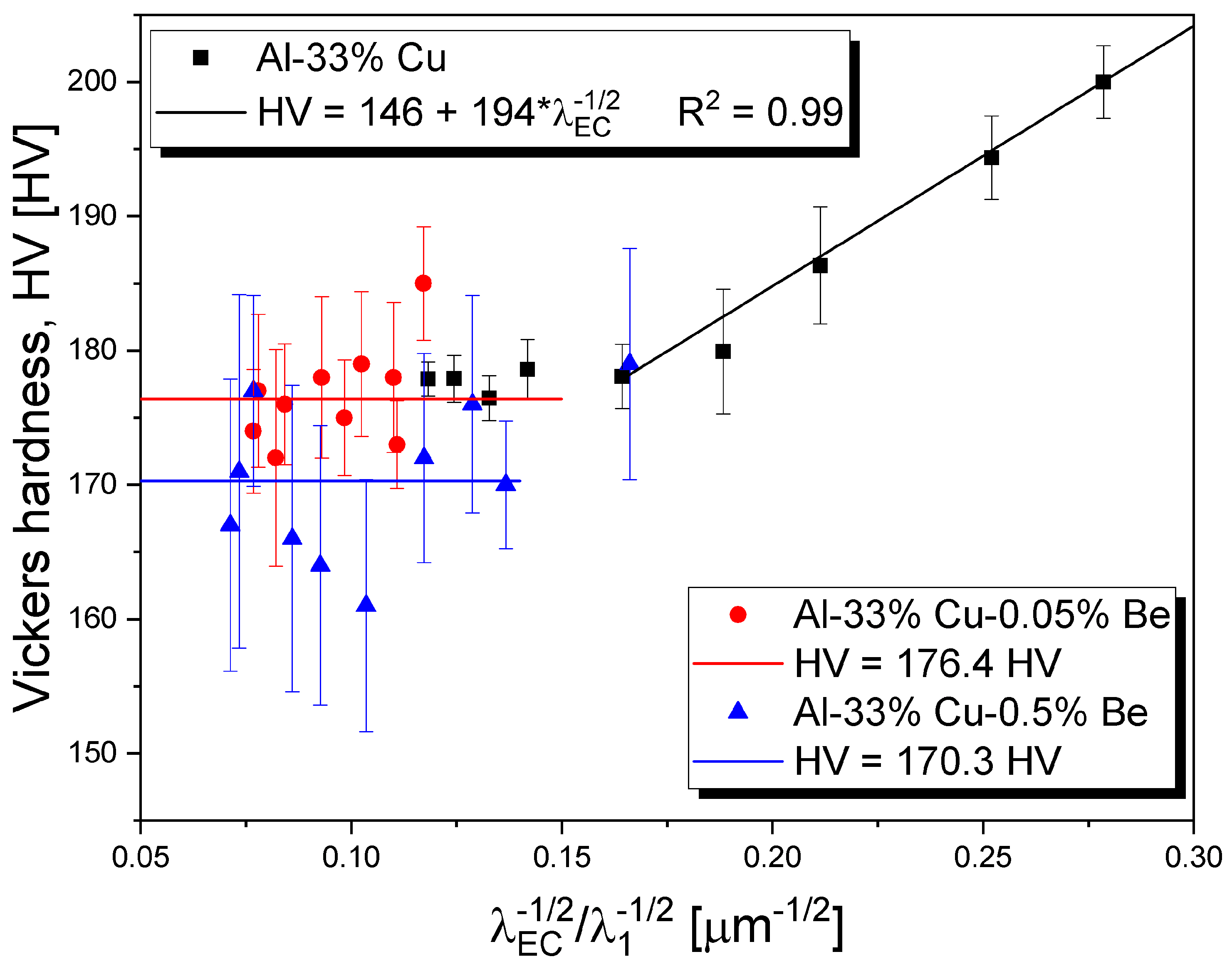
3.5. Microstructural Growth Laws
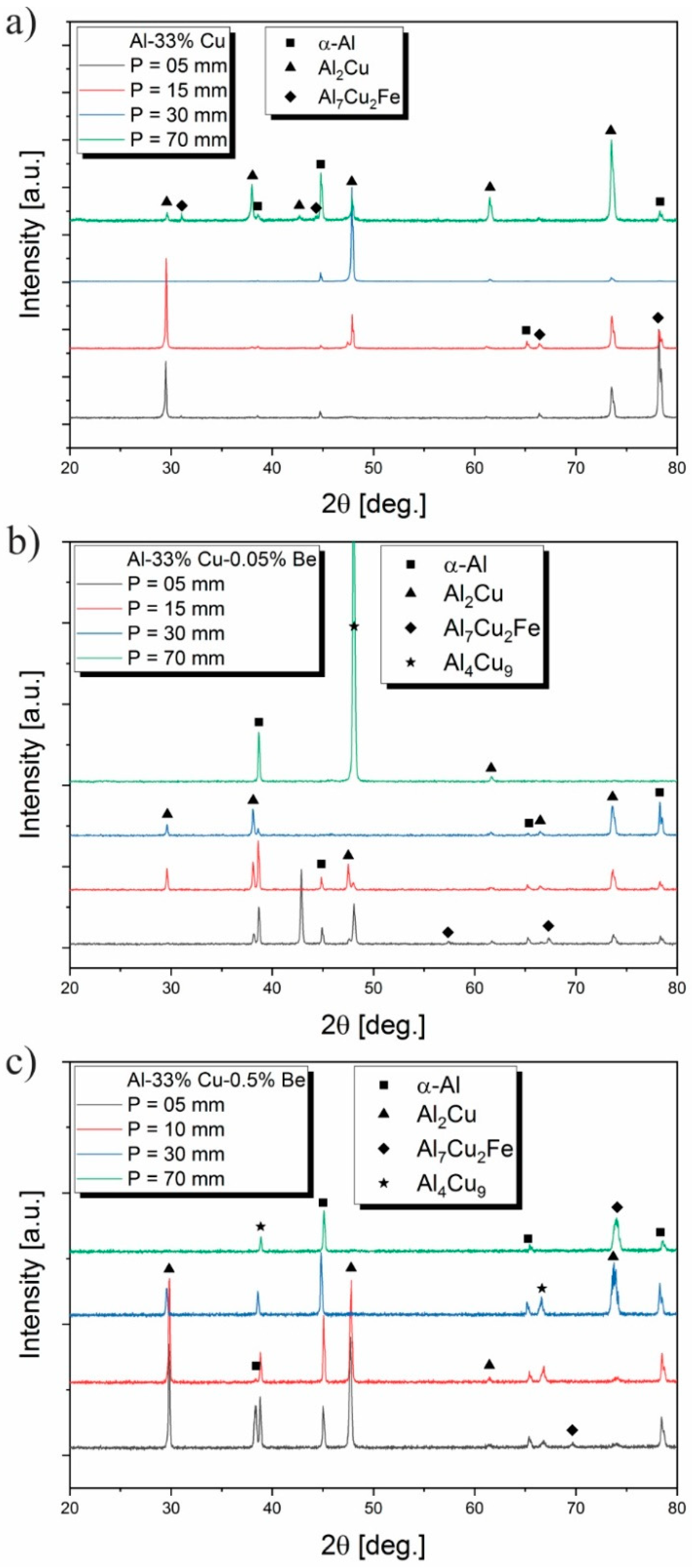
3.6. Microstructural Phases
3.7. Hardness
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantor, B.; Assender, H.; Grant, P. Aerospace Materials; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Kaufman, J.F.; Rooy, E.L. Aluminum Alloy Castings: Properties, Processes, and Applications; ASM International: Materials Park, OH, USA, 2004. [Google Scholar]
- Chen, H.-L.; Chen, Q.; Engström, A. Development and applications of the TCAL aluminum alloy database. Calphad 2018, 62, 154–171. [Google Scholar] [CrossRef]
- Deschamps, A.; Tancret, F.; Benrabah, I.-E.; De Geuser, F.; Van Landeghem, H.P. Combinatorial approaches for the design of metallic alloys. C. R. Phys. 2018, 19, 737–754. [Google Scholar] [CrossRef]
- Gloria, A.; Montanari, R.; Richetta, M.; Varone, A. Alloys for Aeronautic Applications: State of the Art and Perspectives. Metals 2019, 9, 662. [Google Scholar] [CrossRef]
- Dias, M.; Costa, T.; Rocha, O.; Spinelli, J.E.; Cheung, N.; Garcia, A. Interconnection of thermal parameters, microstructure and mechanical properties in directionally solidified Sn–Sb lead-free solder alloys. Mater. Charac. 2015, 106, 52–61. [Google Scholar] [CrossRef]
- Silva, C.; Barros, A.; Rodrigues, A.V.; Kakitani, R.; Soares, T.; Garcia, A.; Cheung, N. Microstructure and Tensile Strength of an Al-Si-Fe-V Alloy: Vanadium and Solidification Thermal Parameters as Recycling Strategies. Sustainability 2022, 14, 13859. [Google Scholar] [CrossRef]
- Kakitani, R.; Konno, C.; Garcia, A.; Cheung, N. The Effects of Solidification Cooling and Growth Rates on Microstructure and Hardness of Supersaturated Al-7%Si-x%Zn Alloys. J. Mater. Eng. Perform. 2022, 31, 1956–1970. [Google Scholar] [CrossRef]
- Septimio, R.S.; Costa, T.A.; Silva, C.A.P.; Vida, T.A.; Damborenea, J.; Garcia, A.; Cheung, N. Correlation between unsteady-state solidification thermal parameters and microstructural growth of Zn–8 mass% Al and Zn–8 mass% Al–XBi tribological alloys. J. Therm. Anal. Calorim. 2020, 139, 1741–1761. [Google Scholar] [CrossRef]
- Duarte, R.N.; Faria, J.D.; Brito, C.; Veríssimo, N.C.; Cheung, N.; Garcia, A. Length scale of the dendritic microstructure affecting tensile properties of Al–(Ag)–(Cu) alloys. Int. J. Mod. Phys. B 2016, 30, 1550261. [Google Scholar] [CrossRef]
