Effect of Controlled Heat Treatment and Aluminum Additions on the Strengthening of Cu–Ni-Based Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Metallographic Evaluation
2.3. Physical Properties
2.4. Mechanical Testing
3. Results and Discussion
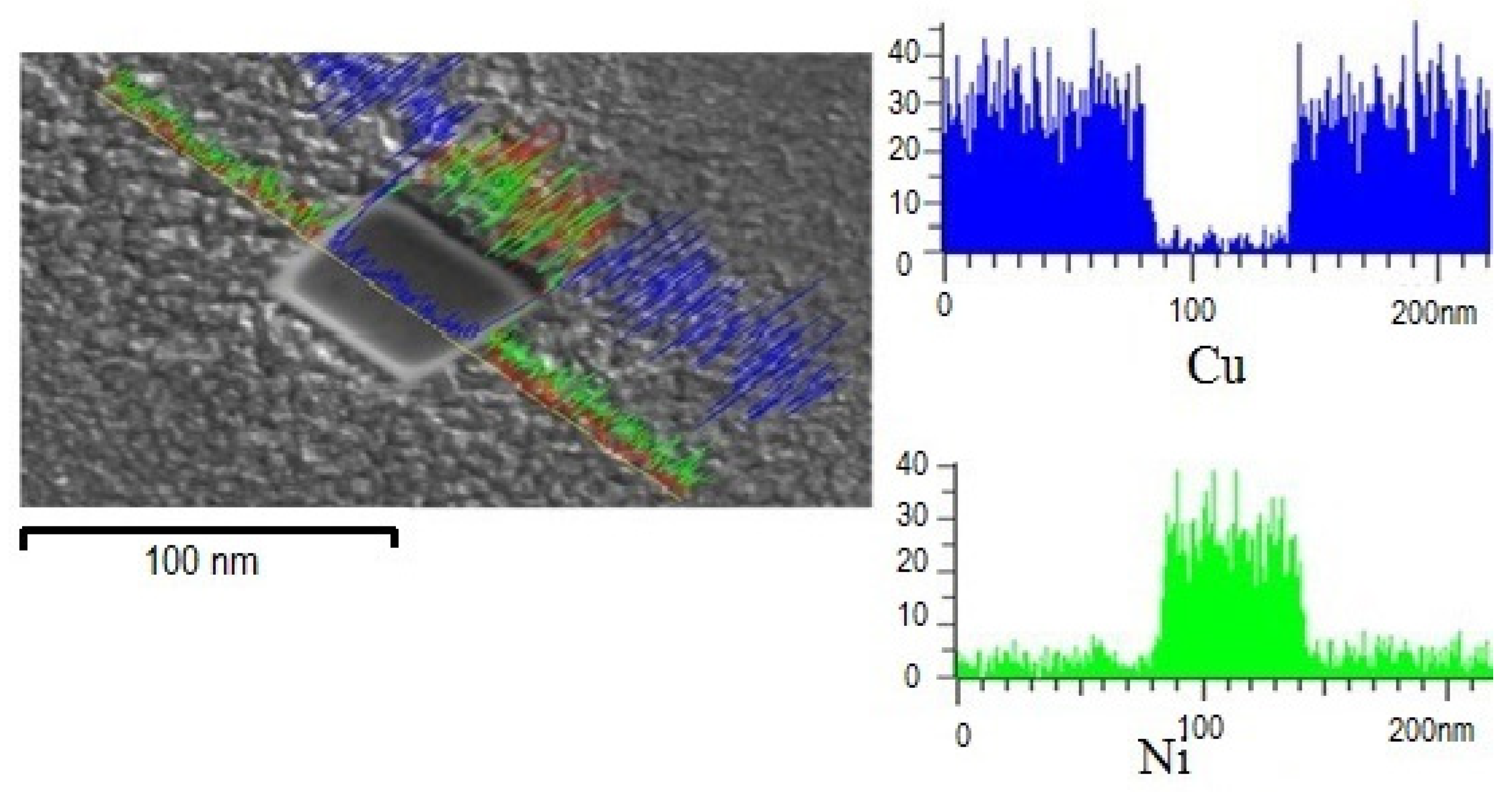
3.1. Microstructural Characterization
Lattice Parameter Analyses
3.2. Physical Properties
Density and Thermal Defects Evaluation
| Alloy Designation | Density by X-ray Diffraction [g/cm3] | Density by He-Pycnometry [g/cm3] | Vacancy Concentration [%] |
|---|---|---|---|
| AB | 9.5601 | 9.5594 | 0.0073 |
| ABTT | 9.5581 | 9.5570 | 0.0115 |
| AT5 | 9.2085 | 9.2011 | 0.0804 |
| AT5TT | 9.2071 | 9.2038 | 0.0358 |
| AT10 | 8.9347 | 8.9307 | 0.0447 |
| AT10TT | 8.9304 | 8.9257 | 0.0526 |
3.3. Mechanical Characterization
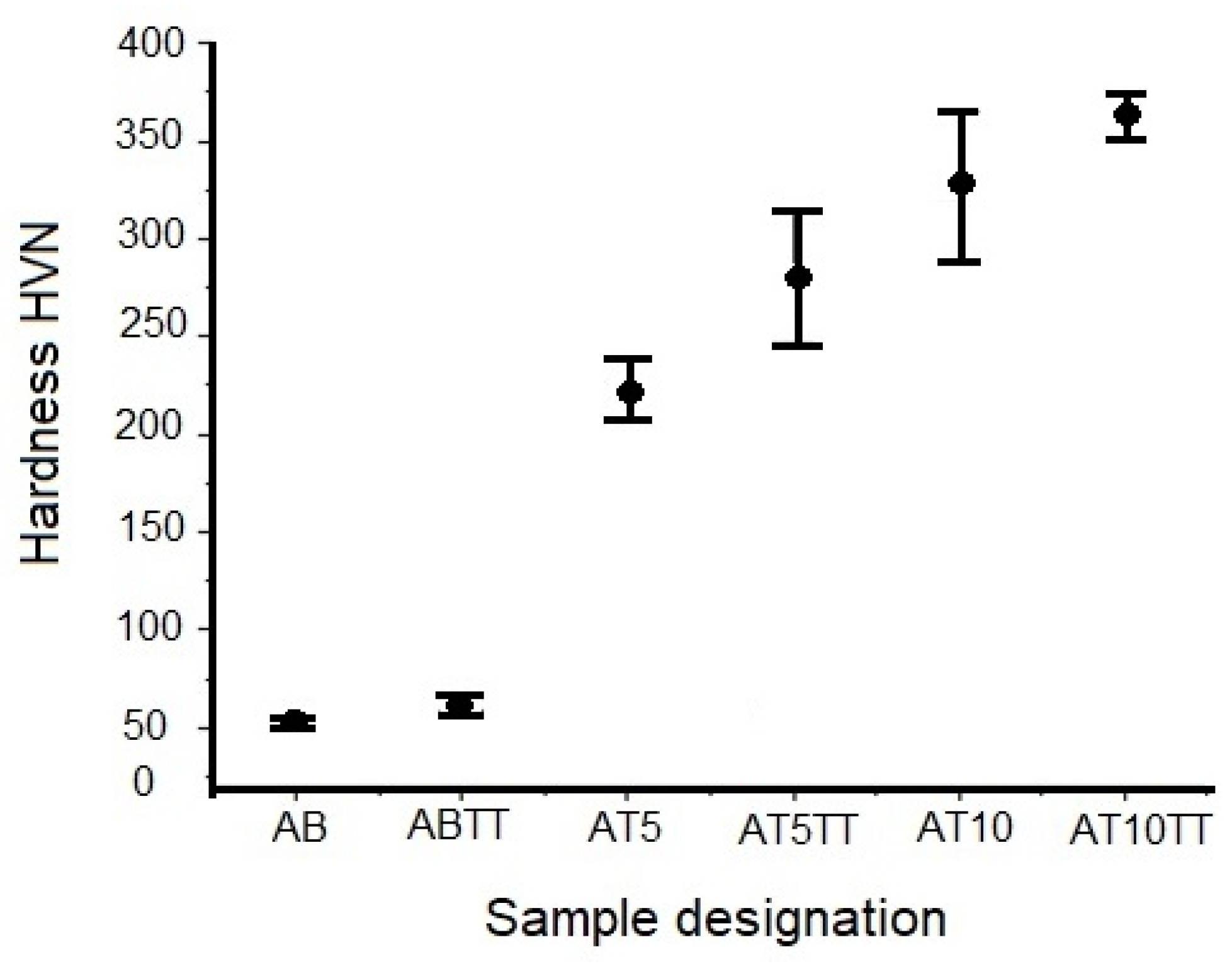
3.3.1. Hardness Evaluation
3.3.2. Compression Tests Evaluation
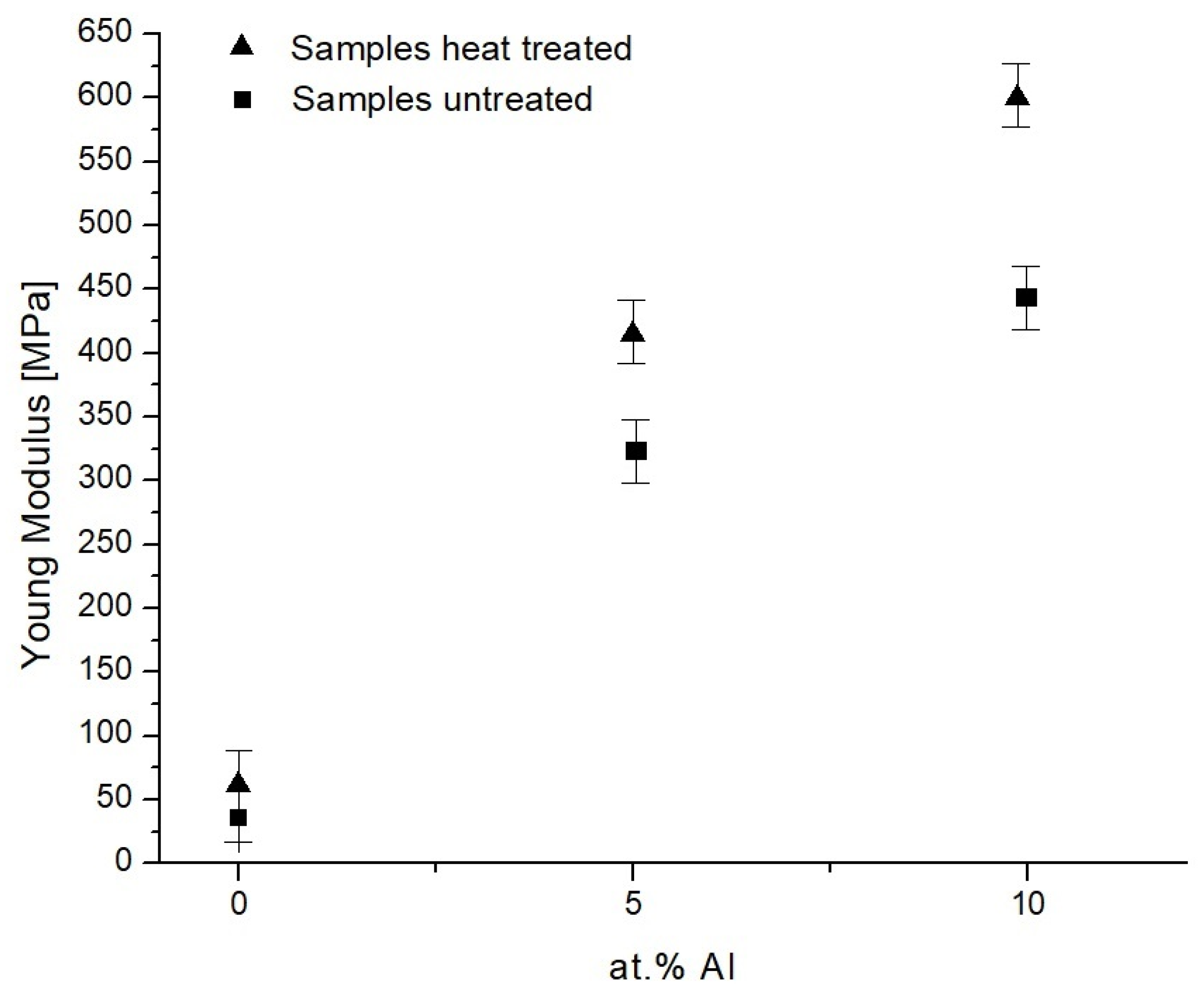
Young’s Modulus Evaluation
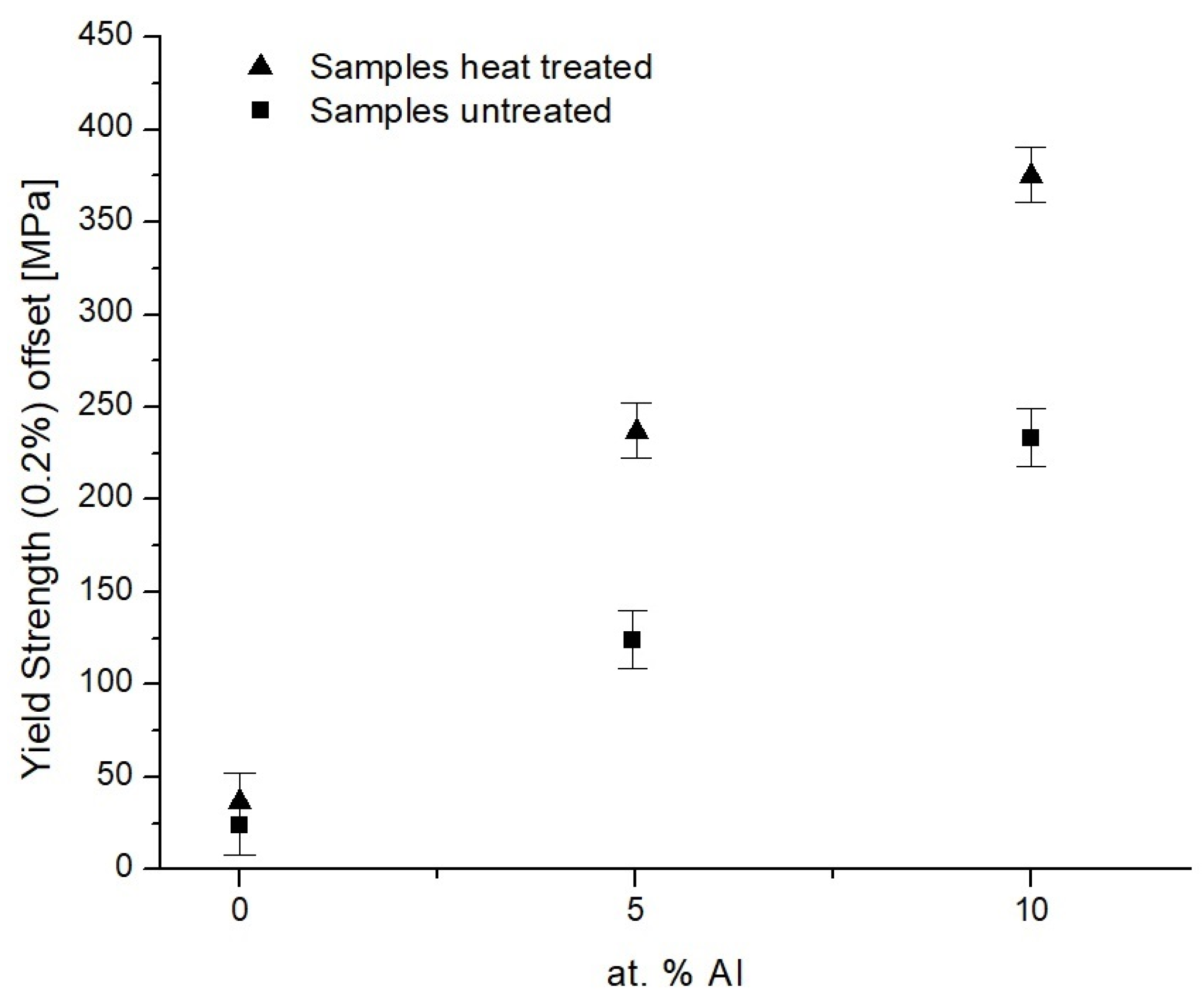
Yield Stress Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grandhi, M.; Nguyen, V.; Liu, Z.; Romo-De-La-cruz, C.-O.; Song, X. Copper-nickel functionally magnetic gradient material fabricated via directed energy deposition. J. Manuf. Process. 2023, 100, 47–54. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Dai, C.; Wang, J.; Chen, X.; Xie, J.M.; Yang, W.W.; Chen, D.L. Effect of aluminum on microstructure and property of Cu–Ni–Si alloys. Mater. Sci. Eng. A 2013, 572, 65–74. [Google Scholar] [CrossRef]
