Selective Separation and Recovery of Li from Spent LiFePO4 Cathode Materials by Oxidation Roasting Followed by Low-Acid Pressure Leaching
Abstract
:1. Introduction
2. Materials and Methods
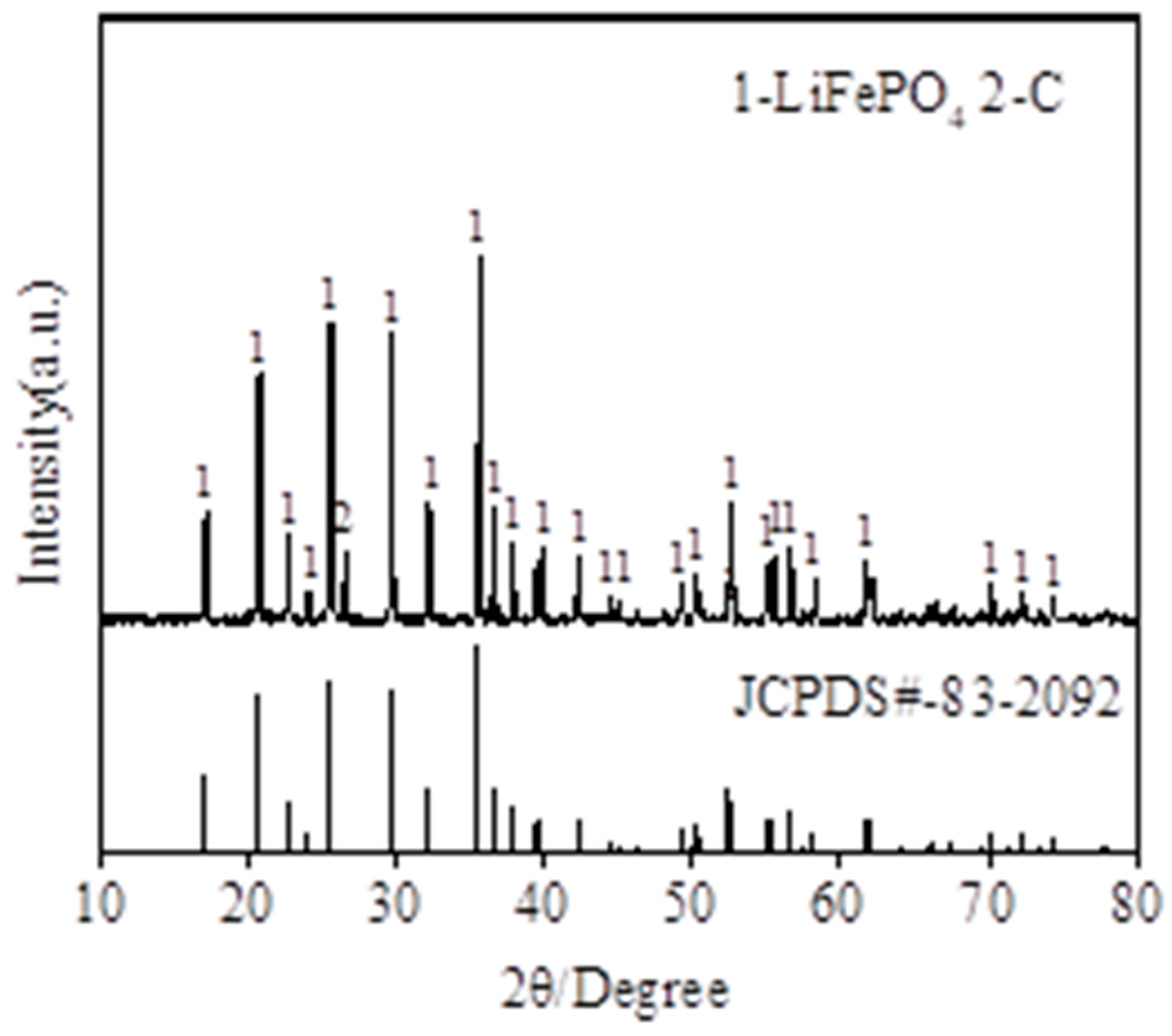
2.1. Materials and Characterization
2.2. Experimental Procedures
2.2.1. Oxidation Roasting
2.2.2. Low-Acid Pressure-Leaching Process
2.2.3. Preparation of Lithium Carbonate
3. Results and Discussion
3.1. Oxidation Roasting of Spent LiFePO4 Cathode Materials
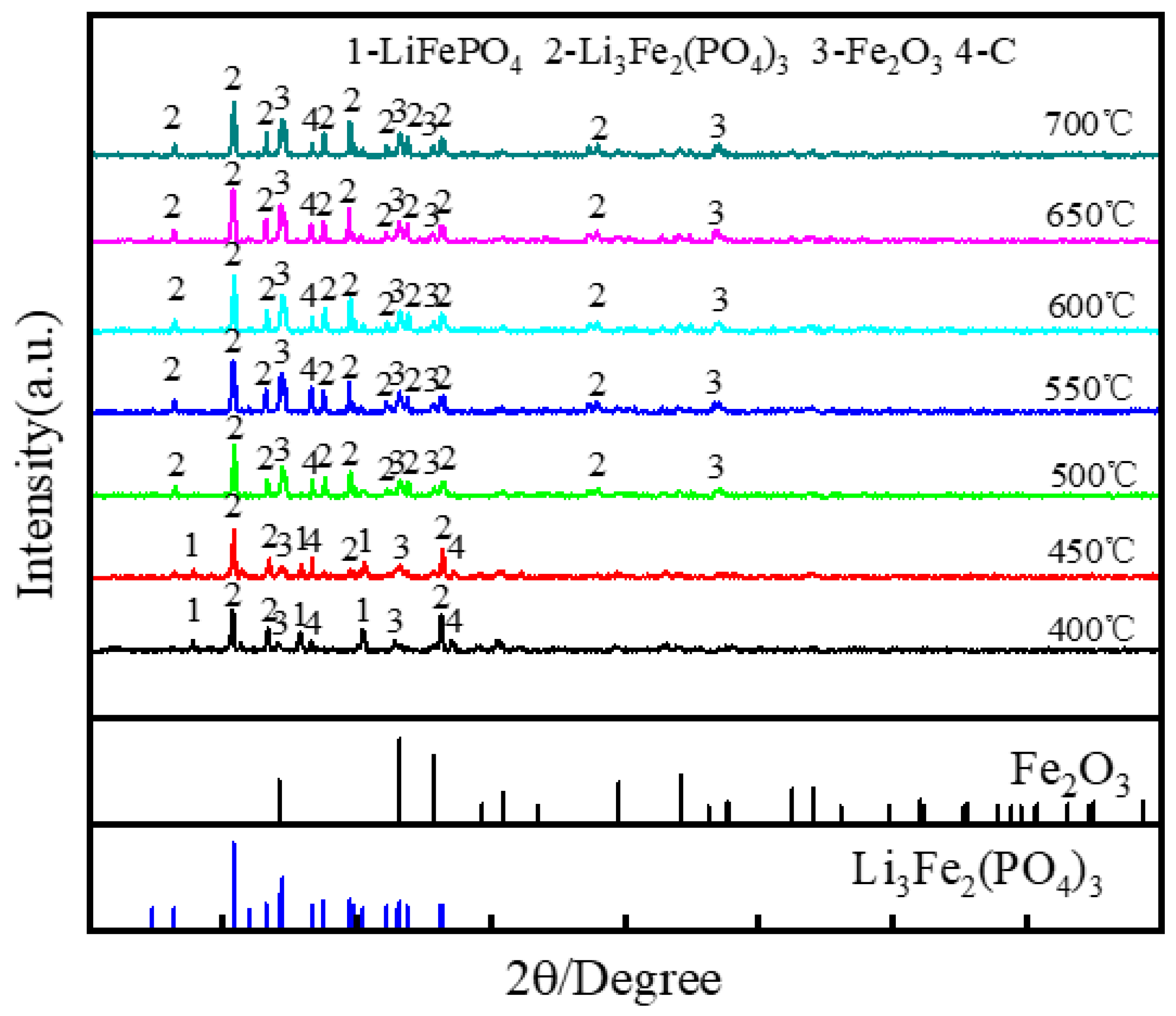
3.1.1. Effect of Roasting Temperature
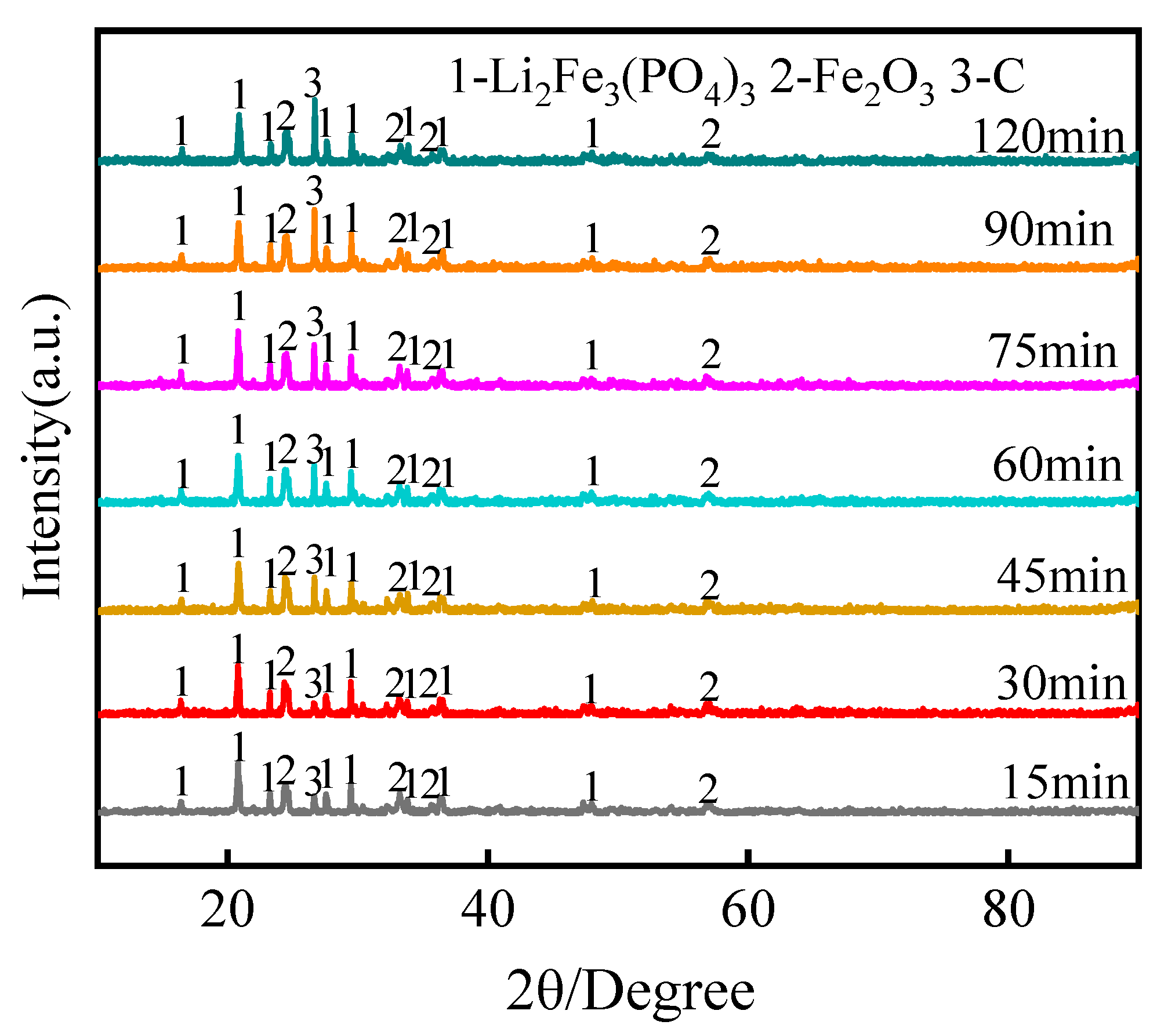
3.1.2. Effect of Roasting Time
3.2. Leaching of Roasted LiFePO4 Cathode Materials
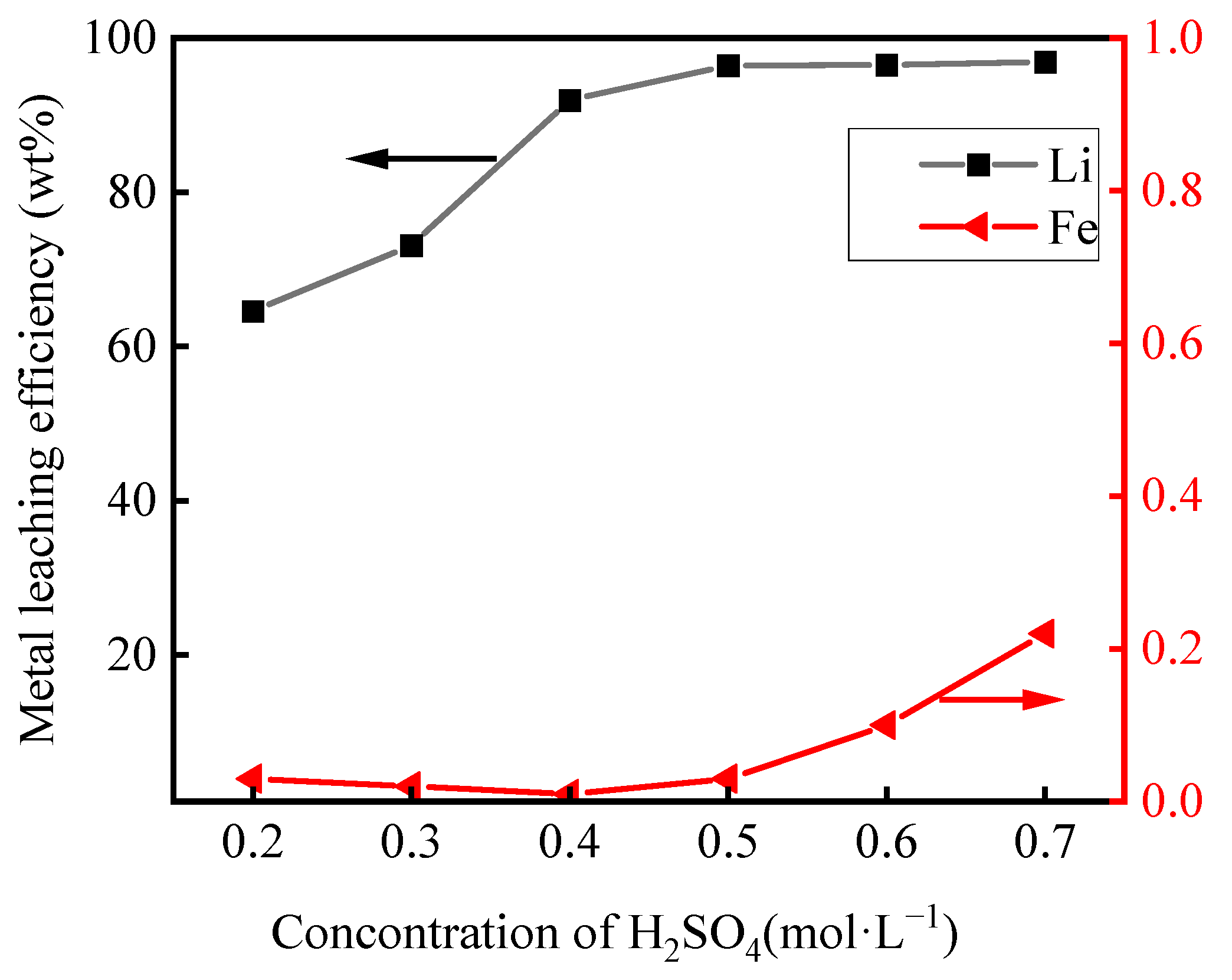
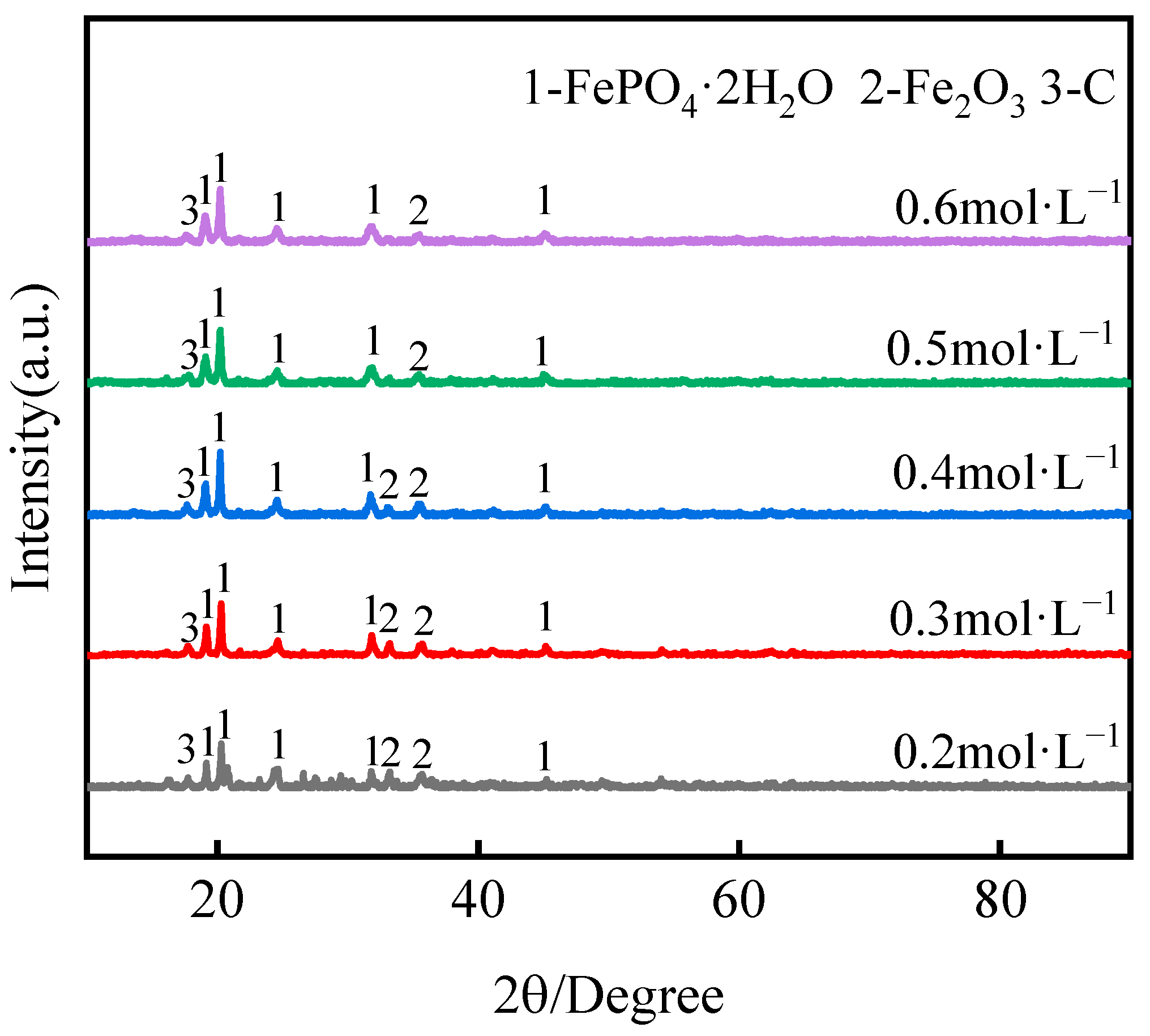
3.2.1. Effect of H2SO4 Concentration
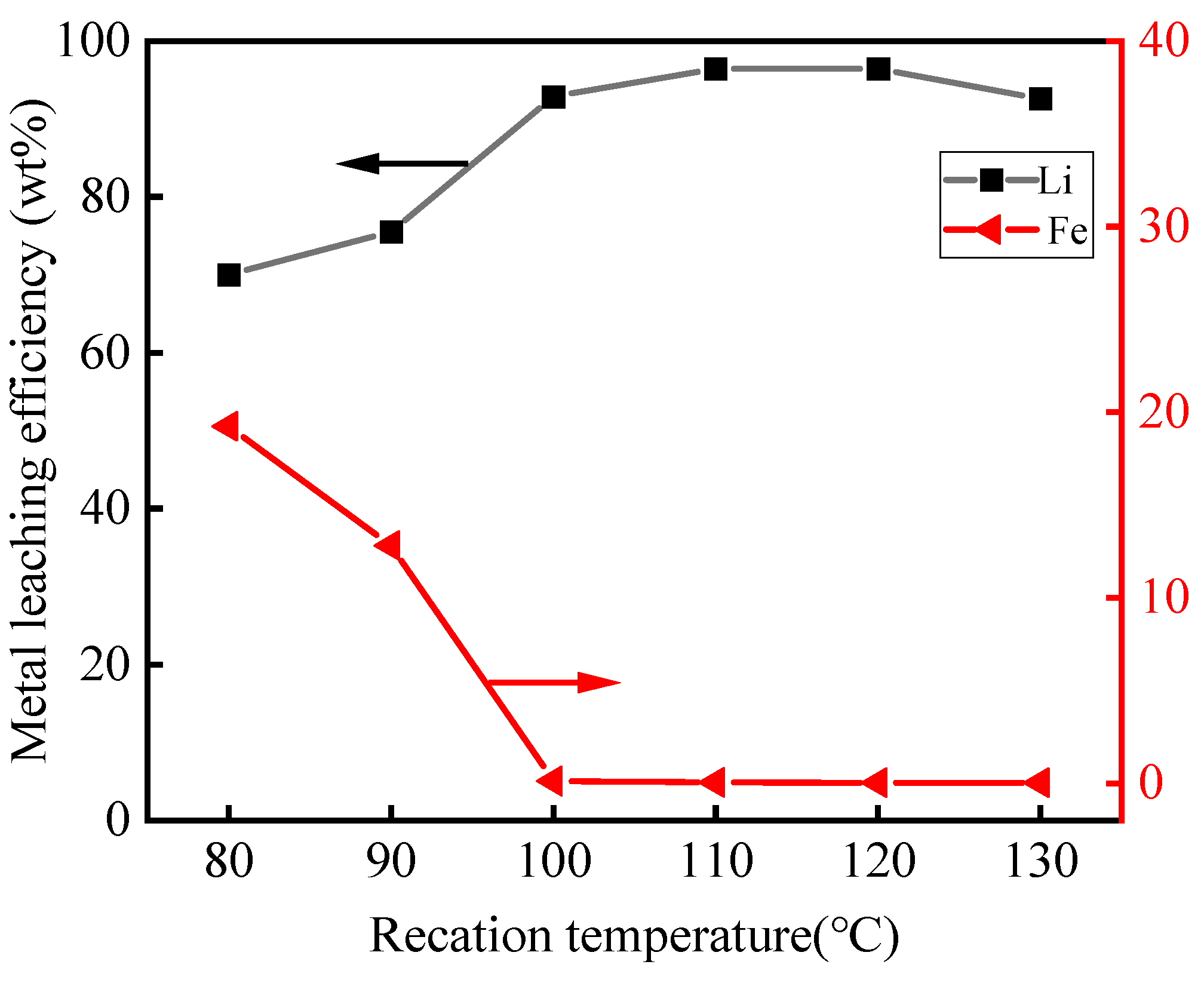
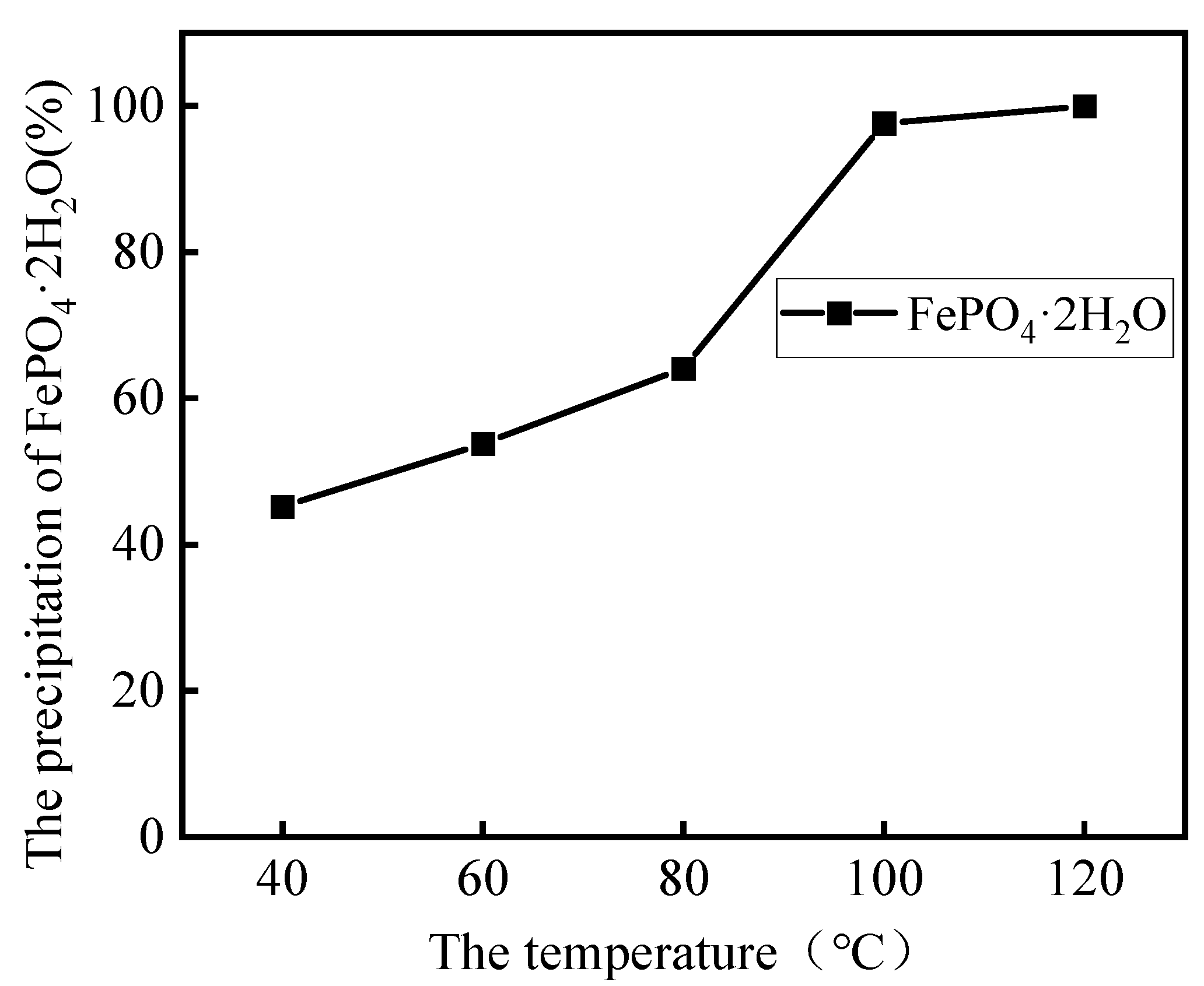
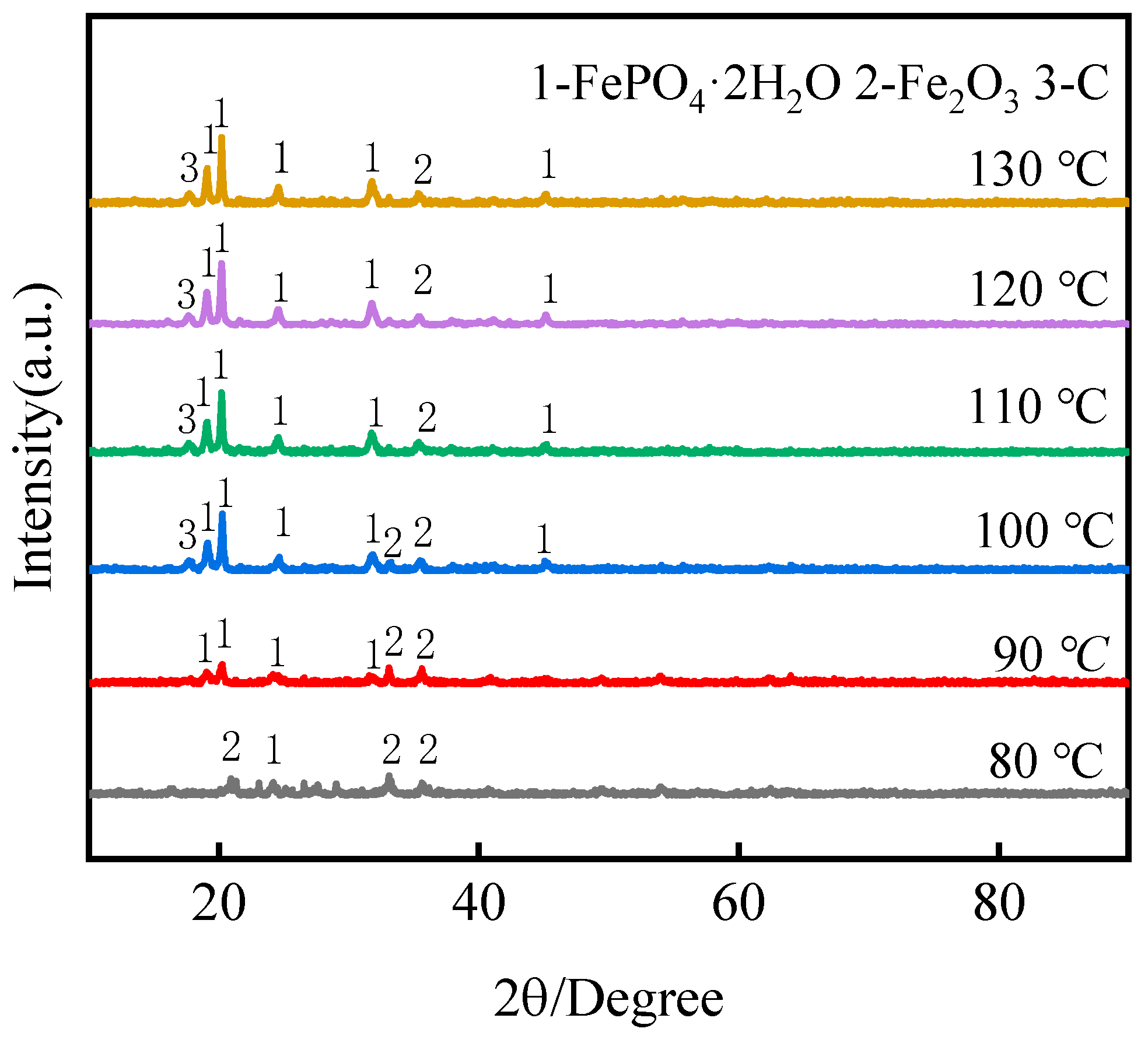
3.2.2. Effect of Leaching Temperature
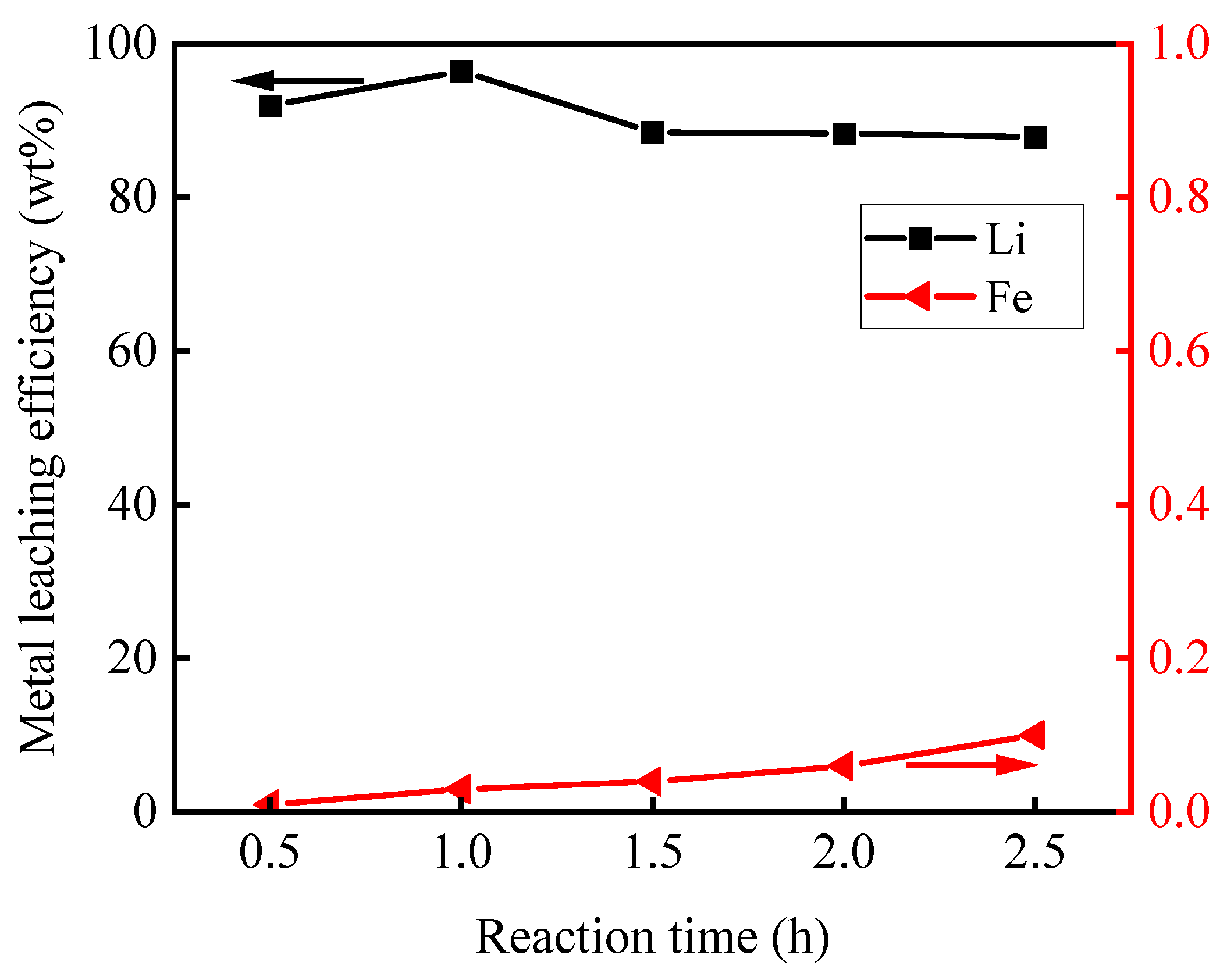
3.2.3. Effect of Leaching Time
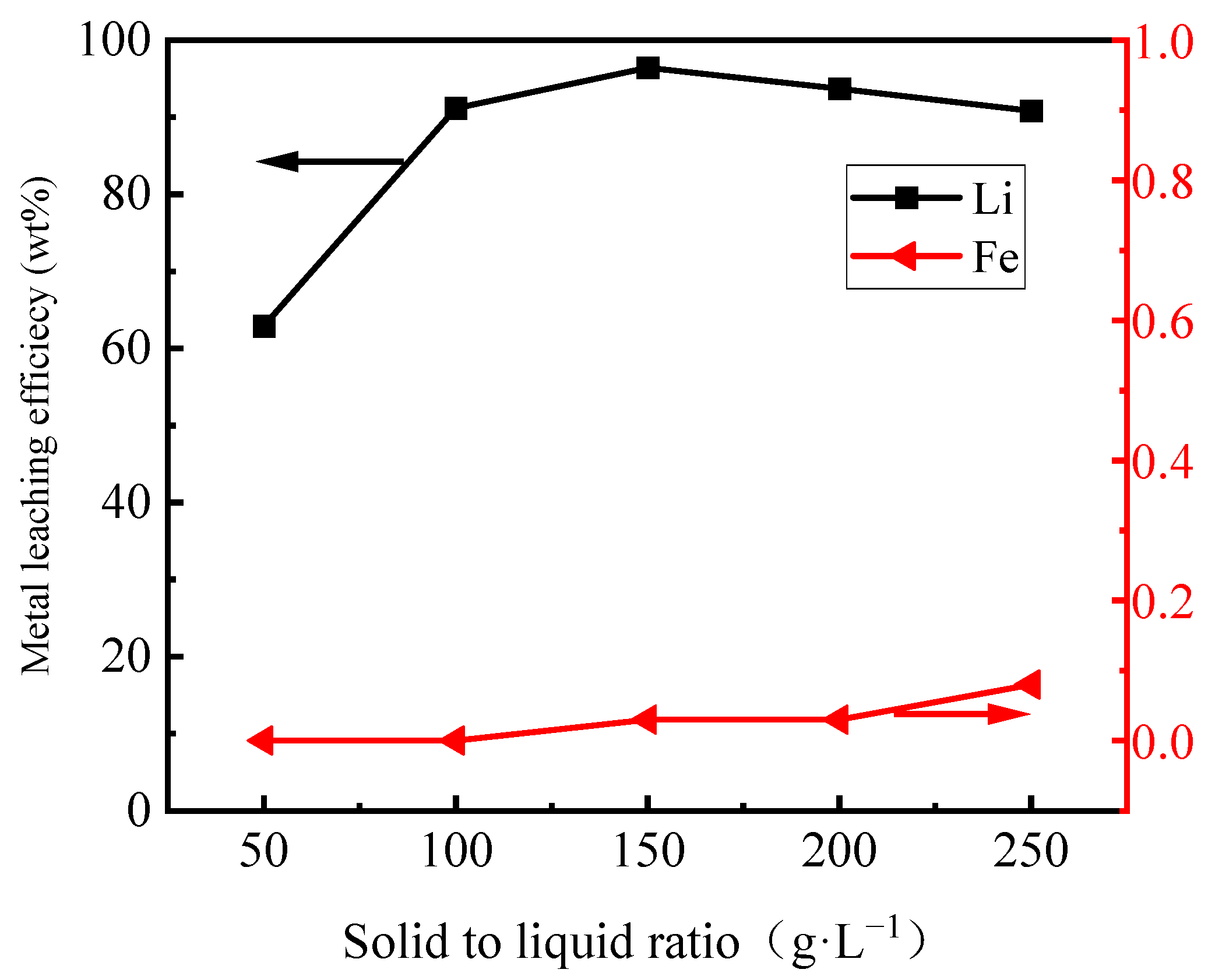
3.2.4. Effect of the Solid-to-Liquor (S/L) Ratio
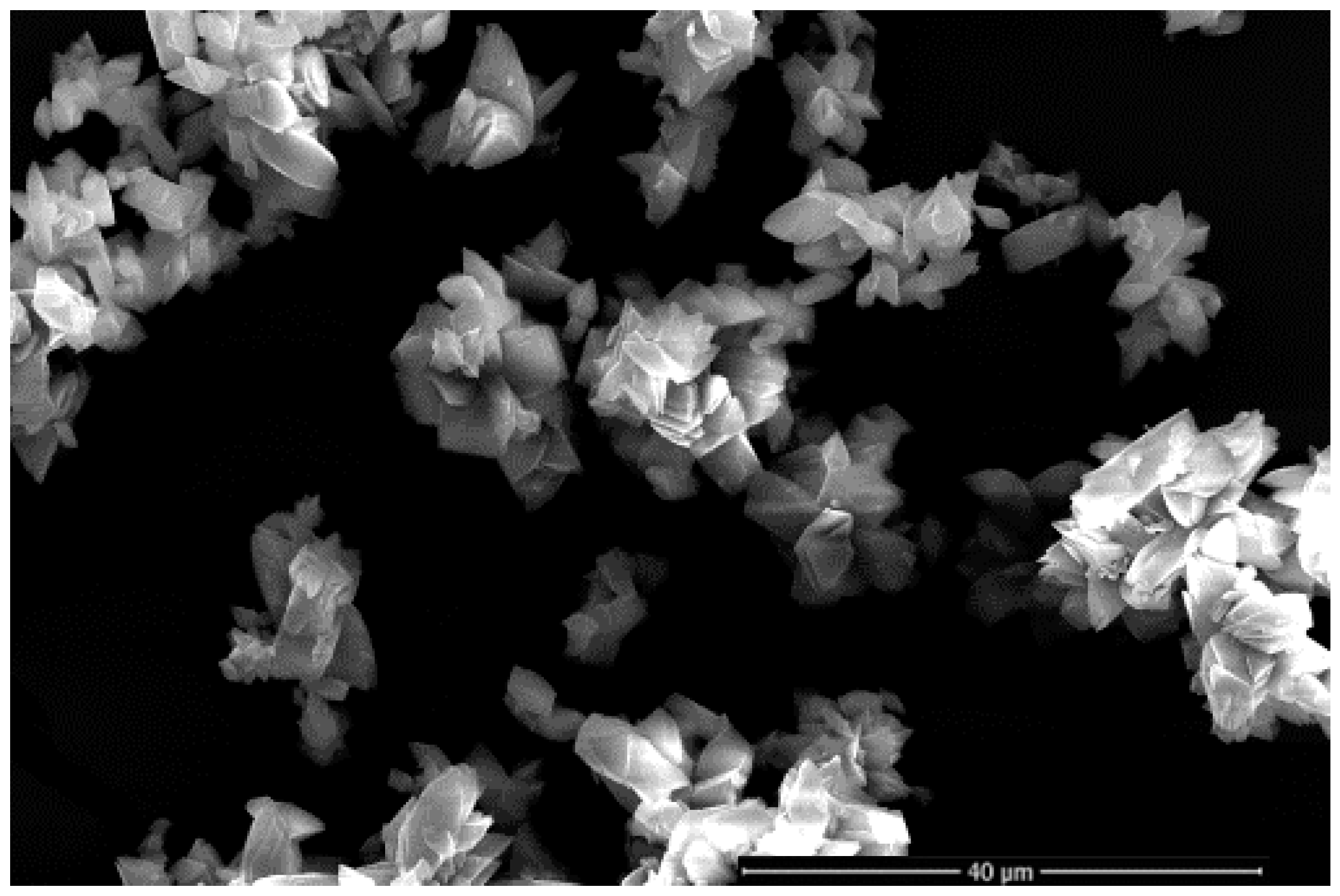
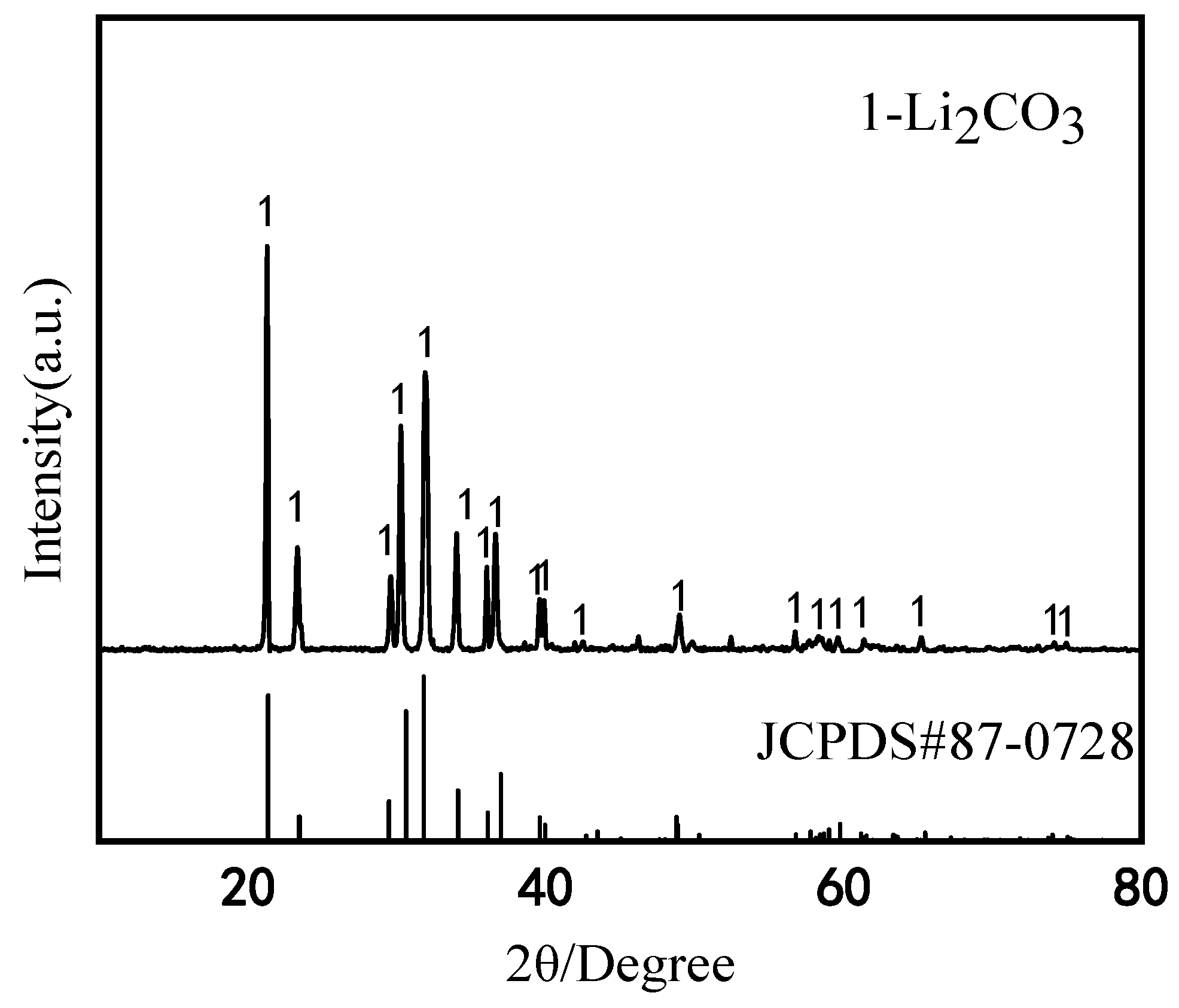
3.3. Li2CO3 Products
3.4. Analysis of Leaching Residues
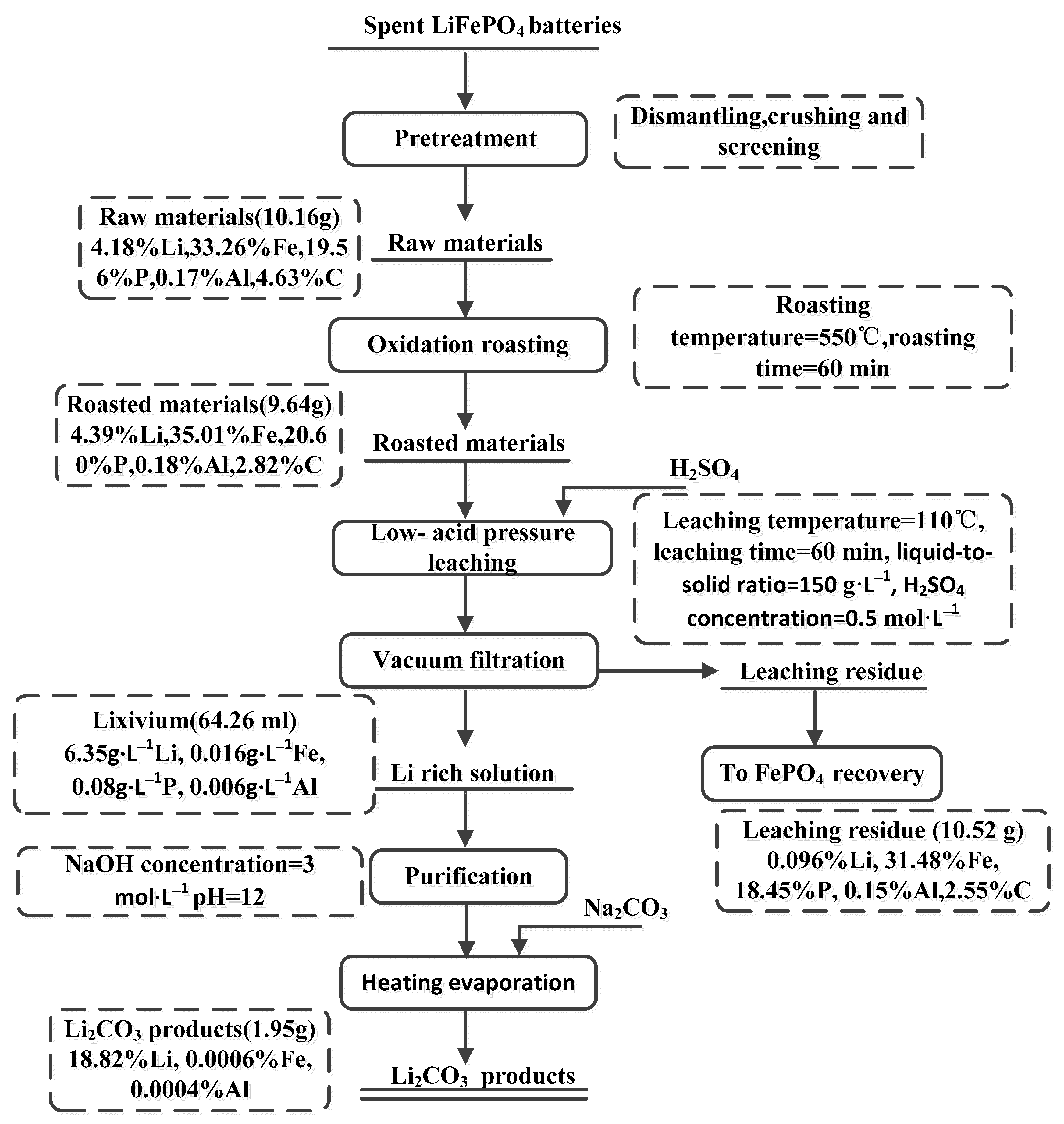
3.5. Flowsheet Development
3.6. The Economic Analysis of the Proposed Process
4. Conclusions
- (1)
- Through the selective recovery of Li and Fe by oxidation roasting following low-acid pressure leaching, the spent LiFePO4 cathode materials were oxidized to Li3Fe2(PO4)3 and Fe2O3, the remaining electrolyte and some of the carbon in the raw materials decomposed, and the selective extraction of Li was achieved by carbonated water leaching. The results showed that it was more effective and efficient in the selective separation of Li and Fe than the conventional method;
