Abstract
The long-period stacking ordered (LPSO) structure, functioning as a strengthening phase in magnesium alloys, plays a pivotal role in compensating for inherent performance limitations. In this study, an as-cast Mg-Gd-Ni-Y alloy, including the LPSO phase, was initially obtained through an ingot metallurgy process. Subsequently, the alloy underwent distinct thermal treatments: annealing at 500 °C for 10 h, and extrusion using an extrusion ratio of 10 at a speed of 5 mm/s. Comparative analysis of the microstructure and corrosion characteristics was performed across these three alloy states. Comprising primarily of α-Mg, LPSO phase, and eutectic structures (ES), the alloy exhibited distinctive microstructural features. Immersion experiments conducted in a 3.5% NaCl solution revealed that the as-cast alloy displayed the highest dissolution rate at various temperatures, from room temperature, to 50 °C, and 70 °C. Following annealing, a reduction in the second phase content within the alloy significantly contributed to the observed decrease in its dissolution rate. Extrusion processes resulted in a denser network structure within the microarchitecture, to some extent impeding the spread of corrosion to some extent. By emloying scanning Kelvin probe force microscopy (SKPFM) and micro-electrochemical testing, it was discerned that predominantly the electrochemical system involving α-Mg and the second phases predominantly dictated the heightened dissolution rate of the alloy. This study presents valuable insights into understanding the dissolution mechanisms and potential strategies for controlling the dissolution performance of magnesium alloys containing the LPSO phase.
1. Introduction
As the lightest engineering structural material, magnesium alloy has the benefits of low density, high specific strength, and good performance in vibration and noise reduction [1,2,3]. With advancements in associated technologies, magnesium alloys have found extensive applications in aerospace, automotive manufacturing, biomedicine, and various other industries [4,5,6]. However, the mechanical characteristics of magnesium alloys, such as strength, ductility and high-temperature creep, have imposed limitations on their broader potential applications [7,8].
In 1994, Luo made a significant discovery by identifying the existence of the long- period stacking order (LPSO) structure while investigating the impact of rare earth elements on the strength of Mg-Zn-Zr alloys [9]. The fundamental structure of the LPSO phase comprises Mg-RE (rare earth element)-X (transition elements such as Zn, Cu, Ni), wherein varying compositions and subsequent heat treatment processes yield diverse morphologies. The arrangement of internal atoms allocates the LPSO phase into structures denoted as 6H, 10H, 14H, 18R, and 24R, with ‘H’ representing a hexagonal lattice and ‘R’ representing a rhombic lattice [10,11]. Notably, the structure of the LPSO phase with the same composition can be altered by heat treatment. For instance, the 18R structure of the LPSO phase will change to a 14H structure after annealing at 773 K [12,13,14,15]. Further investigations have underscored that the LPSO phase can enhance the mechanical properties of magnesium alloys at room and high temperatures through mechanisms such as fine grain strengthening and dispersion strengthening [16,17,18]. This unique attribute significantly enhances the overall utility and performance of magnesium alloys.
As an impurity element in Mg alloys, trace addition of Ni would accelerate the degradation of anti-corrosion performance. Mg2Ni stands out as the primary form of nickel found in magnesium alloys. Studies have demonstrated that as the Ni content increases, there is a corresponding rise in corrosion rates alongside improvements in compressive strength and micro-hardness. This phenomenon is primarily attributed to the augmented presence of secondary phases [19]. Consequently, magnesium alloys incorporating Ni-containing LPSO exhibit accelerated dissolution rates coupled with robust mechanical characteristics, rendering them well-suited for the fabrication of innovative types of fracture-resistant tools.
Ma et al. [20] prepared Mg-Gd-Ni alloys containing the LPSO phase with various morphologies. They investigated the impact of microstructure changes at various extrusion temperatures on the mechanical and degradation properties of the alloys. Their investigation revealed the pivotal role of the LPSO phase as the primary strengthening agent in the alloy. Notably, both bulk-shaped and lamellar configurations of the LPSO phase demonstrated effective contributions to enhancing the material’s strength. The escalated degradation rate observed in the material was attributed to the collective impact of the bulk shaped LPSO phase, lamellar LPSO phase, and the presence of the eutectic phase. Dai et al. [21] investigated the influence of diverse forms and structures of the LPSO phase on its relative corrosion performance. Their findings indicated that with an increase in the LPSO phase content within the material, an early establishment of a corrosion barrier occurred during the dissolution process, consequently reducing the corrosion rate. However, after annealing the network structure comprising the LPSO phase underwent a certain degree of decomposition. Concurrently, the bulk reticular LPSO phase, characterized by specific 18R structures, transformed into a lamellar 14H structure, thereby creating additional paths for corrosion propagation. The synergistic effect of these phenomena ultimately led to an escalation in the corrosion rate. This mechanism has also been validated in various other studies [22]. The delaminated bulk shaped LPSO phase tends to lose its inhibitory effect on corrosion propagation. As the content of the LPSO phase increases, there is a corresponding rise in the proportion of cathode within the electrochemical system, consequently accelerating the overall corrosion process.
Although there have been several studies on the effect of the LPSO phase on the cor-rosion behavior of magnesium alloys, little research has focused on the comparison of different processes (casting, hot working and machining) on the microstructure and cor-rosion behavior of magnesium alloy, thus clarifying the influence of the LPSO phase on the dissolution mechanism of Mg alloy. This study aims to elucidate the influence of the LPSO phase on the dissolution mechanism of Mg alloy. In this paper, Mg-Gd-Ni alloys containing the LPSO phase were synthesized. An in situ corrosion test, in conjunction with a micro-electrochemical method, was conducted to investigate the entire corrosion pro-cess, enabling a detailed discussion of the corrosion mechanism.
2. Materials and Methods
Mg-5Gd-6Ni-1Y (wt.%) alloy was prepared by the casting method using pure Mg, Ni, Mg-Gd and Mg-Y master alloys as raw materials. These materials were melted at 750 °C under the protection of a CO2 and SF6 atmosphere. After melting is completed, the mixture was stirred to ensure uniformity and subsequently held at 720 °C for 20 min. Upon reaching a homogeneous liquid state, the melt was cast into a pre-heated (200 °C) steel mold with an inner diameter of 100 mm upon cooling to 700 °C. An inductively coupled plasma atomic emission spectrometer (ICP-AES, Shimadzu ICPE-9810, Shimadzu, Colombia, MD, USA) was used to determine the precise chemical compositions shown in Table 1. The as-cast alloy material was named 561. Subsequently, two distinct alloys were derived from the ‘561’ as-cast alloy. The first underwent annealing at 500 °C for 10 h, forming the as-annealed alloy (A561). Meanwhile, the second underwent extrusion processing at a ratio of 10 and a speed of 5 mm/s, leading to the creation of the as-extruded alloy (E561).

Table 1.
Chemical compositions of the alloys determined by ICP.
Microstructural characterization was conducted using a Metallographic microscope (OLYMPUS, BX53M, Olympus IMS, Tokyo, Japan), Scanning Electron Microscope (SEM, ZEISS MERLIN COMPACT, UFZ, Leipzig, Germany) equipped with Energy Dispersive Spectroscopy (EDS, Oxford instruments, Abingdon, UK), and Transmission Electron Microscopy (TEM, FEI Talos F200X, Thermo Fisher Scientific, Waltham, MA, USA). Specimens intended for TEM analysis were initially sliced to a thickness of 0.5 mm and subsequently ground down to 200 μm. These slices were then punched to 3 mm in diameter and thinned further using ion polishing techniques (Gatan 691, Gatan, Pleasanton, CA, USA). In order to ascertain the phase compositions of the alloy, XRD equipment (Rigaku SmartLab, Rigaku Corporation, Tokyo, Japan) was used at 45 kV and 200 mA. The scanning step was 2°/min in the range (e.g., 2θ angle) from 20° to 80°.
The corrosion properties of the experimental materials were investigated by immer-sion and electrochemical experiments. Immersion experiments involved subjecting sam-ples sized 20 × 20 × 20 mm to a 3.5 wt.% NaCl solution, whose dissolution rates were meticulously examined at various temperatures, including room temperature, 50 °C, and 70 °C. These experiments provided crucial insights into the material’s response to corro-sive environments across different thermal conditions. Electrochemical experiments were conducted in 3.5 wt.% NaCl solution at room temperature utilizing an electrochemical workstation (GAMRY, Reference 600+, Warminster, PA, USA). A meticulously calibrated standard three-electrode system was established, with a saturated calomel electrode (SCE) as the reference electrode. Open circle potential test (OCP) was carried out for 1800 s., Potentiodynamic polarization was carried out at a scan rate of 1 mV·s−1, at a potential range of −300 mV to +700 mV relative to the OCP. Icorr is calculated according to the fol-lowing formula. These meticulous electrochemical analyses provided comprehensive in-sights into the material’s corrosion behavior and electrochemical response under con-trolled conditions, facilitating a nuanced understanding of its performance in corrosive environments.