- Schneider, M.S.; Kad, B.; Kalantar, D.H.; Remington, B.A.; Kenik, E.; Jarmakani, H.; Meyers, M.A. Laser shock compression of copper and copper–aluminum alloys. Int. J. Impact Eng. 2005, 32, 473–507. [Google Scholar] [CrossRef]
- Devi Janani, R.; Salman, S.A.; Pavithra Priyadharshini, K.; Karthik, V. Effect of composition on the stacking fault energy of copper-nickel alloys using molecular dynamics simulations. Mater. Today Proc. 2021, 39, 1796–1800. [Google Scholar] [CrossRef]
- McFadden, S.X.; Mishra, R.S.; Valiev, R.Z.; Zhilyaev, A.P.; Mukherjee, A.K. Low-temperature superplasticity in nanostructured nickel and metal alloys. Nature 1999, 398, 684–686. [Google Scholar] [CrossRef]
- Brandstetter, S.; Zhang, K.; Escuadro, A.; Weertman, J.R.; Van Swygenhoven, H. Grain coarsening during compression of bulk nanocrystalline nickel and copper. Scr. Mater. 2008, 58, 61–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Tian, B.-H.; Liu, Y.; Li, R.-Q.; Xu, Q.-Q. Hot deformation behavior and processing map of Cu–Ni–Si–P alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 2341–2347. [Google Scholar] [CrossRef]
- Suzuki, S.; Shibutani, N.; Mimura, K.; Isshiki, M.; Waseda, Y. Improvement in strength and electrical conductivity of Cu–Ni–Si alloys by aging and cold rolling. J. Alloys Compd. 2006, 417, 116–120. [Google Scholar] [CrossRef]
- Naghash, A.R.; Etsell, T.H.; Xu, S. XRD and XPS Study of Cu−Ni Interactions on Reduced Copper−Nickel−Aluminum Oxide Solid Solution Catalysts. Chem. Mater. 2006, 18, 2480–2488. [Google Scholar] [CrossRef]
- Fu, H.; Song, S.; Zhuo, L.; Zhang, Z.; Xie, J. Enhanced mechanical properties of polycrystalline Cu–Al–Ni alloy through grain boundary orientation and composition control. Mater. Sci. Eng. A 2016, 650, 218–224. [Google Scholar] [CrossRef]
