Effect of Microstructure Modifications on Stress Corrosion Endurance of 15-5 PH Stainless Steel Formed by Wire Laser Additive Manufacturing (WLAM)
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Tested Samples
2.2. Age Hardening Heat Treatment
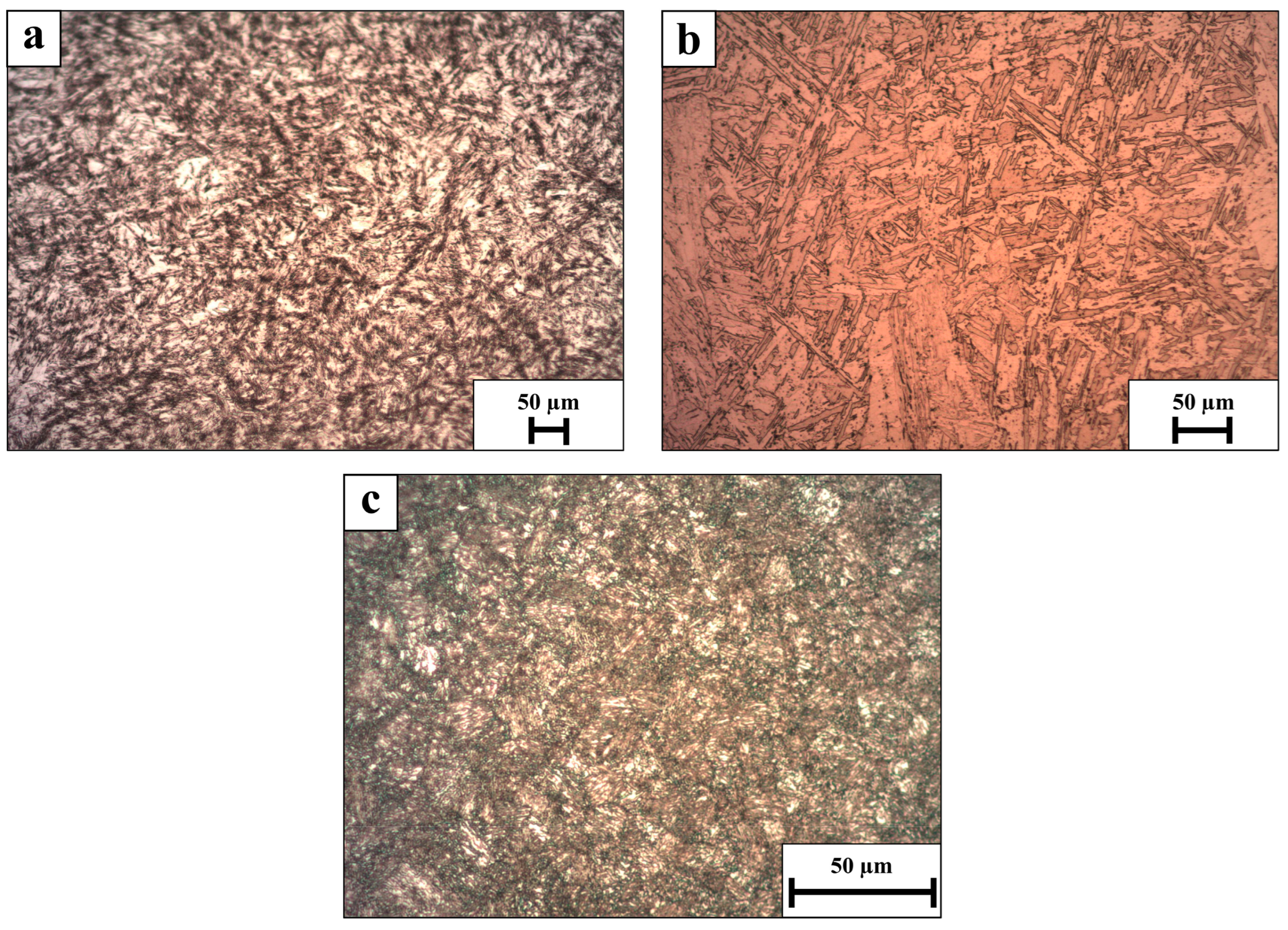
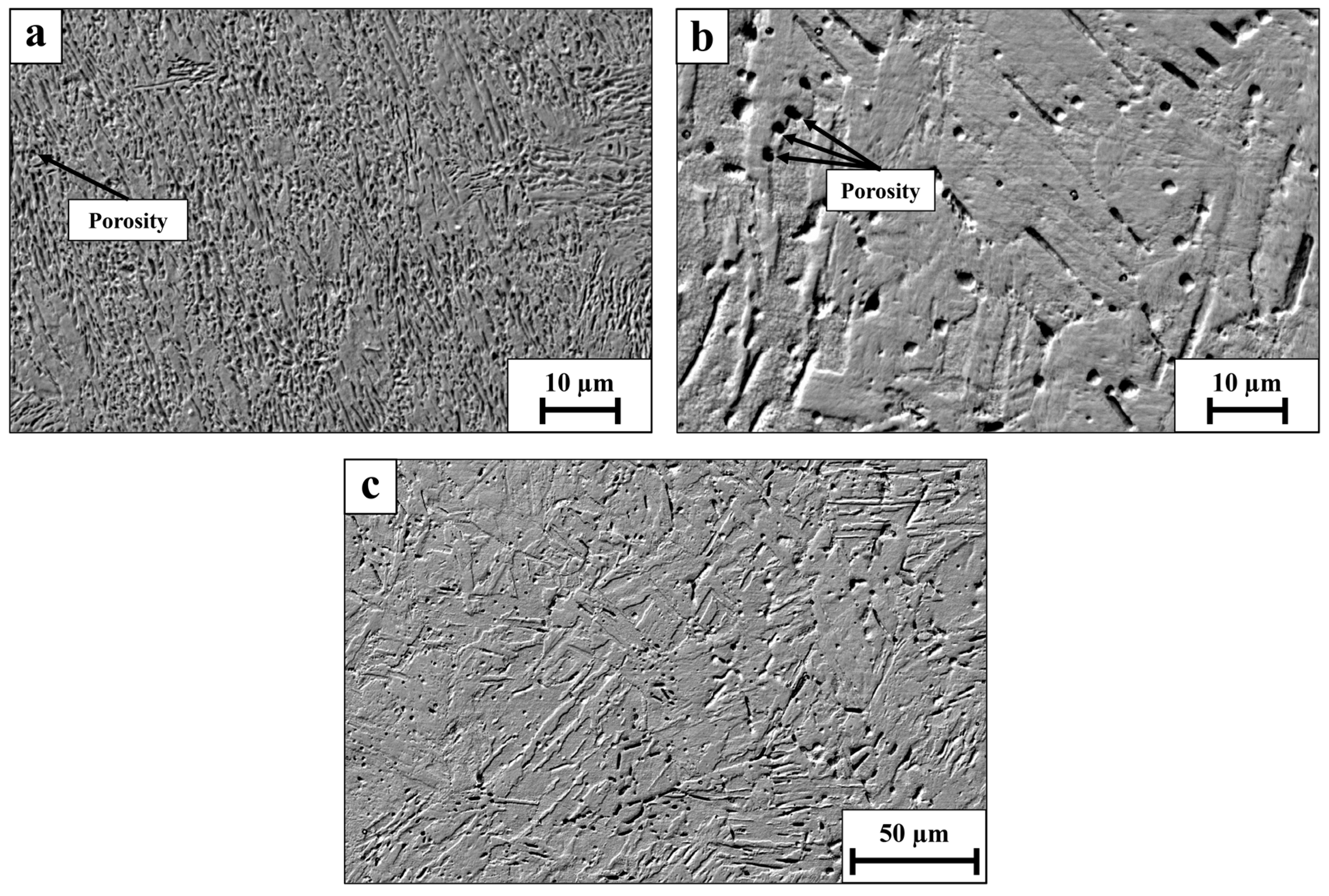
2.3. Microstructure Assessment
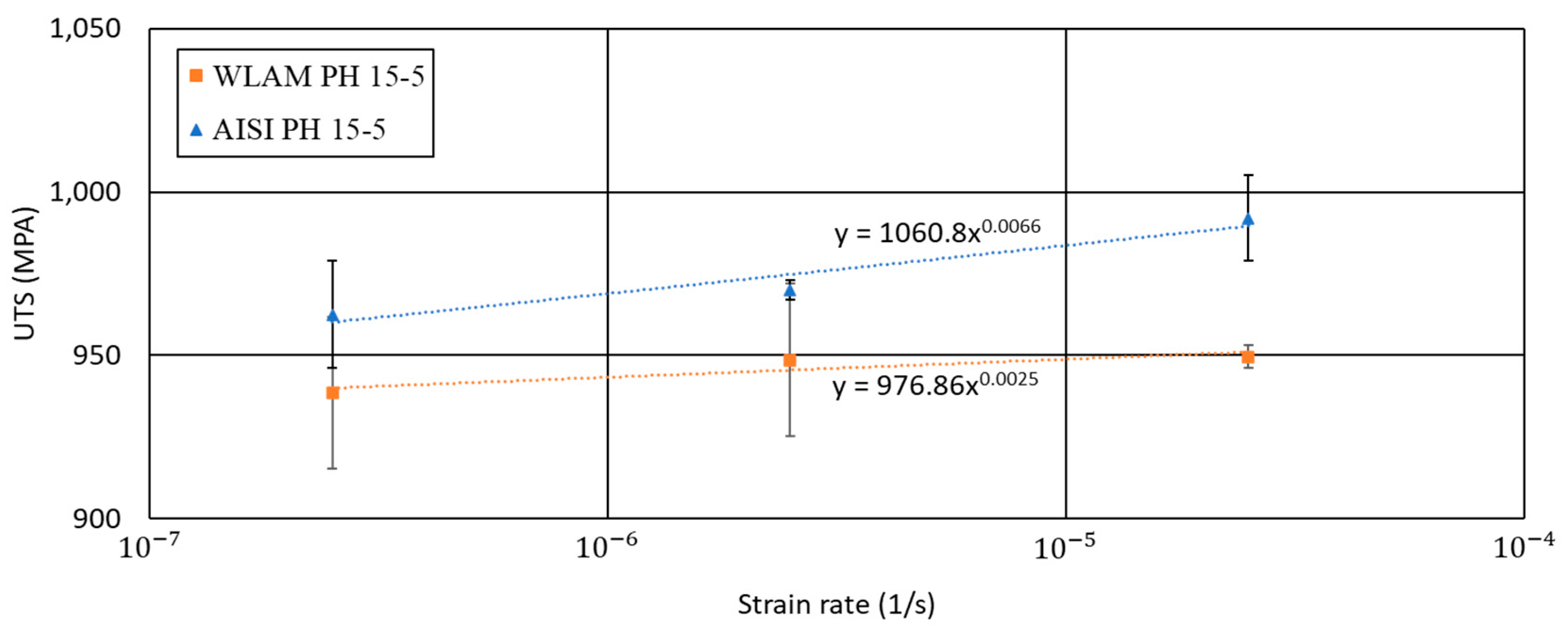
2.4. Mechanical Properties
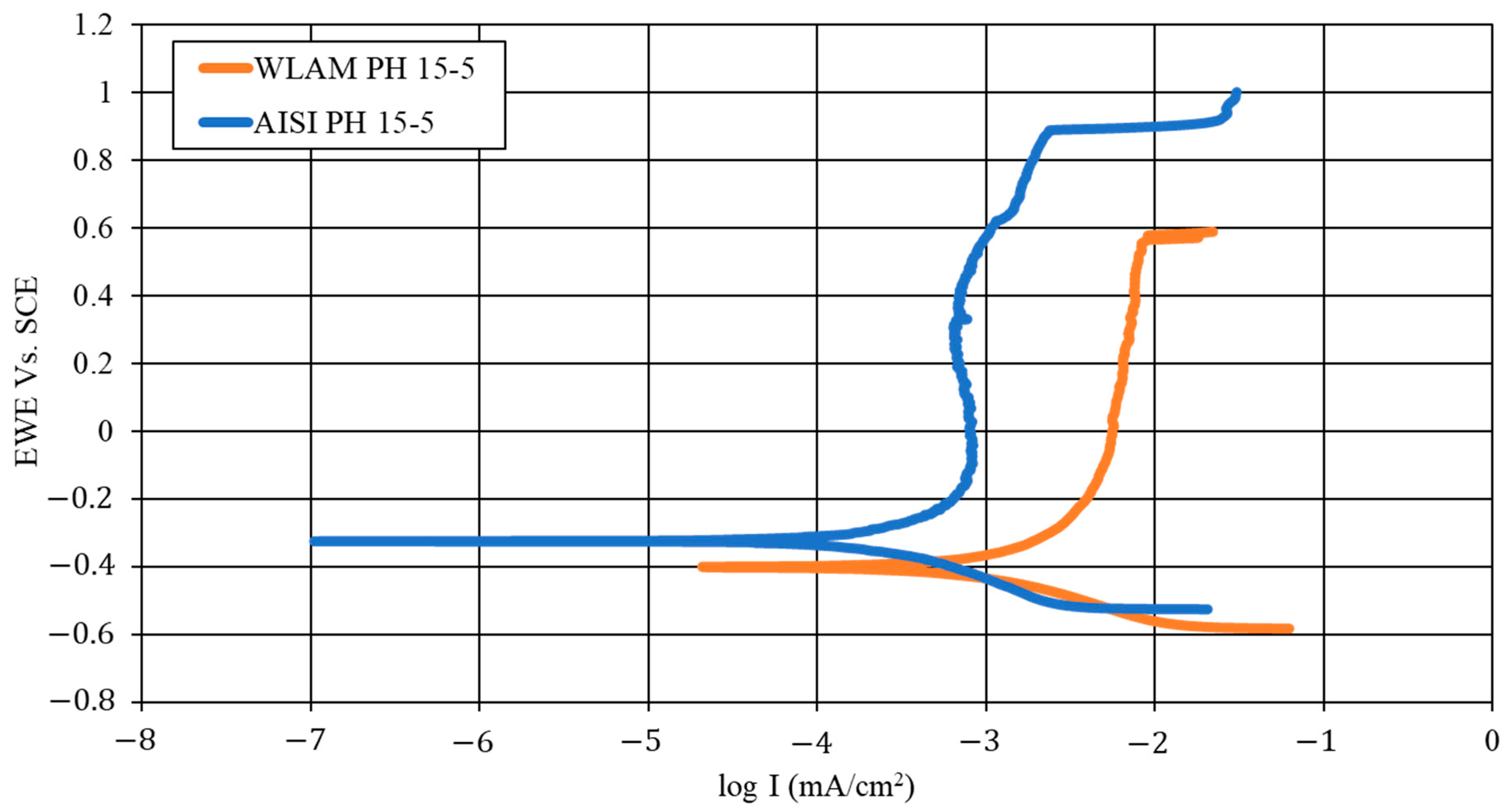
2.5. Electrochemical Analysis
2.6. Stress Corrosion Examination
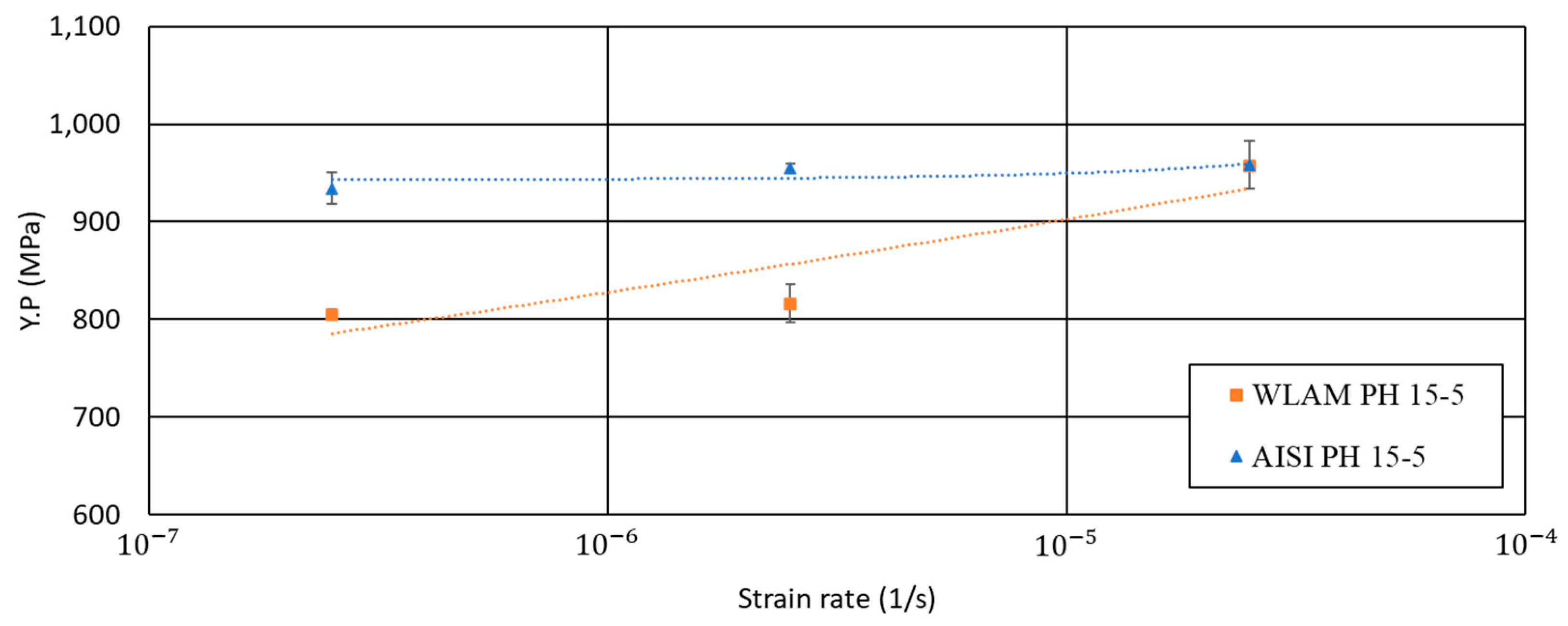
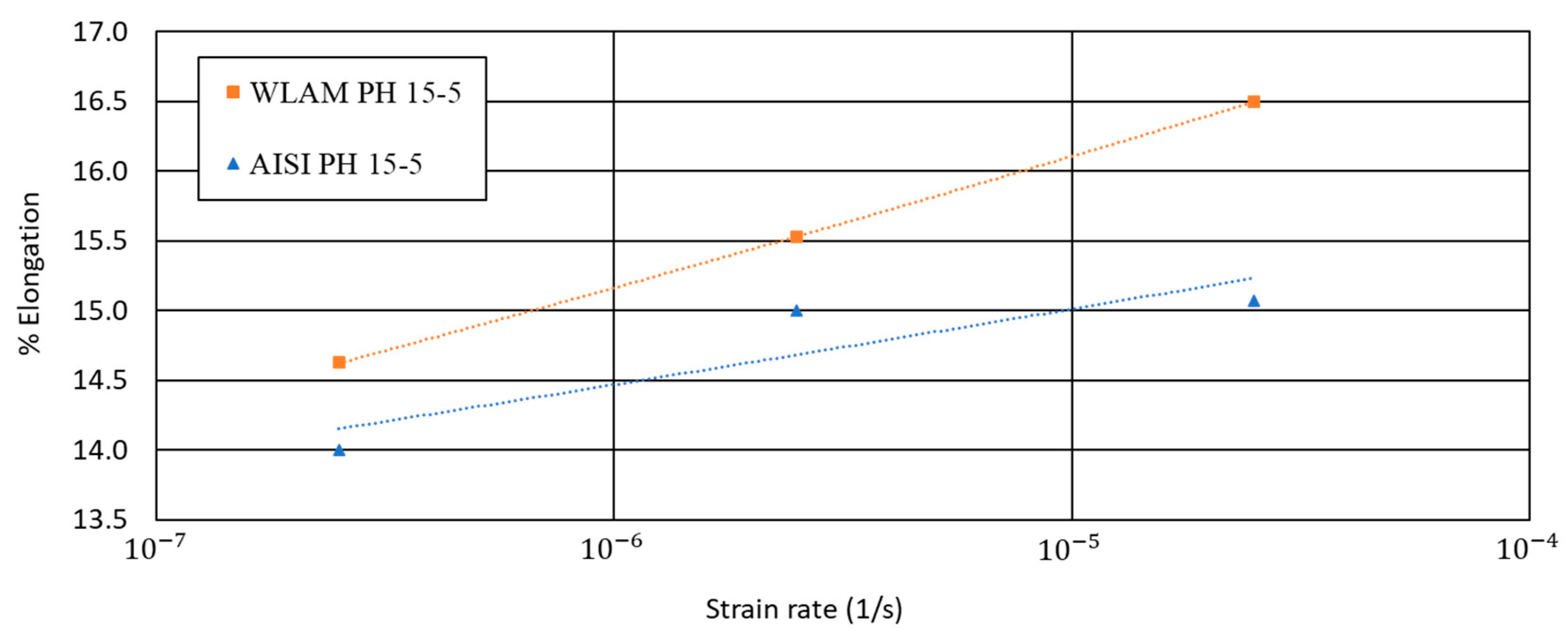
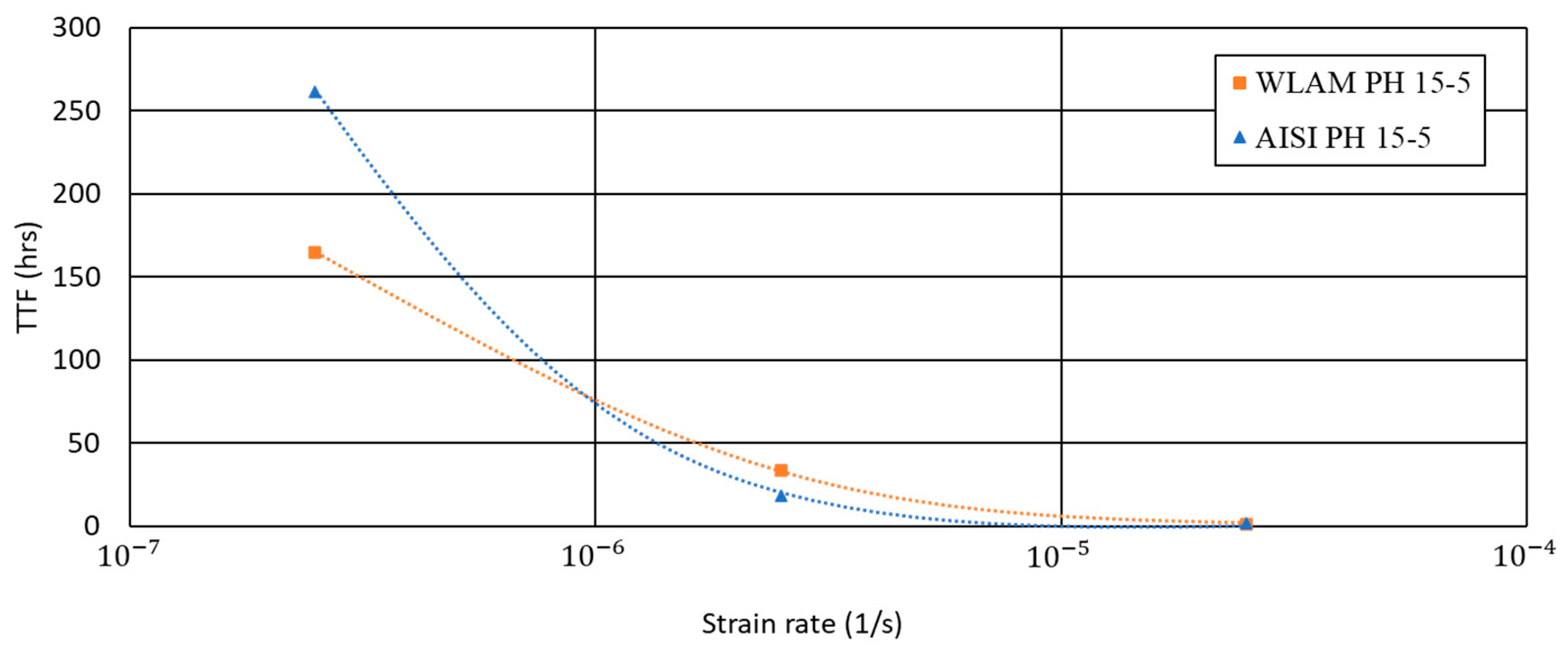
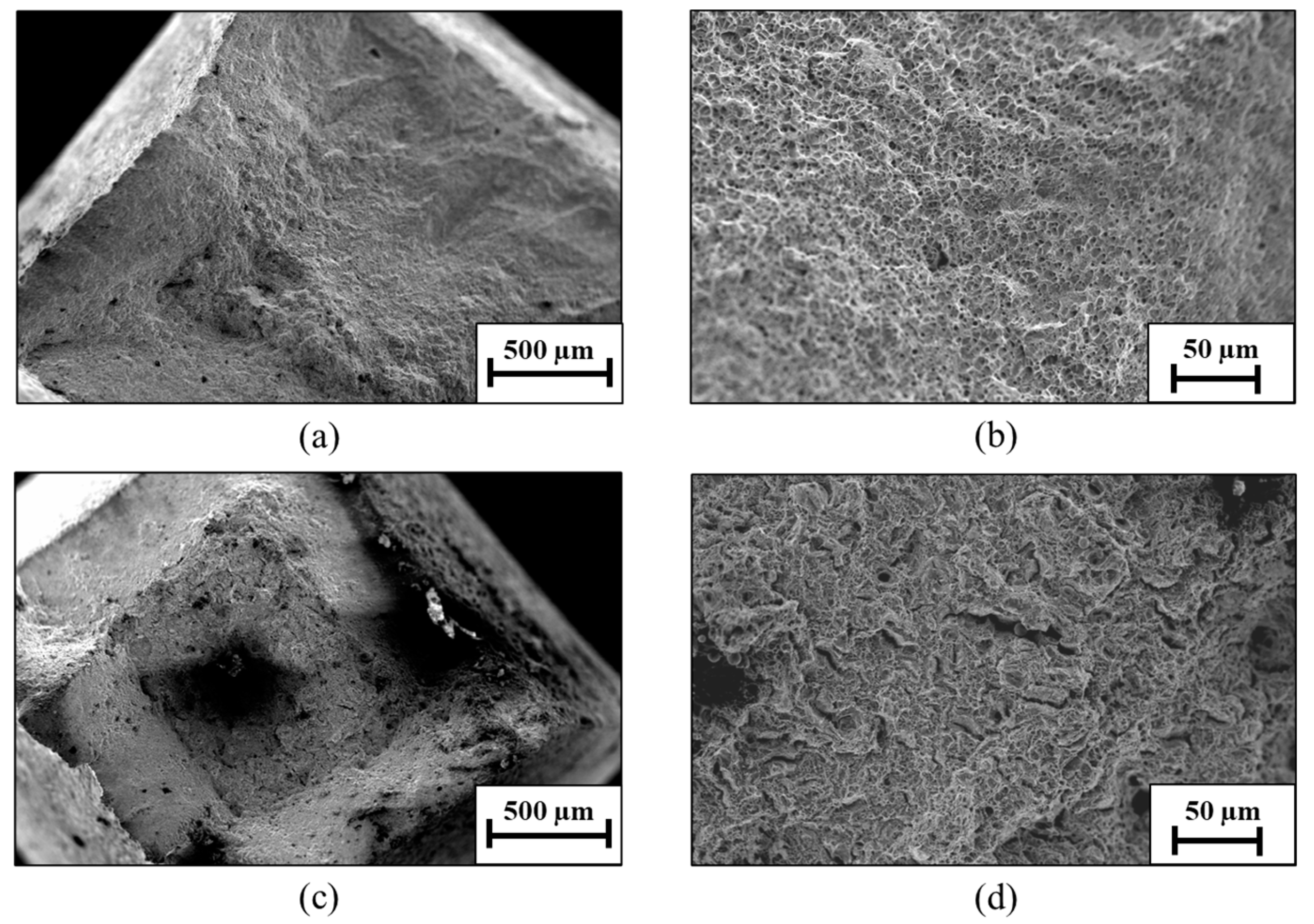
3. Results
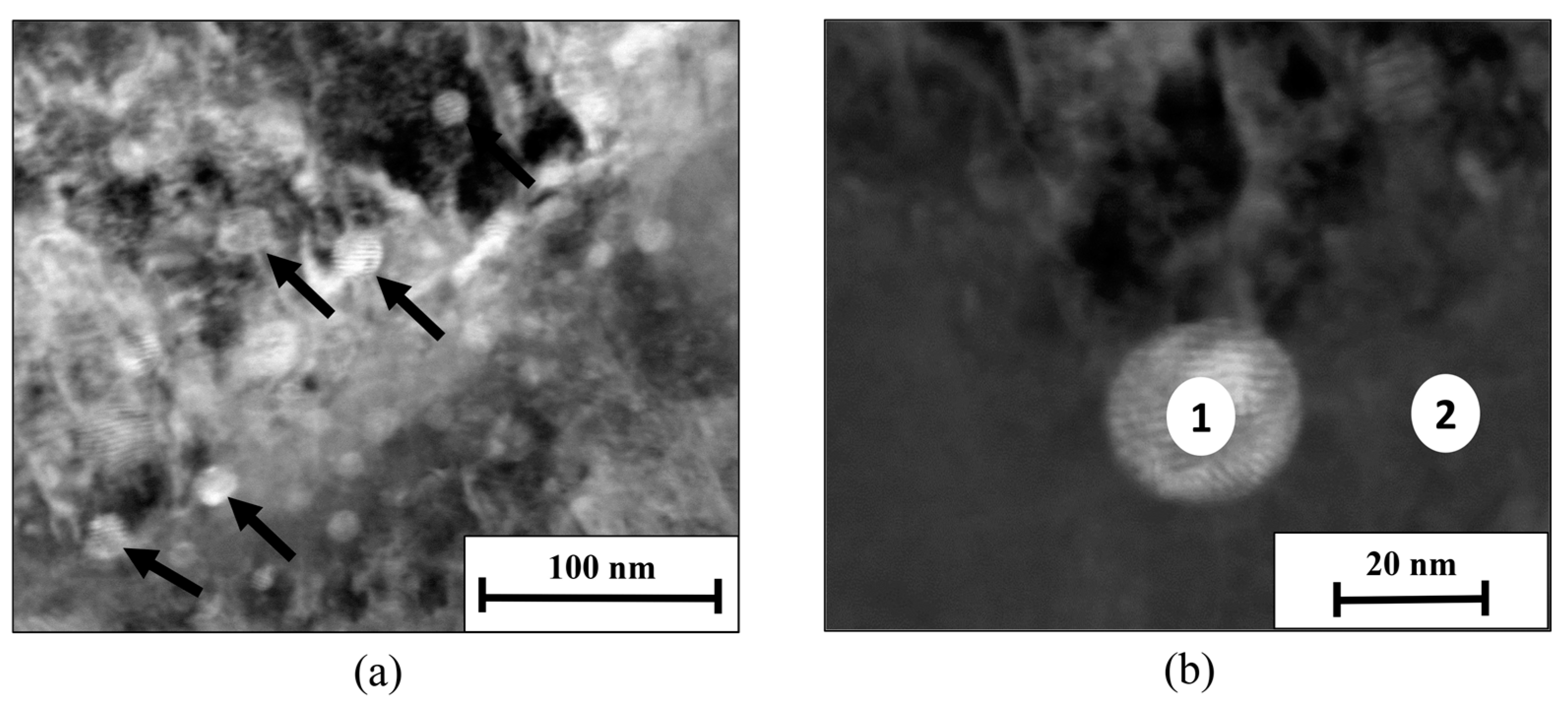
| Point of Measurement | Fe (wt%) | Cr (wt%) | Ni (wt%) | Cu (wt%) | Dominant Phase |
|---|---|---|---|---|---|
| 1 | 45.56 | 10.65 | 2.05 | 36.90 | Main precipitate ε-Cu phase |
| 2 | 78.44 | 17.23 | 2.78 | 0.55 | PH 15-5 matrix |
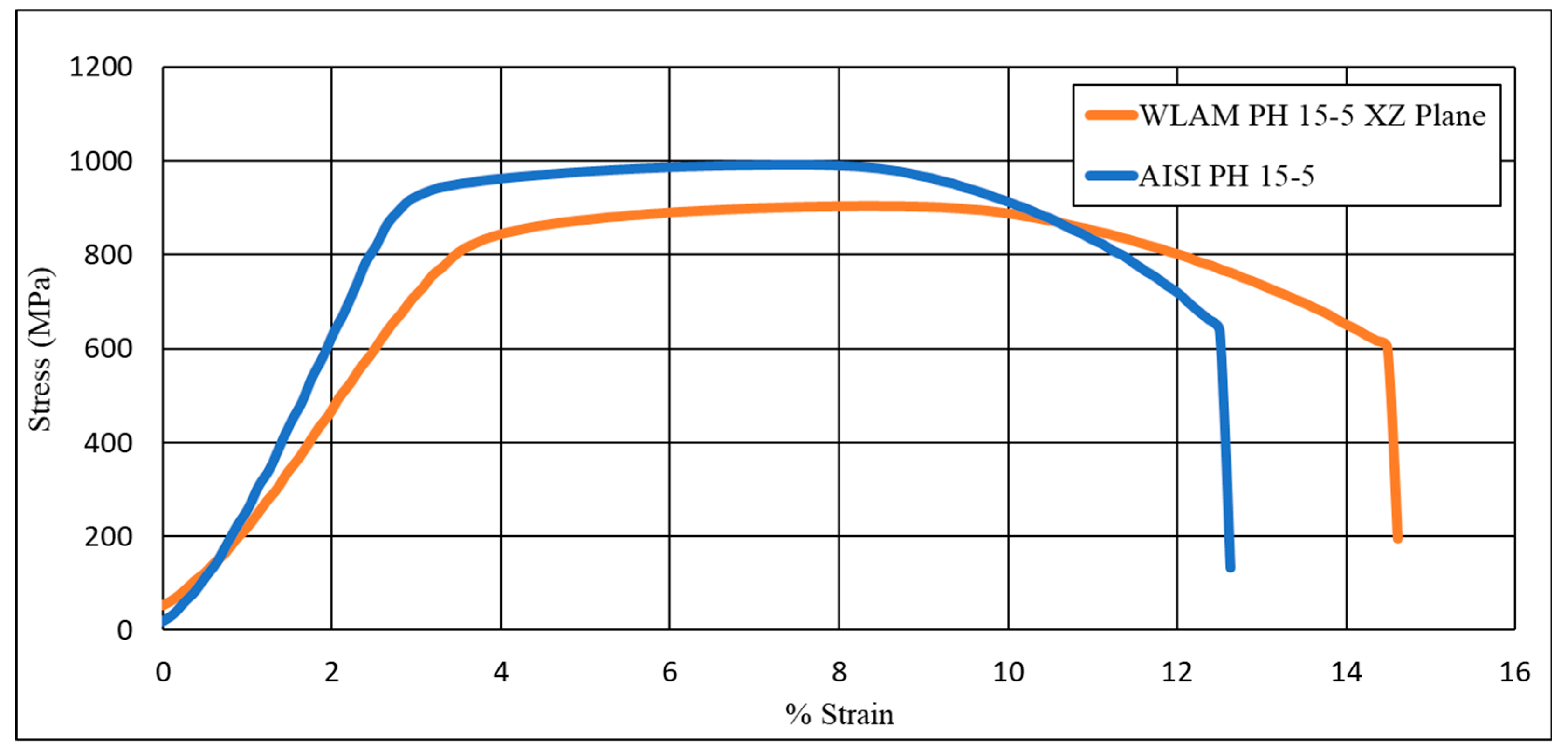
| UTS | Y. P | %Elongation | Hardness (HRC) | |
|---|---|---|---|---|
| WLAM PH 15-5 | 939 ± 25 | 789 ± 5 | 16 ± 1.5 | 30.1 ± 0.5 |
| AISI PH 15-5 | 958 ± 30 | 847 ± 18 | 11 ± 0.8 | 35.6 ± 3.7 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, D.; Pan, Z.; Cuiuri, D.; Li, H. Wire-feed additive manufacturing of metal components: Technologies, developments and future interests. Int. J. Adv. Manuf. Technol. 2015, 81, 465–481. [Google Scholar] [CrossRef]
