Diffusion Welding of Surface Treated Alloy 800H
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Treatment and Diffusion Welding
2.3. Microstructural Evaluation
2.4. Tensile Test
3. Results
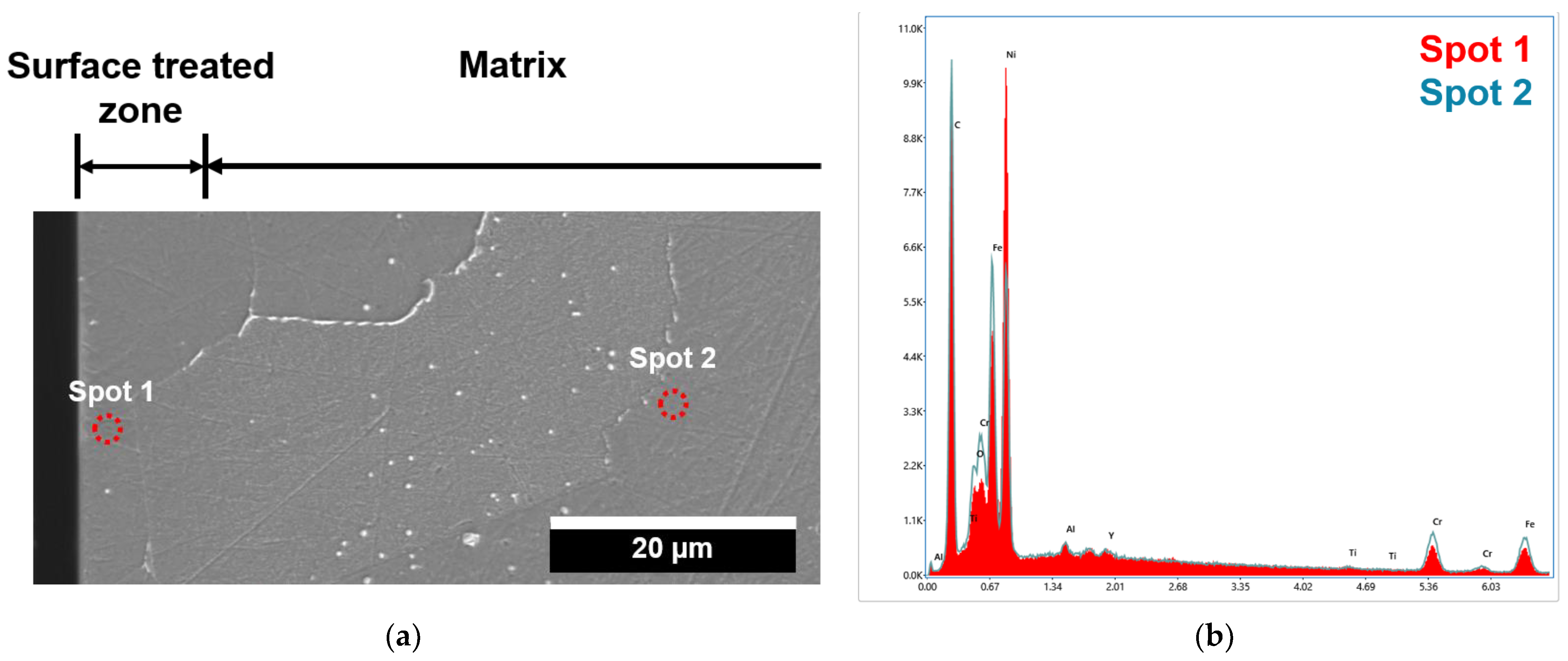
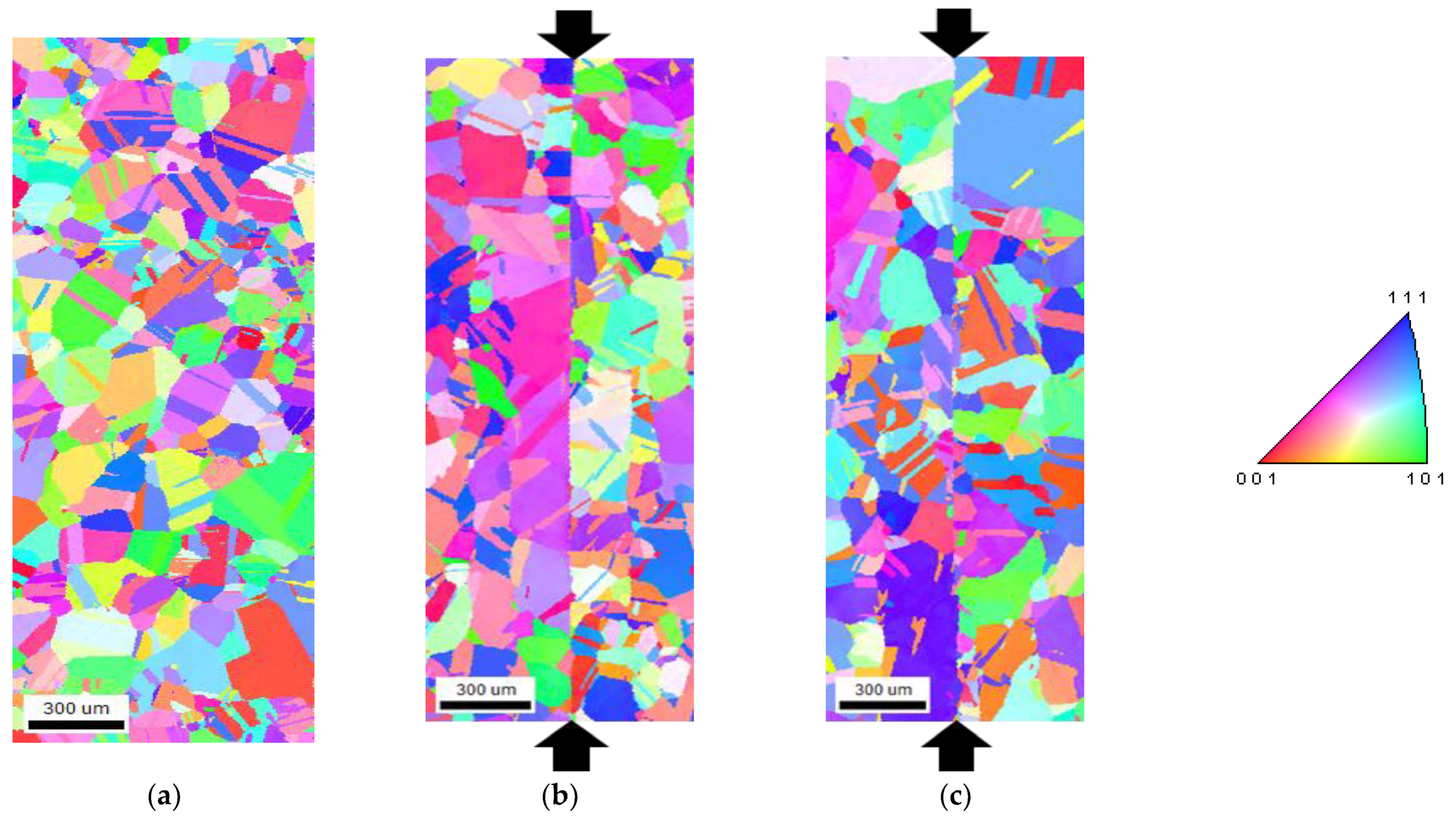
3.1. Microstructural Feature
3.2. Tensile Behavior
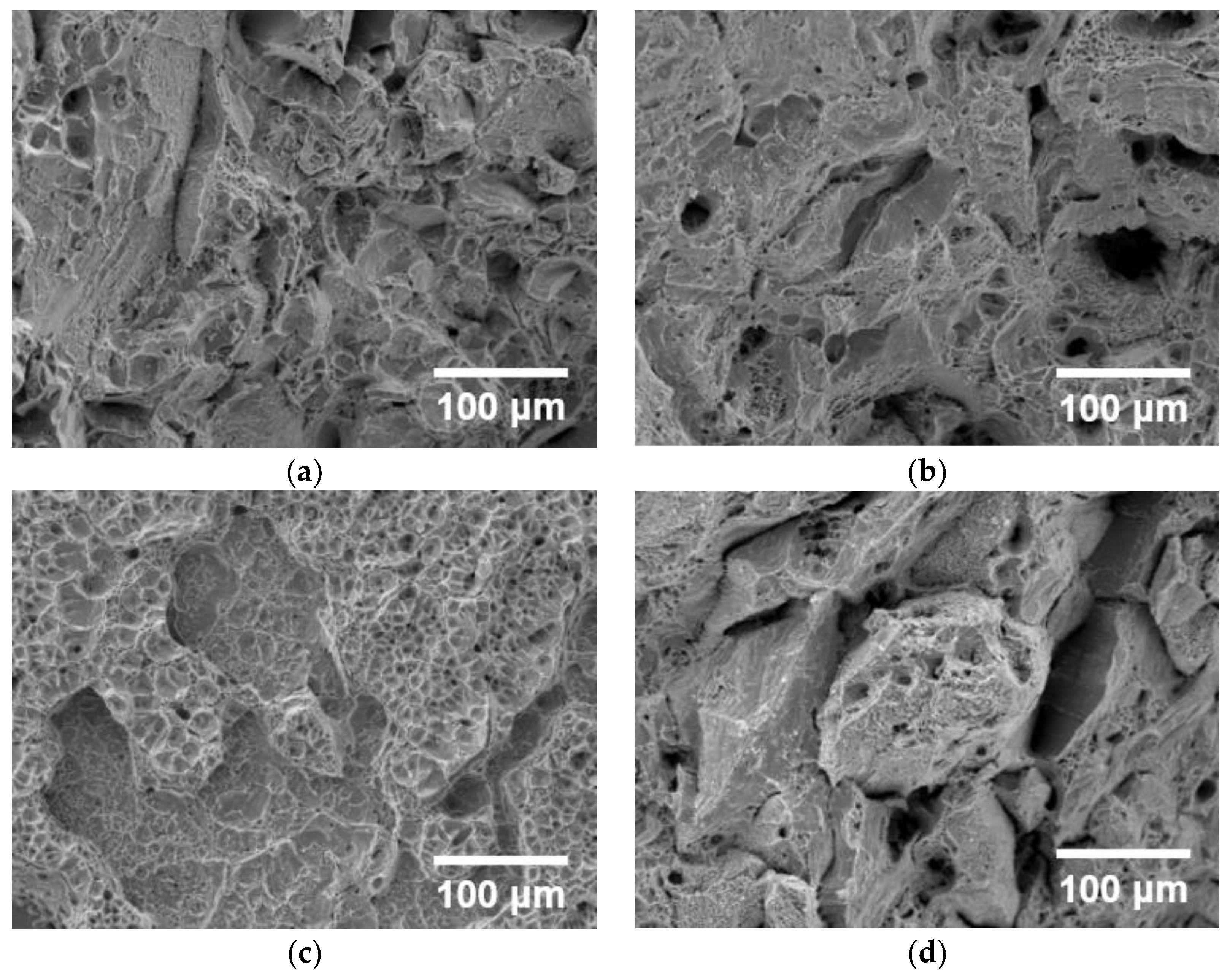
3.3. Fractography