An in situ approach was employed to comprehensively study the entire corrosion process of the experimental materials. In order to enhance the observation of the corrosion process, a 0.001% NaCl solution was selected to deliberately decelerate the corrosion rate. The entire sample was fully immersed in the solution to isolate the influence of the liquid film on the corrosion process. The localized volta potential distribution of the sample was scanned and analyzed by the atomic force microscope with a Kelvin probe (AFM-KPFM, Bruker Dimension Icon), and the scan parameters were meticulously set to a scan height and frequency of 80 nm and 0.4 Hz, respectively.
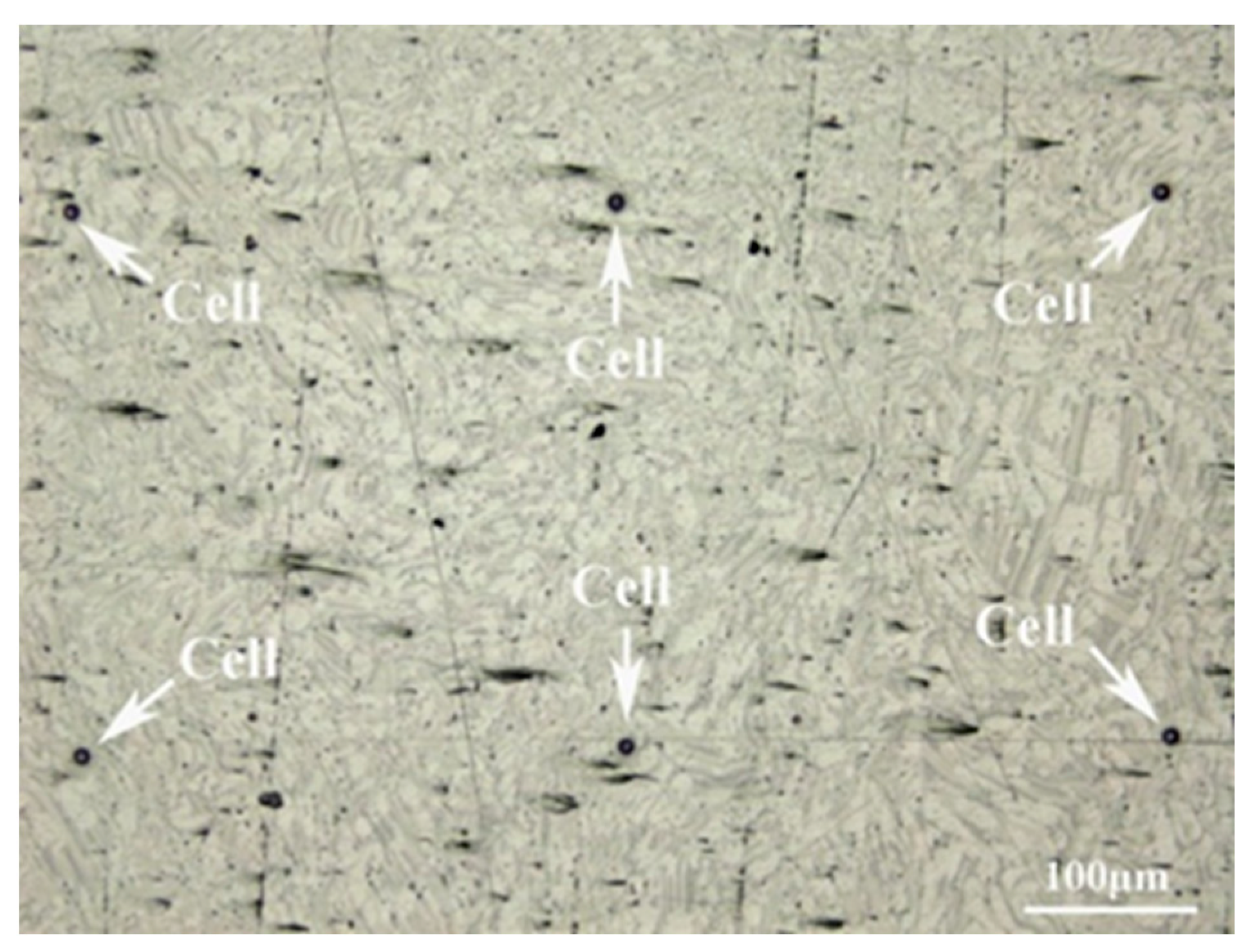
The corrosion mechanism of the sample was studied by micro-electrochemical characterization with an electrochemical workstation (GAMRY, Reference 600+). After being cleaned with ethanol and deionized water, the photoresist was applied to the surface of the polished sample in a specific array using the photolithographic mask technique. The array pattern consisted of holes with a diameter of 10 μm and a pitch of 500 μm, which acted as a part of the micro-electrochemical cell [23,24]. Figure 1 depicts the surface of the treated sample. Since the previous results showed that the experimental material has a high rate of corrosion in NaCl solution, the micro-electrochemical test was adjusted to use a Na2SO4 solution with a concentration of 0.001 mol/L and a silver chloride electrode was utilized. The potentiodynamic polarization was performed over a potential range of −250 mV to +250 mV relative to the OCP at a scan rate of 1 mV·s−1.
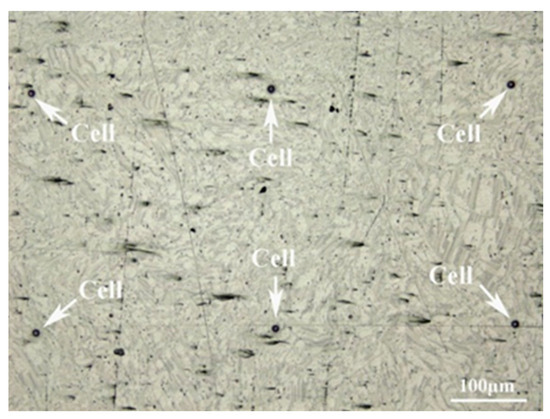
Figure 1.
Optical morphology of the treated sample.
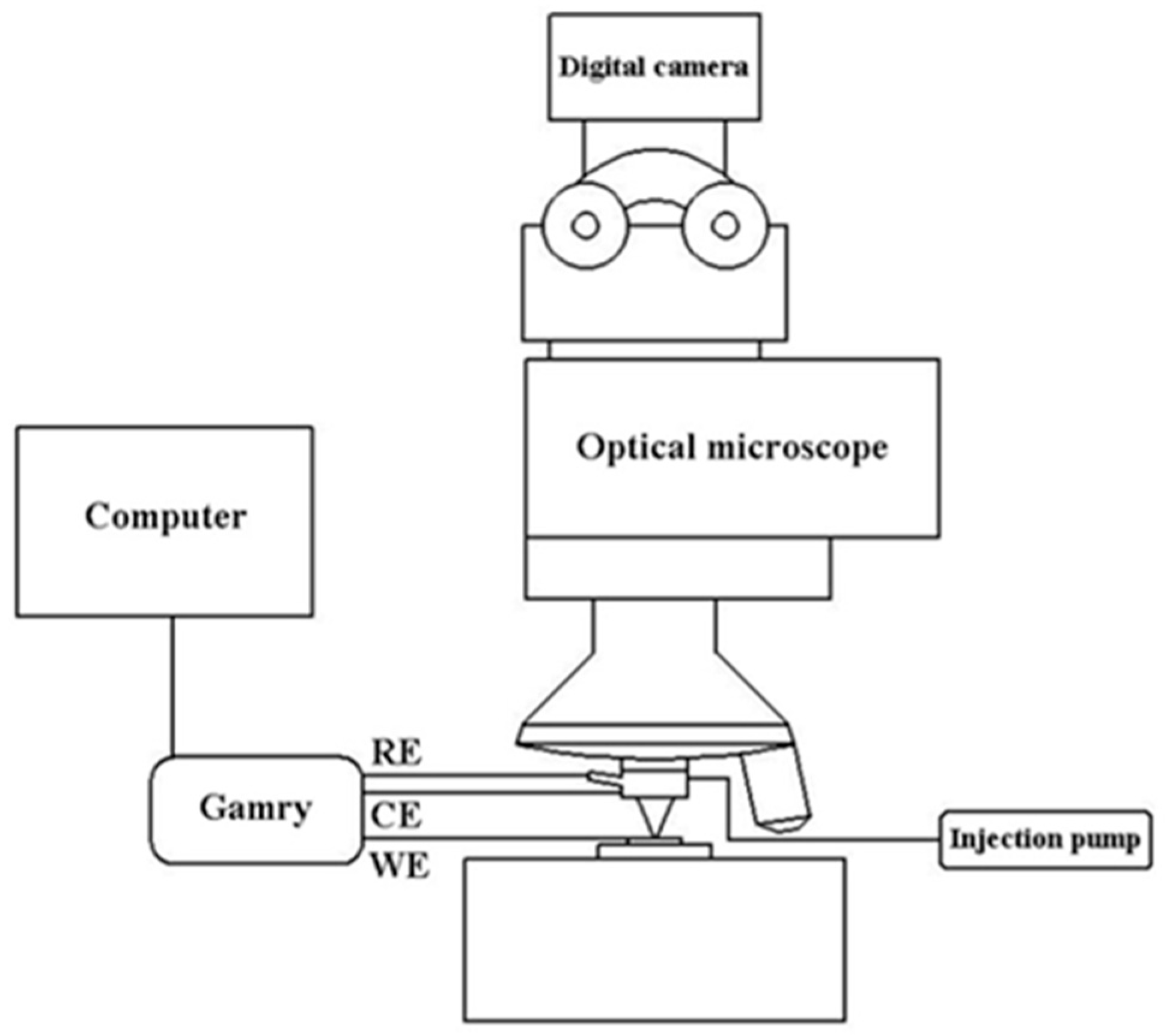
The automated test platform for high throughput microelectrochemical characteriza-tion of metallic materials was developed by Lai [25], and its schematic diagram is shown in Figure 2. First, control the setup by choosing an interesting area with a microscope and an XYZ stage, then inject the desired volume of electrolyte by syringe pump before the working electrode (WE) surface approaches contact with the capillary tip, followed by setting the parameters to start electrochemical tests. Ag-AgCl and Pt electrodes were used as the reference (RE) and counter electrodes (CE), respectively.