- Rodrigues, A.V.; Lima, T.S.; Vida, T.A.; Brito, C.; Garcia, A.; Cheung, N. Microstructure and Tensile/Corrosion Properties Relationships of Directionally Solidified Al-Cu-Ni Alloys. Met. Mater. Int. 2018, 24, 1058–1076. [Google Scholar] [CrossRef]
- Ferreira, I.L.; Garcia, A.; Nestler, B. On Macrosegregation in Ternary Al-Cu-Si Alloys: Numerical and Experimental Analysis. Scr. Mater. 2004, 50, 407–411. [Google Scholar] [CrossRef]
- Bertelli, F.; Freitas, E.S.; Cheung, N.; Arenas, M.A.; Conde, A.; Damborenea, J.; Garcia, A. Microstructure, tensile properties and wear resistance correlations on directionally solidified Al-Sn-(Cu; Si) alloys. J. Alloys Compd. 2017, 695, 3621–3631. [Google Scholar] [CrossRef]
- Houska, C. Beryllium in Aluminum and Magnesium alloys. Met. Mater. 1988, 4, 100–104. [Google Scholar]
- Karov, J.; Youdelis, W.V. Precipitation in Al–3Cu–0·1Be. Mater. Sci. Technol. 1987, 3, 1–6. [Google Scholar] [CrossRef]
- Yuan, Z.; Guoa, Z.; Xiong, S.M. Effect of as-cast microstructure heterogeneity on aging behavior of a high-pressure die-cast A380 alloy. Mater. Charact. 2018, 135, 278–286. [Google Scholar] [CrossRef]
- Çadırlı, E.; Nergiz, E.; Kaya, H.; Büyük, U.; Şahin, M.; Gündüz, M. Effect of growth velocity on microstructure and mechanical properties of directionally solidified 7075 alloy. Int. J. Cast Metal. Res. 2020, 33, 11–23. [Google Scholar] [CrossRef]
- Gündüz, M.; Çadirli, E. Directional Solidification of Aluminium-Copper Alloys. Mater. Sci. Eng. A 2002, 327, 167–185. [Google Scholar] [CrossRef]
- Kakitani, R.; Gouveia, G.L.; Garcia, A.; Cheung, N.; Spinelli, J.E. Thermal analysis during solidification of an Al–Cu eutectic alloy: Interrelation of thermal parameters, microstructure and hardness. J. Therm. Anal. Calorim. 2019, 137, 983–996. [Google Scholar] [CrossRef]
- Jafari, H.; Amiryavari, P. The effects of zirconium and beryllium on microstructure evolution, mechanical properties and corrosion behaviour of as-cast AZ63 alloy. Mater. Sci. Eng. A 2016, A654, 161–168. [Google Scholar] [CrossRef]
- Rodrigues, A.V.; Kakitani, R.; Oliveira, R.; Barros, A.; Brito, C.; Garcia, A.; Cheung, N. Two-Phase Dendrite and Bimodal Structure in an Al-Cu-Ni Alloy: Their Roles in Hardness. J. Mater. Eng. Perform. 2022, 31, 3704–3715. [Google Scholar] [CrossRef]
