Effect of Barnacles on the Corrosion Behavior of 304 Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Specimens
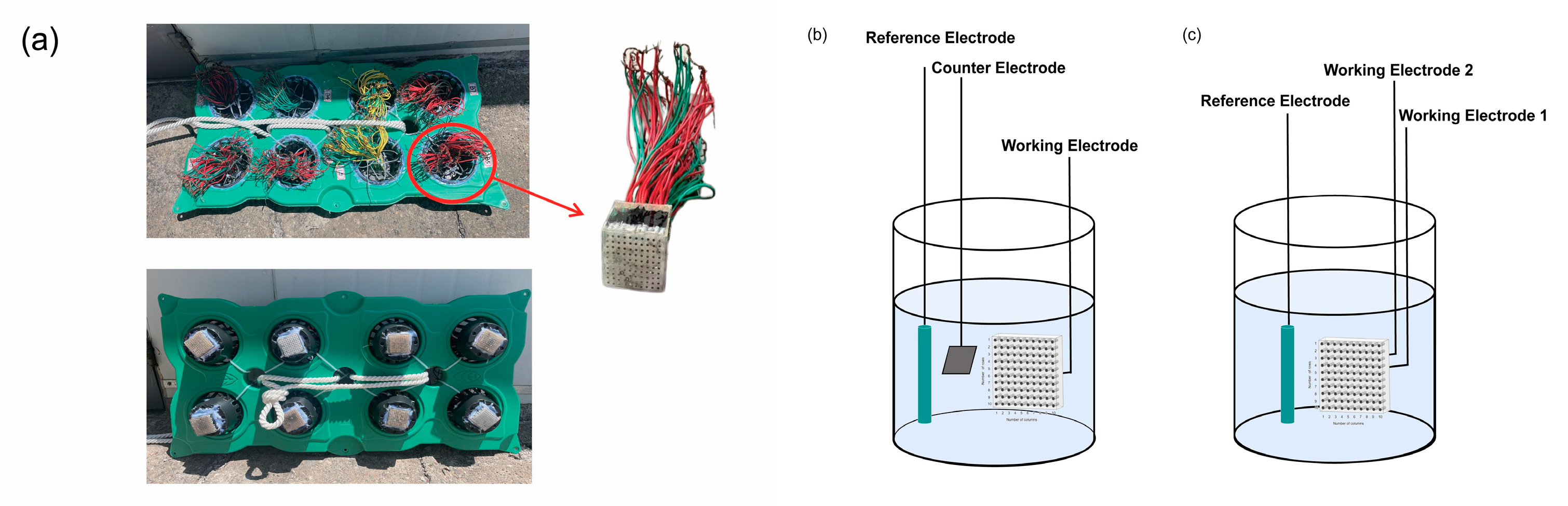
2.2. Field Exposure Experiment
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Electrochemical Distribution Pattern of Array Electrodes Affected by Fouling Organisms
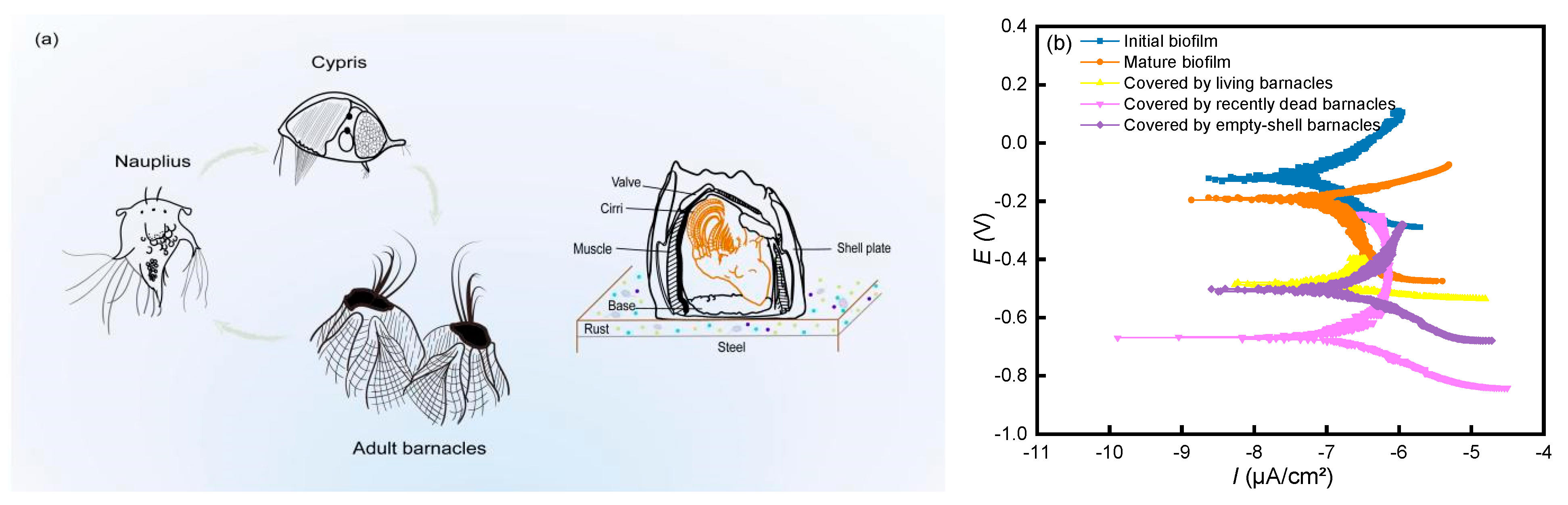
3.2. Corrosion Characteristics of Microelectrodes at Different Barnacle Growth Stages
4. Conclusions
- The severity of corrosion in 304SS decreases and subsequently increases with increasing immersion time due to the influences of marine fouling organisms. The inhibition of corrosion in metals is most effective during the mature biofilm stage, whereas corrosion is most severe during the empty shell stage.
