Pt and Al Recovery from a Spent Pt/Al2O3 Catalyst via an Integrated Soda Roasting–Alkaline Leaching–Carbonization Process
Abstract
:1. Introduction
2. Experimental Work
2.1. Materials and Reagents
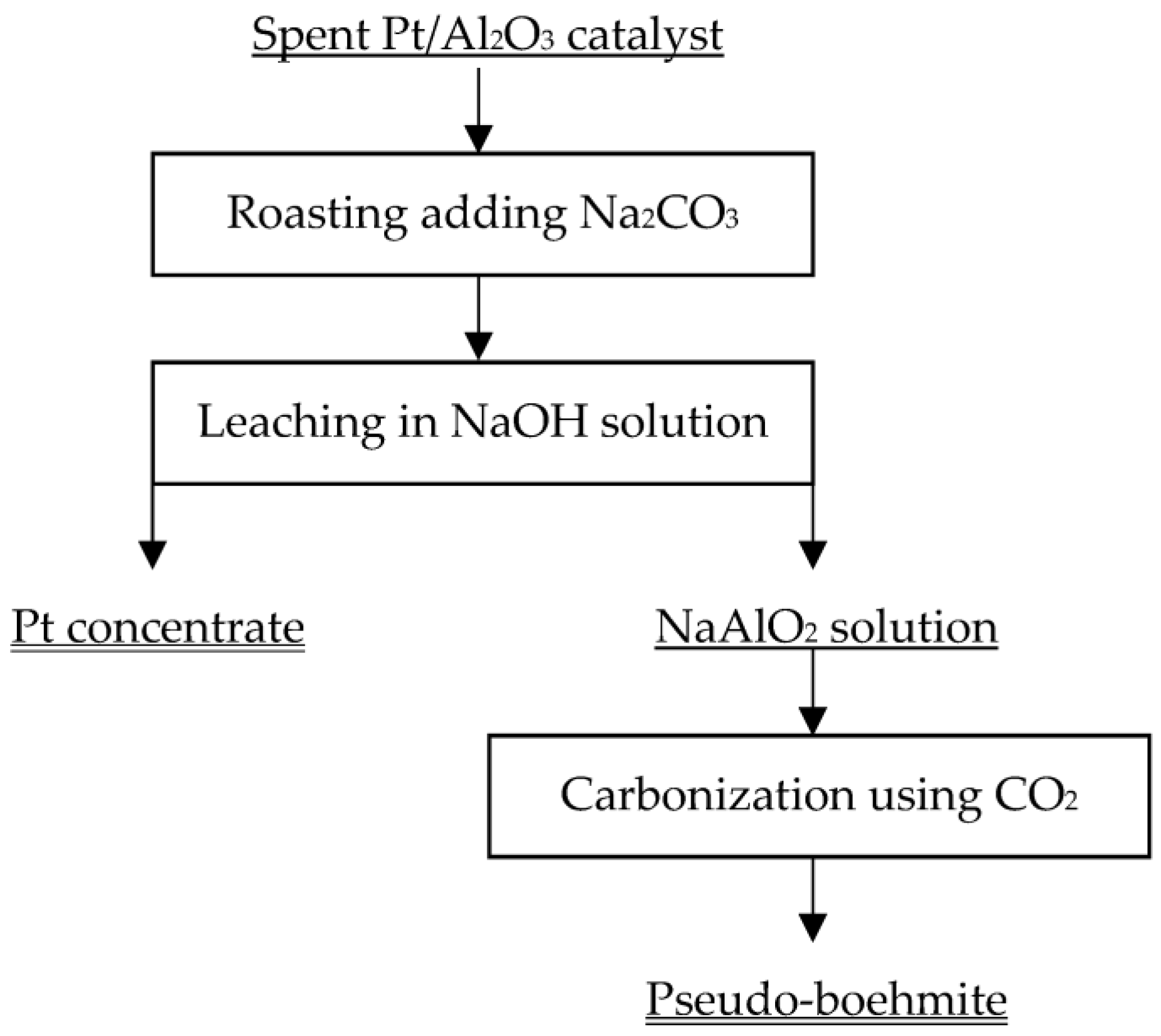
2.2. Experimental Methods
2.3. Analytical Methods
3. Results and Discussion
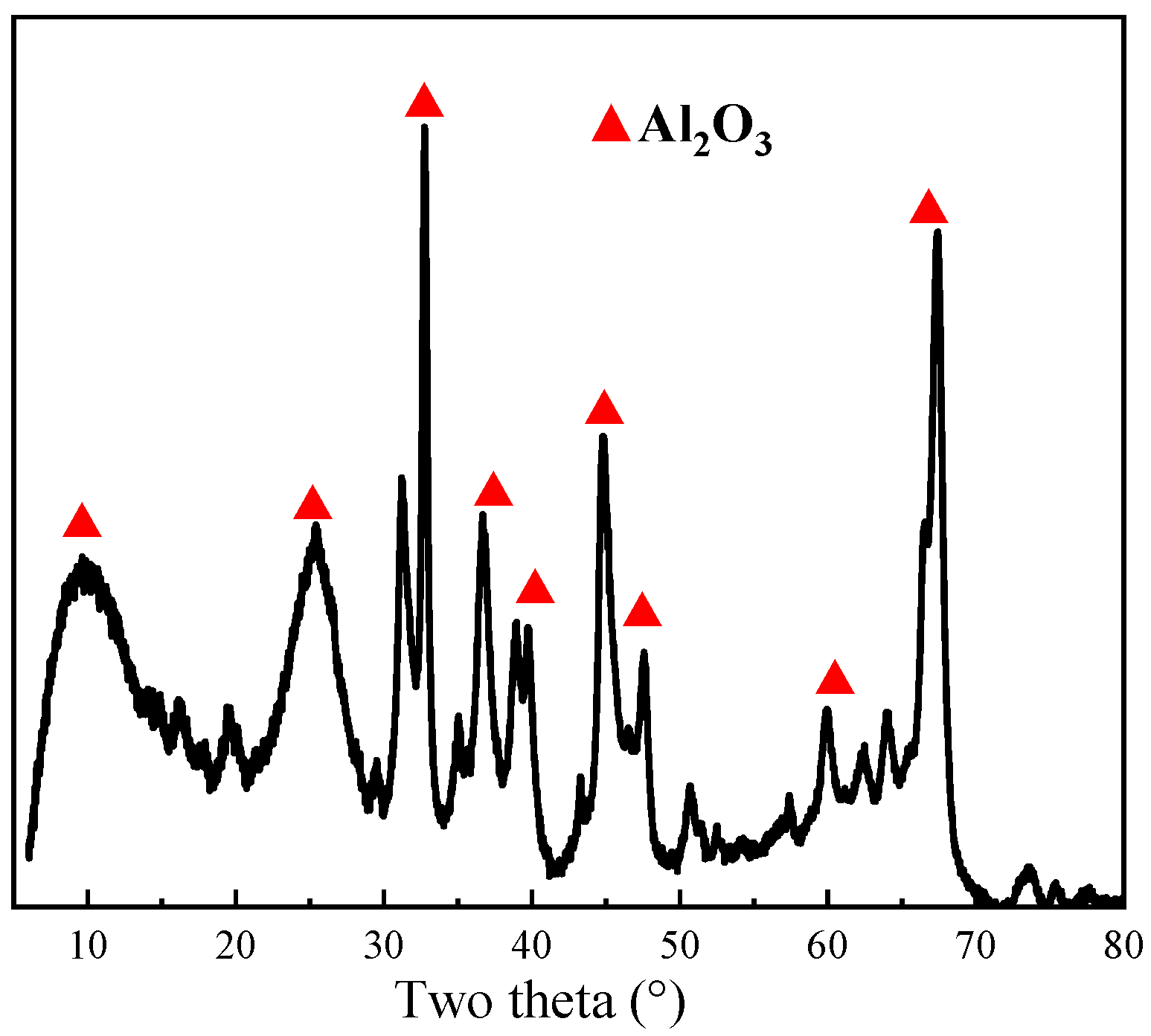
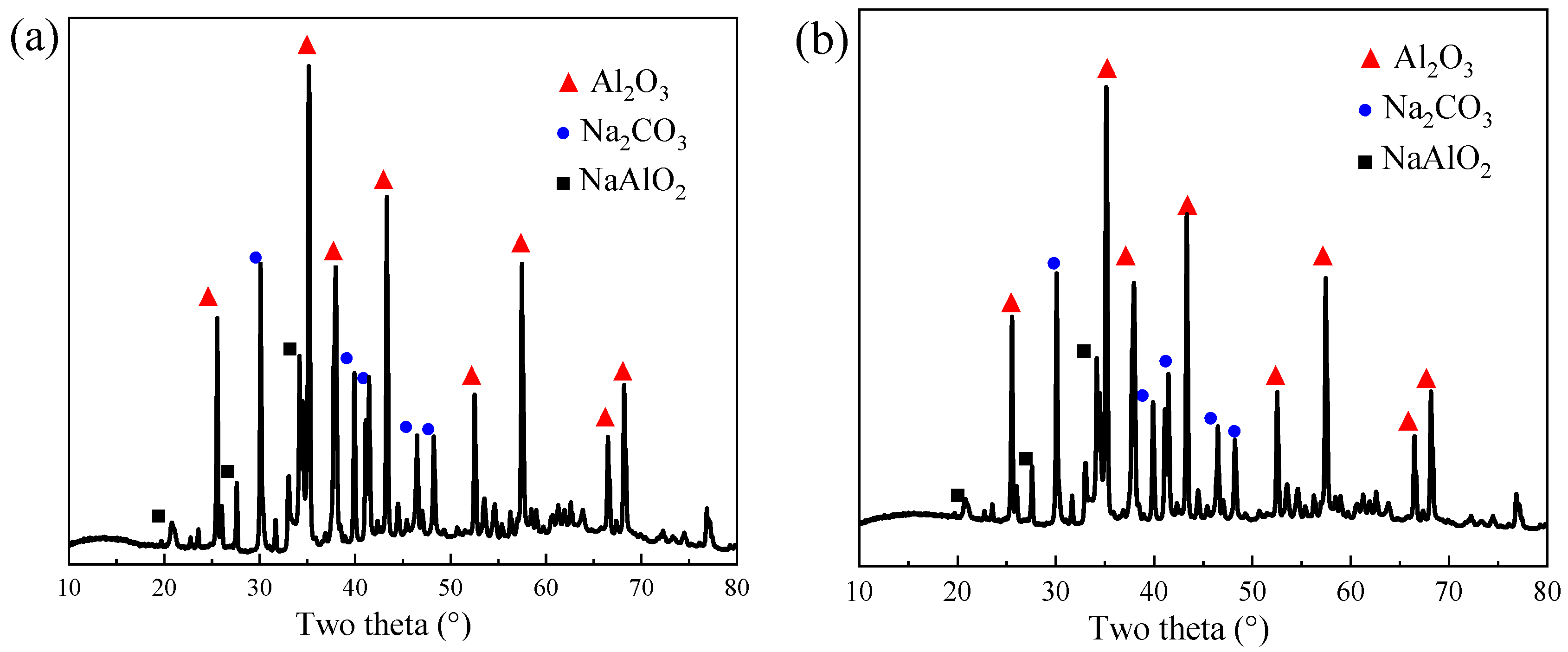
3.1. Soda Roasting of Spent Pt/Al2O3 Catalyst
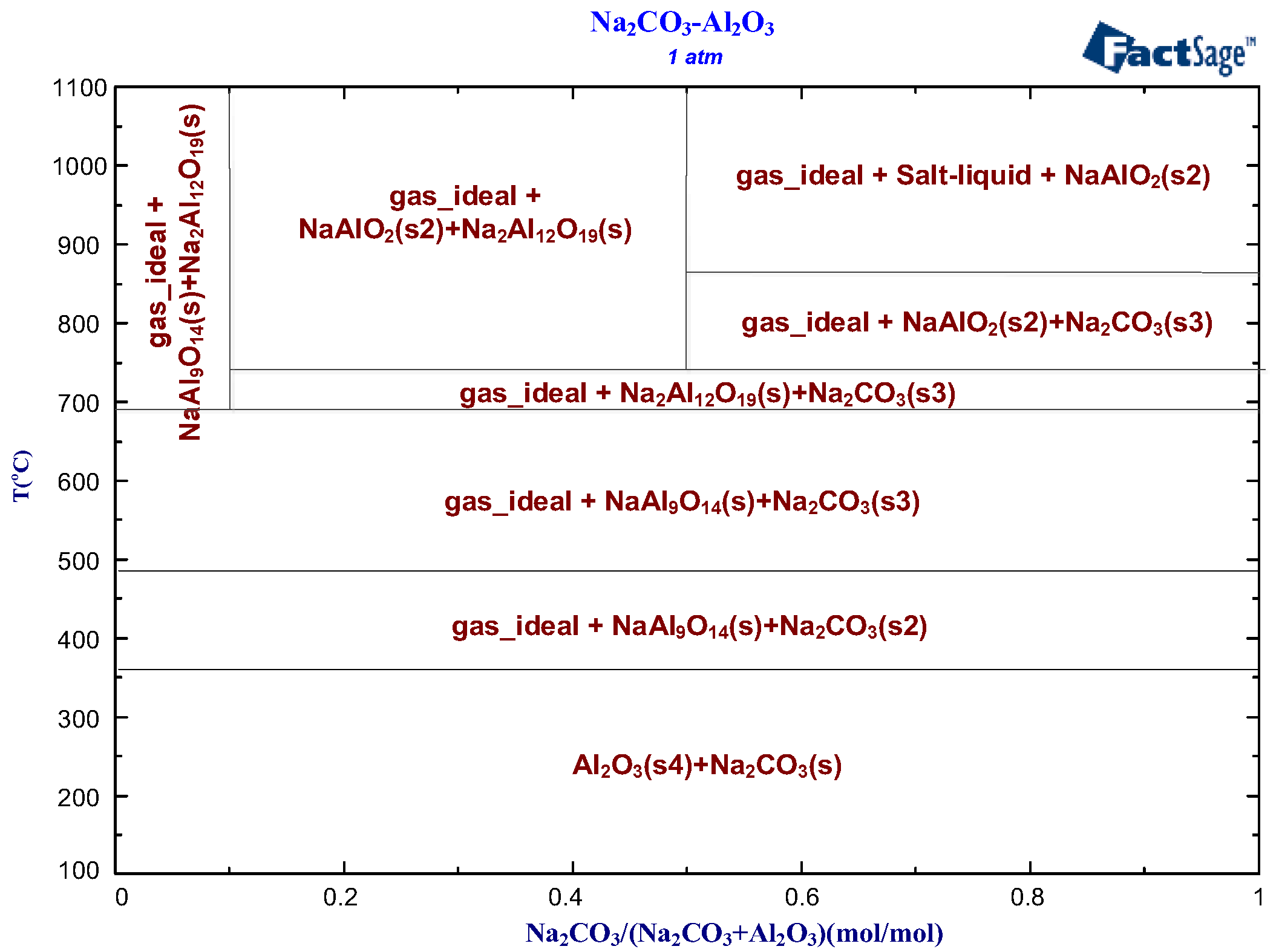
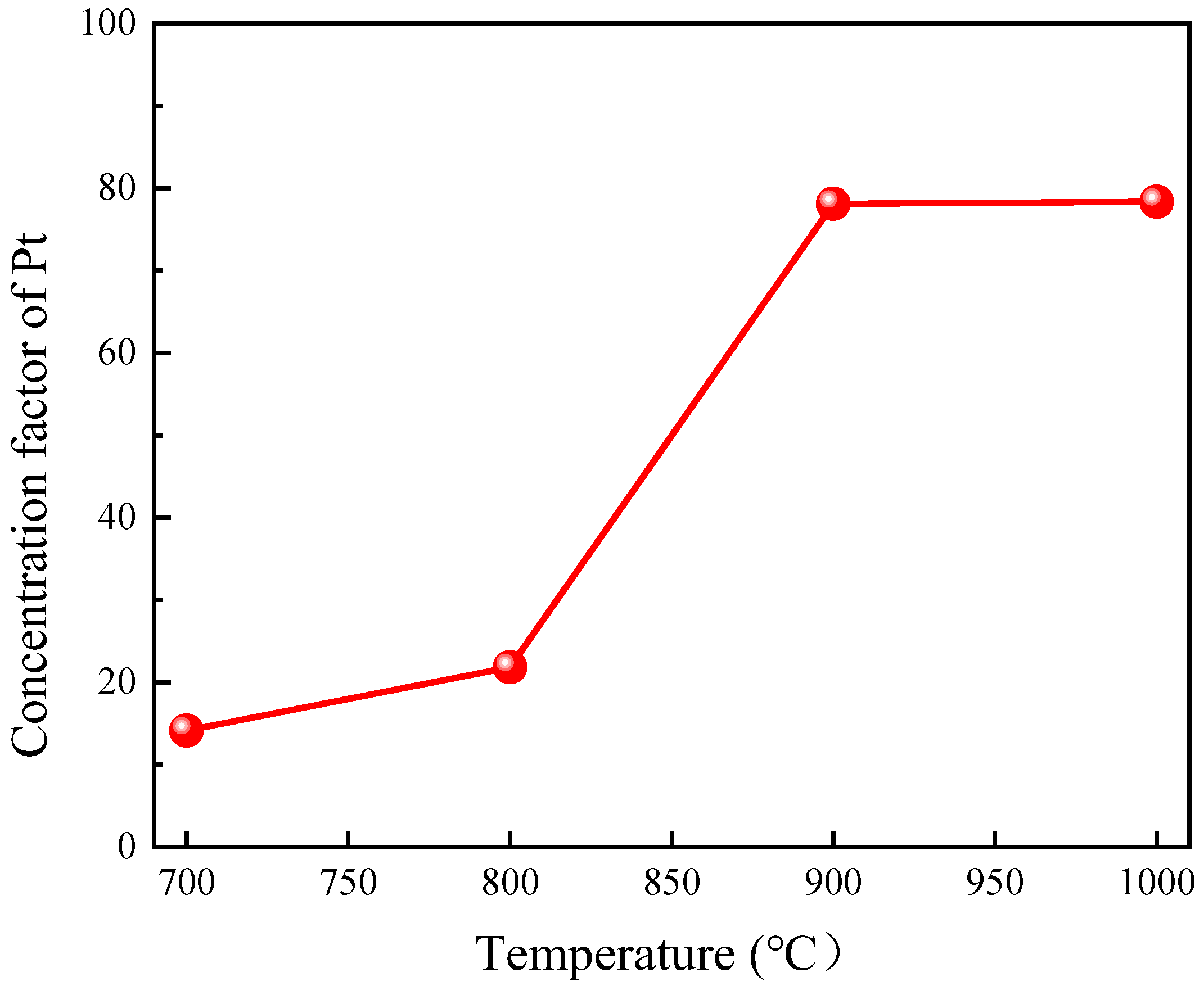
3.1.1. Effect of Roasting Temperature
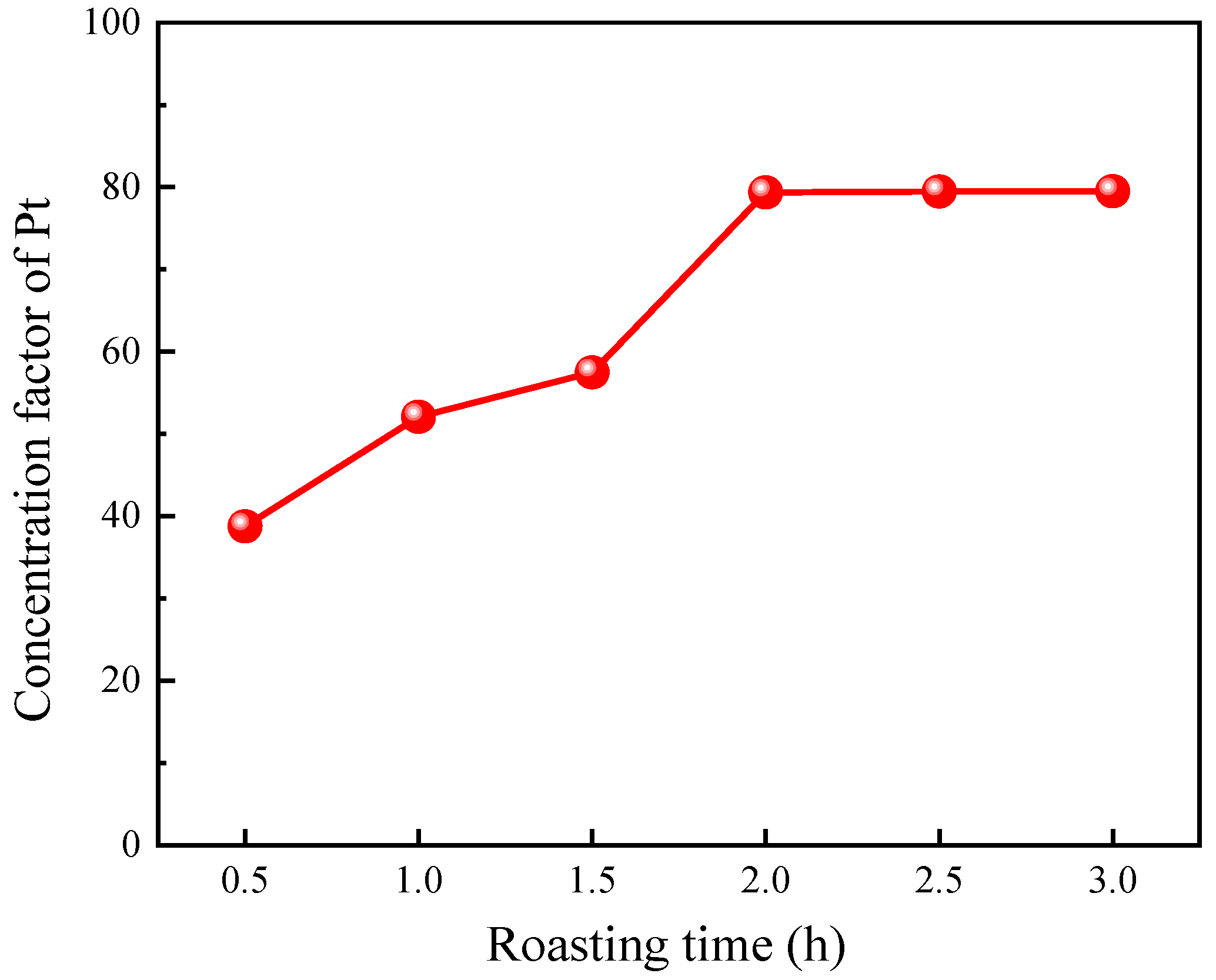
3.1.2. Effect of Roasting Time
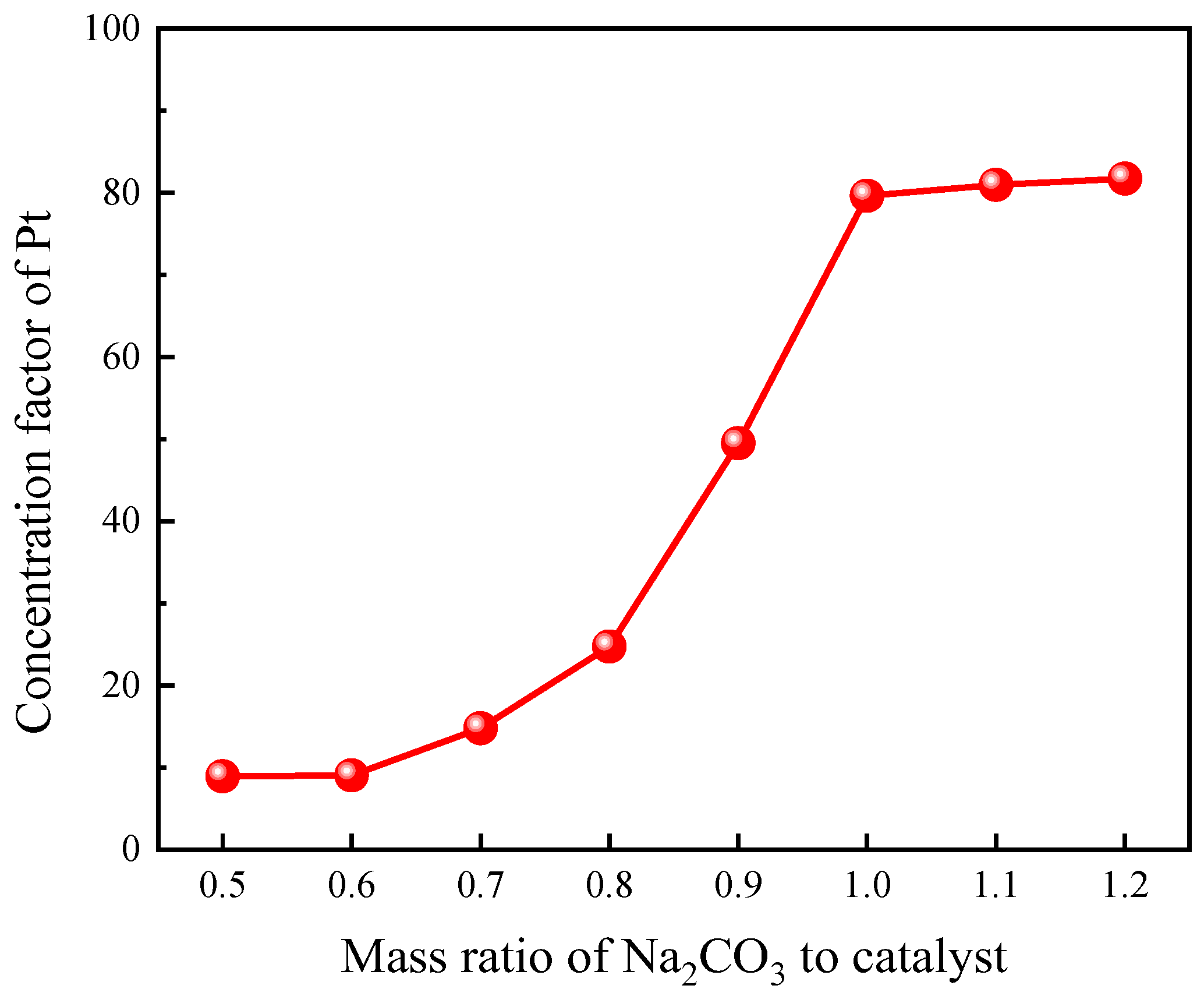
3.1.3. Effect of Na2CO3 Dosage
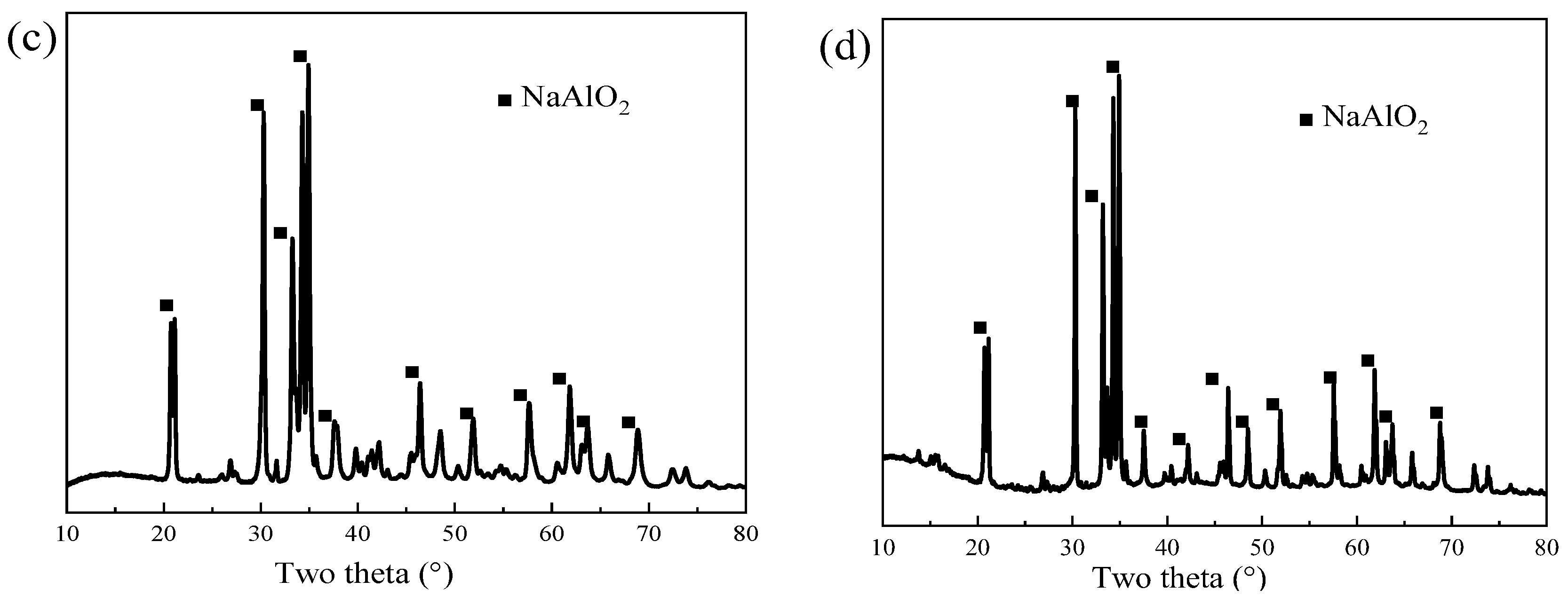
3.2. Alkaline Leaching of Roasted Residue
3.2.1. Effect of Leaching Temperature
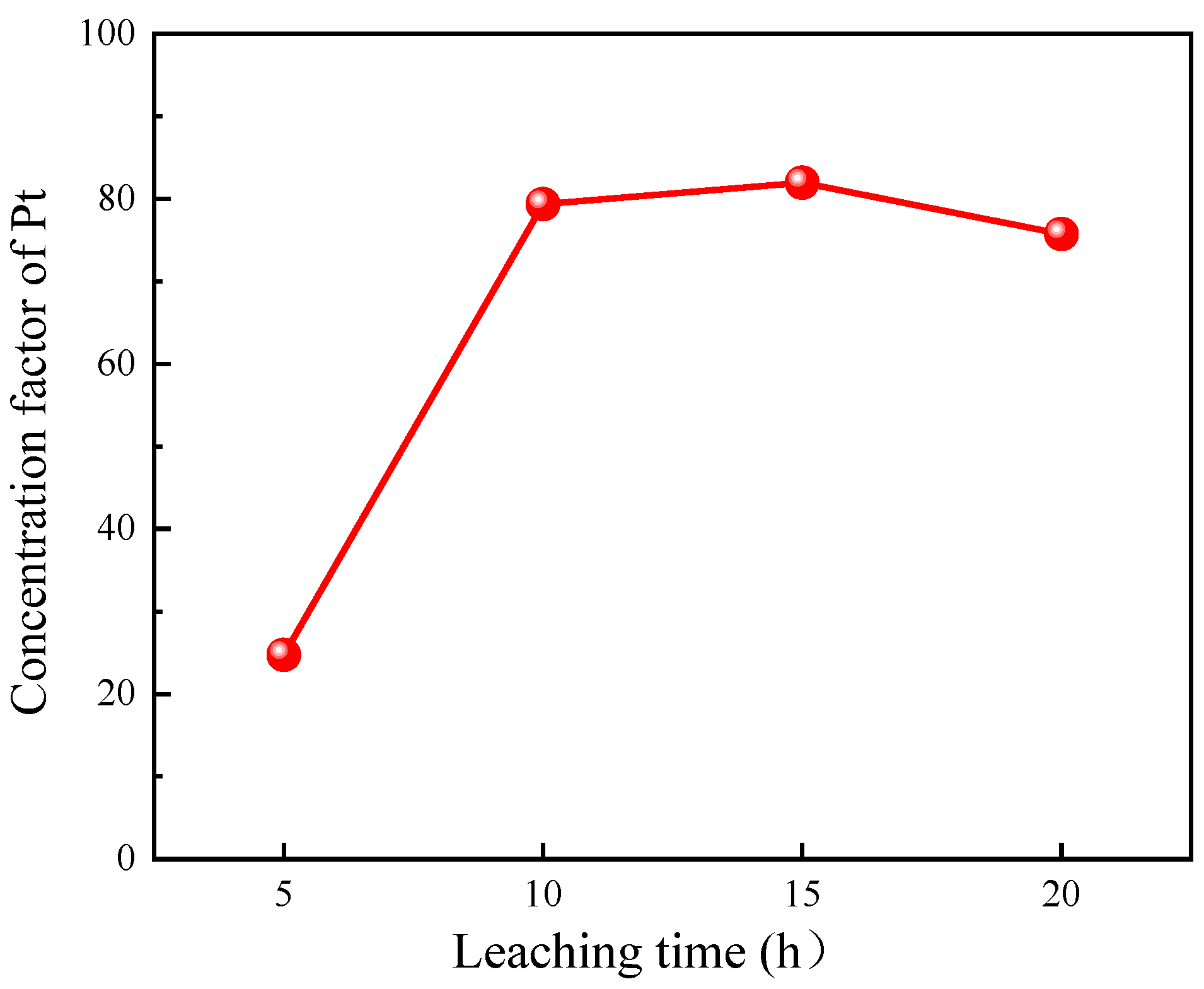
3.2.2. Effect of Leaching Time
3.2.3. Effect of Liquid-to-Solid Ratio
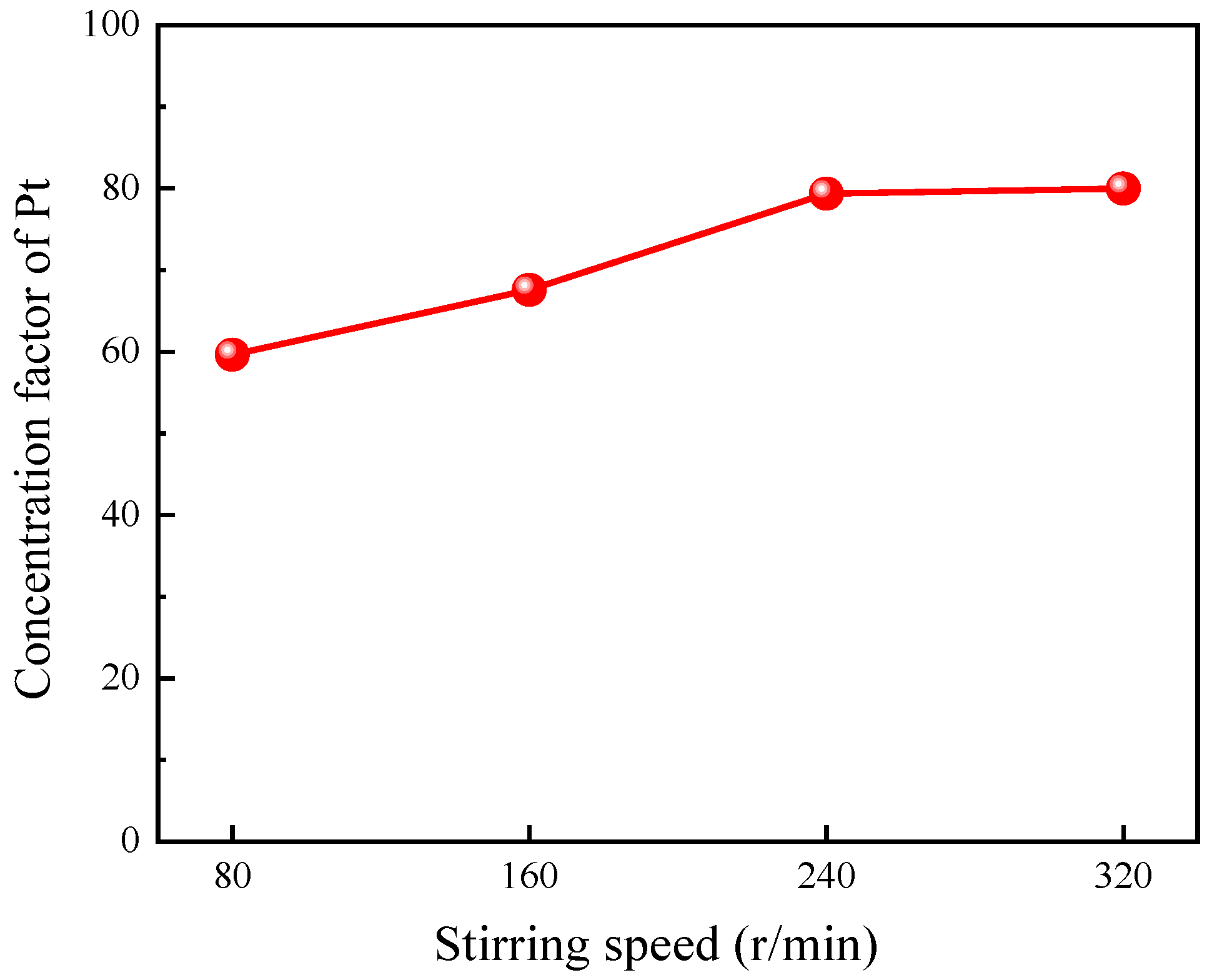
3.2.4. Effect of Stirring Speed
3.2.5. Effect of NaOH Concentration
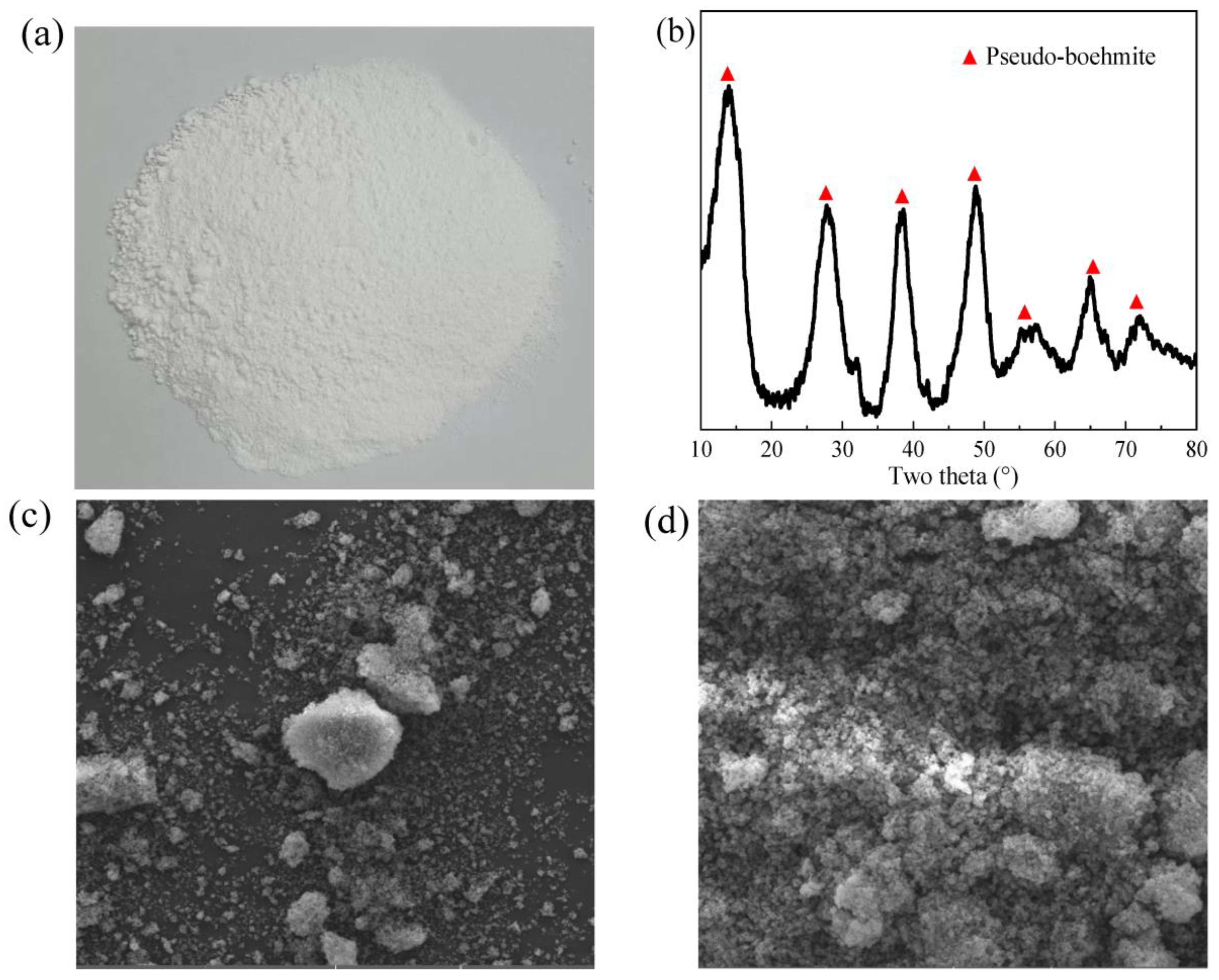