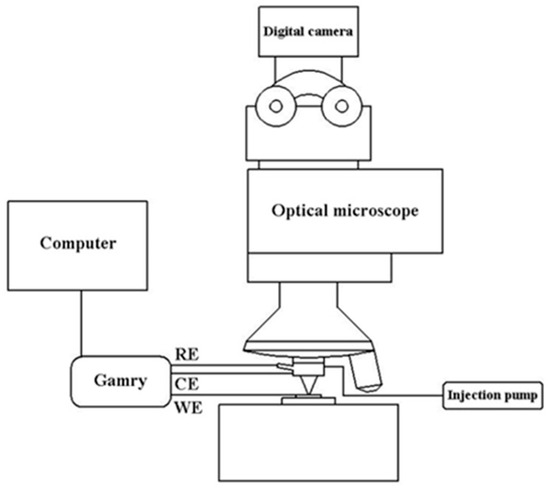
Figure 2.
The schematic diagram of the automated test platform for Micro Electrochemical characterization of metallic materials.
3. Results and Discussion
3.1. Microstructure
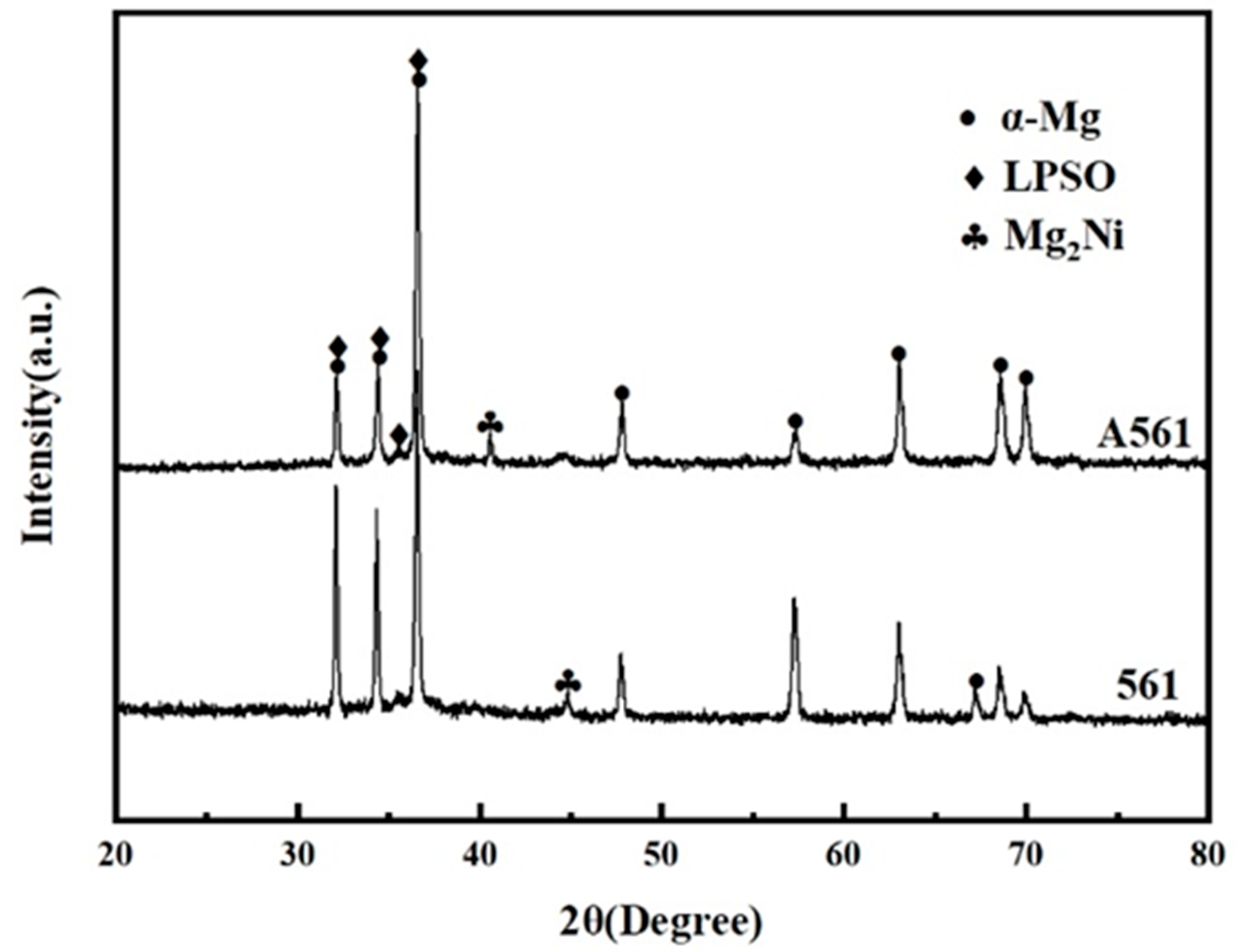
Figure 3 shows the XRD patterns of as-cast and heat-treated Mg-Gd-Ni-Y alloys. Because the diffraction patterns of E561 did not change obviously after extrusion, only the XRD patterns of the two alloys are given here. It can be seen that the alloy is mainly composed of α-Mg, Mg2Ni and the LPSO phase.
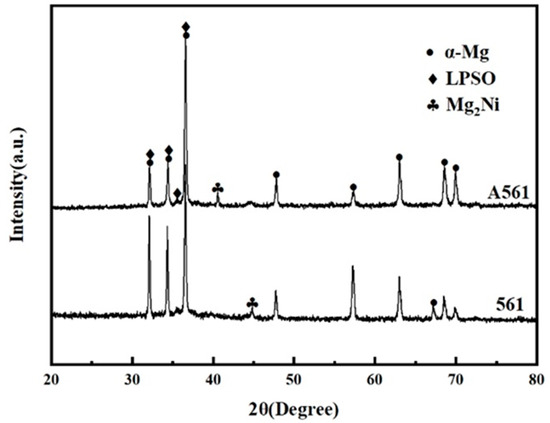
Figure 3.
XRD pattern of Mg-6Gd-7Ni-1Y alloys.