- (2)
- Under optimal conditions, by a low-acid pressure-leaching process using 0.5 mol·L−1 H2SO4 at 110 °C with a 150 g·L−1 S/L ratio, >96% Li was extracted, and, within 60 min, the leaching process could be adapted to a wide range of pH values. The reaction temperature played a significant role in the selective recovery of Li and Fe from roasted materials and the phase of FePO4·2H2O in leaching residues;
- (3)
- Oxidation roasting following the pressure-leaching process showed the advantages of higher efficiency, shorter reaction time, and lower acid concentrations needed. Economic and energy consumption analyses clearly show that this recycling process has great potential to become industrially viable. It has very good potential as an alternative process for spent LiFePO4 recycling.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, W.H.; Guo, Y.; Shang, Z.; Zhang, Y.C.; Xu, S.M. A review on comprehensive recycling of spent power lithium-ion battery in China. Transportation 2022, 11, 100155. [Google Scholar] [CrossRef]
- Fan, M.C.; Zhao, Y.; Kang, Y.Q.; Wozny, J.; Liang, Z.; Wang, J.X.; Zhou, G.M.; Li, B.H.; Tavajohi, N.; Kang, F.Y. Room-temperature extraction of individual elements from charged spent LiFePO4 batteries. Rare Met. 2022, 41, 1595–1604. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Yao, Y.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. A green and effective room-temperature recycling process of LiFePO4 cathode materials for lithium-ion batteries. Waste Manag. 2019, 85, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Zhang, R.; Wang, Y.; Shu, H. Hydrothermal preparation and performance of LiFePO4 by using Li3PO4 recovered from spent cathode scraps as Li source. Waste Manag. 