- The results of the biofilm formation stages show that the galvanic current and potential distributions of the initial biofilm are uneven. With the prolongation of immersion time, both the thickness and uniformity of the biofilm increase. Mature biofilms have a certain inhibitory effect on corrosion, allowing the corrosion rate to be reduced.
- The different growth stages of barnacles have different corrosion mechanisms on 304SS: structurally intact barnacles protect metals well. The dense structure of the calcareous shell and glue can effectively limit the transmission of external corrosive factors. Although the metal covered by recently dead barnacles is affected by the decomposition of corrosive bacteria, the overall structure is still intact, as is that covered by living barnacles. The shell plays a role in limiting the transfer of external substances to some extent, reducing the concentration of oxygen reaching the metal surface and affecting the diffusion rate of oxygen.
- The galvanic current and potential distribution roughly match the bioattachment site. Barnacle attachment behavior has some corrosive effect on the metal in the surrounding area.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunt, J.D.; Zakeri, B.; de Barros, A.G.; Filho, W.L.; Marques, A.D. Buoyancy Energy Storage Technology: An energy storage solution for islands, coastal regions, offshore wind power and hydrogen compression. J. Energy Storage 2021, 40, 102746. [Google Scholar] [CrossRef]
- Barooni, M.; Ashuri, T.; Sogut, D.V.; Wood, S. Floating Offshore Wind Turbines: Current Status and Future Prospects. Energies 2023, 16, 2. [Google Scholar] [CrossRef]
- Adedipe, O.; Brennan, F.; Kolios, A. Review of corrosion fatigue in offshore structures: Present status and challenges in the offshore wind sector. Renew. Sustain. Energy Rev. 2016, 61, 141–154. [Google Scholar] [CrossRef]
- Kirchgeorg, T.; Weinberg, I.; Hornig, M.; Baier, R.; Schmid, M.J. Emissions from corrosion protection systems of offshore wind farms: Evaluation of the potential impact on the marine environment. Mar. Pollut. Bull. 2018, 136, 257–268. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Huang, Y.; Zhou, H.; Wu, Y. Macrofouling organisms: Protection or damage of steel in marine environments? Corros. Sci. 2023, 212, 110928. [Google Scholar] [CrossRef]
- Védie, E.; Brisset, H.; Briand, J.F.; Bressy, C. Bioinspiration and microtopography as nontoxic strategies for marine bioadhesion control. Adv. Mater. Interfaces 2021, 8, 2100994. [Google Scholar] [CrossRef]
- Delgado, A.; Power, S.; Richards, C.; Daly, P.; BriciuBurghina, C. Establishment of an antifouling performance index derived from the assessment of biofouling on typical marine sensor materials. Sci. Total Environ. 2023, 887, 164059. [Google Scholar] [CrossRef]
- Relini, G.; Tixi, F.; Relini, M.; Torchia, G. The macrofouling on offshore platforms at Ravenna. Int. Biodeterior. Biodegrad. 1998, 41, 41–55. [Google Scholar] [CrossRef]
- Al-Muhanna, K.; Habib, K. Corrosion behavior of different alloys exposed to continuous flowing seawater by electrochemical impedance spectroscopy (EIS). Desalination 2010, 250, 404–407. [Google Scholar] [CrossRef]
- Noor, N.M.; Yahaya, N.; Abdullah, A. Microbiologically influenced corrosion of X-70 carbon steel by desulfovibrio vulgaris. Adv. Sci. Lett. 2012, 13, 312–316. [Google Scholar] [CrossRef]
- Zulkafli, R.; Othman, N.K.; Yaakob, N. Localised corrosion of API 5L X65 carbon steel in marine environments: The role of sulfate-reducing bacteria (SRB). J. Bio-Tribo-Corros. 2022, 9, 12. [Google Scholar] [CrossRef]