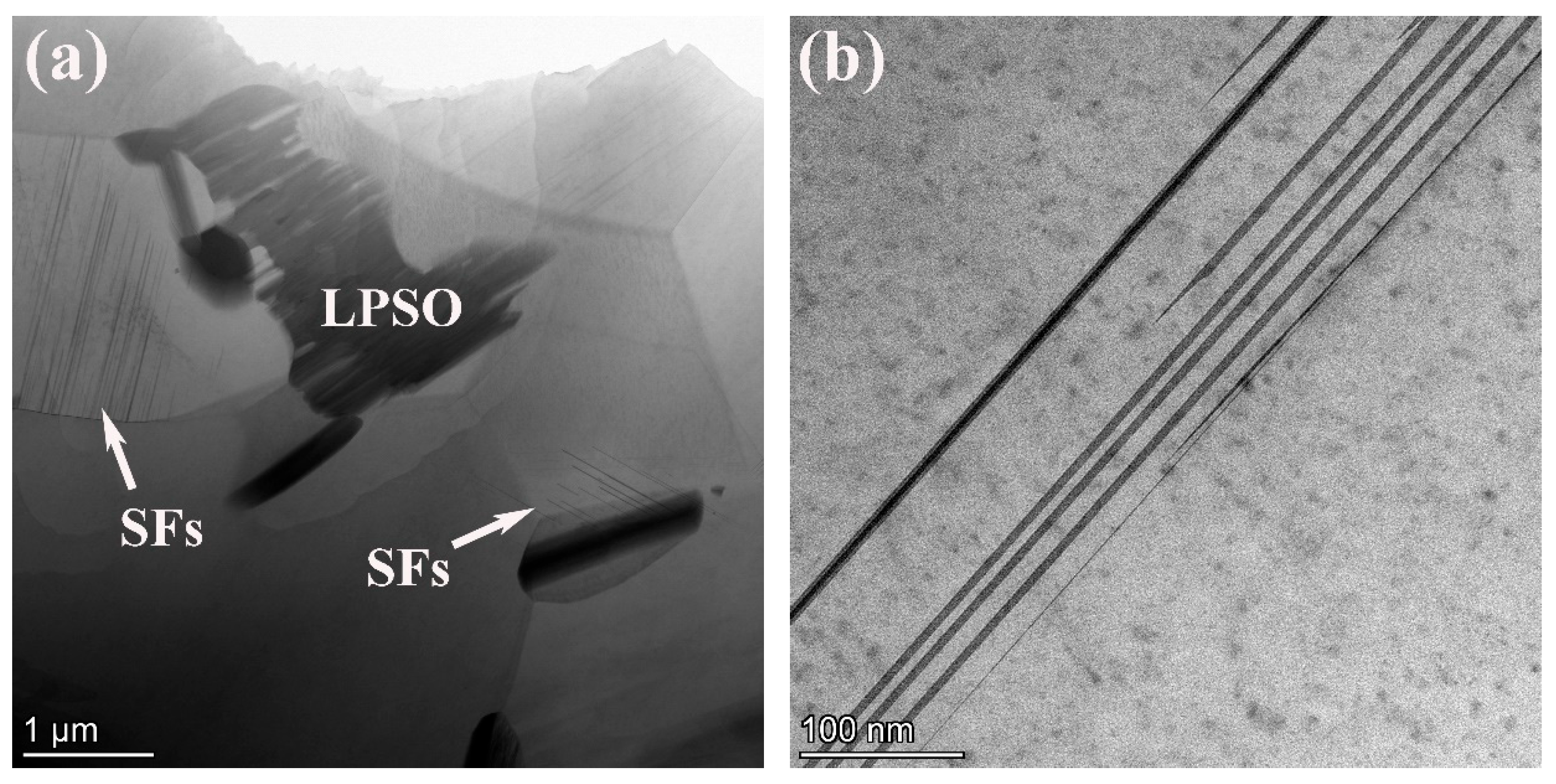
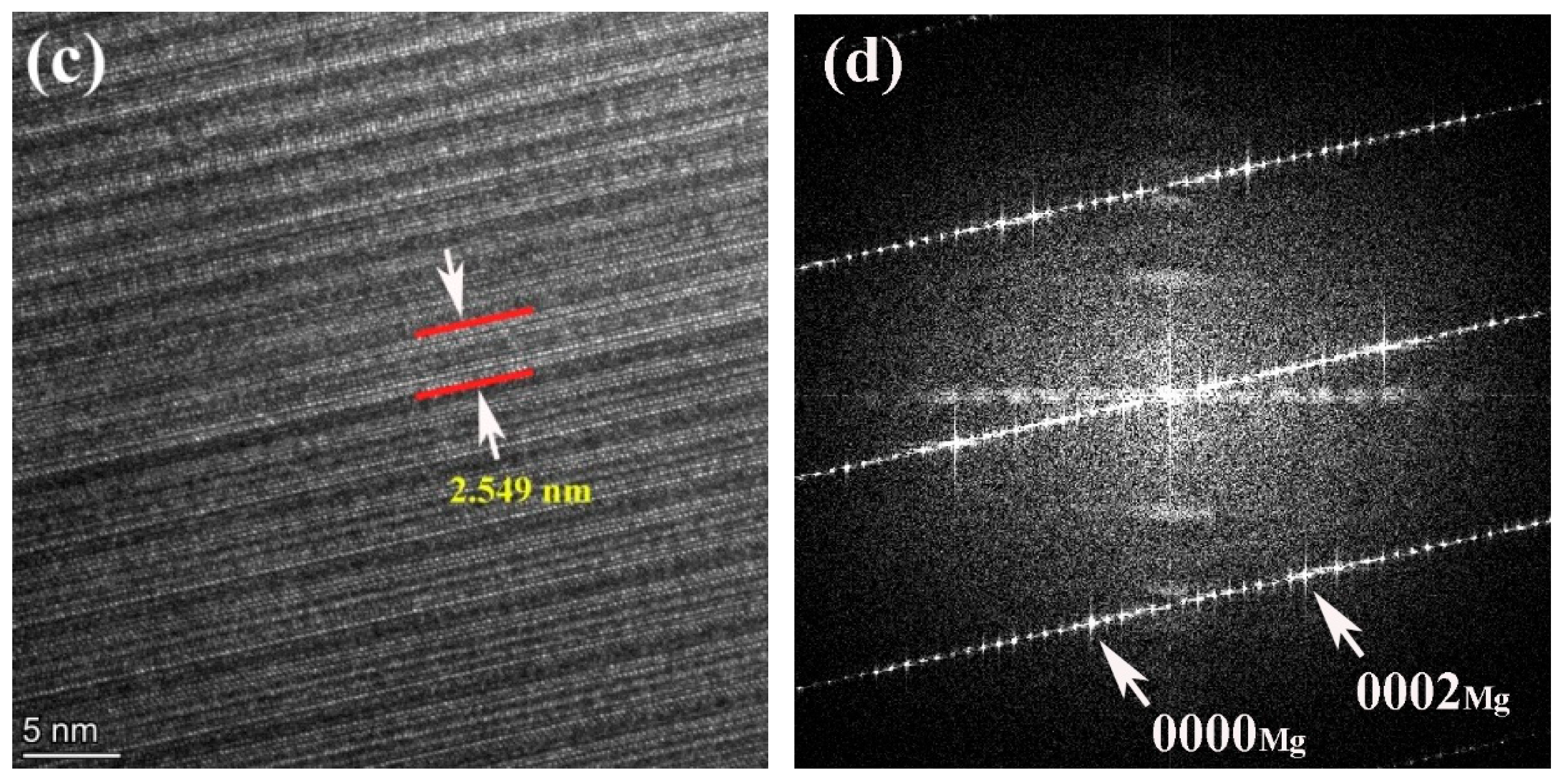
To confirm the structures of the LPSO phase, the 561 alloy was characterized by TEM, as shown in Figure 4. The observed LPSO phase was identified as the 14H type. From Figure 4a, a parallel distribution of fine, needle-like structures, which are distributed near grain boundaries with different phase relationships, can be observed. These structures are identified stacking faults (SFs) in magnesium alloys, representing crucial prerequisites for the formation of LPSO phases.
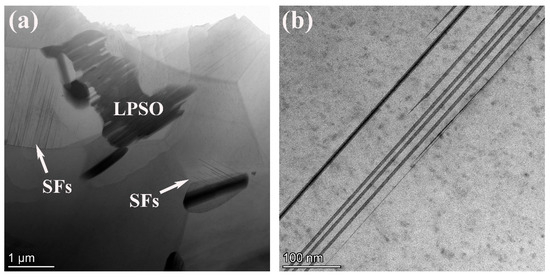
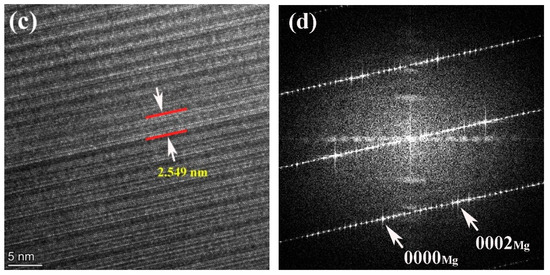
Figure 4.
STEM image (a), HAADF-STEM image (b), high-resolutionin image (c) and corresponding SAED pattern (d) of the 561 alloy.