2018, 78, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Aravindan, V. Burgeoning prospects of spent lithium-ion batteries in multifarious applications. Adv. Energy Mater. 2018, 8, 1802303. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of spent lithium-ion battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Wu, J.; Mackenzie, A.; Sharma, N. Recycling lithium-ion batteries: Adding value with multiple lives. Green Chem. 2020, 22, 2244–2254. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.; Zhou, M.; Wang, Q. A redox targeting-based material recycling strategy for spent lithium ion batteries. Energy Environ. Sci. 2019, 12, 2672–2677. [Google Scholar] [CrossRef]
- Song, X.; Hu, T.; Liang, C.; Long, H.L.; Zhou, L.; Song, W.; You, L.; Wu, Z.S.; Liu, J.W. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method. RSC Adv. 2017, 7, 4783. [Google Scholar] [CrossRef]
- Wang, L.H.; Li, J.; Zhou, H.M.; Huang, Z.Q.; Tao, S.D.; Zhai, B.K.; Liu, L.Q.; Hu, L.S. Regeneration cathode material mixture from spent lithium iron phosphate batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 9283–9290. [Google Scholar] [CrossRef]
- Li, X.L.; Zhang, J.; Song, D.W.; Song, J.S.; Zhang, L.Q. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Sources 2017, 345, 78–84. [Google Scholar] [CrossRef]
- Li, H.; Xing, S.; Liu, Y.; Li, F.; Guo, H.; Kuang, G. Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system. ACS Sustain. Chem. Eng. 2017, 5, 8017–8024. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, L.; Wang, W.; Liu, Y.; Ma, Q.; Mu, D.; Li, R.; Dai, C. Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Adv. 2016, 6, 43613–43625. [Google Scholar] [CrossRef]