- Bassis, M.; Kotliar, A.; Koltiar, R.; Ron, T.; Leon, A.; Shirizly, A.; Aghion, E. The effect of a slow strain rate on the stress corrosion resistance of austenitic stainless steel produced by the wire laser additive manufacturing process. Metals 2021, 11, 1930. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Farshidianfar, M.; Gerlich, A.; Nosko, M.; Trembošová, V.; Khajepour, A. Effects of laser additive manufacturing on microstructure and crystallographic texture of austenitic and martensitic stainless steels. Addit. Manuf. 2019, 31, 100915. [Google Scholar] [CrossRef]
- Lass, E.A.; Stoudt, M.R.; Williams, M.E. Additively Manufactured Nitrogen-Atomized 17-4 PH Stainless Steel with Mechanical Properties Comparable to Wrought. Met. Mater. Trans. A 2019, 50, 1619–1624. [Google Scholar] [CrossRef]
- Chen, W.; Luan, J.; Xu, L.; Han, Y.; Zhao, L.; Kai, J.-J.; Xiao, B.; Jing, H. Reversed austenite in additively manufactured martensitic stainless steel. Mater. Sci. Eng. A 2022, 834, 142597. [Google Scholar] [CrossRef]
- Long, J.; Wang, M.; Zhao, W.; Zhang, X.; Wei, Y.; Ou, W. High-power wire arc additive manufacturing of stainless steel with active heat management. Sci. Technol. Weld. Join. 2022, 27, 256–264. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, D.; Zhou, Z.; Wang, W.; Hu, B.; Xie, Y.; Xiong, Z.; Hu, D. Effect of laser power on the microstructure and properties of additive manufactured 17-4 PH stainless steel in different fabrication atmosphere. Mater. Sci. Eng. A 2022, 839, 142846. [Google Scholar] [CrossRef]
- Morales, C.; Merlin, M.; Fortini, A.; Fortunato, A. Direct energy depositions of a 17-4 PH stainless steel: Geometrical and microstructural characterizations. Coatings 2023, 13, 636. [Google Scholar] [CrossRef]