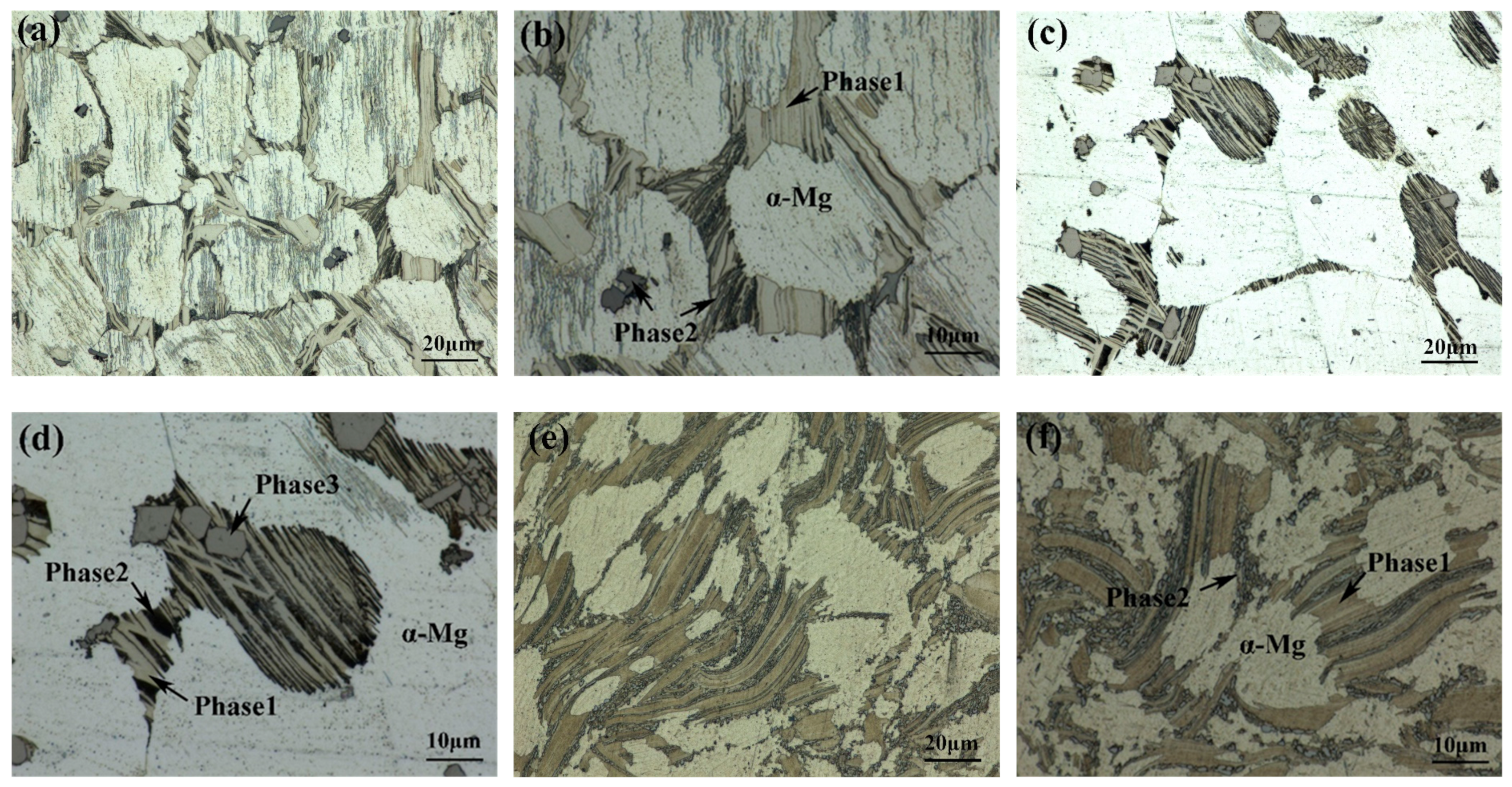
The optical microstructure of as-cast 561 (Figure 5a) shows that the alloy contains two different types of second phases in addition to α-Mg. Phase 1, denoted by a brighter color, is dispersed across the matrix surface by interconnected networks. Meanwhile, Phase 2, appearing darker, exhibits dispersion as finer strips and particles, often superim-posed on Phase 1. Additionally, isolated blocks of Phase 2 are observed within the matrix. Notably, corrosion marks with pronounced directivity can be observed on the surface of the specimen. Previous studies [15] have shown that the LPSO phases with a 14H structure have a particular orientation, with precipitates within the same grain align-ing parallel. However, owing to differing grain orientations, the corrosion-induced strip marks exhibit varying distributions along different directions. It can be seen from Figure 5c,d that after annealing at 500 °C for 10 h, the network structure decomposes, and the second phase transforms into an island-like structure that exists in the matrix inde-pendently. Notably, within A561, the count of distinct secondary phases increases from two to three. As for E561, the microstructure still retains the as-cast network structure, but the massive second phase is broken up into tiny dots, and the lamellar structure formed by these fragments has a parallel orientation relationship among themselves.