- Yang, Y.X.; Zheng, X.H.; Cao, H.B.; Zhao, C.L.; Lin, X.; Ning, P.G.; Zhang, Y.; Jin, W.; Sun, Z. A closed-loop process for selective metal recovery from spent lithium iron phosphate batteries through mechanochemical activation. ACS Sustain. Chem. Eng. 2017, 5, 9972–9980. [Google Scholar] [CrossRef]
- Yan, T.T.; Zhong, S.W.; Zhou, M.M.; Guo, X.M.; Hu, J.W.; Wang, F.F.; Zeng, F.T.; Zuo, S.C. High efficiency method for recycling lithium from spent LiFePO4 cathode. Nanotechnol. Rev. 2020, 9, 1586–1593. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, J.L.; Yang, C.; Ma, L.L.; Chen, Y.Q.; Wang, C.Y. Selective recovery of lithium from spent LiFePO4 battery via a self-catalytic air oxidation method. Chem. Eng. J. 2023, 460, 141805. [Google Scholar] [CrossRef]
- Liu, K.; Wang, M.M.; Zhang, Q.Z.; Xu, Z.B.; Labianca, C.; Komarek, M.; Gao, B.; Tsang, C.W. A perspective on the recovery mechanisms of spent lithium iron phosphate cathode materials in different oxidation environments. J. Hazard. Mater. 2023, 445, 130502. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, J.L.; Wang, D.D.; Jing, Q.K.; Chen, Y.Q.; Wang, C.Y. Facile and efficient recovery of lithium from spent LiFePO4 batteries via air oxidation–water leaching at room temperature. Green Chem. 2022, 24, 152. [Google Scholar] [CrossRef]
- Zhang, B.L.; Qu, X.; Chen, X.; Liu, D.X.; Zhao, Z.Q.; Xie, H.W.; Wang, D.H.; Yin, H.Y. A sodium salt-assisted roasting approach followed by leaching for recovering spent LiFePO4 batteries. J. Hazard. Mater. 2022, 424, 127586. [Google Scholar] [CrossRef]
- Yang, Y.X.; Meng, X.Q.; Cao, H.B.; Lin, X.; Liu, C.M.; Sun, Y.; Zhang, Y.; Sun, Z. Selective recovery of lithium from spent lithium iron phosphate batteries: A sustainable process. Green Chem. 2018, 20, 3121–3133. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Zhang, X.; Bian, Y.; Xue, Q.; Wu, J.; Wu, F.; Chen, R. Selective recovery of Li and Fe from spent lithium-ion batteries by an environmentally friendly mechanochemical approach. ACS Sustain. Chem. Eng. 2018, 6, 11029–11035. [Google Scholar] [CrossRef]