4. Discussion
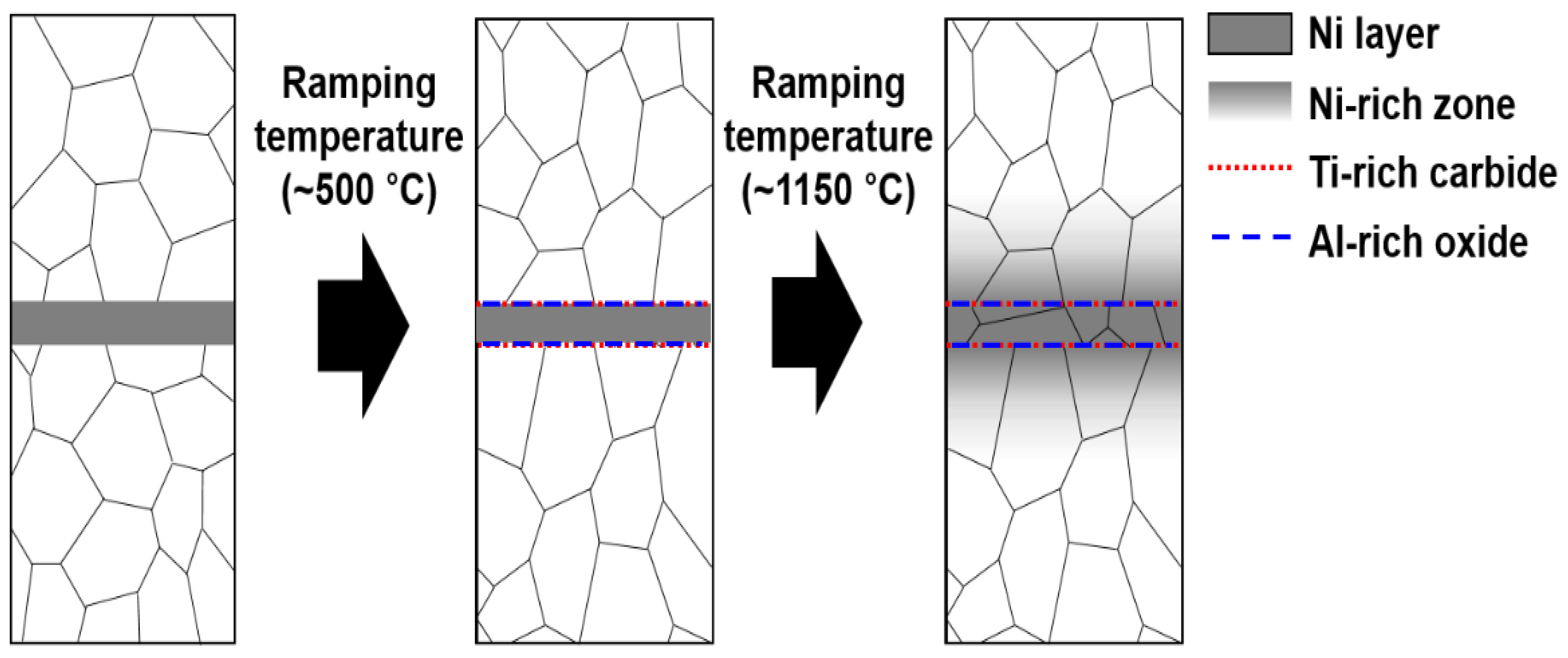
4.1. Dissolution of Secondary Precipitates into the Matrix
4.2. The Difference with the Ni Interlayer Insertion
5. Conclusions
- The surface treatment of Alloy 800H transformed the chemical composition at/near the surface. The Ni concentration was the maximum at the surface, and the thickness of the surface treated zone was ~10 µm.
- The grain boundary bulging at/near the interface occurred due to a reduction of Ti-rich carbides. The sparsely dispersed ~100 nm sized Ti-rich carbides and Al-rich oxides insignificantly influenced the grain boundary bulging across the interface.
- The chemical compositions of the constitutive elements were homogenized during the diffusion welding process. The chemical compositions at/near the interface satisfied the ASTM specifications.
- The tensile properties of the diffusion weldment were comparable to those that Alloy 800H underwent with the same thermo-mechanical process up to 700 °C.
- The specimens were fractured in a ductile manner. The location of failure was random in the gauge section. Macroscopic deformation was observed on the fracture surface.
