Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
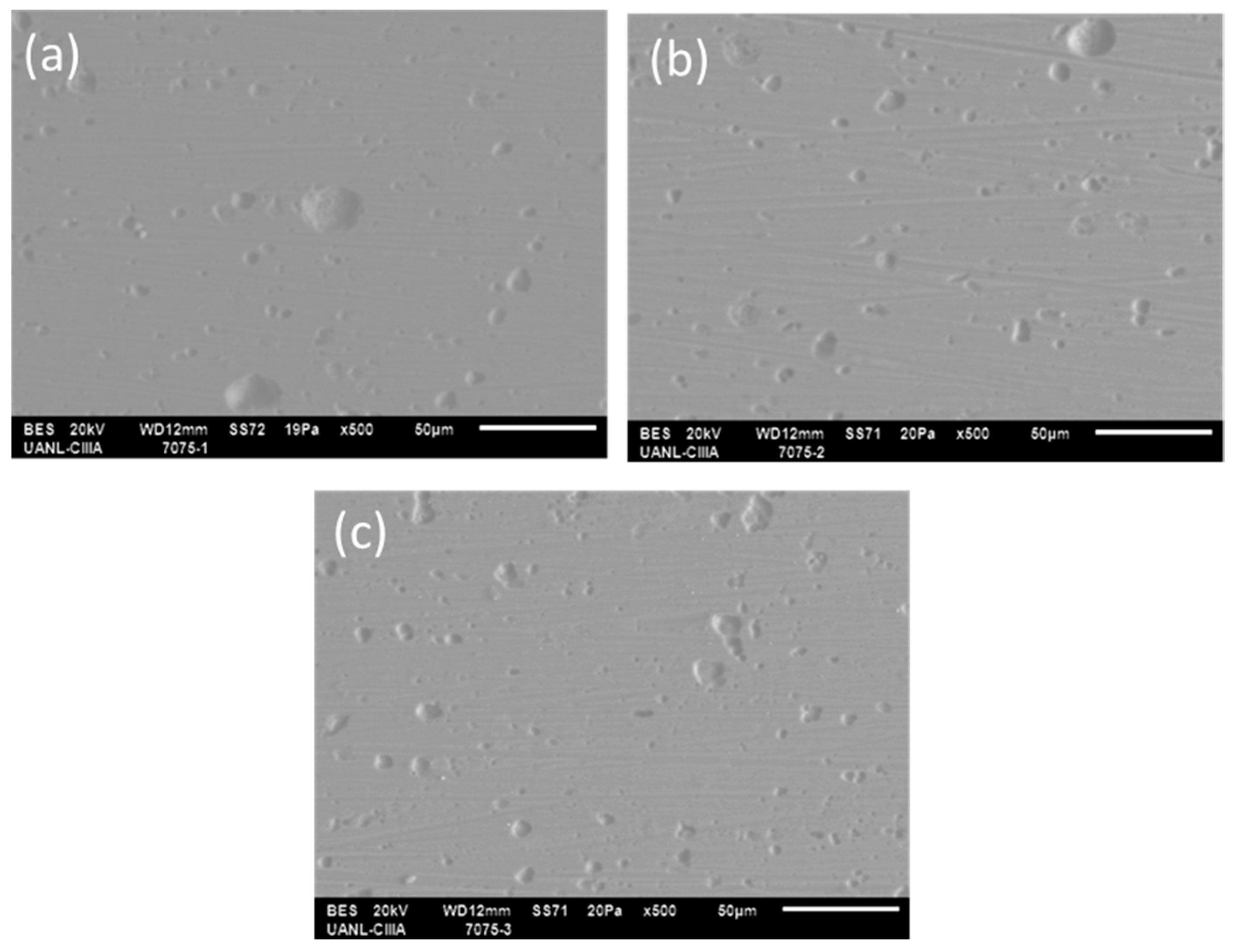
3.1. SEM Superficial Analysis
3.2. Electrochemical Noise
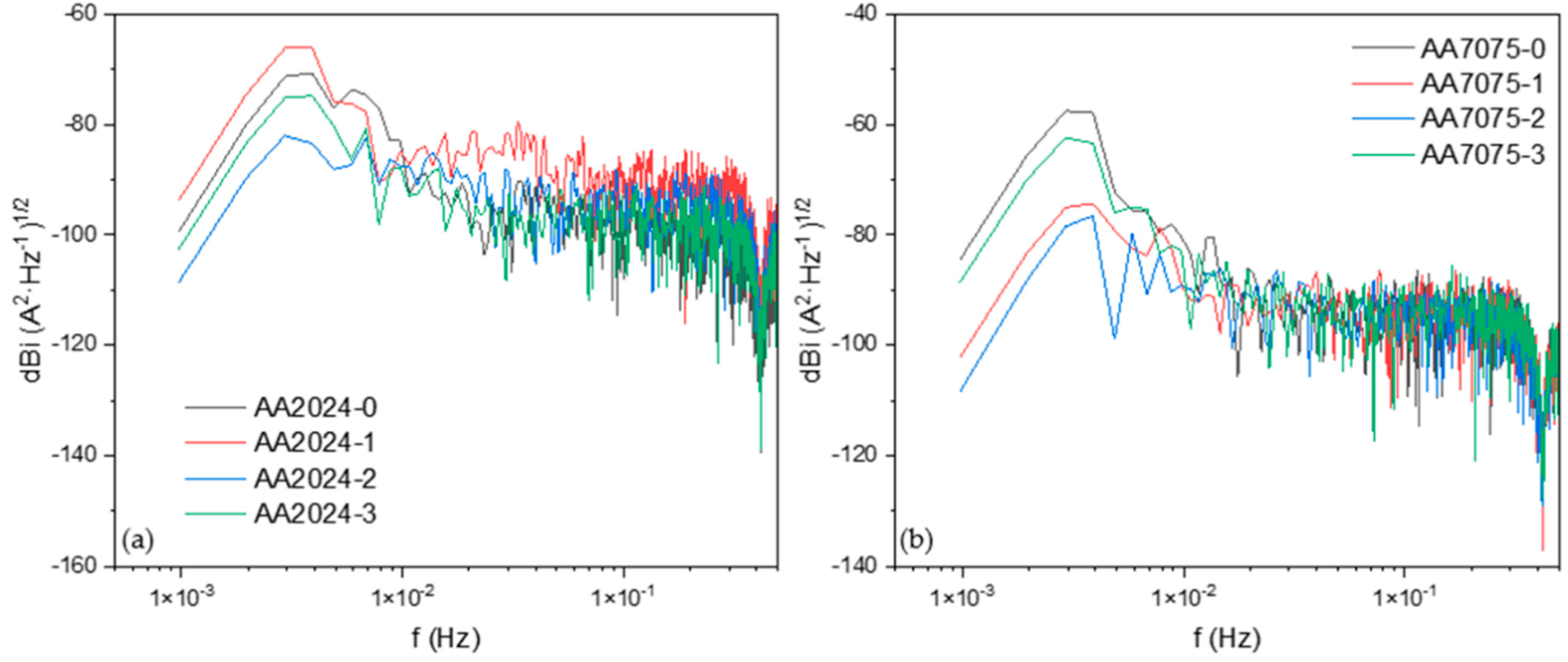
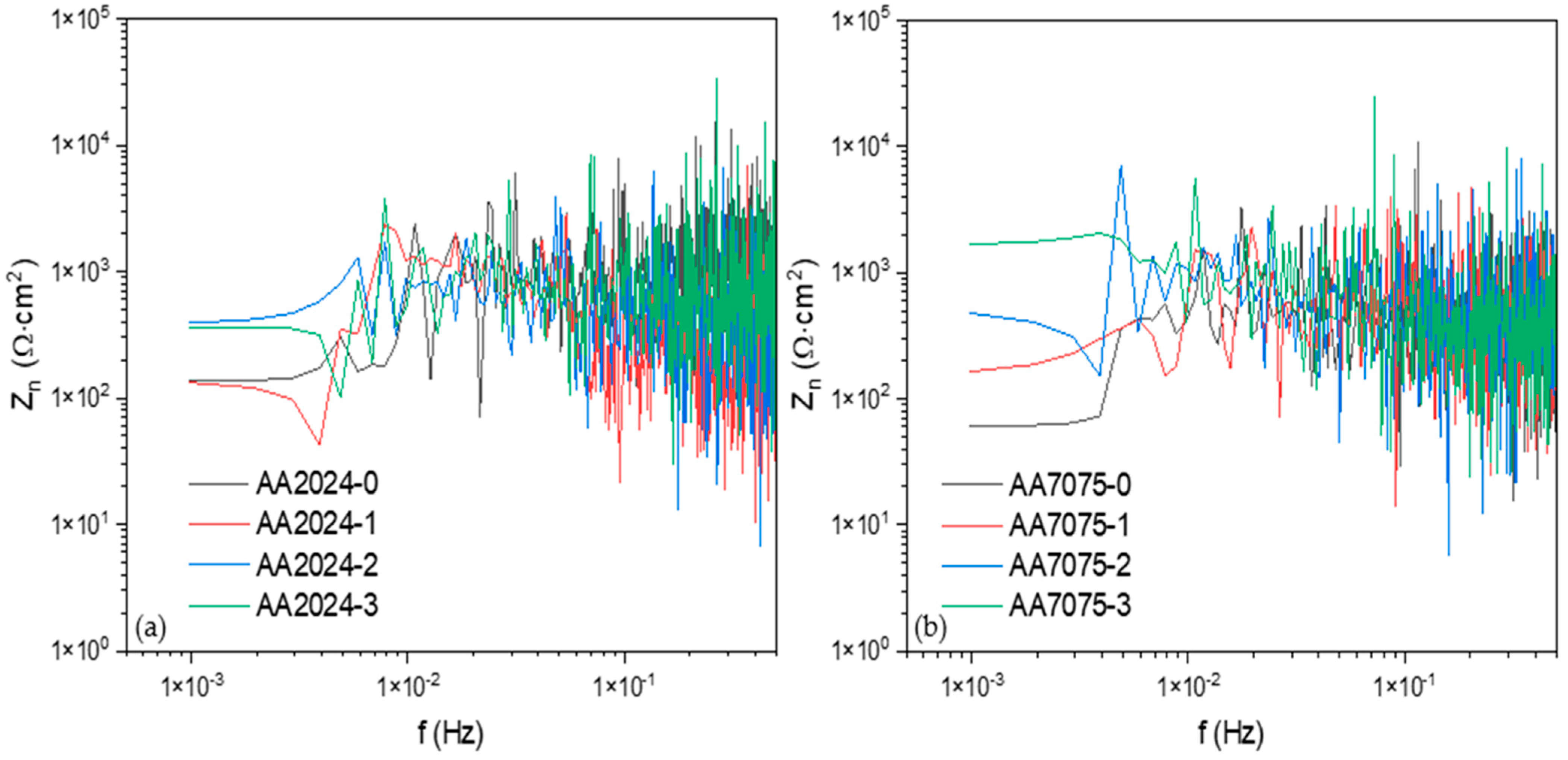
3.2.1. Frequency Domain Analysis: Power Spectral Density (PSD) and Noise Impedance (Zn)