- Leon, A.; Shirizly, A.; Aghion, E. Corrosion behavior of AlSi10Mg alloy produced by additive manufacturing (AM) vs. Its counterpart gravity cast alloy. Metals 2016, 6, 148. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Wilson, J.M.; Piya, C.; Shin, Y.C.; Zhao, F.; Ramani, K. Remanufacturing of turbine blades by laser direct deposition with its energy and environmental impact analysis. J. Clean. Prod. 2014, 80, 170–178. [Google Scholar] [CrossRef]
- Bhushan, B.; Caspers, M. An overview of additive manufacturing (3D printing) for microfabrication. Microsyst. Technol. 2017, 23, 1117–1124. [Google Scholar] [CrossRef]
- Koike, R.; Unotoro, I.; Kakinuma, Y.; Aoyama, T.; Oda, Y.; Kuriya, T.; Fujishima, M. Evaluation for mechanical characteristics of Inconel625–SUS316L joint produced with direct energy deposition. Procedia Manuf. 2017, 14, 105–110. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.L.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. Comput.-Aided Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Lofaj, F.; Kvetková, L.; Shen, Z. Transformation of austenite to duplex austenite-ferrite assembly in annealed stainless steel 316L consolidated by laser melting. J. Alloy. Compd. 2015, 633, 463–469. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Tuck, C.; Ashcroft, I.; Maskery, I.; Everitt, N.M. On the Precipitation Hardening of Selective Laser Melted AlSi10Mg. Met. Mater. Trans. A 2015, 46, 3337–3341. [Google Scholar] [CrossRef]
- Ahmetshin, R.G.; Fedorov, V.V.; Kostikov, K.S.; Martyushev, N.V.; Ovchinnikov, V.A.; Razin, A.V.; Yakovlev, A.N. SLS setup and its working procedure. Key Eng. Mater. 2016, 685, 477–481. [Google Scholar] [CrossRef]
- Ahn, D.-G. Directed Energy Deposition (DED) Process: State of the Art. Int. J. Precis. Eng. Manuf. Technol. 2021, 8, 703–742. [Google Scholar] [CrossRef]