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takeda, T.; Kunitomi, K.; Horie, T.; Iwata, K. Feasibility Study on the Applicability of a Diffusion-Welded Compact Intermediate Heat Exchanger to Next-Generation High Temperature Gas-Cooled Reactor. Nucl. Eng. Des. 1997, 168, 11–21. [Google Scholar] [CrossRef]
- Clark, D.E.; Mizia, R.E.; Glazoff, M.V.; Sabharwall, P.; McKellar, M.G. Diffusion Welding of Alloys for Molten Salt Service—Status Report; INL/EXT-12-24589 Rev. 1; Idaho National Laboratory: Idaho Falls, ID, USA, 2012. [CrossRef]
- Nestell, J.; Sham, T.L. ASME Code Considerations for the Compact Heat Exchanger; ORNL/TM-2015/401; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2015. [CrossRef]
- Ohsumi, M.; Kiyotou, S.I.; Sakamoto, M. The Application of Diffusion Welding to Aircraft Titanium Alloy. Trans. Iron Steel Inst. Japan 1985, 25, 513–520. [Google Scholar] [CrossRef]
- Chaturvedi, M.C. (Ed.) Welding and Joining of Aerospace Materials; Woodhead Publishing: Sawston, UK, 2012. [Google Scholar]
- Xiong, J.; Peng, Y.; Samiuddin, M.; Yuan, L.; Li, J. Common Mechanical Properties of Diffusion Bonded Joints and Their Corresponding Microstructure Features. J. Mater. Eng. Perform. 2020, 29, 3277–3286. [Google Scholar] [CrossRef]
- Sah, I.; Kim, D.; Lee, H.J.; Jang, C. The Recovery of Tensile Ductility in Diffusion-Bonded Ni-base Alloys by Post-Bond Heat Treatments. Mater. Des. 2013, 47, 581–589. [Google Scholar] [CrossRef]
- Hong, S.; Kim, S.H.; Jang, C.; Sah, I. The Effect of Post-Bond Heat Treatment on Tensile Property of Diffusion Bonded Austenitic Alloys. Trans. Korean Soc. Mech. Eng. A 2015, 39, 1221–1227. [Google Scholar] [CrossRef]
- Sah, I.; Hwang, J.B.; Hong, S.I.; Kim, E.S.; Kim, M.H. Effect of Heat Treatment on the Diffusion-Bonded Ni-Base Alloy Hastelloy X. Korean J. Met. Mater. 2017, 55, 114–123. [Google Scholar] [CrossRef]
- Sah, I.; Hwang, J.B.; Kim, W.G.; Kim, E.S.; Kim, M.H. High-Temperature Mechanical Behaviors of Diffusion-Welded Alloy 617. Nucl. Eng. Des. 2020, 364, 110617. [Google Scholar] [CrossRef]
- Mizia, R.E.; Clark, D.E.; Glazoff, M.V.; Lister, T.E.; Trowbridge, T.L. Progress Report for Diffusion Welding of the NGNP Process Application Heat Exchangers; INL/EXT-11-21817 Revision 1; Idaho National Laboratory: Idaho Falls, ID, USA, 2011. [CrossRef]
- Mizia, R.E.; Clark, D.E.; Glazoff, M.V.; Lister, T.E.; Trowbridge, T.L. Optimizing the Diffusion Welding Process for Alloy 800H: Thermodynamic, Diffusion Modeling, and Experimental Work. Metall. Mater. Trans. A 2013, 44, 154–161. [Google Scholar] [CrossRef]
- Mahajan, H.P.; Elbakhshwan, M.; Beihoff, B.C.; Hassan, T. Mechanical and Microstructural Characterization of Diffusion Bonded 800H. In Proceedings of the Pressure Vessels and Piping Conference, Online, 3 August 2020. [Google Scholar] [CrossRef]
- An, T.; Subramanian, G.O.; Jang, C. Effect of Post-Bond Heat Treatment on the Diffusion Bonding Properties of Alloy 800H with Ni-Foil Interlayer. In Proceedings of the Transactions of the Korean Nuclear Society Virtual Autumn Meeting, Online, 21–22 October 2021; Available online: https://www.kns.org/files/pre_paper/46/21A-138-%EC%95%88%ED%83%9C%EC%A0%95 (accessed on 21 October 2021).