3.3. Pseudo-Boehmite Preparation with Leached Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vincenzo, P.; Concetta, R.; Marta, C.; Simona, R.; Eugenio, M.; Giovanni, F.; Marco, M. Platinum based catalysts in the water gas shift reaction: Recent advances. Metals 2020, 10, 866–932. [Google Scholar]
- Andrey, Y.; Sai, K.P.; Srecko, S.; Dominic, F.; Dmitriy, V.; Bernd, F.; Peter, P. Towards understanding the cathode process mechanism and kinetics in Molten LiF–AlF3 during the treatment of spent Pt/Al2O3 catalysts. Metals 2021, 11, 1431–1444. [Google Scholar]
- Ashvin, L.K.; Renu, S.; Parivesh, C.; Prakash, D.V. Syngas production by carbon dioxide reforming of methane over Pt/Al2O3 and Pt/ZrO2-SiO2 catalysts. Chem. Eng. Sci. 2022, 249, 117347. [Google Scholar]
- Xu, C.; Liu, Y.L.; Singh, B.; Yi, S.Y.; Qin, G.W.; Li, S. Interface modulation of Pt/Al2O3 catalyst and their roles in thermal stability. Surf. Interfaces 2022, 33, 102276. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhou, Y.L.; Qiao, B.T.; Pan, X.L.; Wang, C.J.; Cao, L.R.; Li, L.; Lin, J.; Wang, X.D. Enhanced stability of Pt/Al2O3 modified by Zn promoter for catalytic dehydrogenation of ethane. J. Energy Chem. 2020, 51, 14–20. [Google Scholar] [CrossRef]