- Ding, D.; Pan, Z.; Cuiuri, D.; Li, H. A multi-bead overlapping model for robotic wire and arc additive manufacturing (WAAM). Robot. Comput. Manuf. 2015, 31, 101–110. [Google Scholar] [CrossRef]
- Bassis, M.; Ron, T.; Leon, A.; Kotliar, A.; Kotliar, R.; Shirizly, A.; Aghion, E. The Influence of Intralayer Porosity and Phase Transition on Corrosion Fatigue of Additively Manufactured 316L Stainless Steel Obtained by Direct Energy Deposition Process. Materials 2022, 15, 5481. [Google Scholar] [CrossRef] [PubMed]
- Ayed, A.; Bras, G.; Bernard, H.; Michaud, P.; Balcaen, Y.; Alexis, J. Additive Manufacturing of Ti6Al4V with Wire Laser Metal Deposition Process. In Materials Science Forum; Trans Tech Publications, Ltd.: Bach, Switzerland, 2021; Volume 1016, pp. 24–29. [Google Scholar]
- Gisario, A.; Kazarian, M.; Martina, F.; Mehrpouya, M. Metal additive manufacturing in the commercial aviation industry: A review. J. Manuf. Syst. 2019, 53, 124–149. [Google Scholar] [CrossRef]
- Heralic, A. Towards Full Automation of Robotized Laser Metal-Wire Deposition. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2009. [Google Scholar]
- Abadi, S.M.A.N.R.; Mi, Y.; Kisielewicz, A.; Sikström, F.; Choquet, I. Influence of laser-wire interaction on heat and metal transfer in directed energy deposition. Int. J. Heat Mass Transf. 2023, 205, 123894. [Google Scholar] [CrossRef]
- Chen, X.; Gussev, M.; Balonis, M.; Bauchy, M.; Sant, G. Emergence of micro-galvanic corrosion in plastically deformed austenitic stainless steels. Mater. Des. 2021, 203, 109614. [Google Scholar] [CrossRef]
- Pellegrini, A.; Lavecchia, F.; Guerra, M.G.; Galantucci, L.M. Influence of aging treatments on 17-4 PH stainless steel parts realized using material extrusion additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2023, 126, 163–178. [Google Scholar] [CrossRef]