- Grandfield, J.; Eskin, D.G.; Bainbridge, I. Direct-Chill Casting of Light Alloys: Science and Technology; Wiley: New York, NY, USA, 2013; pp. 144–254. [Google Scholar]
- Rocha, O.L.; Siqueira, C.A.; Garcia, A. Heat Flow Parameters Affecting Dendrite Spacings during Unsteady-State Solidification of Sn-Pb and Al-Cu Alloys. Metall. Mater. Trans. A 2003, 34A, 995–1006. [Google Scholar] [CrossRef]
- Rozman, N.; Medved, J.; Zupanic, F. Microstructural evolution in Al-Mn-Cu-(Be) alloys. Philos. Mag. 2011, 91, 4230–4246. [Google Scholar] [CrossRef]
- Barros, A.; Cruz, C.; Garcia, A.; Cheung, N. Corrosion behavior of an Al-Sn-Zn alloy: Effects of solidification microstructure characteristics. J. Mater. Res. Technol. 2021, 12, 257–263. [Google Scholar] [CrossRef]
- Kakitani, R.; Cruz, C.B.; Lima, T.S.; Brito, C.; Garcia, A.; Cheung, N. Transient directional solidification of a eutectic Al-Si-Ni alloy: Macrostructure, microstructure, dendritic growth and hardness. Materialia 2019, 7, 100358. [Google Scholar] [CrossRef]
- Brito, C.; Costa, T.A.; Vida, T.A.; Bertelli, F.; Cheung, N.; Spinelli, J.E.; Garcia, A. Characterization of Dendritic Microstructure, Intermetallic Phases, and Hardness of Directionally Solidified Al-Mg and Al-Mg-Si Alloys. Metall. Mater. Trans. A 2015, 46A, 3342–3355. [Google Scholar] [CrossRef]
- Kakitani, R.; Reyes, R.V.; Garcia, A.; Spinelli, J.E.; Cheung, N. Relationship between spacing of eutectic colonies and tensile properties of transient directionally solidified Al-Ni eutectic alloy. J. Alloys Compd. 2018, 733, 59–68. [Google Scholar] [CrossRef]
- Silva, C.A.P.; Kakitani, R.; Canté, M.V.; Brito, C.; Garcia, A.; Spinelli, J.E.; Cheung, N. Microstructure, phase morphology, eutectic coupled zone and hardness of Al-Co alloys. Mater. Charact. 2020, 169, 110617. [Google Scholar] [CrossRef]
- Jackson, K.A.; Hunt, J.D. Binary Eutectic Solidification. Trans. Met. Soc. AIME 1966, 6, 843–852. [Google Scholar]
- Wilde, J.D.; Froyen, L. Two-phase planar and regular lamellar coupled growth along the univariant eutectic reaction in ternary alloys: An analytical approach and application to the Al–Cu–Ag system. J. Appl. Phys. 2005, 97, 113515. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Yang, C.; Zhang, D.; Wang, Z. Effect of Si on Fe-rich intermetallic formation and mechanical properties of heat-treated Al-Cu-Mn-Fe alloys. J. Mater. Res. 2018, 33, 898–911. [Google Scholar] [CrossRef]