- Peng, C.; Liu, F.P.; Wang, Z.; Wilson, B.P.; Lundström, M. Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. J. Power Sources 2019, 415, 179–188. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, Z.D.; Hua, D.; Gua, H.N.; Wang, N. Theoretical-molar Fe3+ recovering lithium from spent LiFePO4 batteries: An acid-free, efficient, and selective process. J. Hazard. Mater. 2020, 396, 122707. [Google Scholar] [CrossRef]
- He, K.; Zhang, Z.Y.; Zhang, F.S. A green process for phosphorus recovery from spent LiFePO4 batteries by transformation of delithiated LiFePO4 crystal into NaFeS2. J. Hazard. Mater. 2020, 395, 122614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Hu, J.T.; Liu, Y.B.; Jing, Q.K.; Yang, C.; Chen, Y.Q.; Wang, C.Y. Sustainable and facile method for the selective recovery of lithium from cathode scrap of spent LiFePO4 batteries. ACS Sustain. Chem. Eng. 2019, 7, 5626–5631. [Google Scholar] [CrossRef]
- Wang, Y.Q.; An, N.; Wen, L.; Wang, L.; Jiang, X.T.; Hou, F.; Yin, Y.X.; Liang, J. Recent progress on the recycling technology of Li-ion batteries. J. Energy Chem. 2021, 55, 391–419. [Google Scholar] [CrossRef]
- Liu, K.; Tan, Q.Y.; Liu, L.L.; Li, J.H. Acid-Free and Selective Extraction of Lithium from Spent Lithium Iron Phosphate Batteries via a Mechanochemically Induced Isomorphic Substitution. Environ. Sci. Technol. 2019, 53, 9781–9788. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, L.L.; Tan, Q.Y.; Li, J.H. Selective extraction of lithium from a spent lithium iron phosphate battery by mechanochemical solid-phase oxidation. Green Chem. 2021, 23, 1344–1352. [Google Scholar] [CrossRef]