- Cheruvathur, S.; Lass, E.A.; Campbell, C.E. Additive Manufacturing of 17-4 PH Stainless Steel: Post-processing Heat Treatment to Achieve Uniform Reproducible Microstructure. JOM 2016, 68, 930–942. [Google Scholar] [CrossRef]
- Giganto, S.; Martínez-Pellitero, S.; Barreiro, J.; Zapico, P. Influence of 17-4 PH stainless steel powder recycling on properties of SLM additive manufactured parts. J. Mater. Res. Technol. 2022, 16, 1647–1658. [Google Scholar] [CrossRef]
- Murr, L.E.; Martinez, E.; Hernandez, J.; Collins, S.; Amato, K.N.; Gaytan, S.M.; Shindo, P.W. Microstructures and properties of 17-4 PH stainless steel fabricated by selective laser melting. J. Mater. Res. Technol. 2012, 1, 167–177. [Google Scholar] [CrossRef]
- Leo, P.; Nobile, R.; Barreiro, J.; Bagheri, S.; Mele, C. Precipitation hardening stainless steel samples processed by additive manufacturing: Process parameters and thermo mechanical treatments effects on microstructure and corrosion resistance. Opt. Laser Technol. 2022, 156, 108547. [Google Scholar] [CrossRef]
- Guo, C.; Hu, R.; Chen, F. Microstructure and performances for 15-5 PH stainless steel fabricated through the wire-arc additive manufacturing technology. Mater. Technol. 2020, 36, 831–842. [Google Scholar] [CrossRef]
- Nong, X.; Zhou, X.; Wang, Y.; Yu, L.; Li, J. Effects of geometry, location, and direction on microstructure and mechanical properties of 15–5PH stainless steel fabricated by directed energy deposition. Mater. Sci. Eng. A 2021, 821, 141587. [Google Scholar] [CrossRef]