- Payne, J.; Welsh, G.; Christ, R.J., Jr.; Nardiello, J.; Papazian, J.M. Observations of fatigue crack initiation in 7075-T651. Int. J. Fatigue 2010, 32, 247–255. [Google Scholar] [CrossRef]
- Birbilis, N.; Cavanaugh, M.K.; Buchheit, R.G. Electrochemical behavior and localized corrosion associated with Al7Cu2Fe particles in aluminum alloy 7075-T651. Corros. Sci. 2006, 48, 4202–4215. [Google Scholar] [CrossRef]
- Jiang, N.; Gao, X.; Zheng, Z. Microstructure evolution of aluminum-lithium alloy 2195 undergoing commercial production. Trans. Nonferrous Met. Soc. China 2010, 20, 740–745. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, C.; Xu, C.; Chen, K. Microstrutures and Tensile Fracture Behavior of 2219 Wrought Al-Cu Alloys with Different Impurity of Fe. Metals 2021, 11, 174. [Google Scholar] [CrossRef]
- Karov, J.; Youdelis, W.V. Solubility of beryllium in CuAl2. Mater. Sci. Technol. 1987, 3, 394–395. [Google Scholar] [CrossRef]
- Amirkhanlou, S.; Ji, S. Casting lightweight stiff aluminum alloys: A review. Crit. Rev. Solid State Mater. Sci. 2019, 45, 171–186. [Google Scholar] [CrossRef]
- Bowden, D.; Pokross, C.; Kaczynski, D.; London, G.; Starke, E.A.; Sanders, T.H.; Cassada, W.A. Characterization of aluminium-beryllium alloy sheet. Mater. Sci. Forum 2000, 331–337, 901–906. [Google Scholar] [CrossRef]
- Zupanič, F. Extracting electron backscattering coefficients from backscattered electron micrographs. Mater. Charact. 2010, 61, 1335–1341. [Google Scholar] [CrossRef]
- Okamoto, H. Supplemental Literature Review of Binary Phase Diagrams: Ag-Ni, Al-Cu, Al-Sc, C-Cr, Cr-Ir, Cu-Sc, Eu-Pb, H-V, Hf-Sn, Lu-Pb, Sb-Yb, and Sn-Y. J. Phase Equilib. Diffus. 2013, 34, 493–505. [Google Scholar] [CrossRef]
- Kaiser, M.S.; Sabbir, S.H.; Kabir, M.S.; Soummo, M.R.; Al Nur, M. Study of Mechanical and Wear Behaviour of Hyper-Eutectic Al-Si Automotive Alloy Through Fe, Ni and Cr Addition. Mater. Res. 2018, 21. [Google Scholar] [CrossRef]