- Martínez, C.; Briones, F.; Rojas, P.; Aguilar, C.; Guzman, D.; Ordoñez, S. Microstructural and mechanical characterization of copper, nickel, and Cu-based alloys obtained by mechanical alloying and hot pressing. Mater. Lett. 2017, 209, 509–512. [Google Scholar] [CrossRef]
- Tassaoui, K.; Damej, M.; Molhi, A.; Berisha, A.; Errili, M.; Ksama, S.; Mehmeti, V.; El Hajjaji, S.; Benmessaoud, M. Contribution to the corrosion inhibition of Cu-30Ni copper-nickel alloy by 3-amino-1,2,4-triazole-5-thiol (ATT) in 3% NaCl solution. Experimental and theoretical study (DFT, MC and MD). Int. J. Corros. Scale Inhib. 2022, 11, 221–244. [Google Scholar] [CrossRef]
- Gonçalves, R.S.; Azambuja, D.S.; Serpa Lucho, A.M. Electrochemical studies of propargyl alcohol as corrosion inhibitor for nickel, copper, and copper/nickel (55/45) alloy. Corros. Sci. 2002, 44, 467–479. [Google Scholar] [CrossRef]
- Shao, G.; Gao, Y.; Wu, J.; Liu, P.; Zhang, K.; Li, W.; Ma, F.; Zhou, H.; Chen, X. Effect of Fe/Mn content on mechanical and corrosion properties of 90/10 copper–nickel alloy. Mater. Corros. 2022, 73, 1085–1098. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Stokes, K.R.; Walsh, F.C. Electrochemistry of non-aged 90–10 copper–nickel alloy (UNS C70610) as a function of fluid flow: Part 1: Cathodic and anodic characteristics. Electrochim. Acta 2007, 52, 1889–1898. [Google Scholar] [CrossRef]