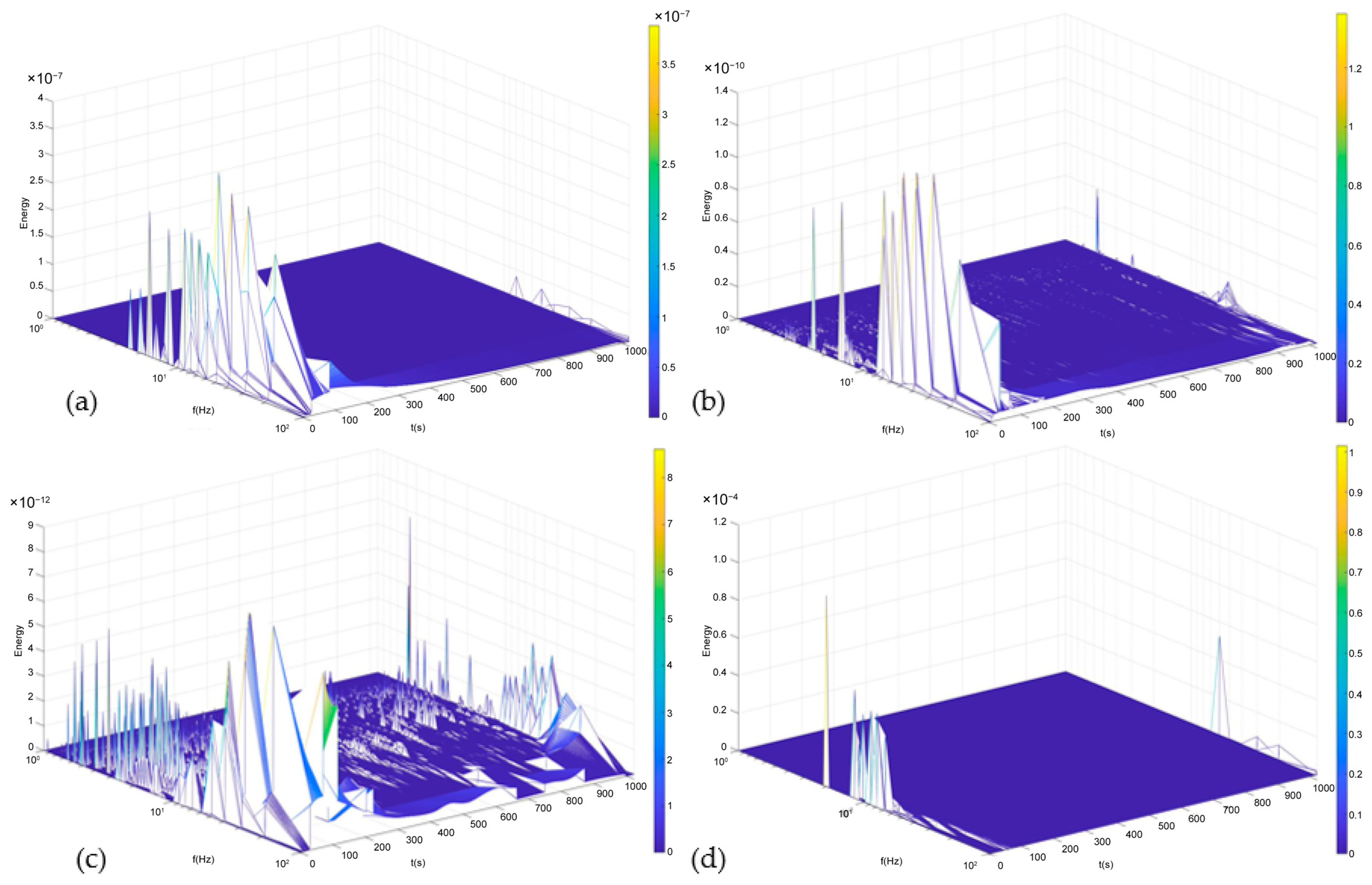
3.2.2. Time–Frequency Domain Analysis: Hilbert–Huang Transform ANALYSIS
3.3. Time Domain Analysis—Chaos Theory
3.3.1. Signal Processing
3.3.2. Correlation Dimension
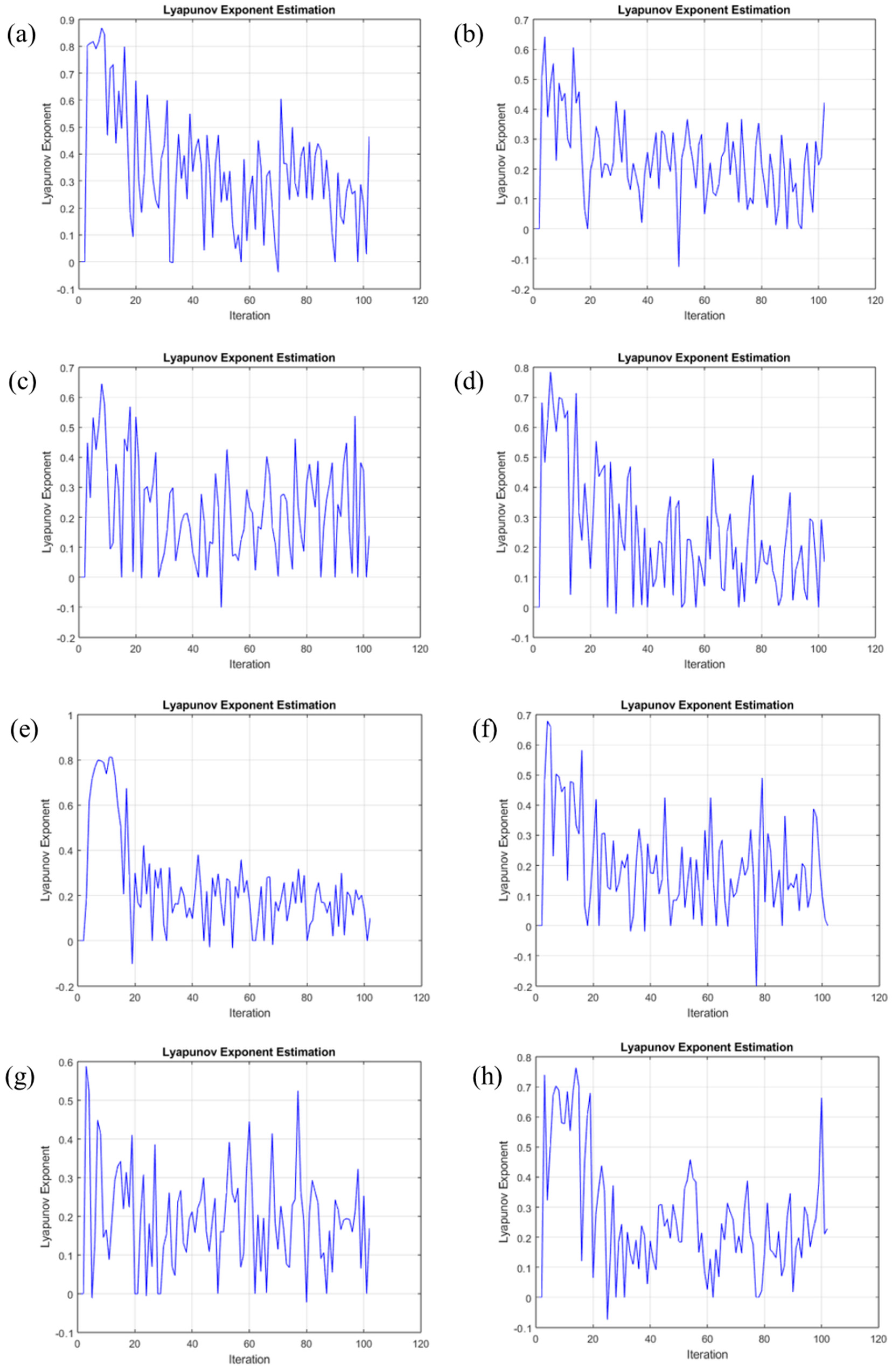
3.3.3. Average Lyapunov Exponent
4. Discussion
5. Conclusions
- The result indicated that the AA2024-0, AA2024-1, and AA2024-2 samples and the AA7075-2 and AA7075-3 samples exhibit mixed corrosion via the Lyapunov constant, with a notable inclination towards localized corrosion when analyzed using the PSD and HHT methods. The surface is not homogenous, and the corrosion process is predominately localized in specific zones.