- Ghaffari, M.; Nemani, A.V.; Nasiri, A. Microstructural evolution and mechanical performance after precipitation hardening of PH 13-8Mo martensitic stainless steel fabricated by wire arc additive manufacturing. Materialia 2022, 24, 101507. [Google Scholar] [CrossRef]
- Moniruzzaman, F.M.; Shakil, S.I.; Shaha, S.K.; Kacher, J.; Nasiri, A.; Haghshenas, M.; Hadadzadeh, A. Study of direct aging heat treatment of additively manufactured PH13–8Mo stainless steel: Role of the manufacturing process, phase transformation kinetics, and microstructure evolution. J. Mater. Res. Technol. 2023, 24, 3772–3787. [Google Scholar] [CrossRef]
- Brown, B.; Newkirk, J.; Liou, F. Absorption of Nitrogen during Pulsed Wave L-PBF of 17-4 PH Steel. Materials 2021, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Mukherjee, S.; Kumar, C.S.; Nath, A.K. Effects of heat treatment on microstructure, mechanical and corrosion properties of 15-5 PH stainless steel parts built by selective laser melting process. J. Manuf. Process. 2020, 50, 279–294. [Google Scholar] [CrossRef]
- Kaya, A.; Uzan, P.; Eliezer, D.; Aghion, E. Electron microscopical investigation of as cast AZ 91 D alloy. Mater. Sci. Technol. 2000, 16, 1001–1006. [Google Scholar] [CrossRef]
- Shaffer, D.; Wilson-Heid, A.; Keist, J.; Beese, A.; Palmer, T. Impact of retained austenite on the aging response of additively manufactured 17-4 PH grade stainless steel. Mater. Sci. Eng. A 2021, 817, 141363. [Google Scholar] [CrossRef]
- Meredith, S.; Zuback, J.; Keist, J.; Palmer, T. Impact of composition on the heat treatment response of additively manufactured 17–4 PH grade stainless steel. Mater. Sci. Eng. A 2018, 738, 44–56. [Google Scholar] [CrossRef]
- ASTM G129-21; Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking. Astm International: West Conshohocken, PA, USA.