- Rohini, S.Z.; Prakash, D.V. Hydrogen production by aqueous-phase reforming of macroalgal biomass using a Pt Al2O3 catalyst. Ind. Eng. Chem. Res. 2023, 62, 17451–17460. [Google Scholar]
- Xie, S.H.; Zhang, X.; Xu, P.; Hatcher, B.; Liu, Y.X.; Ma, L.; Ehrlich, S.N.; Hong, S.; Liu, F.D. Effect of surface acidity modulation on Pt/Al2O3 single atom catalyst for carbon monoxide oxidation and methanol decomposition. Catal. Today 2022, 402, 149–160. [Google Scholar] [CrossRef]
- Abo Atia, T.; Wouters, W.; Monforte, G.; Spooren, J. Microwave chloride leaching of valuable elements from spent automotive catalysts: Understanding the role of hydrogen peroxide. Resour. Conserv. Recy. 2021, 166, 105349. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Paukszta, D.; Regel-Rosocka, M. Hydrometallurgical recovery of platinum group metals from spent automotive converters. Physicochem. Probl. Miner. Process. 2021, 57, 83–94. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, T.; Xu, B.; Li, Q.; Yang, Y.B. Gold recovery from pregnant thiosulfate solution by ion exchange resin: Synergistic desorption behaviors and mechanisms. Sep. Purif. Technol. 2023, 323, 12448. [Google Scholar] [CrossRef]