- In the analysis conducted using the PSD slope, only AA2024-2 presented a uniform corrosion value (−6); meanwhile, the rest of the samples presented values of localized corrosion.
- The AA7075-3 sample presented the highest corrosion resistance (1667 Ω·cm2), and the AA2024-1 sample presented the lowest corrosion resistance with 132 Ω·cm2.
- The AA2024-0 sample, in contrast, exhibited a lower correlation dimension, implying less complex or self-replicating corrosion processes. This potentially indicates a more uniform or straightforward corrosion behavior, which may contribute to localized corrosion tendencies. The result obtained using the HHT method matched the result obtained using the correlation dimension.
- Among the AA7075 alloy samples, it is noteworthy that only the AA7075-1 and AA7075-3 samples exhibited heightened passivity, as indicated by their higher correlation dimensions.
- The successful application of the chaos theory in our study underscores its efficacy and capacity for an in-depth analysis of electrochemical noise results. It has proven to be instrumental in providing detailed insights into the nature and mechanisms of corrosion processes.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okene, S.U.; Abdullahi, M.; Gana, A. Corrosion Improvement of AA7075 through Precipitation Hardness and Anodization. Mater. Today Proc. 2021, 38, 729–736. [Google Scholar] [CrossRef]
- Totten, G.; Tiryankioglu, M.; Kessler, O. Encyclopedia of Aluminum and Its Alloys; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351045636. [Google Scholar]
- Campbell, F.C. Manufacturing Technology for Aerospace Structural Materials; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–600. [Google Scholar] [CrossRef]
- Mouritz, A.P. Introduction to Aerospace Materials; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9781855739468. [Google Scholar]
- Acuña-González, N.; García-Ochoa, E.; González-Sánchez, J. Assessment of the Dynamics of Corrosion Fatigue Crack Initiation Applying Recurrence Plots to the Analysis of Electrochemical Noise Data. Int. J. Fatigue 2008, 30, 1211–1219. [Google Scholar] [CrossRef]
- García, E.; Hernández, M.A.; Rodríguez, F.J.; Genescá, J.; Boerio, F.J. Oscillation and Chaos in Pitting Corrosion of Steel. Corrosion 2003, 59, 50–58. [Google Scholar] [CrossRef]
- Markworth, A.J.; Stringer, J.; Rollins, R.W. Deterministic Chaos Theory and Its Applications to Materials Science. MRS Bull. 1995, 20, 20–28. [Google Scholar] [CrossRef]
- Rosenstein, M.T.; Collins, J.J.; De Luca, C.J. A Practical Method for Calculating Largest Lyapunov Exponents from Small Data Sets. Phys. D Nonlinear Phenom. 1993, 65, 117–134. [Google Scholar] [CrossRef]
- Terada, M.; Queiroz, F.M.; De Aguiar, D.B.D.S.; Ayusso, V.H.; Costenaro, H.; Olivier, M.; De Melo, H.G.; Costa, I. Corrosion resistance of tartaric-sulfuric acid anodized AA2024-T3 sealed with Ce and protected with hybrid sol–gel coating. Surf. Coat. Technol. 2019, 372, 422–426. [Google Scholar] [CrossRef]
- Bensalah, W.; Elleuch, K.; Feki, M.; Depetris-Wery, M.; Ayedi, H.F. Optimization of Tartaric/Sulphuric Acid Anodizing Process Using Doehlert Design. Surf. Coat. Technol. 2012, 207, 123–129. [Google Scholar] [CrossRef]
- Cottis, R.A. Interpretation of Electrochemical Noise Data. Corrosion 2001, 57, 265–285. [Google Scholar] [CrossRef]
- Bahena, D.; Rosales, I.; Sarmiento, O.; Guardián, R.; Menchaca, C.; Uruchurtu, J. Electrochemical Noise Chaotic Analysis of NiCoAg Alloy in Hank Solution. Int. J. Corros. 2011, 2011, 491564. [Google Scholar] [CrossRef]
- Critchlow, G.W.; Yendall, K.A.; Bahrani, D.; Quinn, A.; Andrews, F. Strategies for the Replacement of Chromic Acid Anodising for the Structural Bonding of Aluminium Alloys. Int. J. Adhes. Adhes. 2006, 26, 419–453. [Google Scholar] [CrossRef]
- Idusuyi, N.; Fayomi, O.S. Performance Study of Aluminium–Zinc Anode using Noise Resistance, Pitting Index and Recurrence Plots from Electrochemical Noise Data. J. Bio Tribo Corros. 2021, 7, 56. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Men, H.; Sun, B.; Zhao, X.; Li, X.; Liu, J.; Xu, Z. Dynamics of Stainless Steel Corrosion Based on the Theory of Phase Space Reconstruction and Chaos. Anti-Corrosion Methods Mater. 2016, 63, 214–225. [Google Scholar] [CrossRef]