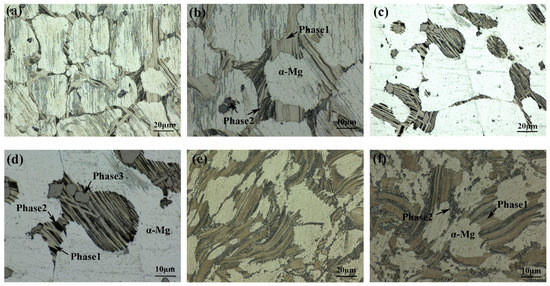
Figure 5.
Optical Microstructure of 561 (a,b), A561 (c,d), and E561 (e,f).
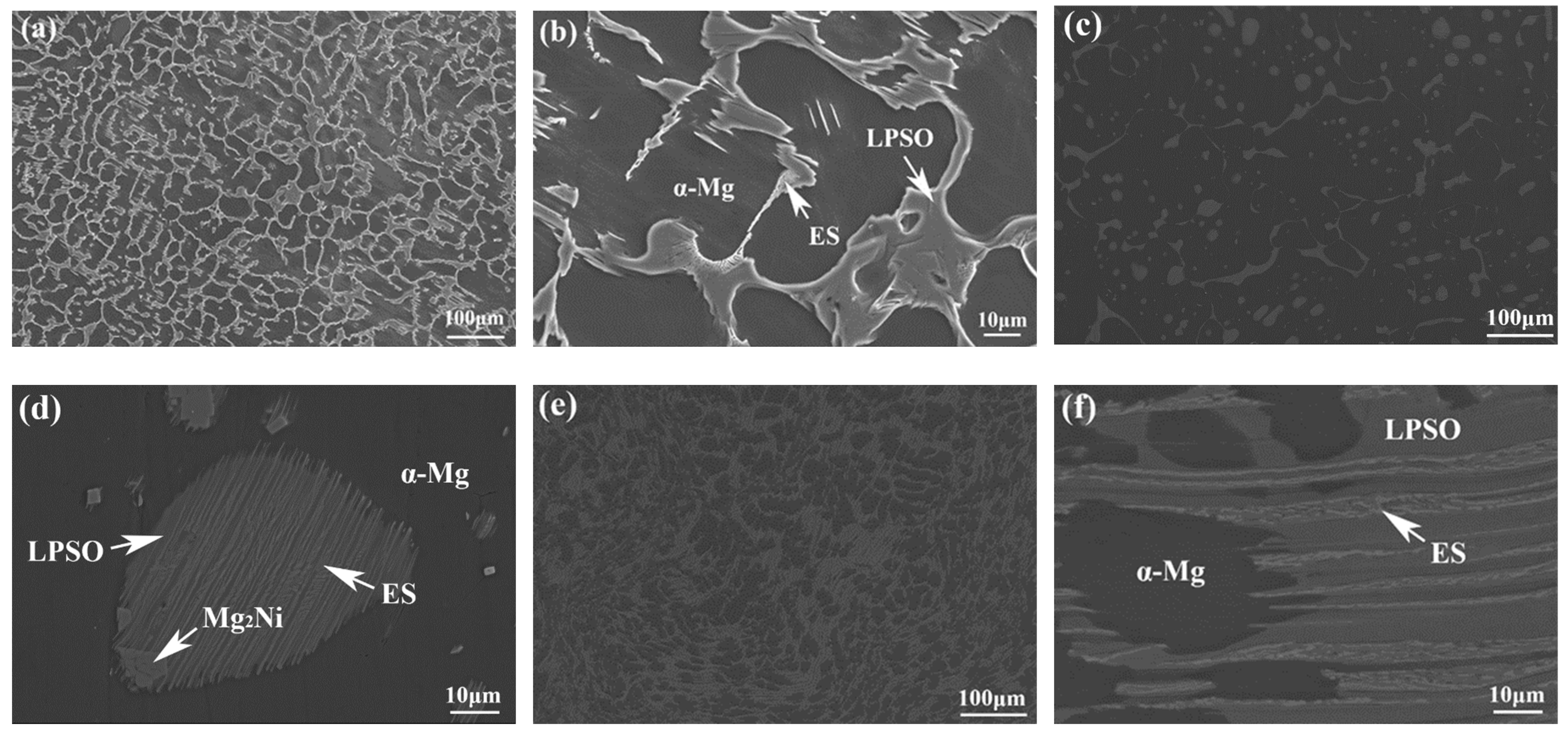
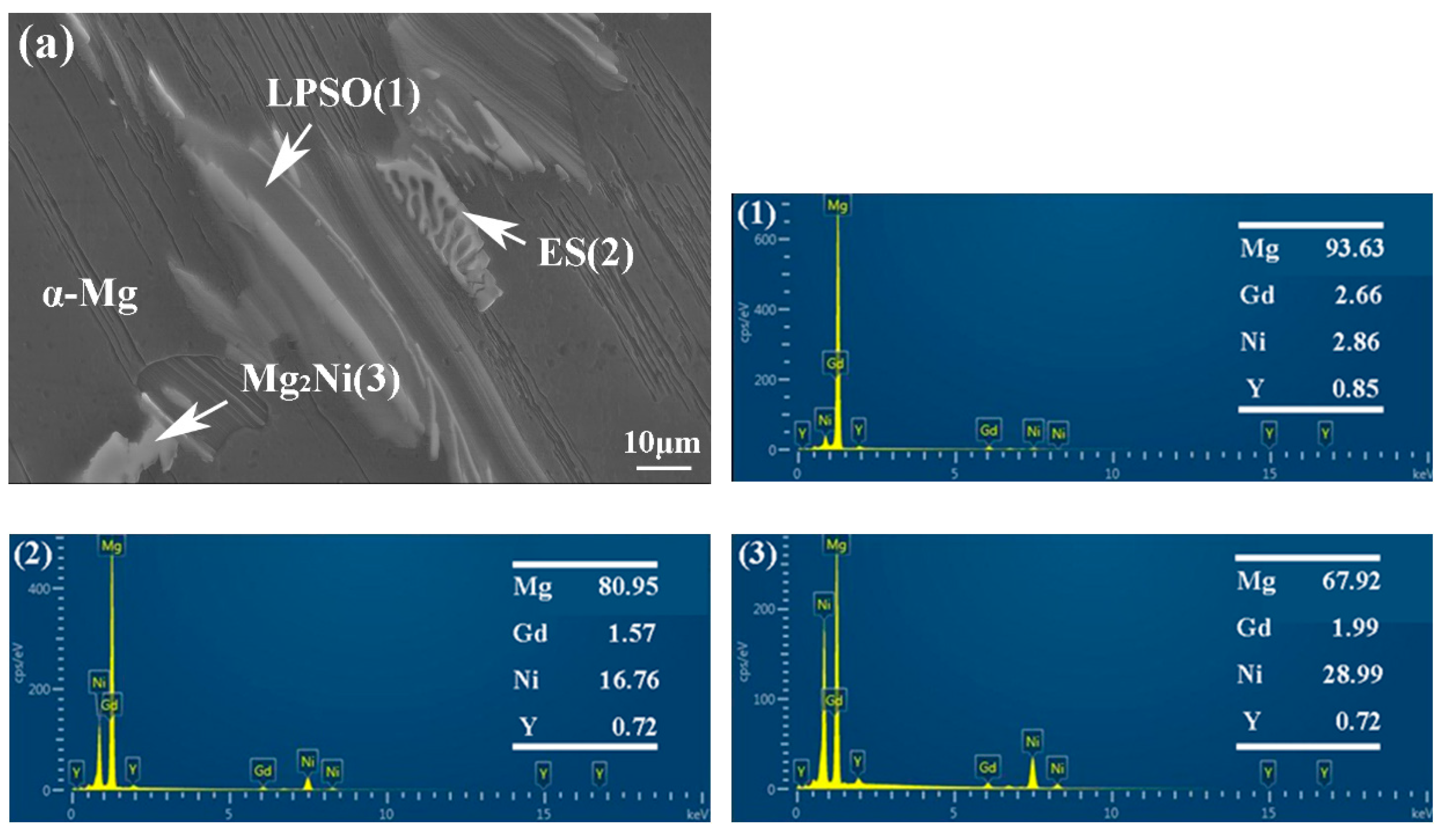
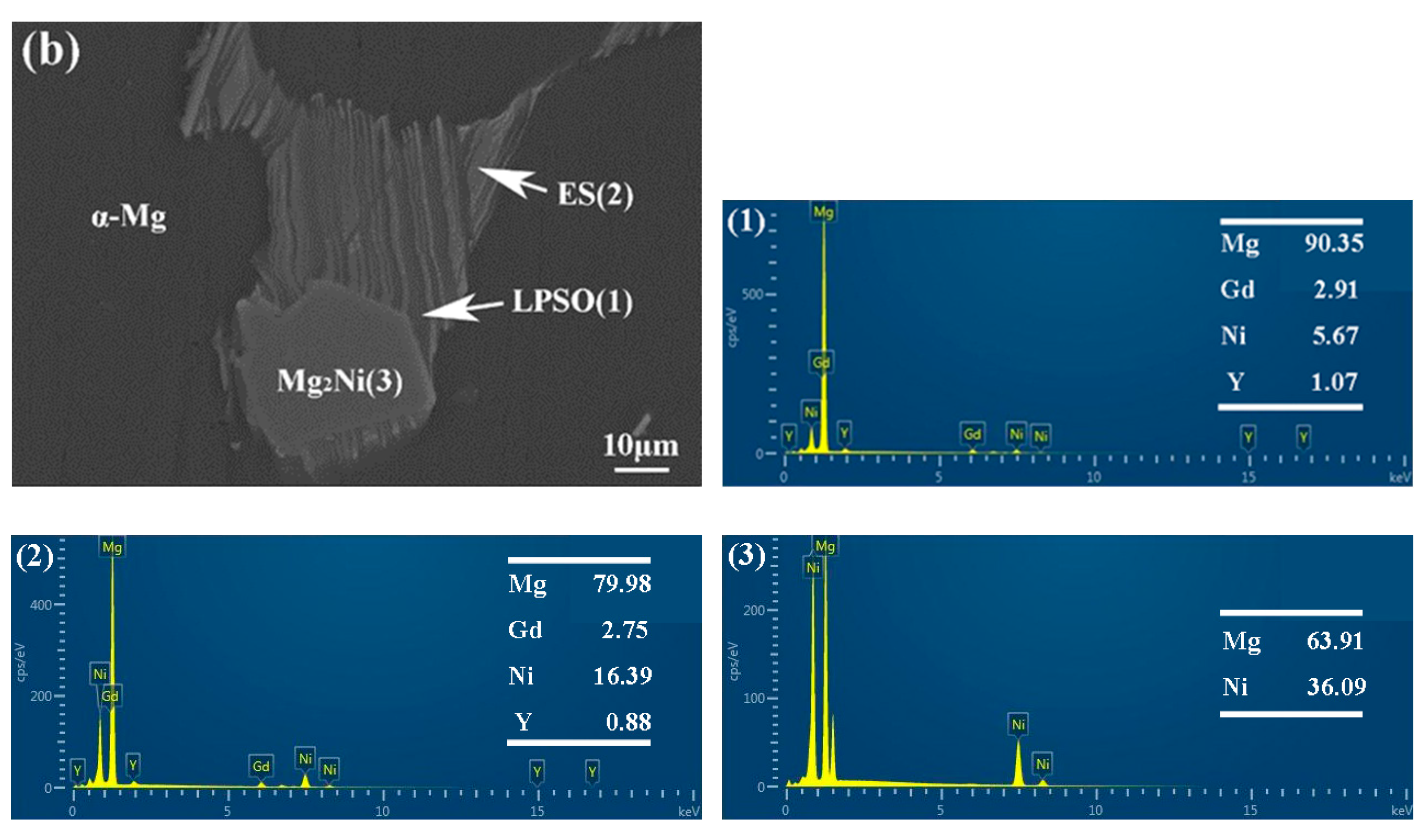
The morphology and composition of the second phase are analyzed further by SEM in Figure 6 and Figure 7. Based on the statistical results by ImagePro, the content of the second phase within the 561, A561, and E561 is determined to be 22.44%, 14.21% and 41.47%, respectively. The EDS pattern in Figure 7 indicates that the network structure in the alloy is the LPSO phase, which transforms into island structures distributed separately on the matrix after annealing as the Ni content decreases. Compared with the LPSO phase, the Ni content in the other second phase is higher, reaching around 16%. According to the XRD results and related reports [26,27], these products are likely eutectic structures (ES) formed by the Mg matrix, LPSO phase and Mg2Ni phase. Upon annealing, a notable transformation is observed in Figure 6d, depicting the breakdown and transformation of the ES, along with certain LPSO phases, into bulk Mg2Ni structures.
