From Bauxite as a Critical Material to the Required Properties of Cast Aluminum Alloys for Use in Electro Automotive Parts
Abstract
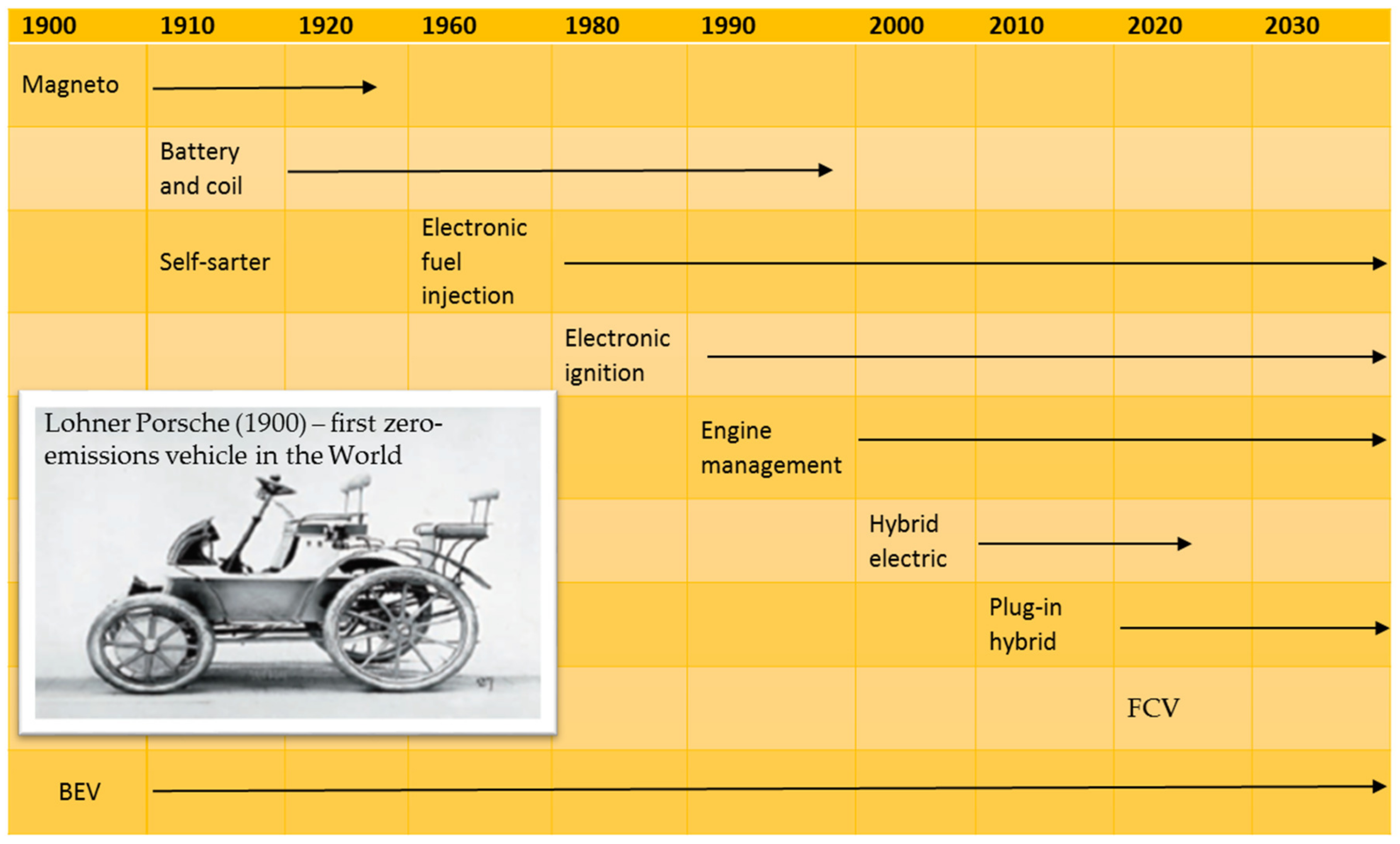
:1. Introduction
2. Aluminum Cast Alloys
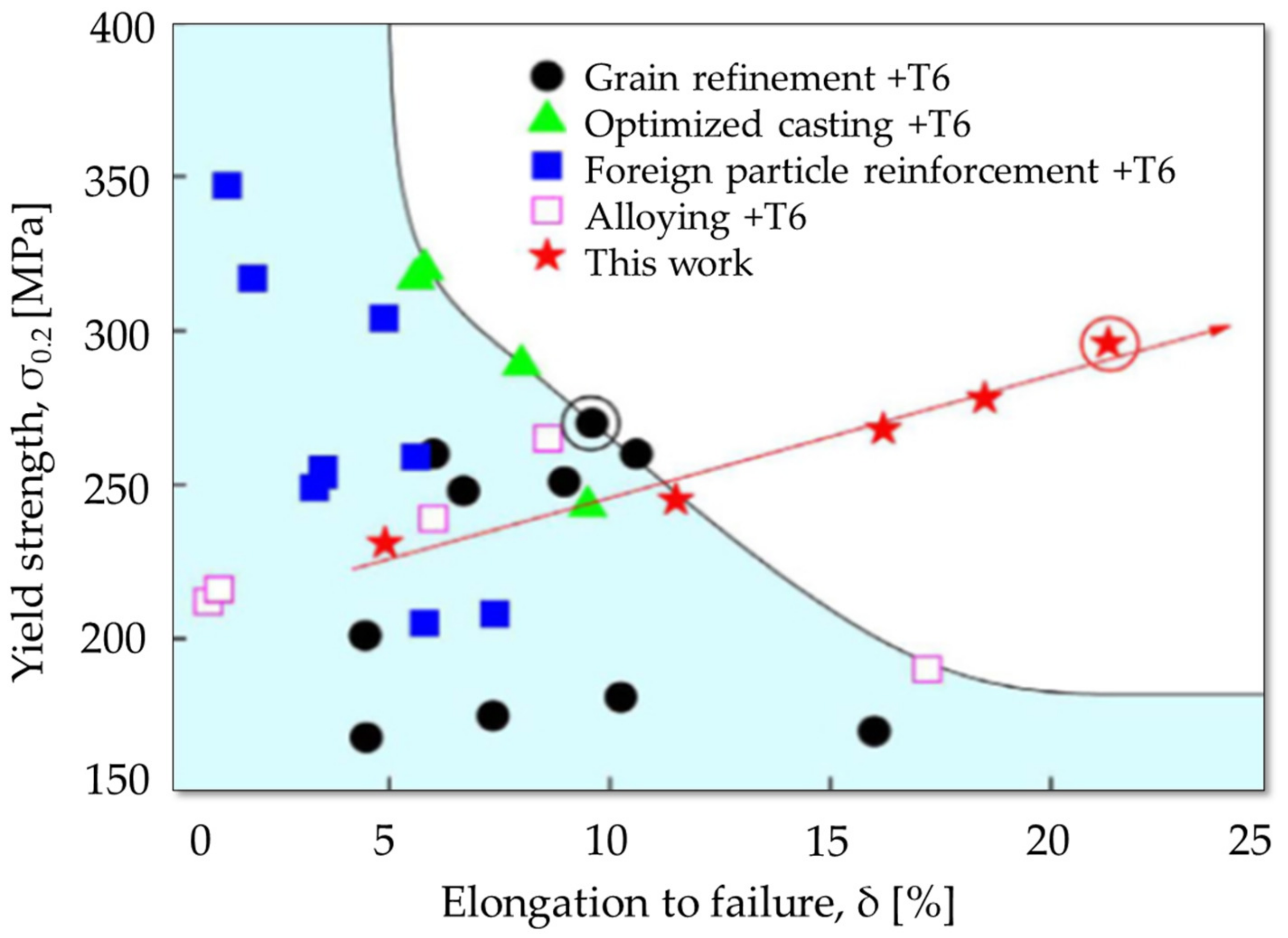
3. Mechanical Properties
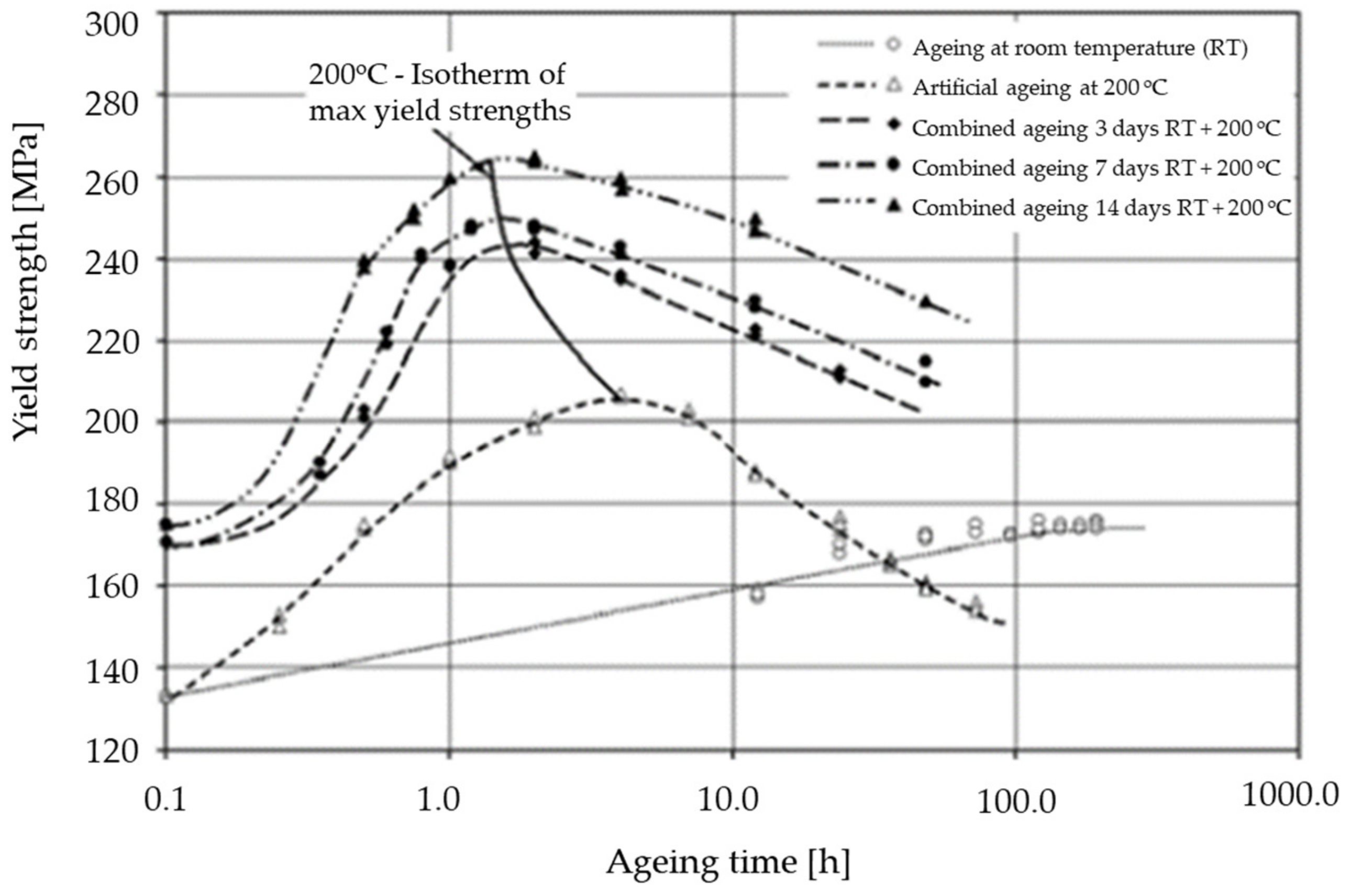
4. Improvement of Strength through Selection of Appropriate Heat Treatment Process