- Mahajan, H.P.; Lima, L.M.A.; Hassan, T. Mechanical and Microstructural Performance Evaluation of Diffusion Bonded Alloy 800H for Very High Temperature Nuclear Service. J. Eng. Mater. Technol. 2022, 144, 021008. [Google Scholar] [CrossRef]
- Rozman, K.A.; Carl, M.A.; Kapoor, M.; Doğan, Ö.N.; Hawk, J.A. Creep Performance of Transient Liquid Phase Bonded Haynes 230 Alloy. Mater. Sci. Eng. A 2019, 768, 138477. [Google Scholar] [CrossRef]
- Malekan, A.; Farvizi, M.; Mirsalehi, S.E.; Saito, N.; Nakashima, K. Holding Time Influence on Creep Behavior of Transient Liquid Phase Bonded Joints of Hastelloy X. Mater. Sci. Eng. A 2020, 772, 138694. [Google Scholar] [CrossRef]
- Hu, M.; Li, C.; Guo, Q.; Han, X.; Ding, R.; Li, H.; Xia, X.; Liu, Y. Improving Creep Property and Deformation Mechanism of Transient Liquid Phase Bonded Polycrystalline Ni3Al-Based Superalloy with Post Bond Heat Treatment. Mater. Sci. Eng. A 2023, 874, 145078. [Google Scholar] [CrossRef]
- Sah, I.; Kim, E.S. Enhanced Joint Integrity of Diffusion-Welded Alloy 617 by Controlling the Micro-Chemistry near the Surface. Mater. Today Commun. 2021, 29, 102770. [Google Scholar] [CrossRef]
- Lei, X.W.; Li, D.Y.; Zhang, X.H.; Liang, T.X. Effect of Solid Solution Elements on Solubility Products of Carbides and Nitrides in Austenite: Thermodynamic Calculations. Metall. Mater. Trans. A 2019, 50, 4445–4461. [Google Scholar] [CrossRef]
- ASTM B409-22; Standard Specification for Nickel-Iron-Chromium Alloy Plate, Sheet, and Strip. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM E8/E8M-22; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM E21-20; Standard Test Methods for Elevated Temperature Tension Tests of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2021.
- Bassford, T.H.; Hosier, J.C. Production and Welding Technology of Some High-Temperature Nickel Alloys in Relation to Their Properties. Nucl. Technol. 1984, 66, 35–43. [Google Scholar] [CrossRef]
- Wright, J.K.; Carroll, L.J.; Cabet, C.; Lillo, T.M.; Benz, J.K.; Simpson, J.A.; Lloyd, W.R.; Chapman, J.A.; Wright, R.N. Characterization of Elevated Temperature Properties of Heat Exchanger and Steam Generator Alloys. Nucl. Eng. Des. 2012, 251, 252–260. [Google Scholar] [CrossRef]
- Wright, J.K.; Simpson, J.A.; Wright, R.N.; Carroll, L.J.; Sham, T.L. Strain Rate Sensitivity of Alloys 800H and 617. In Proceedings of the ASME 2013 Pressure Vessels and Piping Conference, Paris, France, 14–18 July 2013. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, C.; Zhang, C.; Di, H.; Huang, G.; Liu, Q. Influence of Dynamic Strain Aging on the Mechanical Properties and Microstructural Evolution for Alloy 800H during Hot Deformation. Mater. Sci. Eng. A 2018, 724, 37–44. [Google Scholar] [CrossRef]
- Broek, D. A Study on Ductile Fracture; NLR–TR 71021 U; National Aerospace Laboratory NLR: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Wada, T.; Wada, H.; Elliott, J.F.; Chipman, J. Thermodynamics of the Fcc Fe-Ni-C and Ni-C Alloys. Metall. Mater. Trans. B 1971, 2, 2199–2208. [Google Scholar] [CrossRef]
- Rahmel, A.; Grabke, H.J.; Steinkusch, W. Carburization—Introductory survey. Mater. Corros. 1998, 49, 221–225. [Google Scholar] [CrossRef]