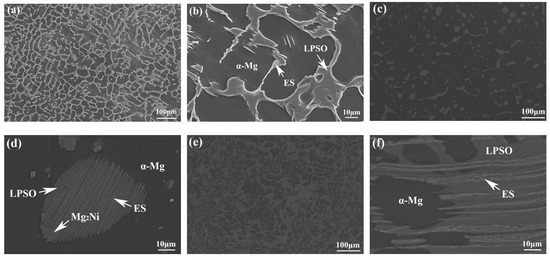
Figure 6.
SEM microstructure of 561 (a,b), A561 (c,d), and E561 (e,f).
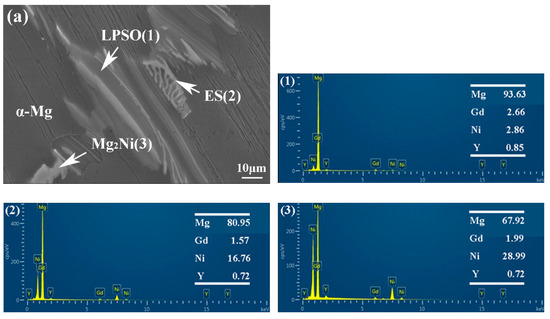
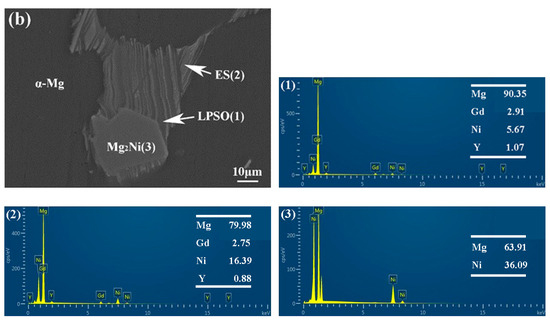
Figure 7.
EDS analysis of 561 (a) and A561 (b) (at.%).
3.2. Corrosion Properties
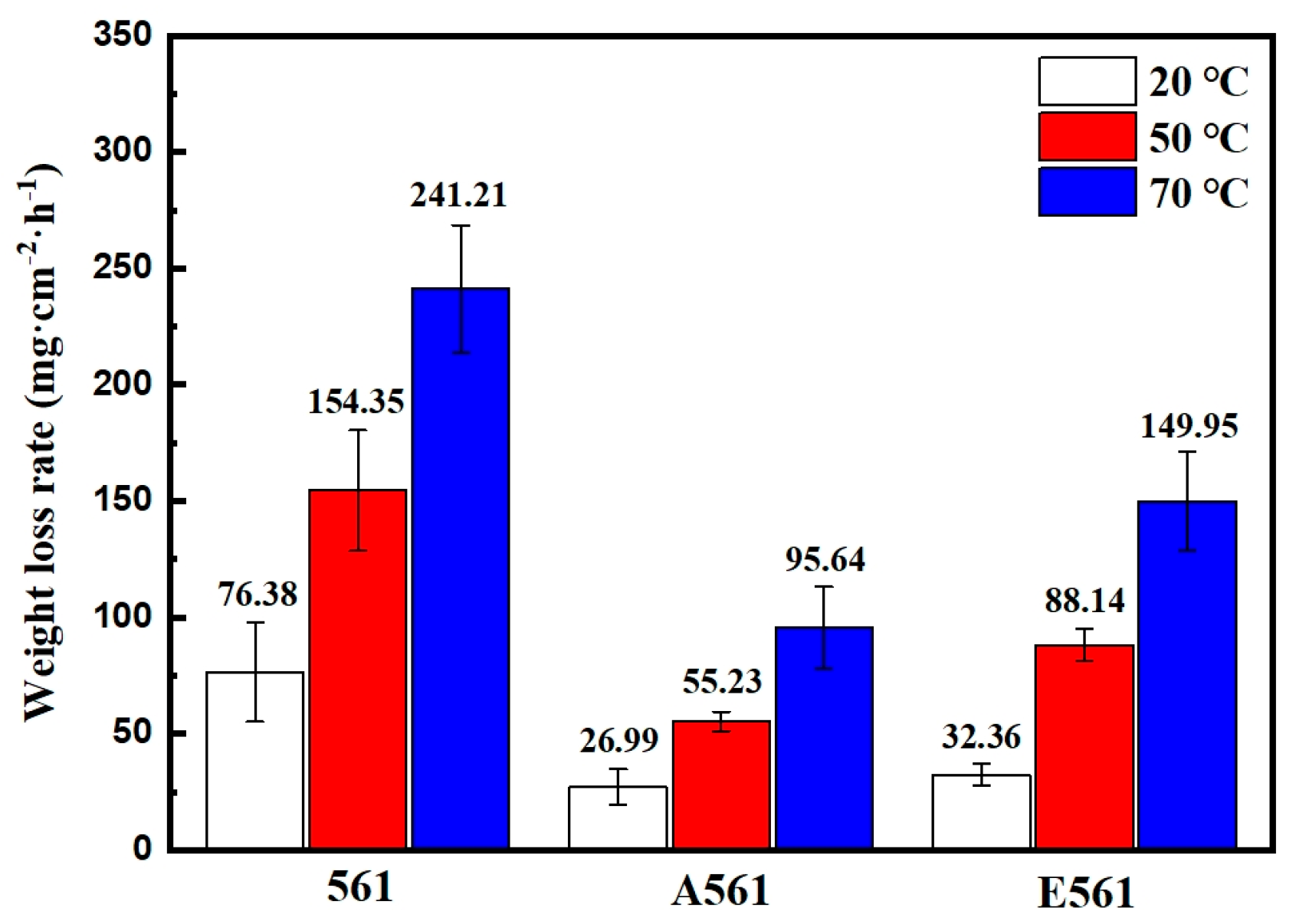
Figure 8 is a comparison chart of the dissolution rates at different temperatures of three alloy materials in a 3.5% NaCl solution. It can be seen that the dissolution rate of the three alloys increases greatly with the increase in temperature, mainly due to the strong galvanic corrosion caused by Mg2Ni. The as-cast alloy 561 has the highest dissolution rate across all tests, peaking at 241.21 mg·cm−2·h−1 at 70 °C. The dissolution rate of annealed alloy A561 is only about a third of the as-cast alloy. Previous results showed that the second phase’s content dropped by 8.2% after annealing, which is the main reason for the decrease in the dissolution rate in the alloy A561. Furthermore, the extruded alloy E561 also exhibited a notable decrease in dissolution rate compared to the as-cast 561 alloy, but remained higher than A561.
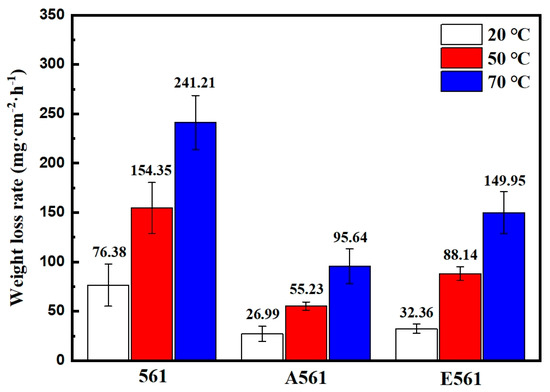
Figure 8.
Degradation rates measured by weight loss at different temperatures.