- Zai, L.; Zhang, C.; Wang, Y.; Guo, W.; Wellmann, D.; Tong, X.; Tian, Y. Laser powder bed fusion of precipitation-hardened martensitic stainless steels: A review. Metals 2020, 10, 255. [Google Scholar] [CrossRef]
- Sun, Y.; Hebert, R.J.; Aindow, M. Effect of heat treatments on microstructural evolution of additively manufactured and wrought 17-4 PH stainless steel. Mater. Des. 2018, 156, 429–440. [Google Scholar] [CrossRef]
- Wang, D.; Chi, C.; Wang, W.; Li, Y.; Wang, M.; Chen, X.; Chen, Z.; Cheng, X.; Xie, Y. The effects of fabrication atmosphere condition on the microstructural and mechanical properties of laser direct manufactured stainless steel 17-4 PH. J. Mater. Sci. Technol. 2019, 35, 1315–1322. [Google Scholar] [CrossRef]
- Brennan, M.C.; Keist, J.S.; Palmer, T.A. Defects in metal additive manufacturing processes. J. Mater. Eng. Perform. JMEPEG 2021, 30, 4808–4818. [Google Scholar] [CrossRef]
- Brauer, G. Journal of the less-commoii metals *3r nitrides, carronitrides and oxynitrides of niobium. J. Less-Common Met. 1960, 2, 131–137. [Google Scholar] [CrossRef]
- Ščetinec, A.; Klobčar, D.; Nagode, A.; Vuherer, T.; Bračun, D.; Trdan, U. Optimisation of precipitation hardening for 15-5 PH martensitic stainless steel produced by wire arc directed energy deposition. Sci. Technol. Weld. Join. 2023, 28, 558–568. [Google Scholar] [CrossRef]
- Itzhak, D.; Aghion, E. An anodic behaviour study of an analogical sintered system of austenitic stainless steel in H2SO4 solution. Corros. Sci. 1984, 24, 145–149. [Google Scholar] [CrossRef]
- Itzhak, D.; Aghion, E. Corrosion behavior of hot-pressed austenitic stainless steel in H2SO4 solution at room temperature. Corros. Sci. 1983, 23, 1085–1094. [Google Scholar] [CrossRef]
- Olabisi, O.; Khuraibut, Y.; Jarragh, A.; Mathew, A. Identifying key performance indicators for corrosion in oilfield water handling systems. In Proceedings of the Corrosion 2014, San Antonio, TA, USA, 9–13 March 2014. [Google Scholar]
- Avula, I.; Arohi, A.C.; Kumar, C.S.; Sen, I. Microstructure, Corrosion and Mechanical Behavior of 15-5 PH Stainless Steel Processed by Direct Metal Laser Sintering. J. Mater. Eng. Perform. 2021, 30, 6924–6937. [Google Scholar] [CrossRef]
- Vunnam, S.; Saboo, A.; Sudbrack, C.; Starr, T.L. Effect of powder chemical composition on the as-built microstructure of 17-4 PH stainless steel processed by selective laser melting. Addit. Manuf. 2019, 30, 100876. [Google Scholar] [CrossRef]
- Niu, J.; Cui, B.; Jin, H.; Yan, J.; Meng, W.; Min, C.; Xu, D. Effect of Post-Weld Aging Temperature on Microstructure and Mechanical Properties of Weld Metal of 15-5 PH Stainless Steel. J. Mater. Eng. Perform. 2020, 29, 7026–7033. [Google Scholar] [CrossRef]