- Jaume, J.; Marques, M.J.F.; Délia, M.L.; Basséguy, R. Surface modification of 5083 aluminum-magnesium induced by marine microorganisms. Corros. Sci. 2022, 194, 109934. [Google Scholar] [CrossRef]
- Vignoli Machado, T.; Atz Dick, P.; Knörnschild, G.H.; Dick, L.F.P. The effect of different carboxylic acids on the sulfuric acid anodizing of AA2024. Surf. Coat. Technol. 2020, 383, 125283. [Google Scholar]
- Del Olmo, R.; Mohedano, M.; Visser, P.; Matykina, E.; Arrabal, R. Flash-PEO coatings loaded with corrosion inhibitors on AA2024. Surf. Coat. Technol. 2020, 402, 126317. [Google Scholar]
- Ofoegbu, S.U.; Fernandes, F.A.; Pereira, A.B. The sealing step in aluminum anodizing: A focus on sustainable strategies for enhancing both energy efficiency and corrosion resistance. Coatings 2020, 10, 226. [Google Scholar]
- Attolico, M.A.; Casavola, C.; Moramarco, V.; Renna, G.; Furfari, D.; Buss, D. Influence of tartaric-sulfuric acid anodic film on four-point bending fatigue behavior of AA 7050-T7451 samples. Fatigue Fract. Eng. Mater. Struct. 2022, 45, 3716–3730. [Google Scholar] [CrossRef]
- Greisiger, H.; Schauer, T. On the Interpretation of the Electrochemical Noise Data for Coatings. Prog. Org. Coatings 2000, 39, 31–36. [Google Scholar] [CrossRef]
- Mubarok, M.; Sutarno, W.; Wahyudi, S. Effects of Anodizing Parameters in Tartaric-Sulphuric Acid on Coating Thickness and Corrosion Resistance of Al 2024 T3 Alloy. J. Miner. Mater. Charact. Eng. 2015, 3, 3. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B.; Zeino, A.; Umoren, S.A. Electrochemical Noise (EN) Technique: Review of Recent Practical Applications to Corrosion Electrochemistry Research. J. Adhes. Sci. Technol. 2019, 33, 1453–1496. [Google Scholar]
- Xia, D.; Shi, S.; Behnamian, Y.; Hu, W.; Cheng, Y.F.; Luo, J.; Huet, F. Review—Electrochemical Noise Applied in Corrosion Science: Theoretical and Mathematical Models towards Quantitative Analysis. J. Electrochem. Soc. 2020, 167, 081507. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.Z.; Behnamian, Y. Detection of Corrosion Degradation Using Electrochemical Noise (EN): Review of Signal Processing Methods for Identifying Corrosion Forms. Corros. Eng. Sci. Technol. 2016, 51, 527–544. [Google Scholar] [CrossRef]
- Chen, A.; Cao, F.; Liao, X.; Liu, W.; Zheng, L.; Zhang, J.; Cao, C. Study of Pitting Corrosion on Mild Steel during Wet–Dry Cycles by Electrochemical Noise Analysis Based on Chaos Theory. Corros. Sci. 2013, 66, 183–195. [Google Scholar] [CrossRef]
- Skokos, C.; Gottwald, G.A.; Laskar, J. Erratum to: Chaos Detection and Predictability; Skokos, C., Gottwald, G.A., Laskar, J., Eds.; Lecture Notes in Physics; Springer: Berlin/Heidelberg, Germany, 2016; p. 915. ISBN 978-3-662-48408-1. [Google Scholar]
- Jirón-Lazos, U.; Corvo, F.; De la Rosa, S.C.; García-Ochoa, E.M.; Bastidas, D.M.; Bastidas, J.M. Localized Corrosion of Aluminum Alloy 6061 in the Presence of Aspergillus Niger. Int. Biodeterior. Biodegrad. 2018, 133, 17–25. [Google Scholar] [CrossRef]
- Stringer, J.; Markworth, A.J. Applications of Deterministic Chaos Theory to Corrosion. Corros. Sci. 1993, 35, 751–760. [Google Scholar] [CrossRef]
- Bertocci, U.; Gabrielli, C.; Huet, F.; Keddam, M.; Rousseau, P. Noise Resistance Applied to Corrosion Measurements: II. Experimental Tests. J. Electrochem. Soc. 1997, 144, 37–43. [Google Scholar] [CrossRef]
- ASTM E3-95; Standard Practice for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 1995.
- ASTM E-407-16; Standard Practice for Microetching Metals and Alloys. ASTM International: West Conshohocken, PA, USA, 2016.
- Villegas-Tovar, J.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Baltazar-Zamora, M.A.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderón, F. Electrochemical Corrosion Behavior of Passivated Precipitation Hardening Stainless Steels for Aerospace Applications. Metals 2023, 13, 835. [Google Scholar] [CrossRef]
- ASTM G199-09; Standard Guide for Electrochemical Noise Measurement. ASTM International: West Conshohocken, PA, USA, 2020.