- Liu, W. Rapid MIC attack on 2205 duplex stainless steel pipe in a yacht. Eng. Fail. Anal. 2014, 42, 109–120. [Google Scholar] [CrossRef]
- Antony, P.J.; Chongdar, S.; Kumar, P.; Raman, R. Corrosion of 2205 duplex stainless steel in chloride medium containing sulfate-reducing bacteria. Electrochim. Acta. 2007, 52, 3985–3994. [Google Scholar] [CrossRef]
- Xu, D.; Xia, J.; Zhou, E.; Zhang, D.; Zhang, D.; Yang, C.; Li, Q.; Lin, H.; Li, X.; Yang, K. Accelerated corrosion of 2205 duplex stainless steel caused by marine aerobic Pseudomonas.aeruginosa biofilm. Bioelectrochemistry 2017, 113, 1–8. [Google Scholar] [CrossRef]
- Huang, L.; Chang, W.; Zhang, D.; Huang, Y.; Li, Z. Acceleration of corrosion of 304 stainless steel by outward extracellular electron transfer of Pseudomonas aeruginosa biofilm. Corros. Sci. 2022, 199, 110159. [Google Scholar] [CrossRef]
- Palanichamy, S.; Subramanian, G. Hard foulers induced crevice corrosion of HSLA Steel in the Coastal Waters of the Gulf of Mannar (Bay of Bengal). J. Mar. Sci. Appl. 2014, 13, 117–126. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Wang, X.; Xu, Y.; Cai, F. Effects of oyster as macrofouling organism on corrosion mechanisms of a high-strength low-alloy steel. Corros. Sci. 2022, 207, 110580. [Google Scholar] [CrossRef]
- Neville, A.; Hodgkiess, T. Localised effects of macrofouling species on electrochemical corrosion of corrosion resistant alloys. Br. Corros. J. 2000, 35, 54–59. [Google Scholar] [CrossRef]
- Sangeetha, R.; Kumar, R.; Venkatesan, R.; Doble, M.; Vedaprakash, L.; Lakshmi, K. Understanding the structure of the adhesive plaque of Amphibalanus reticulatus. Mater. Sci. Eng. 2010, 30, 112–119. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Lim, C.S.; Teo, S.L.M.; Hu, X.; Pang, J. Macrofouling induced localized corrosion of stainless steel in Singapore seawater. Corros. Sci. 2017, 129, 152–160. [Google Scholar] [CrossRef]
- Patel, B.; Crisp, D. The influence of temperature on the breeding and the moulting activities of some warm-water species of operculate barnacles. J. Mar. Biol. Assoc. UK 1960, 39, 667–680. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Zhuo, W.; Liu, W.; Li, Z.; Tang, M. The characteristic patterns of macrofaunal fouling assemblages in nearshore waters of the South China Sea. J. Ocean. Univ. China 2018, 17, 1142–1148. [Google Scholar] [CrossRef]
- Li, Y.; Ning, C. Latest research progress of marine microbiological corrosion and bio-fouling, and new approaches of marine anti-corrosion and anti-fouling. Bioact. Mater. 2019, 4, 189–195. [Google Scholar] [CrossRef]
- Hu, J.; Shangguan, J.; Deng, P.; Feng, Q.; Wang, G.; Wang, P. Effect of barnacle adhesion on corrosion behavior of Q235 steel. J. Chin. Soc. Corros. Prot. 2023, 43, 1145–1150. [Google Scholar]
- Venkatnarayanan, S.; Murthy, P.S.; Kirubagaran, R.; Venugopalan, V.P. Effect of chlorination on barnacle larval stages: Implications for biofouling control and environmental impact. Int. Biodeterior. Biodegrad. 2016, 109, 141–149. [Google Scholar] [CrossRef]
- Liang, C.; Strickland, J.; Ye, Z.; Wu, W.; Hu, B.; Rittschof, D. Biochemistry of barnacle adhesion: An updated review. Front. Mar. Sci. 2019, 6, 565. [Google Scholar] [CrossRef]
- Lou, Y.; Chang, W.; Cui, T.; Wang, J.; Qian, H.; Ma, L.; Hao, X.; Zhang, D. Microbiologically influenced corrosion inhibition mechanisms in corrosion protection: A review. Bioelectrochemistry 2021, 141, 107883. [Google Scholar] [CrossRef]