- Tapoglou, N.; Clulow, J.; Curtis, D. Increased shielding of a Direct Energy Deposition process to enable Deposition of reactive materials; an investigation into Deposition of 15-5 PH Stainless Steel, Inconel 718 and Ti-6Al-4V. CIRP J. Manuf. Sci. Technol. 2022, 36, 227–235. [Google Scholar] [CrossRef]


| Tested Alloy | Cr | Ni | Cu | Mn | Si | Nb | C | Fe |
|---|---|---|---|---|---|---|---|---|
| WLAM PH 15-5 | 14.31 ± 0.19 | 5.17 ± 0.38 | 3.46 ± 0.45 | 0.65 ± 0.23 | 0.59 ± 0.04 | 0.51 ± 0.15 | 0.06 | Bal. |
| AISI PH 15-5 | 15.00 | 5.50 | 4.50 | 1.00 | 1.00 | 0.45 | 0.06 | Bal. |
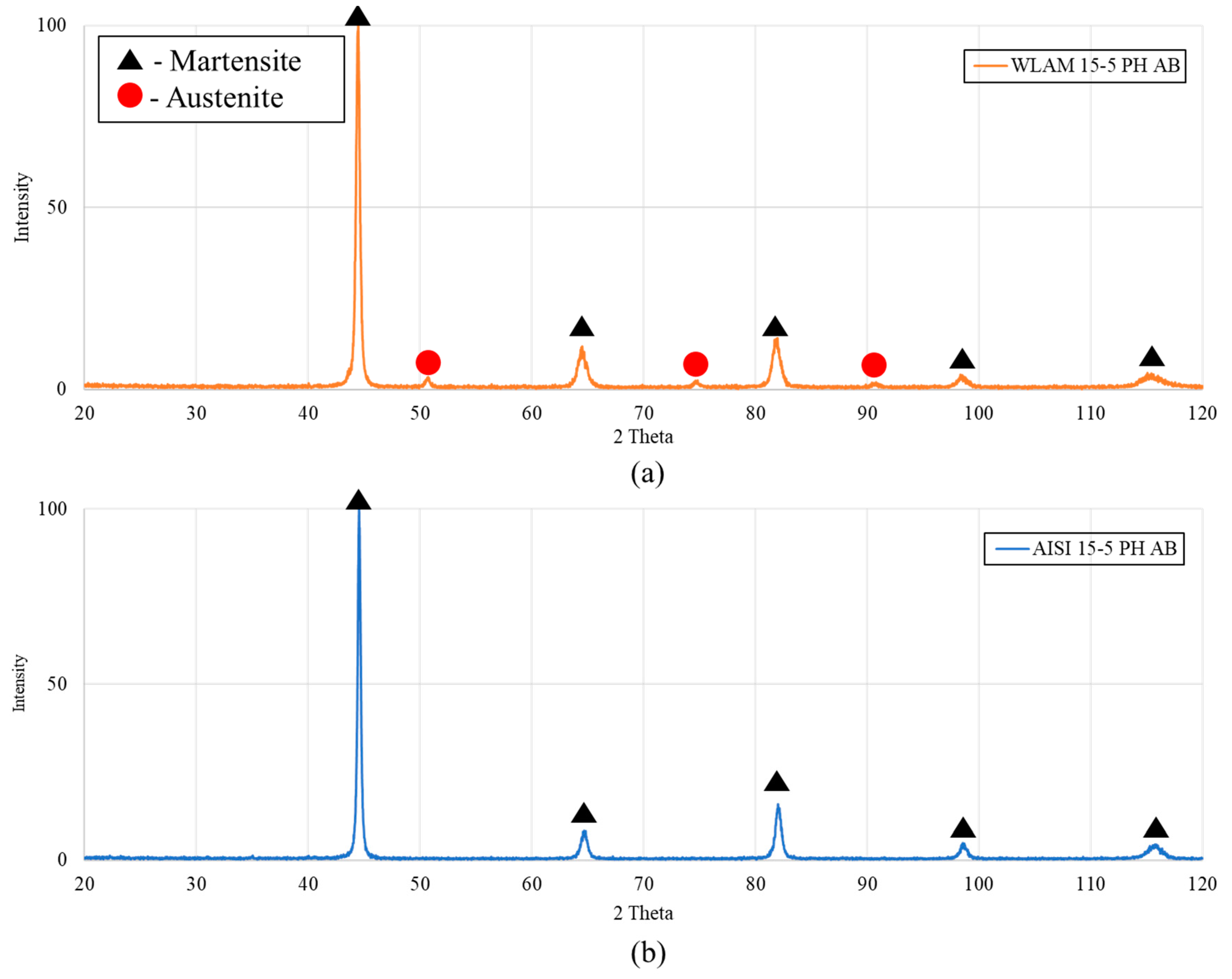
| Material | Austenite | BCC/BCT Martensite |
|---|---|---|
| WLAM 15-5 PH—As built | 18% | 82% |
| AISI 15-5 PH—As built | 1% | 99% |
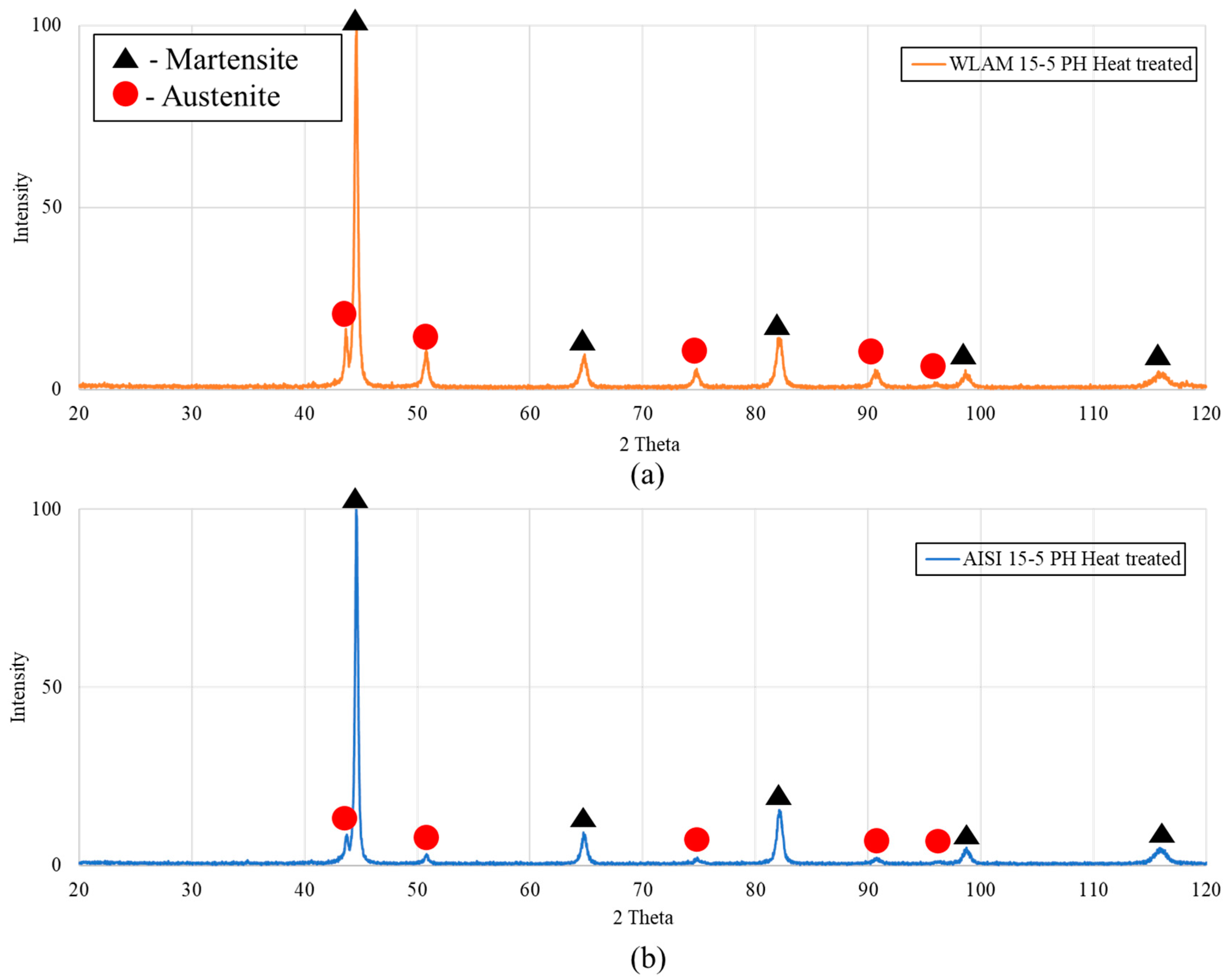
| WLAM 15-5 PH—Heat treated | 54% | 46% |
| AISI 15-5 PH—Heat treated | 11% | 89% |
| N (wt%) | C (wt%) | Cr (wt%) | Fe (wt%) | Nb (wt%) |
|---|---|---|---|---|
| 20.8 | 10.3 | 4.5 | 3.6 | 54.7 |
| Material | Ecorr (V) | Icorr (µA/cm2) | Corrosion Rate (mmpy) | βa (mV) | βc (mV) |
|---|---|---|---|---|---|
| WLAM PH 15-5 | −0.45 ± 0.04 | 1.34 ± 0.18 | 0.015 ± 0.002 | 193.30 ± 60.77 | 46.33 ± 10.90 |
| AISI PH 15-5 | −0.31 ± 0.04 | 1.18 ± 0.24 | 0.003 ± 0.002 | 80.23 ± 22.72 | 57.27 ± 12.81 |
| Material | a2 | R1 (Ω) | R2 (KΩ) | Q2 (F.S^(a−1)) | a2 |
|---|---|---|---|---|---|
| WLAM PH 15-5 | 0.73 ± 0.01 | 16.20 ± 0.08 | 3 ± 0.5 | 0.00018 ± 0.00002 | 0.73 ± 0.01 |
| AISI PH 15-5 | 0.60 ± 0.01 | 30.43 ± 0.22 | 352 ± 83 | 0.00011 ± 0.00002 | 0.60 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassis, M.; Ron, T.; Shirizly, A.; Aghion, E. Effect of Microstructure Modifications on Stress Corrosion Endurance of 15-5 PH Stainless Steel Formed by Wire Laser Additive Manufacturing (WLAM). Metals 2023, 13, 1748. https://doi.org/10.3390/met13101748
Bassis M, Ron T, Shirizly A, Aghion E. Effect of Microstructure Modifications on Stress Corrosion Endurance of 15-5 PH Stainless Steel Formed by Wire Laser Additive Manufacturing (WLAM). Metals. 2023; 13(10):1748. https://doi.org/10.3390/met13101748
Chicago/Turabian StyleBassis, Maxim, Tomer Ron, Amnon Shirizly, and Eli Aghion. 2023. "Effect of Microstructure Modifications on Stress Corrosion Endurance of 15-5 PH Stainless Steel Formed by Wire Laser Additive Manufacturing (WLAM)" Metals 13, no. 10: 1748. https://doi.org/10.3390/met13101748
APA StyleBassis, M., Ron, T., Shirizly, A., & Aghion, E. (2023). Effect of Microstructure Modifications on Stress Corrosion Endurance of 15-5 PH Stainless Steel Formed by Wire Laser Additive Manufacturing (WLAM). Metals, 13(10), 1748. https://doi.org/10.3390/met13101748