- Martinez, S.; Metikoš-Huković, M. The inhibition of copper–nickel alloy corrosion under controlled hydrodynamic condition in seawater. J. Appl. Electrochem. 2006, 36, 1311–1315. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Babić, R.; Škugor, I.; Grubač, Z. Copper–nickel alloys modified with thin surface films: Corrosion behaviour in the presence of chloride ions. Corros. Sci. 2011, 53, 347–352. [Google Scholar] [CrossRef]
- Melchers, R.E. Temperature Effect on Seawater Immersion Corrosion of 90:10 Copper-Nickel Alloy. Corrosion 2001, 57, 440–451. [Google Scholar] [CrossRef]
- Shams El Din, A.M.; El Dahshan, M.E.; Taj El Din, A.M. Dissolution of copper and copper-nickel alloys in aerated dilute HCl solutions. Desalination 2000, 130, 89–97. [Google Scholar] [CrossRef]
- Okulov, I.; Kühn, U.; Marr, T.; Freudenberger, J.; Soldatov, I.; Schultz, L.; Oertel, C.G.; Skrotzki, W.; Eckert, J. Microstructure and mechanical properties of new composite structured Ti–V–Al–Cu–Ni alloys for spring applications. Mater. Sci. Eng. A 2014, 603, 76–83. [Google Scholar] [CrossRef]
- Chikova, O.A.; Chezganov, D.S.; Tsepelev, V.S.; Yu Ilyin, V. SEM investigation of the microstructure of cast CuNiAlCoFe equiatomic high entropy alloy. IOP Conf. Ser. Mater. Sci. Eng. 2019, 699, 012007. [Google Scholar] [CrossRef]
- Svirid, A.E.; Afanasiev, S.V.; Davydov, D.I.; Kuranova, N.N.; Makarov, V.V.; Pushin, V.G.; Ustyugov, Y.M. Microstructure and Mechanical Behavior of Cu-Al-Ni-B Alloys with Thermoelastic Martensitic Transformation. Metals 2023, 13, 967. [Google Scholar] [CrossRef]
- Pushin, V.; Kuranova, N.; Svirid, A.E.; Ustyugov, Y. Mechanical Behavior and Structural Characterization of a Cu-Al-Ni-Based Shape-Memory Alloy Subjected to Isothermal Uniaxial Megaplastic Compression. Materials 2022, 15, 3713. [Google Scholar] [CrossRef] [PubMed]