- Dong, Z.L.; Jiang, T.; Xu, B.; Wu, J.T.; Li, Q.; Yang, Y.B. A comparative study of electrodeposition and sodium dithionite reduction for recovering gold in gold-rich solution from the adsorption of thiosulfate solution by ion exchange resin. Sep. Purif. Technol. 2024, 328, 125053. [Google Scholar] [CrossRef]
- Jha, M.K.; Lee, J.C.; Kim, M.S.; Jeong, J.; Kim, B.S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. [Google Scholar] [CrossRef]
- Ding, Y.J.; Zheng, H.D.; Zhang, S.G.; Liu, B.; Wu, B.Y.; Jian, Z.M. Highly efficient recovery of platinum, palladium, and rhodium from spent automotive catalysts via iron melting collection. Resour. Conserv. Recycl. 2020, 155, 104644. [Google Scholar] [CrossRef]
- Grumett, P. Precious metal recovery from spent catalysts. Platin. Met. Rev. 2003, 47, 162–166. [Google Scholar]
- Marinho, R.S.; da Silva, C.N.; Afonso, J.C.; da Cunha, J.W. Recovery of platinum, tin and indium from spent catalysts in chloride medium using strong basic anion exchange resins. J. Hazard. Mater. 2011, 192, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- De Sá Pinheiro, A.A.; de Lima, T.S.; Campos, P.C.; Afonso, J.C. Recovery of platinum from spent catalysts in a fluoride-containing medium. Hydrometallurgy 2004, 74, 77–84. [Google Scholar] [CrossRef]
- Sun, P.P.; Lee, M.S. Separation of Pt from hydrochloric acid leaching solution of spent catalysts by solvent extraction and ion exchange. Hydrometallurgy 2011, 110, 91–98. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Zhang, Z.H. Extraction of platinum from waste Pt-Al2O3 catalysts with NaClO3 oxidation. Precious Met. 2006, 27, 6–9. [Google Scholar]
- Kim, M.S.; Kim, E.Y.; Jeong, J.; Lee, J.C.; Kim, W. Recovery of platinum and palladium from the spent petroleum catalysts by substrate dissolution in sulfuric acid. Mater. Trans. 2010, 51, 1927–1933. [Google Scholar] [CrossRef]
- Barakat, M.A.; Mahmoud, M.H.H. Recovery of platinum from spent catalyst. Hydrometallurgy 2004, 72, 179–184. [Google Scholar] [CrossRef]