5. Improvement of Strength through Selection of Appropriate Alloying Elements
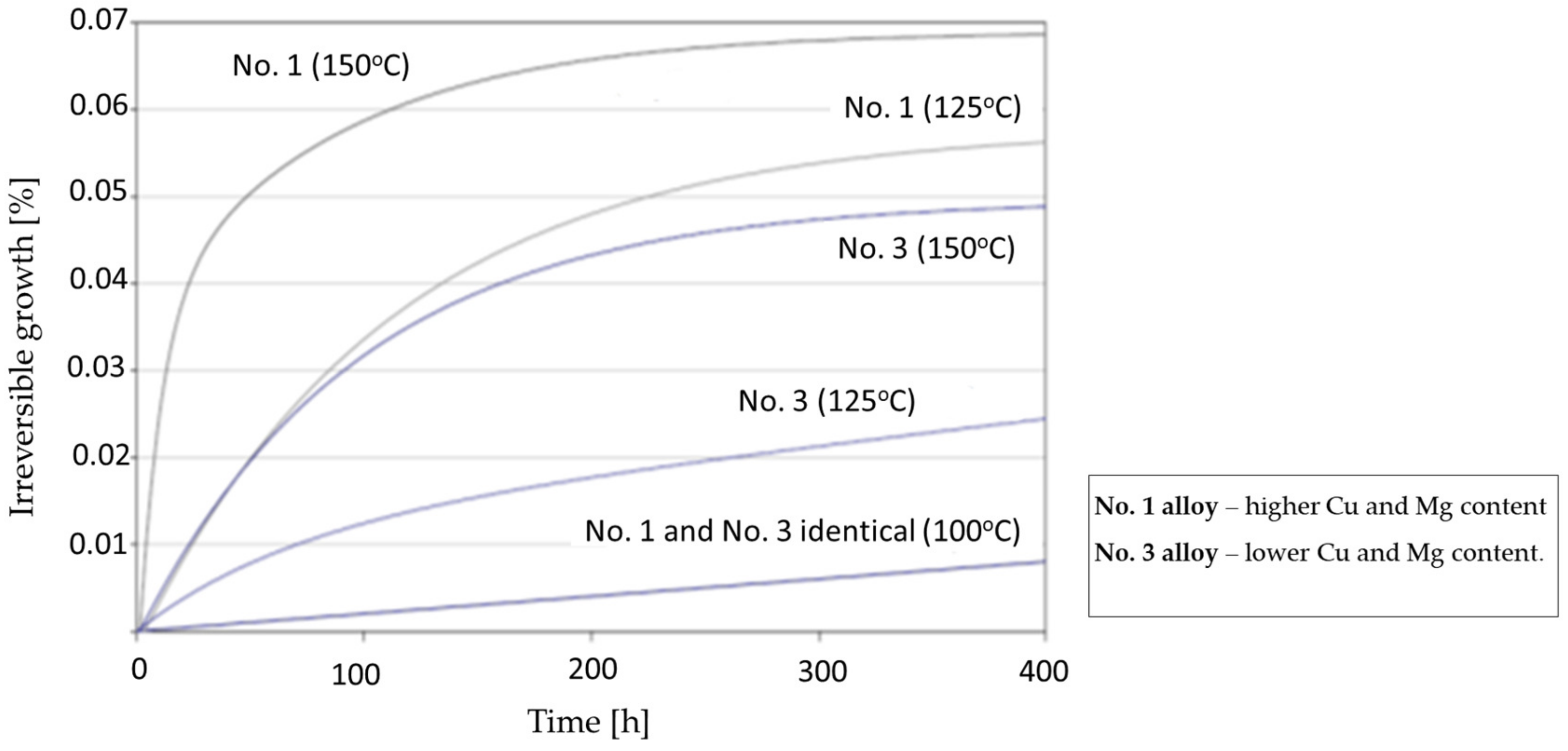
6. Dimensional Stability
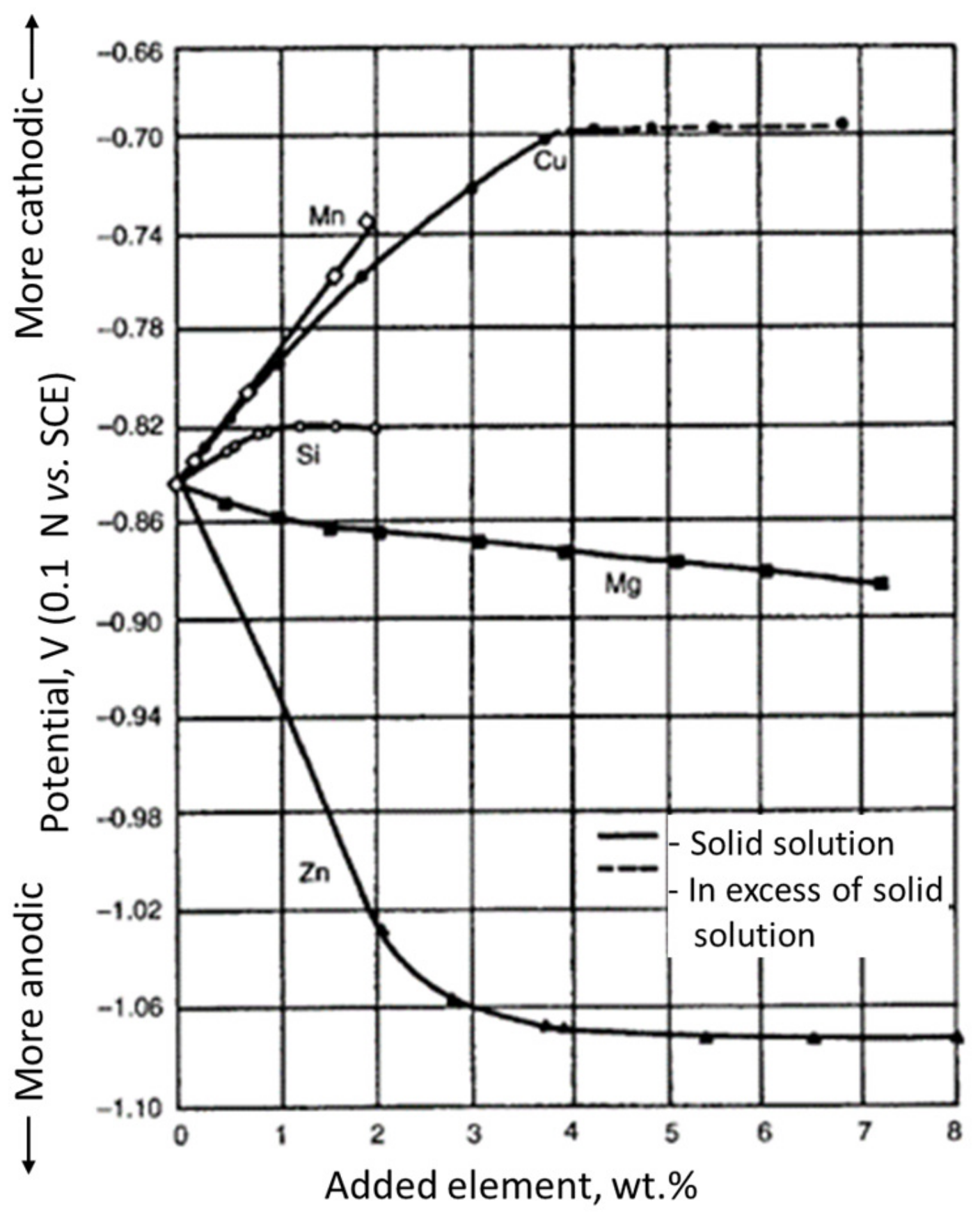
7. Corrosion Resistance
8. Electromagnetic Compatibility
9. Crashworthiness
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vračar, R.; Živković, Ž. Extractive Metallurgy of Aluminum; Naučna Knjiga: Belgrade, Serbia, 1993; p. 314. [Google Scholar]