- Jie, Y.F.; Yang, S.H.; Shi, P.F.; Chang, D.; Fang, G.; Mo, C.X.; Ding, J.; Liu, Z.Q.; Lai, Y.Q.; Chen, Y.M. Thermodynamic Analysis and Experimental Investigation of Al and F Removal from Sulfuric Acid Leachate of Spent LiFePO4 Battery Powder. Metals 2021, 11, 1641. [Google Scholar] [CrossRef]
- Bi, H.J.; Zhu, H.B.; Zu, L.; Gao, Y.; Gao, S.; Peng, J.L.; Li, H.B. Low-temperature thermal pretreatment process for recycling inner core of spent lithium iron phosphate batteries. Waste Manag. Res. 2020, 39, 146–155. [Google Scholar] [CrossRef]
- Lin, E.Y.; Rahmawati, A.; Ko, J.H.; Liu, J.C. Extraction of yttrium and europium from waste cathode-ray tube (CRT) phosphor by subcritical water. Sep. Purif. Technol. 2018, 192, 166–175. [Google Scholar] [CrossRef]
- Jie, Y.F.; Yang, S.H.; Li, Y.; Zhao, D.Q.; Lai, Y.Q.; Chen, Y.M. Oxidizing Roasting Behavior and Leaching Performance for the Recovery of Spent LiFePO4 Batteries. Minerals 2020, 10, 949. [Google Scholar] [CrossRef]
- Jie, Y.F.; Yang, S.H.; Li, Y.; Hu, F.; Zhao, D.Q.; Chang, D.; Lai, Y.Q.; Chen, Y.M. Waste Organic Compounds Thermal Treatment and Valuable Cathode Materials Recovery from Spent LiFePO4 Batteries by Vacuum Pyrolysis. ACS Sustain. Chem. Eng. 2020, 8, 19084–19095. [Google Scholar] [CrossRef]
- Liu, F.P.; Porvalib, A.; Wang, J.L.; Wang, H.Q.; Peng, C.; Wilsonb, B.P.; Lundström, M. Recovery and separation of rare earths and boron from spent Nd-Fe-B magnets. Miner. Eng. 2020, 145, 106097. [Google Scholar] [CrossRef]
- SAC. Methods for Chemical Analysis of Lithium Carbonate, Lithium Hydroxide Monohydrate and Lithium Chloride; Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Xiao, C.; Zeng, L.; Li, Y.B.; Xiao, L.S. Thermodynamic analysis on removing iron by phosphate precipitation. Chin. J. Nonferrous Met. 2018, 28, 637–643. [Google Scholar]