- Eashwar, M.; Subramanian, G.; Chandrasekaran, P.; Balakrishnan, K. Mechanism for barnacle-induced crevice corrosion in stainless steel. Corros. Sci. 1992, 48, 608–612. [Google Scholar] [CrossRef]
- Cai, F.; Huang, Y.; Xing, S.; Xu, Y.; Zhao, X. Characteristics and mechanisms of low-alloy high-strength steel corrosion behavior under barnacle adhesion based on a comparison experiment. Corros. Sci. 2023, 217, 111146. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Z.; Gao, P.; Liu, P. Selection of aquatic plants for phytoremediation of heavy metal in electroplate wastewater. Acta Physiol. Plant. 2012, 35, 355–364. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, D.; Moradi, M.; Yang, C.; Jin, Y. Inhibiting corrosion of aluminum alloy 5083 through Vibrio species biofilm. Corros. Sci. 2021, 180, 109188. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Cai, F.; Lu, D.; Wang, X. Study on corrosion behavior and mechanism of AISI 4135 steel in marine environments based on field exposure experiment. Sci. Total Environ. 2022, 830, 154864. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Karunamoorthy, L.; Doble, M.; Kumar, R.; Venkatesan, R. Barnacle adhesion on natural and synthetic substrates: Adhesive structure and composition. Int. J. Adhes. Adhes. 2013, 41, 140–143. [Google Scholar] [CrossRef]
- Sangeetha, R.; Kumar, R.; Doble, M.; Venkatesan, R. Barnacle cement: An etchant for stainless steel 316L? Colloids Surf. B Biointerfaces 2010, 79, 524–530. [Google Scholar] [CrossRef] [PubMed]


| Element wt (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Material | C | Si | Mn | P | S | Cr | Ni | N |
| 304SS | 0.007 | 0.075 | 2 | 0.045 | 0.03 | 19.5 | 8 | 0.1 |
| Ecorr (mV) | Icorr (μA/cm2) | ||
|---|---|---|---|
| 7 d | Initial biofilm | −110.9 | 0.18448 |
| 15 d | Mature biofilm | −191.3 | 0.0677 |
| 30 d | Covered by living barnacles | −485.4 | 0.06815 |
| 90 d | Covered by recently dead barnacles | −668.8 | 0.07946 |
| 90 d | Covered by empty-shell barnacles | −504.8 | 0.14688 |
| Rs | CPEf-T | CPEf-P | Rf | CPEdl-T | CPEdl-P | Rct | W-R | W-T | W-P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ω·cm2 | F·cm−2Sn | Ω·cm2 | F·cm−2Sn | Ω·cm2 | Ω−1cm−2S0.5 | |||||
| Initial biofilm | 18.25 | 2.5309 × 10−5 | 0.81991 | 444.8 | 1.1927 × 10−5 | 0.81325 | 427,810 | / | / | / |
| Mature biofilm | 134.3 | 8.1691 × 10−6 | 0.80371 | 34,791 | 1.7814 × 10−6 | 0.9716 | 783,660 | / | / | / |
| Covered by living barnacles | 24.45 | 2.5163 × 10−8 | 0.97669 | 1977 | 3.321 × 10−7 | 0.796 | 429.7 | 1839 | 0.07892 | 0.44781 |
| Covered by recently dead barnacles | 18.22 | 1.0082 × 10−8 | 0.9192 | 2061 | 4.3046 × 10−7 | 0.75041 | 363.3 | 2320 | 0.15487 | 0.3735 |
| Covered by empty-shell barnacles | 22.91 | / | / | / | 2.9092 × 10−6 | 0.65084 | 322 | 748.9 | 0.10615 | 0.41022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, P.; Shangguan, J.; Hu, J.; Geng, B.; Wang, P. Effect of Barnacles on the Corrosion Behavior of 304 Stainless Steel. Metals 2023, 13, 1649. https://doi.org/10.3390/met13101649
Deng P, Shangguan J, Hu J, Geng B, Wang P. Effect of Barnacles on the Corrosion Behavior of 304 Stainless Steel. Metals. 2023; 13(10):1649. https://doi.org/10.3390/met13101649
Chicago/Turabian StyleDeng, Peichang, Juyu Shangguan, Jiezhen Hu, Baoyu Geng, and Peilin Wang. 2023. "Effect of Barnacles on the Corrosion Behavior of 304 Stainless Steel" Metals 13, no. 10: 1649. https://doi.org/10.3390/met13101649
APA StyleDeng, P., Shangguan, J., Hu, J., Geng, B., & Wang, P. (2023). Effect of Barnacles on the Corrosion Behavior of 304 Stainless Steel. Metals, 13(10), 1649. https://doi.org/10.3390/met13101649