- Samaniego-Gámez, P.O.; Almeraya-Calderon, F.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Lopez-Leon, L.D.; Silva Vidaurri, L.G.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Corrosion Behavior of AA2055 Aluminum-Lithium Alloys Anodized in the Presence of Sulfuric Acid Solution. Coatings 2021, 11, 1278. [Google Scholar] [CrossRef]
- Xia, D.H.; Behnamian, Y. Electrochemical noise: A review of experimental setup, instrumentation and DC removal. Russ. J. Electrochem. 2015, 51, 593–601. [Google Scholar] [CrossRef]
- Cerezo, H.R.; Tiburcio, C.G.; Miramontes, J.A.C.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Almeraya-Calderon, F. Electrochemical characterization of Al–Li alloys AA2099 and AA2055 for aeronautical applications: Effect of thermomechanical treatments. J. Solid State Electrochem. 2023, 27, 3101–3117. [Google Scholar] [CrossRef]
- Li, L.; Qiao, Y.; Zhang, L.; Ma, A.; Ma, R.; Zheng, Y. Understanding the Corrosion Behavior of Nickel–Aluminum Bronze Induced by Cavitation Corrosion Using Electrochemical Noise: Selective Phase Corrosion and Uniform Corrosion. Materials 2023, 16, 669. [Google Scholar] [CrossRef] [PubMed]
- Lentka, Ł; Smulko, J. Methods of Trend Removal in Electrochemical Noise Data—Overview. Measurement 2019, 131, 569–581. [Google Scholar] [CrossRef]
- Moradi, M.; Rezaei, M. Electrochemical noise analysis to evaluate the localized anti-corrosion properties of PP/graphene oxide nanocomposite coatings. J. Electroanal. Chem. 2022, 921, 116665. [Google Scholar] [CrossRef]
- Mehdipour, M.; Naderi, R.; Markhali, B.P. Electrochemical Study of Effect of the Concentration of Azole Derivatives on Corrosion Behavior of Stainless Steel in H2SO4. Prog. Org. Coatings 2014, 77, 1761–1767. [Google Scholar] [CrossRef]
- Coakley, J.; Vorontsov, V.A.; Littrell, K.C.; Heenan, R.K.; Ohnuma, M.; Jones, N.G.; Dye, D. Nanoprecipitation in a Beta-Titanium Alloy. J. Alloys Compd. 2015, 623, 146–156. [Google Scholar] [CrossRef]
- Kroll, R.; Kearns, P.; Usman, B.J.; Zhou, X.; Engelberg, D.L. A novel approach to determine cathodic passivation characteristics and semiconducting properties of pure aluminum 99.5 wt% and aluminum alloy 7075-T6 with an electrochemical pen electrode. Corros. Sci. 2023, 211, 110898. [Google Scholar] [CrossRef]
- Butler, J.A.V. Studies in Heterogeneous Equilibria. Part II.—The Kinetic Interpretation of the Nernst Theory of Electromotive Force. Trans. Faraday Soc. 1924, 19, 729–733. [Google Scholar] [CrossRef]
- Lafront, A.M.; Safizadeh, F.; Ghali, E.; Houlachi, G. Study of the Copper Anode Passivation by Electrochemical Noise Analysis Using Spectral and Wavelet Transforms. Electrochim. Acta 2010, 55, 2505–2512. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Méndez-Ramírez, C.T.; Baltazar-Zamora, M.Á.; Estupinán-López, F.; Bautista-Margulis, R.G.; Cuevas-Rodríguez, J.; los Rios, J.P.F.-D.; Almeraya-Calderón, F. Corrosion of Titanium Alloys Anodized Using Electrochemical Techniques. Metals 2023, 13, 476. [Google Scholar] [CrossRef]
- Bertocci, U.; Huet, F. Noise Analysis Applied to Electrochemical Systems. Corrosion 1995, 51, 131–144. [Google Scholar] [CrossRef]
- Oliveira, N.T.C.; Guastaldi, A.C. Electrochemical Stability and Corrosion Resistance of Ti–Mo Alloys for Biomedical Applications. Acta Biomater. 2009, 5, 399–405. [Google Scholar] [CrossRef]
- Adamek, G.; Pałka, K.; Jakubowicz, J. Corrosion Properties of Ti Scaffolds Prepared with Sucrose as a Space Holder. Solid. State Phenom. 2015, 227, 519–522. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Thampuran, R.; Teoh, S.H. The Influence of Pore Morphology on Corrosion. Corros. Sci. 1998, 40, 547–556. [Google Scholar] [CrossRef]
- Homborg, A.M.; Cottis, R.A.; Mol, J.M.C. An Integrated Approach in the Time, Frequency and Time-Frequency Domain for the Identification of Corrosion Using Electrochemical Noise. Electrochim. Acta 2016, 222, 627–640. [Google Scholar] [CrossRef]