- Jafarifar, D.; Daryanavard, M.R.; Sheibani, S. Ultrafast microwave-assisted leaching for recovery of platinum from spent catalyst. Hydrometallurgy 2005, 78, 166–171. [Google Scholar] [CrossRef]
- Fu, J.G. Recovering platinum in spent catalyst from petrol reforming. China Nonferr. Metall. 2006, 2, 43–45. [Google Scholar]
- Ding, Y.J.; Zheng, X.Y.; Wu, B.Y.; Liu, B.; Zhang, S.G. Highly porous ceramics production using slags from smelting of spent automotive catalysts. Resour. Conserv. Recycl. 2021, 166, 105373. [Google Scholar] [CrossRef]
- Du, A.R. Research on the Recycling of Aluminum and Remaking Carrier from Alkaline Solution of Spent Pd/Al2O3 Catalysts; Central South University: Changsha, China, 2018. [Google Scholar]
- Zhou, H.D.; Liu, Y.B.; Ma, B.Z.; Wang, C.Y.; Chen, Y.Q. Strengthening extraction of lithium and rubidium from activated α-spodumene concentrate via sodium carbonate roasting. J. Ind. Eng. 2023, 123, 248–259. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.B.; Yang, W.J.; Ma, B.Z.; Chen, Y.Q.; Wang, C.Y. Phase transformation and roasting kinetics of cobalt-rich copper sulfide ore in oxygen atmosphere assisted by sodium sulfate. J. Ind. Eng. 2022, 116, 217–228. [Google Scholar] [CrossRef]