- Gow, N.N.; Lozej, G.P. Bauxite. Geosci. Can. 1993, 20, 9–16. [Google Scholar]
- Michi, R.; Plotkowski, A.; Shyam, A.; Dehoff, R.; Baby, S. Towards high-temperature applications of aluminum alloys enabled by additive manufacturing. Int. Mater. Rev. 2022, 67, 298–345. [Google Scholar] [CrossRef]
- Czerwinski, F. Current Trends in Automotive Lightweighting Strategies and Materials. Materials 2021, 14, 6631. [Google Scholar] [CrossRef] [PubMed]
- Grauers, A.; Sarasini, S.; Karlström, M. Why electromobility and what is it? In Systems Perspectives on Electro Mobility; Chalmers University of Technology: Gothenburg, Sweden, 2013; pp. 10–21. [Google Scholar]
- Nieuwenhuis, P. The challenge of decarbonizing the car. In Paving the Road to Sustainable Transport, Governance and Innovation in Low-Carbon Vehicles; Nilsson, M., Hillman, K., Rickne, A., Magnusson, T., Eds.; Routledge Studies in Ecological Economics; Routledge: London, UK, 2012; Available online: https://www.book2look.com/embed/9781136316609 (accessed on 23 August 2023).
- Available online: https://natural-resources.canada.ca/our-natural-resources/minerals-mining/minerals-metals-facts/aluminum-facts/20510 (accessed on 23 August 2023).