- Loginova, I.S.; Sazerat, M.V.; Loginov, P.A.; Pozdniakov, A.V.; Popov, N.A.; Solonin, A.N. Evaluation of Microstructure and Hardness of Novel Al-Fe-Ni Alloys with High Thermal Stability for Laser Additive Manufacturing. JOM 2020, 72, 3744–3752. [Google Scholar] [CrossRef]
- Zhu, J.H.; Pike, L.M.; Liu, C.T.; Liaw, P.K. Point defects in binary Laves phase alloys. Acta Mater. 1999, 47, 2003–2018. [Google Scholar] [CrossRef]
- Tyagunov, A.; Milder, O.; Tarasov, D.; Tyagunov, G. LLT structural changes in metal liquids as a basis for a thermal treatment technology of melts: A review. Phase Transit. 2021, 94, 1–22. [Google Scholar] [CrossRef]
- Okulov, I.V.; Volegov, A.S.; Attar, H.; Bönisch, M.; Ehtemam-Haghighi, S.; Calin, M.; Eckert, J. Composition optimization of low modulus and high-strength TiNb-based alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 866–871. [Google Scholar] [CrossRef]
- Alexey, E.S.; Elena, S.B.; Artemy, V.P.; Vladimir, G.P. Comparative Electron-Microscopic Study of Shape Memory Alloys of Systems Cu-Ni-Al and Ni-Mn-Al. KnE Eng. 2019, 4, 215–222. [Google Scholar] [CrossRef]
- Ding, H.; Xiao, Y.; Bian, Z.; Wu, Y.; Yang, H.; Wang, H.; Wang, H. Design, microstructure and thermal stability of a novel heat-resistant Al-Fe-Ni alloy manufactured by selective laser melting. J. Alloys Compd. 2021, 885, 160949. [Google Scholar] [CrossRef]
- Svirid, A.E.; Pushin, V.G.; Kuranova, N.N.; Belosludtseva, E.S.; Pushin, A.V.; Lukyanov, A.V. The Effect of Plastification of Cu–14Al–4Ni Alloy with the Shape Memory Effect in High-Temperature Isothermal Precipitation. Tech. Phys. Lett. 2020, 46, 118–121. [Google Scholar] [CrossRef]