- Strogatz, S.; Friedman, M.; Mallinckrodt, A.J.; McKay, S. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering. Comput. Phys. 1994, 8, 532. [Google Scholar] [CrossRef]
- Xia, D.; Shi, S.; Wang, J.; Shi, J.; Bi, H.; Gao, Z. Determination of corrosion types from electrochemical noise by phase space reconstruction theory. Electrochem. Commun. 2012, 15, 88–92. [Google Scholar] [CrossRef]
- Kennel, M.B.; Brown, R.; Abarbanel, H.D.I. Determining Embedding Dimension for Phase-Space Reconstruction Using a Geometrical Construction. Phys. Rev. A 1992, 45, 3403. [Google Scholar] [CrossRef]
- Curioni, M.; Skeldon, P.; Koroleva, E.; Thompson, G.E.; Ferguson, J. Role of Tartaric Acid on the Anodizing and Corrosion Behavior of AA 2024 T3 Aluminum Alloy. J. Electrochem. Soc 2009, 156, C147. [Google Scholar] [CrossRef]
- Surganov, V.F.; Gorokh, G.G. Anodic Oxide Cellular Structure Formation on Aluminum Films in Tartaric Acid Electrolyte. Mater. Lett. 1993, 17, 121–124. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Liao, Y.; Chen, X.; Zhang, C.; Wu, H.; Wang, Z.; Huang, W. Effect of Anodizing Parameters on Film Morphology and Corrosion Resistance of AA2099 Aluminum-Lithium Alloy. J. Electrochem. Soc. 2016, 163, C369–C376. [Google Scholar] [CrossRef]
- Li, J.; Du, C.W.; Liu, Z.Y.; Li, X.G.; Liu, M. Effect of Microstructure on the Corrosion Resistance of 2205 Duplex Stainless Steel. Part 2: Electrochemical Noise Analysis of Corrosion Behaviors of Different Microstructures Based on Wavelet Transform. Constr. Build. Mater. 2018, 189, 1294–1302. [Google Scholar] [CrossRef]
- Jáquez-Munõz, J.M.; Gaona-Tiburcio, C.; Cabral, J.A.; Lara-Banda, M.; Estupinãn-López, F.H.; Zambrano, P.; Almeraya-Calderón, F. Corrosion Behavior of Titanium Alloys Using Electrochemical Noise. ECS Trans. 2021, 101, 167. [Google Scholar] [CrossRef]
- Arellano-Pérez, J.H.; Escobar-Jiménez, R.F.; Granados-Lieberman, D.; Gómez-Aguilar, J.F.; Uruchurtu-Chavarín, J.; Alvarado-Martínez, V.M. Electrochemical Noise Signals Evaluation to Classify the Type of Corrosion Using Synchrosqueezing Transform. J. Electroanal. Chem. 2019, 848, 113249. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; Estupinán-López, F.; López-León, L.D.; Chacón-Nava, J.; Almeraya-Calderón, F. Frequency Analysis of Transients in Electrochemical Noise of Superalloys Waspaloy and Ultimet. Metals 2021, 11, 702. [Google Scholar] [CrossRef]
- Xia, D.; Mao, Y.; Zhu, Y.; Yuan, Q.; Deng, C.; Hu, W. A novel approach used to study the corrosion susceptibility of metallic materials at a dynamic seawater/air interface. Corros. Commun. 2022, 6, 62–66. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, Y.; Zhang, B.; Behnamian, Y.; Xia, D.; Hu, W. A review of micro-scale and atomic-scale corrosion mechanisms of the second phases in aluminum alloys. Trans. Nonferrous Met. Soc. China 2021, 31, 3205–3227. [Google Scholar] [CrossRef]
- Jaquez-Muñoz, J.; Gaona-Tiburcio, C.; Lira-Martinez, A.; Zambrano-Robledo, P.; Maldonado-Bandala, E.; Samaniego-Gamez, O.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderon, F. Susceptibility to Pitting Corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V Alloys for Aeronautical Applications. Metals 2021, 11, 1002. [Google Scholar] [CrossRef]
- Verro, V.; Di Franco, F.; Zaffora, A.; Santamaria, M. Enhancing corrosion resistance of anodized AA7075 alloys by electrodeposition of superhydrophobic coatings. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132040. [Google Scholar] [CrossRef]
- Wallot, S.; Mønster, D. Calculation of Average Mutual Information (AMI) and False-Nearest Neighbors (FNN) for the estimation of embedding parameters of multidimensional time series in Matlab. Front. Psychol. 2019, 9, 1679. [Google Scholar] [CrossRef]
- Wolf, A.; Swift, J.B.; Swinney, H.L.; Vastano, J.A. Determining Lyapunov exponents from a time series. Phys. D Nonlinear Phenom. 1985, 16, 285–317. [Google Scholar] [CrossRef]