- Djurdjevic, M.B.; Feikus, F.J.; Fernandez Gutierrez, R. Invited lecture: Properties of cast aluminum alloys suitable for production of E-mobility components. In Proceedings of the Metallurgical and Materials Engineering Congress of South-East Europe (MMSEE), Belgrade, Serbia, 5–7 June 2019; p. 9. [Google Scholar]
- Steglich, J.; Luisa Marzoli, L.; Zerbin, I. Cast alloys for electric vehicle powertrains. In Proceedings of the European Aluminium Conference by GDA TRIMET Aluminium SE, Düsseldorf, Germany, 25–26 November 2019; pp. 1–18. [Google Scholar] [CrossRef]
- EN 1706; Aluminium and Aluminium Alloys—Castings. European Standard: Brussels, Belgium, March 1998; pp. 1–18. Available online: https://aluminiumtoday.com/news/aluminium-usage-in-cars-surges-as-automotive-industry-shifts-towards-electrification (accessed on 23 August 2023).
- Jolly, M.R. Castings. In Comprehensive Structural Integrity; Milne, I., Ritchie, R.O., Karihaloo, B., Eds.; Elsevier Pergamon: Oxford, UK, 2003. [Google Scholar]
- Kaufman, J.G.; Rooy, E.L. Aluminum Alloy Castings—Properties, Processes and Application; Chapter—Heat Treatment of Aluminum Castings; AFS Schaumburg: Schaumburg, IL, USA, 2004; pp. 61–67. ISBN 0-87170-803-5. [Google Scholar]
- Dang, B.; Zhang, X.; Chen, Y.Z.; Chen, C.X.; Wang, H.T.; Liu, F. Breaking through the strength-ductility trade-off dilemma in Al-Si based casting alloy. Sci. Rep. 2016, 6, 30874. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Lee, S.-J.; Kim, D.-U.; Kim, M.-S. Experimental Investigation on Tensile Properties and Yield Strength Modeling of T5 Heat-Treated Counter Pressure Cast A356 Aluminum Alloys. Metals 2021, 11, 1192. [Google Scholar] [CrossRef]
- Primary Aluminum Alloys for Pressure Die Casting, 4th ed.; Rheinfelden Alloys: Rheinfelden, Germany, 2016; Available online: https://rheinfelden-alloys.eu/wp-content/uploads (accessed on 23 August 2023).
- Pabel, T.; Geier, G.F.; Rockenschaub, H.; Hopfinger, M. Improved mechanical properties of the high pressure die casting alloy AlSi9Cu3(Fe)(Zn) as a result of the combination of natural and artificial ageing. Int. J. Mater. Res. 2007, 98, 516–520. [Google Scholar] [CrossRef]
- Kim, D.; Maeng, H.; Choi, Y.; Choi, H.; Lee, S.J. Constitutive Model of Triple-Step-Aged Al–Mg–Si Alloy Incorporating Precipitation Kinetics. Met. Mater. Int. 2021, 27, 4577–4585. [Google Scholar] [CrossRef]
- Yuxian, L.; Hu, A.; Fu, Y.; Liu, S.; Shen, W.; Hu, H.; Nie, X. Al Alloys and Casting Processes for Induction Motor Applications in Battery-Powered Electric Vehicles: A Review. Metals 2022, 12, 216. [Google Scholar] [CrossRef]
- Lumley, R.N.; O’Donnell, R.G.; Gunasegaram, D.R.; Kittel-Sherri, T.; Gershenzon, M.; Yob, A.C.; Polmear, I.J. The role of alloy composition in the heat treatment of aluminum high pressure die castings. Mater. Sci. Technol. 2008, 26, 1–10. [Google Scholar]
- Caceres, C.H.; Davidson, C.J.; Wang, Q.G.; Griffiths, J.R.; Wang, Q.G. The Effect of Mg on the Microstructure and Mechanical Behavior of Al-Si-Mg Casting Alloys. Metall. Mater. Trans. A Phys. 1999, 30, 2611–2618. [Google Scholar] [CrossRef]
- Abdulwahab, M. The Effect of Chromium and Manganese on the Mechanical Properties and Corrosion Resistance of Al-Si-Fe Alloy in 0.5M HCl Solution. Ph.D. Thesis, Department of Metallurgical Engineering Ahmadu Bello University, Zaria, Nigeria, August 2007. [Google Scholar]
- Ammar, H.R.; Moreau, C.; Samuel, A.M.; Samuel, F. Influences of alloying elements, solution treatment time and quenching media on quality indices of 413-type Al–Si casting alloys. Mater. Sci. Eng. A 2008, 489, 426–438. [Google Scholar] [CrossRef]