- Svirid, A.E.; Pushin, V.G.; Kuranova, N.N.; Makarov, V.V.; Ustyugov, Y.M. Structural and Phase Transformations and Physical and Mechanical Properties of Cu-Al-Ni Shape Memory Alloys Subjected to Severe Plastic Deformation and Annealing. Materials 2021, 14, 4394. [Google Scholar] [CrossRef] [PubMed]
- Svirid, A.E.; Lukyanov, A.V.; Pushin, V.G.; Kuranova, N.N.; Makarov, V.V.; Pushin, A.V.; Uksusnikov, A.N. Application of Isothermal Upset for Megaplastic Deformation of Cu–Al–Ni β Alloys. Tech. Phys. 2020, 65, 1044–1050. [Google Scholar] [CrossRef]
- Cahn, R.W.; Davies, R.G. X-ray evidence for segregation of solute to stacking faults in a copper-aluminium alloy. Philos. Mag. J. Theor. Exp. Appl. Phys. 1960, 5, 1119–1126. [Google Scholar] [CrossRef]
- Marković, I.; Nestorović, S.; Markoli, B.; Premović, M.; Mladenović, S. Study of anneal hardening in cold worked Cu–Au alloy. J. Alloys Compd. 2016, 658, 414–421. [Google Scholar] [CrossRef]
- Pushin, V.G.; Kuranova, N.N.; Svirid, A.E.; Uksusnikov, A.N.; Ustyugov, Y.M. Design and Development of High-Strength and Ductile Ternary and Multicomponent Eutectoid Cu-Based Shape Memory Alloys: Problems and Perspectives. Metals 2022, 12, 1289. [Google Scholar] [CrossRef]
- Svirid, A.E.; Pushin, V.G.; Kuranova, N.N.; Makarov, V.V.; Uksusnikov, A.N. The Effect of Heat Treatment on the Structure and Mechanical Properties of Nanocrystalline Cu–14Al–3Ni Alloy Subjected to High-Pressure Torsion. Phys. Met. Metallogr. 2021, 122, 883–890. [Google Scholar] [CrossRef]