- Elrefaie, F.A.; Smeltzer, W.W. Thermodynamics of Nickel-Aluminum-Oxygen System between 900 and 1400 K. J. Electrochem. Soc. 1981, 128, 2237–2242. [Google Scholar] [CrossRef]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Keown, S.R.; Pickering, F.B. Niobium in Stainless Steels. In Proceedings of the Niobium International Symposium, San Francisco, CA, USA, 8–11 November 1981. [Google Scholar]
- Kajihara, M.; Yoshikawa, T.; Kikuchi, M.; Yamauchi, K. Solubility of NbC in a Ni-16Cr-8Fe alloy. ISIJ Int. 1992, 32, 755–763. [Google Scholar] [CrossRef]
- Vander Voort, G.F. (Ed.) Atlas of Time-Temperature Diagrams for Nonferrous Alloys; ASM International: Tokyo, Japan, 1991. [Google Scholar]
- Neumann, G.; Tuijn, C. Self-Diffusion and Impurity Diffusion in Pure Metals: Handbook of Experimental Data; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Lander, J.J.; Kern, H.E.; Beach, A.L. Solubility and Diffusion Coefficient of Carbon in Nickel: Reaction Rates of Nickel-Carbon Alloys with Barium Oxide. J. Appl. Phys. 1952, 23, 1305–1309. [Google Scholar] [CrossRef]







| Designation | Chemical Compositions [wt.%] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ni | Cr | Al | C | Mn | Si | S | Ti | Cu | P | Remark | |
| UNS N08810 | 39.5 min | 30 min | 19.0 min | 0.15 min | 0.05–0.10 | 1.5 max | 1.0 | 0.015 | 0.15–0.60 | 0.75 | - | - |
| 800H(T) (HH2768AG) | 45.79 | 31.85 | 20.12 | 0.49 | 0.07 | 0.87 | 0.17 | 0.001 | 0.58 | 0.12 | 0.013 | Al + Ti = 1.00 |
| 800H (735400) | 46.83 | 30.18 | 20.43 | 0.49 | 0.07 | 0.98 | 0.42 | 0.000 | 0.54 | 0.45 | 0.022 | Al + Ti = 1.03 |
| Alloy | Designation | Dimension [mm] | Qty | Temperature [°C] | Pressure [MPa] | Time [min] | Vacuum [Torr] | Avg. ΔZ [%] | Post-Weld Heat Treatment | Remark |
|---|---|---|---|---|---|---|---|---|---|---|
| 800H(T) (HH2768AG) | 8NP0 | 50 (L) × 50 (W) × 25 (T) | 2 | 1150 | 10 | 60 | 7.0 × 10−5 | −2.91 | - | Microstructure, tensile |
| 8NP2 | - | 1120 °C 20 h | Tensile | |||||||
| 800H (735400) | 8NB | 110 (L) × 80 (W) × 25 (T) | 4 | 1140 | 7 | 120 | 7.0 × 10−5 | −0.92 | 1120 °C 20 h | Tensile |
| Temperature (°C) | Designation | Yield Strength (YS, MPa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|---|
| 25 | 8AR | 239 | 563 | 50.4 |
| 8TM | 201 | 555 | 44.9 | |
| 8DF | 202 | 553 | 46.7 | |
| 540 | 8AR | 148 | 487 | 53.6 |
| 8TM | 122 | 454 | 44.5 | |
| 8DF | 120 | 450 | 46.6 | |
| 650 | 8AR | 142 | 418 | 42.7 |
| 8TM | 118 | 372 | 40.9 | |
| 8DF | 112 | 376 | 39.1 | |
| 700 | 8AR | 141 | 362 | 37.8 |
| 8TM | 112 | 299 | 40.2 | |
| 8DF | 111 | 312 | 37.3 |
| NC (wt.%) | Matrix | |||||
|---|---|---|---|---|---|---|
| Fe-Cr (Fe-25Ni-20Cr) log[(Nb)(C)] = 4.07 − 8358/T | Ni-Cr (Alloy 600) ln[(Nb)(C0.81)] = 7.5 − 14000/T | |||||
| Solubility Product | Reaction Quotient | NNb, min (wt.%) | Solubility Product | Reaction Quotient | NNb, min (wt.%) | |
| 0.07 | 0.015 | 0.040 | 0.13 | 0.096 | 0.069 | 0.83 |
| 0.14 | 0.081 | 0.077 | 0.12 | 0.47 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-B.; Sa, I.; Kim, E.-S.; Lee, D.-H. Diffusion Welding of Surface Treated Alloy 800H. Metals 2023, 13, 1727. https://doi.org/10.3390/met13101727
Hwang J-B, Sa I, Kim E-S, Lee D-H. Diffusion Welding of Surface Treated Alloy 800H. Metals. 2023; 13(10):1727. https://doi.org/10.3390/met13101727
Chicago/Turabian StyleHwang, Jong-Bae, Injin Sa, Eung-Seon Kim, and Dong-Hyun Lee. 2023. "Diffusion Welding of Surface Treated Alloy 800H" Metals 13, no. 10: 1727. https://doi.org/10.3390/met13101727