- Wang, L.; Makhlouf, M.; Apelian, D. Aluminum Die Casting Alloys—Alloy composition, microstructure, and properties-performance relationship. Int. Mater. Rev. 1995, 40, 221–238. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Samuel, F.; Samuel, A.M.; Doty, H.W. Effects of Individual and Combined Additions of Pb, Bi, and Sn on the Microstructure and Mechanical Properties of Al-10.8Si-2.25Cu-0.3Mg Alloy. Metall. Mater. Trans. A Phys. 2009, 40, 240–254. [Google Scholar] [CrossRef]
- Mao, H.; Bai, X.; Song, F.; Song, Y.; Jia, Z.; Xu, H.; Wang, Y. Effect of Cd on Mechanical Properties of Al-Si-Cu-Mg Alloys under Different Multi-Stage Solution Heat Treatment. Materials 2022, 15, 5101. [Google Scholar] [CrossRef] [PubMed]
- Akopyan, T.K.; Belov, N.A.; Letyagin, N.V.; Milovich, F.O.; Fortuna, A.S. Increased precipitation hardening response in Al-Si-Cu based aluminum casting alloy with In trace addition. Mater. Today Commun. 2021, 27, 102410. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lee, S.-L.; Lee, C.-K.; Lin, J.-C. Effects of Be and Fe on the mechanical and corrosion behaviors of A357 alloys. Mater. Chem. Phys. 2005, 93, 412–419. [Google Scholar] [CrossRef]
- Yaro, S.A.; Aigbodion, V.S. The Effect of Chromium and Manganese Addition on the Corrosion of As-cast Al-Si-Fe-Cu Alloy System in Caustic Soda. Eurasian Chem.-Technol. 2008, 10, 79–84. [Google Scholar] [CrossRef]
- Li, G.J.; Guo, M.-X.; Wang, Y.; Zheng, C.-H.; Zhang, J.-S.; Zhuang, L.-Z. Effect of Ni addition on microstructure and mechanical properties of Al–Mg–Si–Cu–Zn alloys with a high Mg/Si ratio. Int. J. Miner. Metall. Mater. 2019, 26, 740–751. [Google Scholar] [CrossRef]
- Rahimian, M.; Amirkhanlou, S.; Blake, P.; Ji, S. Nanoscale Zr-containing precipitates; a solution for significant improvement of high-temperature strength in Al-Si-Cu-Mg alloys. Mater. Sci. Eng. A 2018, 721, 328–338. [Google Scholar] [CrossRef]
- Ifeanyi, O.B.; Nkem, N.E.; Amaechi, A.F. Effect of Chromium and Molybdenum on the structure and mechanical properties of Al-Si alloys obtained by metal-mold casting. J. Sci. Eng. Res. 2016, 3, 383–389. [Google Scholar]
- Emadi, D.; Prasada Rao, A.K.; Mahfoud, M. Influence of scandium on the microstructure and mechanical properties of A319 alloy. Mater. Sci. Eng. 2010, 527, 6123–6132. [Google Scholar] [CrossRef]
- Ahmad, R.; Asmael, M.B.A. Influence of Lanthanum on Solidification, Microstructure, and Mechanical Properties of Eutectic Al-Si Piston Alloy. J. Mater. Eng. Perform. 2016, 25, 2799–2813. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, J.; Seo, N.; Son, S.B.; Lee, S.J. Effect of Heating Rate on Microstructure and Mechanical Properties in Al 7055. Met. Mater. Int. 2021, 27, 449–455. [Google Scholar] [CrossRef]
- Patarić, A.; Mihailović, M.; Marković, B.; Sokić, M.; Radovanović, A.; Jordović, B. Microstructure as an essential aspect of 7075 aluminum alloy quality influenced by electromagnetic field during continual casting process. Chem. Ind. 2021, 75, 31–37. [Google Scholar] [CrossRef]
- Rohr, A. Dauerfestigkeitserhöhung und Reduktion des irreversiblen Gusswachstums bei Aluminium-Sandgusslegierungen. Ph.D. Thesis, Technischen Universität Berlin, Berlin, Germany, 20 January 2012. [Google Scholar]
- Jacobs, M.H. Corrosion and Corrosion Protection. In TALAT Lecture 1252, Interdisciplinary Research Centre in Materials; The University of Birmingham: Birmingham, UK; European Aluminum Association: London, UK, 1999; p. 17. [Google Scholar]
- Campbell, F.C. Elements of Metallurgy and Engineering Alloys; ASM International Materials Park: Novelty, OH, USA, 2008; pp. 502–508. ISBN 978-0-87170-867-0. [Google Scholar]
- Davis, J.R. Alloying: Understanding the Basics; ASM International: Almere, The Netherlands, 2001; pp. 351–416. Available online: www.asminternational.org (accessed on 23 August 2023).