- Ahmad, S.; Sajal, W.R.; Gulshan, F.; Hasan, M.; Rhamdhani, M.A. Thermodynamic analysis of caustic–roasting of electric arc furnace dust. Heliyon 2022, 8, e11031. [Google Scholar] [CrossRef]
- Li, S.Y.; Bo, P.H.; Kang, L.W.; Guo, H.G.; Gao, W.Y.; Qin, S.J. Activation pretreatment and leaching process of high-alumina coal fly ash to extract lithium and aluminum. Metals 2020, 10, 893. [Google Scholar] [CrossRef]
- Liang, X.; Tang, J.J.; Li, L.S.; Wu, Y.S. Recovery of valuable metals from spent Al2O3-based catalysts by sodium carbonate roasting and water leaching. JOM 2023, 75, 4689–4700. [Google Scholar] [CrossRef]
- Lv, H.; Xie, M.Z.; Wu, Z.G.; Li, L.L.; Yang, R.J.; Han, J.S.; Liu, F.Q.; Zhao, H.L. Effective extraction of the Al element from secondary aluminum dross using a combined dry pressing and alkaline roasting process. Materials 2022, 15, 5686. [Google Scholar] [CrossRef]



| Element | Pt | Al2O3 | Fe2O3 | K2O | S | C |
|---|---|---|---|---|---|---|
| Content (%) | 0.37 | 88.00 | 2.00 | 1.80 | 0.30 | 6.50 |
| Element | Na | Al | Si | Fe | Ca |
|---|---|---|---|---|---|