| Alloy Designation | Chemical Composition wt.% | ||
|---|---|---|---|
| Cu | Ni | Al | |
| AB | 90.68 | 9.32 | -- |
| ABTT | 90.69 | 9.31 | -- |
| AT5 | 78.44 | 19.32 | 2.22 |
| AT5TT | 78.50 | 19.30 | 2.20 |
| AT10 | 75.49 | 19.22 | 4.57 |
| AT10TT | 75.90 | 19.63 | 4.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales-Cadena, I.; Gonzalez-Rodriguez, J.G.; Diaz-Reyes, C.; Guardian-Tapia, R.; Ruiz-Ochoa, J.A.; Ramirez-Arteaga, A.M.; Lopez-Sesenes, R. Effect of Controlled Heat Treatment and Aluminum Additions on the Strengthening of Cu–Ni-Based Alloys. Metals 2023, 13, 1835. https://doi.org/10.3390/met13111835
Rosales-Cadena I, Gonzalez-Rodriguez JG, Diaz-Reyes C, Guardian-Tapia R, Ruiz-Ochoa JA, Ramirez-Arteaga AM, Lopez-Sesenes R. Effect of Controlled Heat Treatment and Aluminum Additions on the Strengthening of Cu–Ni-Based Alloys. Metals. 2023; 13(11):1835. https://doi.org/10.3390/met13111835
Chicago/Turabian StyleRosales-Cadena, Isai, Jose Gonzalo Gonzalez-Rodriguez, Constancio Diaz-Reyes, Rene Guardian-Tapia, Juan Antonio Ruiz-Ochoa, America Maria Ramirez-Arteaga, and Roy Lopez-Sesenes. 2023. "Effect of Controlled Heat Treatment and Aluminum Additions on the Strengthening of Cu–Ni-Based Alloys" Metals 13, no. 11: 1835. https://doi.org/10.3390/met13111835
APA StyleRosales-Cadena, I., Gonzalez-Rodriguez, J. G., Diaz-Reyes, C., Guardian-Tapia, R., Ruiz-Ochoa, J. A., Ramirez-Arteaga, A. M., & Lopez-Sesenes, R. (2023). Effect of Controlled Heat Treatment and Aluminum Additions on the Strengthening of Cu–Ni-Based Alloys. Metals, 13(11), 1835. https://doi.org/10.3390/met13111835