- Silva, C.; Barros, A.; Vida, T.; Garcia, A.; Cheung, N.; Reis, D.A.P.; Brito, C. Assessing Microstructure Tensile Properties Relationships in Al-7Si-Mg Alloys via Multiple Regression. Metals 2022, 12, 1040. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Zhao, Y.; Liu, K.; Hou, H.; Chen, L.-Q. Role of interfacial energy anisotropy in dendrite orientation in Al-Zn alloys: A phase field study. Mater. Des. 2022, 216, 110555. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y. Effects of beryllium in Al-Si-Mg-Ti cast alloy. Mater. Sci. Eng. A 2000, 280, 124–127. [Google Scholar] [CrossRef]



| Element/Master Alloy | Al | Si | Mg | Cu | Fe | Pb | Zn | Ni | Sn | Mn | Co | Be |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Bal. | 0.006 | 0.0011 | 0.01 | 0.073 | 0.006 | 0.005 | - | - | - | - | - |
| Cu | - | - | - | Bal. | - | - | - | 0.008 | 0.009 | 0.008 | - | - |
| Cu-Be | 0.029 | 0.097 | - | Bal. | 0.053 | 0.0028 | - | 0.01 | - | - | 0.014 | 9.83 |
| Alloy | %Be |
|---|---|
| Al-33wt.%Cu-0.05wt.%Be | 0.055 ± 0.02 |
| Al-33wt.%Cu-0.5wt.%Be | 0.60 ± 0.01 |
| Phase | HV0.05 (HV) |
|---|---|
| θ-Al2Cu | 397 ± 61 |
| Al4Cu9 | 824 ± 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.V.; Kakitani, R.; Silva, C.; Giovanetti, L.; Dias, M.; Henein, H.; Garcia, A.; Cheung, N. Influence of Minor Additions of Be on the Eutectic Modification of an Al-33wt.%Cu Alloy Solidified under Transient Conditions. Metals 2023, 13, 94. https://doi.org/10.3390/met13010094
Rodrigues AV, Kakitani R, Silva C, Giovanetti L, Dias M, Henein H, Garcia A, Cheung N. Influence of Minor Additions of Be on the Eutectic Modification of an Al-33wt.%Cu Alloy Solidified under Transient Conditions. Metals. 2023; 13(1):94. https://doi.org/10.3390/met13010094
Chicago/Turabian StyleRodrigues, Adilson Vitor, Rafael Kakitani, Cássio Silva, Leonardo Giovanetti, Marcelino Dias, Hani Henein, Amauri Garcia, and Noé Cheung. 2023. "Influence of Minor Additions of Be on the Eutectic Modification of an Al-33wt.%Cu Alloy Solidified under Transient Conditions" Metals 13, no. 1: 94. https://doi.org/10.3390/met13010094
APA StyleRodrigues, A. V., Kakitani, R., Silva, C., Giovanetti, L., Dias, M., Henein, H., Garcia, A., & Cheung, N. (2023). Influence of Minor Additions of Be on the Eutectic Modification of an Al-33wt.%Cu Alloy Solidified under Transient Conditions. Metals, 13(1), 94. https://doi.org/10.3390/met13010094