- Jing, Q.K.; Zhang, J.L.; Liu, Y.B.; Yang, C.; Ma, B.Z.; Chen, Y.Q.; Wang, C.Y. E-pH Diagrams for the Li-Fe-P-H2O System from 298 to 473 K: Thermodynamic Analysis and Application to the Wet Chemical Processes of the LiFePO4 Cathode Material. J. Phys. Chem. C 2019, 123, 14207–14215. [Google Scholar] [CrossRef]
- Guo, X.; Cao, X.; Huang, G.; Tian, Q.; Sun, H. Recovery of lithium from the effluent obtained in the process of spent lithium-ion batteries recycling. J. Environ. Manag. 2017, 198, 84–89. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Lou, W.B.; Zhang, Y.; Zhang, Y.; Zheng, S.L.; Sun, P.; Wang, X.J.; Qiao, S.; Li, J.Z.; Zhang, Y.; Liu, D.Y.; et al. A facile way to regenerate FePO4∙2H2O precursor from spent lithium iron phosphate cathode powder: Spontaneous precipitation and phase transformation in an acidic medium. J. Alloys Compd. 2021, 856, 158148. [Google Scholar] [CrossRef]







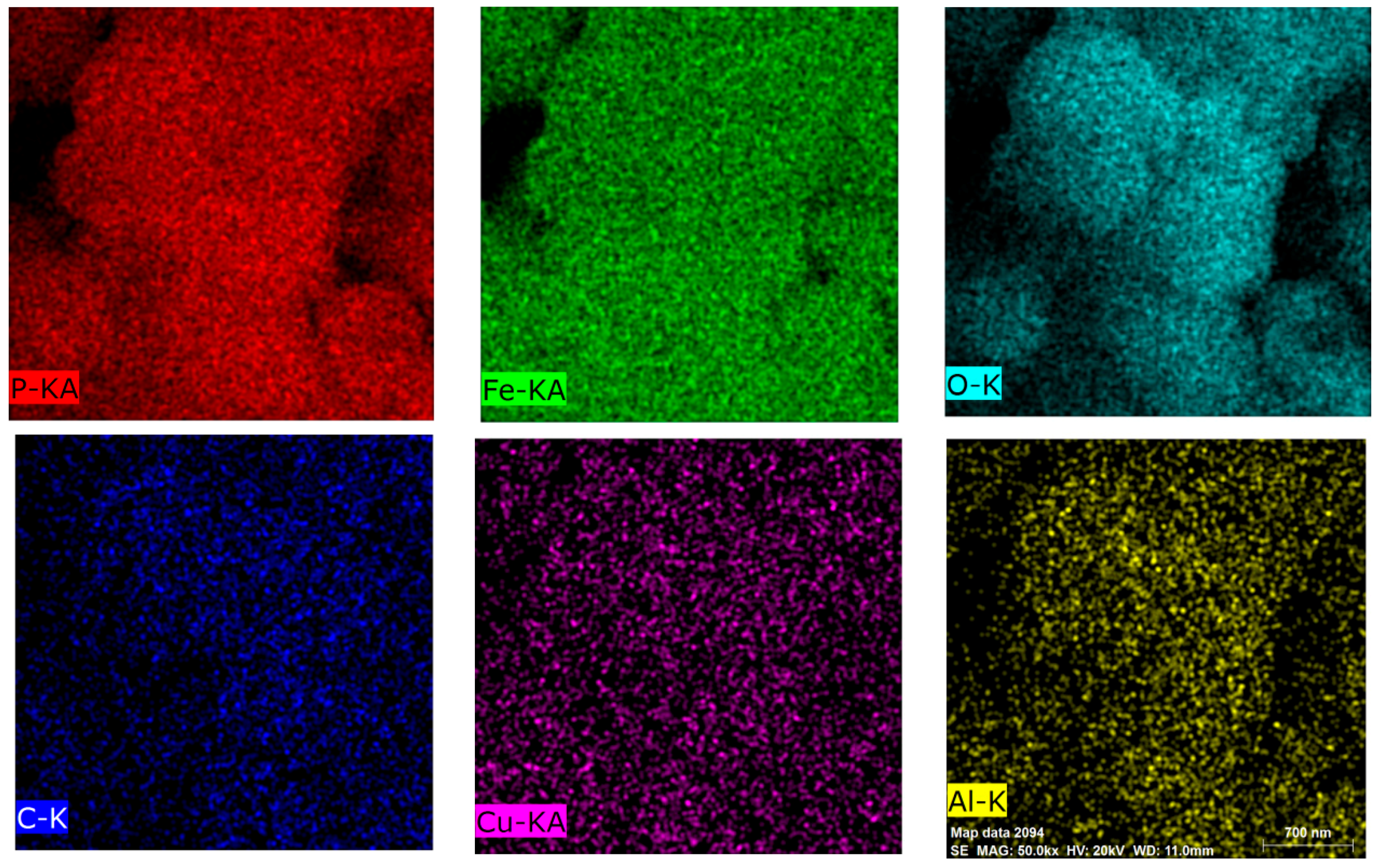
| Elements | Li | Fe | C | P | Cu | Al |
|---|---|---|---|---|---|---|
| wt % | 4.18 | 33.26 | 4.63 | 19.56 | <0.001 | 0.17 |
| Element | Li | Fe | Cu | Al | Mg | Ca |
| Standard Values | 99.5 | 0.001 | 0.0003 | 0.001 | 0.008 | 0.02 |
| Measured Values | 99.7 | 0.0006 | 0.0002 | 0.0004 | - | - |
| Element | Na | K | Pb | SO42− | Cl− | Hydrochloric Acid Insoluble Matter |
| Standard Values | 0.04 | 0.01 | 0.002 | 0.08 | 0.003 | 0.05 |
| Measured Values | 0.03 | - | - | 0.05 | - | 0.01 |
| Samples Description and Units of Elemental Assay | Li | Fe | Al | P | C | Sample Mass (g) or Volume (mL) |
|---|---|---|---|---|---|---|
| Raw materials (%w/w) | 4.18 | 33.26 | 0.17 | 19.56 | 4.63 | 10.16 |
| Roasted materials (%w/w) | 4.39 | 35.01 | 0.18 | 20.60 | 2.82 | 9.64 |
| Lixivium(g·L−1) | 6.35 | 0.016 | 0.006 | 0.08 | - | 64.26 |
| Li2CO3 products (%w/w) | 18.82 | 0.0006 | 0.0004 | - | - | 1.95 |