- Xia, D.; Shi, J.; Gong, W.; Rong-Ji, Z.; Gao, Z.; Wang, J. The Significance of Correlation Dimension Obtained from Electrochemical Noise. Electrochemistry 2012, 80, 907–912. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.; Behnamian, Y.; Gao, Z.; Wu, Z.; Qin, Z.; Xia, D.H. Measuring atmospheric corrosion with electrochemical noise: A review of contemporary methods. Measurement 2019, 138, 54–79. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Xia, D.; Ji, Y.; Zhang, R.; Mao, Y.; Behnamian, Y.; Hu, W.; Birbilis, N. On the localized corrosion of AA5083 in a simulated dynamic seawater/air interface—Part 1: Corrosion initiation mechanism. Corros. Sci. 2023, 213, 110985. [Google Scholar] [CrossRef]
- Deng, C.M.; Xia, D.; Zhang, R.; Behnamian, Y.; Hu, W.; Birbilis, N. On the localized corrosion of AA5083 in a simulated dynamic seawater/air interface—Part 2: Effects of wetting time. Corros. Sci. 2023, 221, 111367. [Google Scholar] [CrossRef]
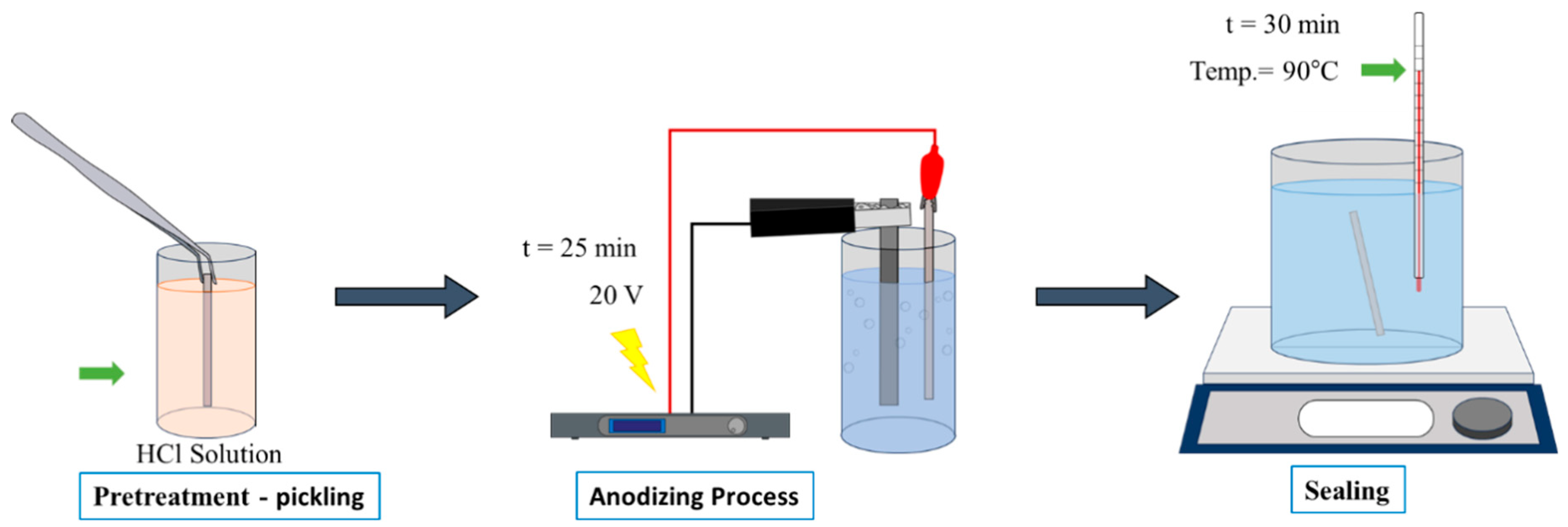
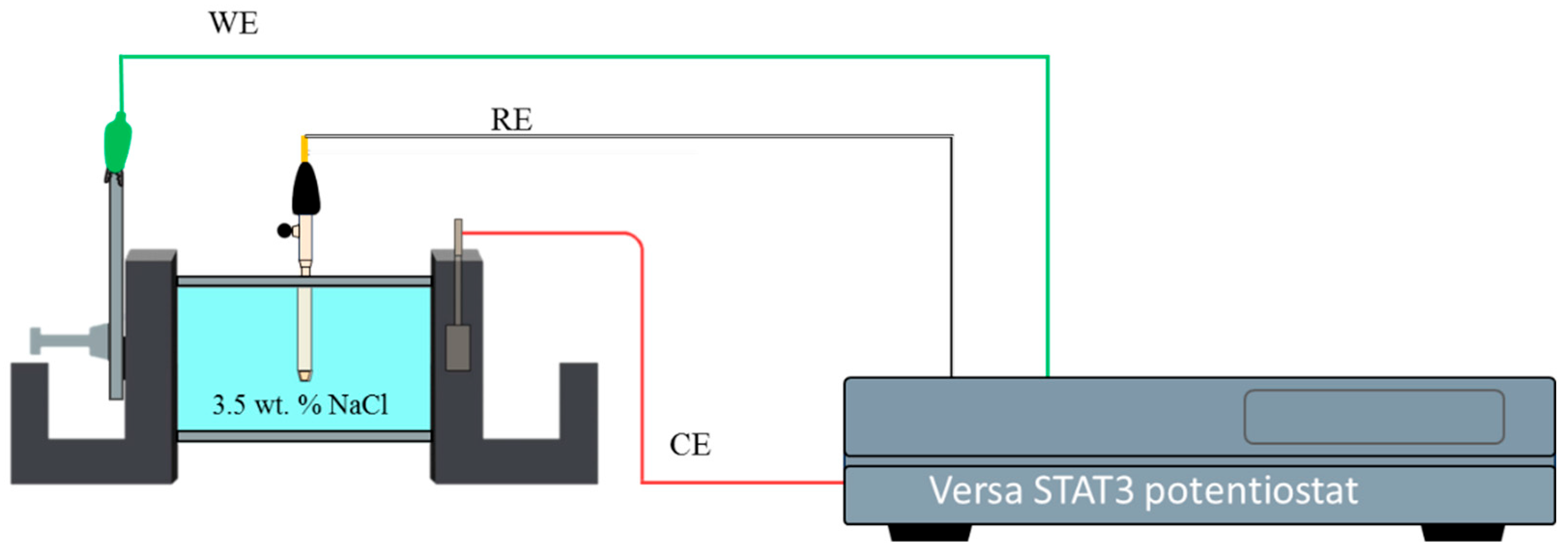


| Alloys | Cu | Zn | W | Fe | Cr | Mn | Pb | Al |
|---|---|---|---|---|---|---|---|---|
| AA2024 | 4.32 | 0.174 | -- | 0.242 | 0.049 | 0.487 | 0.0022 | Balance |
| AA7075 | 1.72 | 5.61 | 0.27 | 0.194 | 0.166 | 0.036 | -- | Balance |
| Alloys | Anodizing Electrolytes (%) | Voltage (V) | Time (min) | Sealing | Nomenclature | ||
|---|---|---|---|---|---|---|---|
| H2SO4 | H3PO4 | C4H6O6 | |||||
| AA2024 | - | - | - | - | - | - | AA2024-0 |
| 5 | 5 | 5 | 20 | 25 | Deionized water at 90 ± 2 °C for 30 min | AA2024-1 | |
| 7.5 | 5 | 10 | AA2024-2 | ||||
| 7.5 | 10 | 5 | AA2024-3 | ||||
| AA7075 | - | - | - | - | - | - | AA7075-0 |
| 5 | 5 | 5 | 20 | 25 | Deionized water at 90 ± 2 °C for 30 min | AA7075-1 | |
| 7.5 | 5 | 10 | AA7075-2 | ||||
| 7.5 | 10 | 5 | AA7075-3 | ||||
| NaCl | |||
|---|---|---|---|
| Sample | Ψ0 (dBi) | Β (dB [A]) | Zn0 (Ω·cm2) |
| AA2024-0 | −99 | −13 | 136 |
| AA2024-1 | −93 | −9 | 132 |
| AA2024-2 | −108 | −6 | 390 |
| AA2024-3 | −102 | −8 | 364 |
| AA7075-0 | −84 | −10 | 60 |
| AA7075-1 | −102 | −7 | 164 |
| AA7075-2 | −108 | −8 | 477 |
| AA7075-3 | −88 | −9 | 1677 |
| AA2024 | AA7075 | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Correlation Dimension | 0.339 | 0.229 | 0.224 | 0.247 | 0.238 | 0.199 | 0.195 | 0.265 |
| AA2024 | AA7075 | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| LE Average | 0.339 | 0.229 | 0.224 | 0.247 | 0.238 | 0.199 | 0.195 | 0.265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Ramos, C.; Olguin-Coca, J.; Lopez-Leon, L.D.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Castañeda-Robles, I.; Jaquez-Muñoz, J.M.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; et al. Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals 2023, 13, 1850. https://doi.org/10.3390/met13111850
Martínez-Ramos C, Olguin-Coca J, Lopez-Leon LD, Gaona-Tiburcio C, Lara-Banda M, Maldonado-Bandala E, Castañeda-Robles I, Jaquez-Muñoz JM, Cabral-Miramontes J, Nieves-Mendoza D, et al. Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals. 2023; 13(11):1850. https://doi.org/10.3390/met13111850
Chicago/Turabian StyleMartínez-Ramos, Cynthia, Javier Olguin-Coca, Luis Daimir Lopez-Leon, Citlalli Gaona-Tiburcio, María Lara-Banda, Erick Maldonado-Bandala, Ivan Castañeda-Robles, Jesús M. Jaquez-Muñoz, Jose Cabral-Miramontes, Demetrio Nieves-Mendoza, and et al. 2023. "Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions" Metals 13, no. 11: 1850. https://doi.org/10.3390/met13111850
APA StyleMartínez-Ramos, C., Olguin-Coca, J., Lopez-Leon, L. D., Gaona-Tiburcio, C., Lara-Banda, M., Maldonado-Bandala, E., Castañeda-Robles, I., Jaquez-Muñoz, J. M., Cabral-Miramontes, J., Nieves-Mendoza, D., & Almeraya-Calderón, F. (2023). Electrochemical Noise Analysis Using Experimental Chaos Theory, Power Spectral Density and Hilbert–Huang Transform in Anodized Aluminum Alloys in Tartaric–Phosphoric–Sulfuric Acid Solutions. Metals, 13(11), 1850. https://doi.org/10.3390/met13111850