| Content (mg·L−1) | 36,999 | 13,184 | 130 | 0.04 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Zhang, C.; Zhao, J.; Wu, Y.; Dong, Z.; Li, Q. Pt and Al Recovery from a Spent Pt/Al2O3 Catalyst via an Integrated Soda Roasting–Alkaline Leaching–Carbonization Process. Metals 2023, 13, 1944. https://doi.org/10.3390/met13121944
Dong H, Zhang C, Zhao J, Wu Y, Dong Z, Li Q. Pt and Al Recovery from a Spent Pt/Al2O3 Catalyst via an Integrated Soda Roasting–Alkaline Leaching–Carbonization Process. Metals. 2023; 13(12):1944. https://doi.org/10.3390/met13121944
Chicago/Turabian StyleDong, Haigang, Chunxi Zhang, Jiachun Zhao, Yuedong Wu, Zhonglin Dong, and Qian Li. 2023. "Pt and Al Recovery from a Spent Pt/Al2O3 Catalyst via an Integrated Soda Roasting–Alkaline Leaching–Carbonization Process" Metals 13, no. 12: 1944. https://doi.org/10.3390/met13121944
APA StyleDong, H., Zhang, C., Zhao, J., Wu, Y., Dong, Z., & Li, Q. (2023). Pt and Al Recovery from a Spent Pt/Al2O3 Catalyst via an Integrated Soda Roasting–Alkaline Leaching–Carbonization Process. Metals, 13(12), 1944. https://doi.org/10.3390/met13121944