- Davoodi, A. Mechanistic Studies of Localized Corrosion of Al Alloys by High Resolution In Situ and Ex-Situ Probing Techniques. Ph.D. Thesis, KTH Chemical Science and Engineering, Stockholm, Sweden, 2007. ISBN 978-91-7178-817-7. [Google Scholar]
- Jegdić, B.; Bobić, B.; Stevanović, M.; Mihailović, M.; Daničić, D.; Kovačina, J.; Radojković, B. Resistance to pit formation and pit growth for different tempers of AA2024 aluminium alloy in presence of benzotriazole. Met. Mater. Int. 2022, 26, 1643–1653. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, B.; Wang, Y.; Dong, T.; Li, J.; Li, G.; Zhao, X.; Liu, J.; Zhang, G. Effect of Cu Content on the Precipitation Behaviors, Mechanical and Corrosion Properties of As-Cast Ti-Cu Alloys. Materials 2022, 15, 1696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, L.; Hu, B.; Chen, B. The effect of Cu addition on corrosion resistance of Al-Si-Mg-Cr alloy. Metals 2023, 13, 795. [Google Scholar] [CrossRef]
- Revie, R.W. Uhlig’s Corrosion Handbook, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Chapter 40. [Google Scholar]

| Casting Process | Advantages | |
|---|---|---|
| High Pressure Die Casting (HPDC) |  |
|
| Low Pressure Die Casting (LPDC) |  |
|
| Core Package System (CPS) |  |
|
| Alloy | As-Cast Mechanical Properties | ||
|---|---|---|---|
| UTS, MPa | YS, MPa | A, % | |
| AlSi9Cu3(Fe) | 240 | 140 | <1 |
| AlSi10Mg | 240 | 140 | 1 |
| AlSi10MgMn | 250 | 120 | 5 |
| AlSi12(Fe) | 240 | 130 | 1 |
| AlSi12Cu1(Fe) | 240 | 140 | 1 |
| Alloying Element | UTS | YS | A | References | |
|---|---|---|---|---|---|
| Major | Si |  |  |  | [18] |
| Cu |  |  |  | [19] | |
| Mg |  |  |  | [20] | |
| Mn | Insignificant | Insignificant |  | [21] | |
| Zn |  |  |  | [22] | |
| Minor | Fe |  |  |  | [23] |
| Pb | Insignificant | Insignificant | Insignificant | [24] | |
| Sn |  |  |  | [25] | |
| In |  |  |  | [26] | |
| Be |  |  |  | [27] | |
| Cr |  |  |  | [28] | |
| Ni |  |  |  | [29] | |
| Zr |  |  |  | [30] | |
| Mo |  |  |  | [31] | |
| Sc |  |  |  | [32] | |
| La |  |  |  | [33] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djurdjevic, M.; Manasijevic, S.; Mihailović, M.; Stopic, S. From Bauxite as a Critical Material to the Required Properties of Cast Aluminum Alloys for Use in Electro Automotive Parts. Metals 2023, 13, 1796. https://doi.org/10.3390/met13111796
Djurdjevic M, Manasijevic S, Mihailović M, Stopic S. From Bauxite as a Critical Material to the Required Properties of Cast Aluminum Alloys for Use in Electro Automotive Parts. Metals. 2023; 13(11):1796. https://doi.org/10.3390/met13111796
Chicago/Turabian StyleDjurdjevic, Mile, Srecko Manasijevic, Marija Mihailović, and Srecko Stopic. 2023. "From Bauxite as a Critical Material to the Required Properties of Cast Aluminum Alloys for Use in Electro Automotive Parts" Metals 13, no. 11: 1796. https://doi.org/10.3390/met13111796
APA StyleDjurdjevic, M., Manasijevic, S., Mihailović, M., & Stopic, S. (2023). From Bauxite as a Critical Material to the Required Properties of Cast Aluminum Alloys for Use in Electro Automotive Parts. Metals, 13(11), 1796. https://doi.org/10.3390/met13111796