| Leaching residue (%w/w) | 0.096 | 31.48 | 0.15 | 18.45 | 2.55 | 10.52 |
| Substance | Price (USD/kg−1) | Consumption (kg) | Economic Benefits (USD) |
|---|---|---|---|
| Raw materials | 3.83 | 500 | −1915 |
| H2SO4 | 0.041 | 162.72 | −6.671 |
| NaOH | 0.82 | 0.47 | −0.3854 |
| Na2CO3 | 0.38 | 36.5 | −13.87 |
| Leaching residue | 0.547 | 517.71 | 283.19 |
| Li2CO3 products | 25.86 | 95.96 | 2481.52 |
| Process | Price (USD/kWh) | Energy Cost (kWh) | Total Price (USD) |
|---|---|---|---|
| Oxidation roasting | 0.109 | 750 | 81.75 |
| Low-acid pressure leaching | 0.109 | 562.5 | 61.3125 |
| Filtering, drying, and sieving | 0.109 | 357.5 | 38.9675 |
| Purification and evaporation | 0.109 | 240 | 26.16 |
| Precipitation | 0.109 | 175 | 19.075 |
| Total | 2085 | 227.265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Shen, C.; Liu, F.; Wang, J. Selective Separation and Recovery of Li from Spent LiFePO4 Cathode Materials by Oxidation Roasting Followed by Low-Acid Pressure Leaching. Metals 2023, 13, 1884. https://doi.org/10.3390/met13111884
Chen Z, Shen C, Liu F, Wang J. Selective Separation and Recovery of Li from Spent LiFePO4 Cathode Materials by Oxidation Roasting Followed by Low-Acid Pressure Leaching. Metals. 2023; 13(11):1884. https://doi.org/10.3390/met13111884
Chicago/Turabian StyleChen, Zaoming, Changquan Shen, Fupeng Liu, and Jinliang Wang. 2023. "Selective Separation and Recovery of Li from Spent LiFePO4 Cathode Materials by Oxidation Roasting Followed by Low-Acid Pressure Leaching" Metals 13, no. 11: 1884. https://doi.org/10.3390/met13111884
APA StyleChen, Z., Shen, C., Liu, F., & Wang, J. (2023). Selective Separation and Recovery of Li from Spent LiFePO4 Cathode Materials by Oxidation Roasting Followed by Low-Acid Pressure Leaching. Metals, 13(11), 1884. https://doi.org/10.3390/met13111884