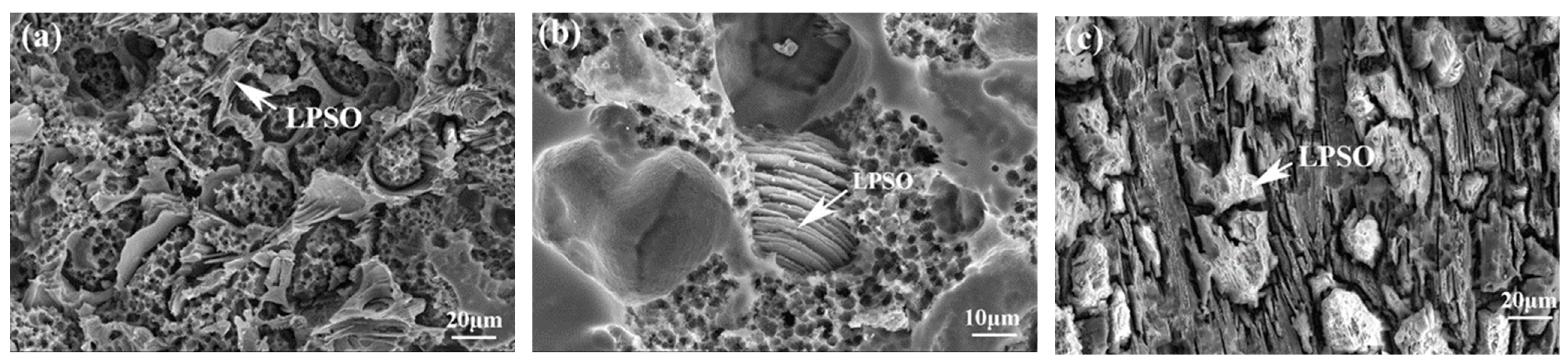
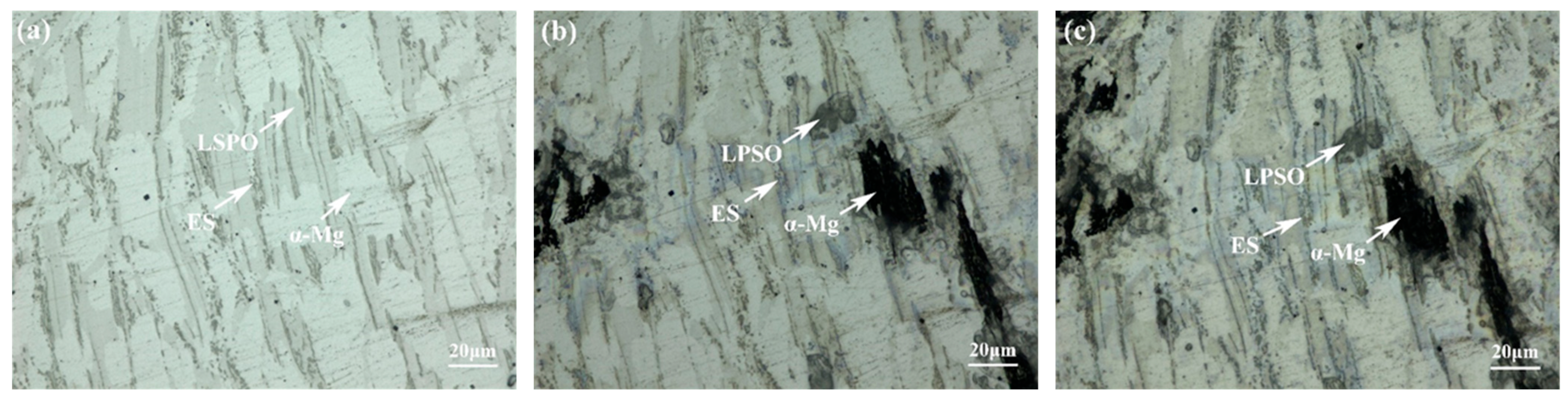
Figure 9 depicts the corrosion surface morphologies of three alloys. In Figure 9a, dis-cernibly, the corrosion exhibits greater severity at the periphery compared to the central area surrounded by the network structure. This indicates a specific potential difference between the matrix and the second phase, and the matrix with the lower potential serves as the anode throughout the corrosion process. Consequently, the proximity to the second phase accentuates the severity of the corrosion. Although the morphology after corrosion varied, a similar phenomenon was also observed in E561 (Figure 9c). In addition, the la-mellar structure of the LPSO phase with the 14H structure was observed in corroded A561 (Figure 9b). These results suggest a correlation between the proximity to the second phase, potential differences, and resulting corrosion severity.
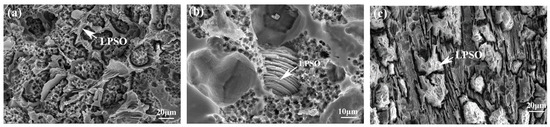
Figure 9.
Corrosion surface morphologies of 561 (a), A561 (b), and E561 (c).
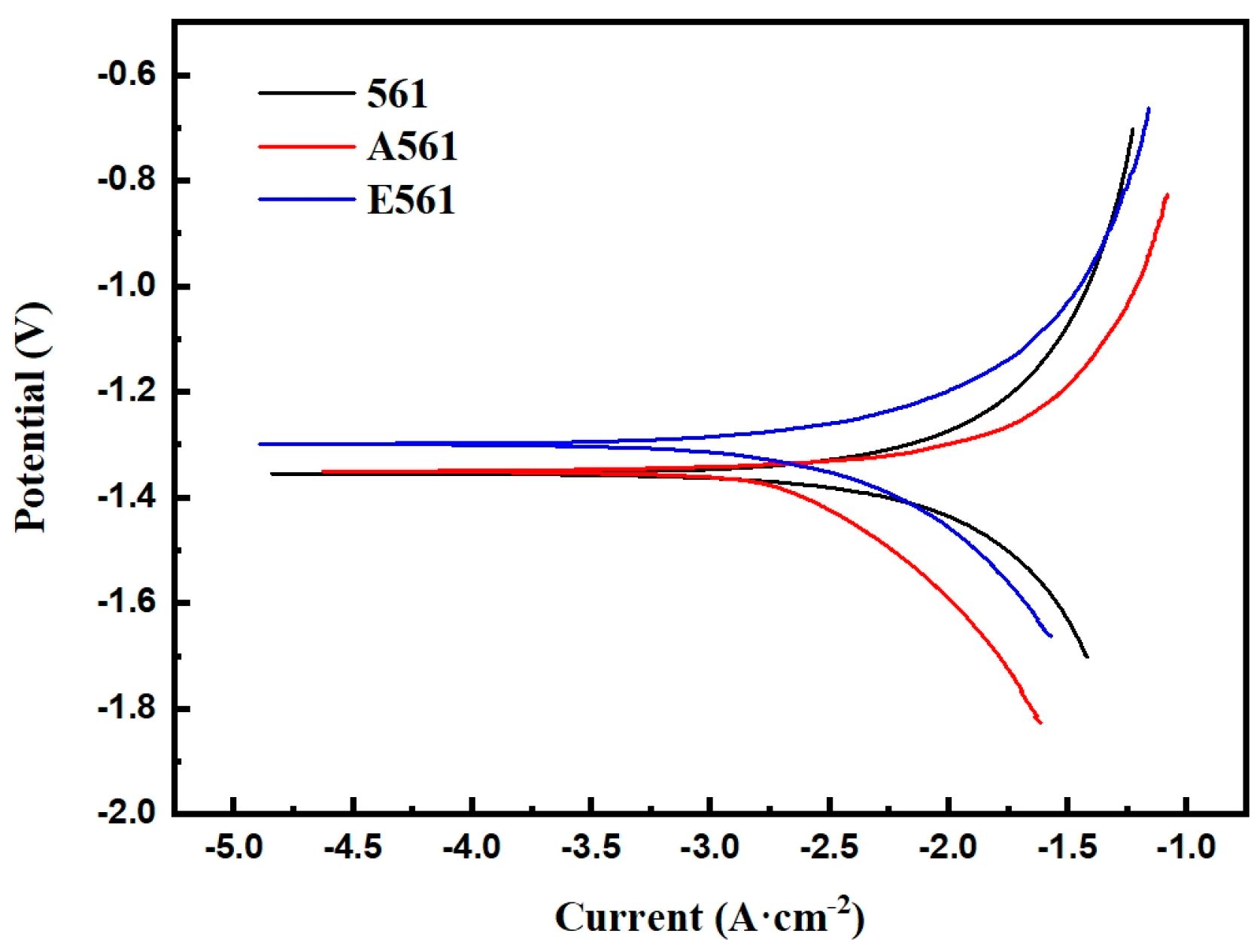
The polarization curve in a 3.5% NaCl solution at 25 °C is shown in Figure 10. It can be seen that the three alloy materials did not exhibit passivation during the corrosion process, which is a continuous corrosion process. This is mainly because the chloride ions in the solution destroy the Mg(OH)2 film generated on the surface of the sample, thereby allowing the corrosion to continue.
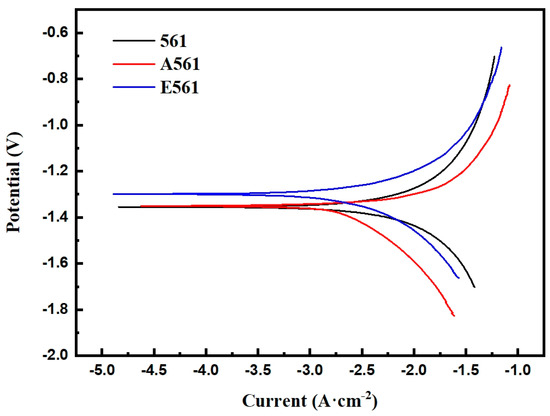
Figure 10.
Potentiodynamic polarization curves of the alloys.
As demonstrated in Table 2, the corrosion potential of the three alloys increases sequentially. Alloy 561 exhibits the highest Ecorr, measured at −1.41 VSCE. However, Ecorr mainly depicts the corrosion resistance, which can not evaluate the corrosion rate. It is the Icorr that determines the corrosion rate. Although alloy A561 shows a lower Ecorr than alloy E561, the latter exhibits a higher Icorr, correlating with a greater corrosion rate. Consistent with the immersion test, the as-cast alloy 561, with the fastest dissolution rate, exhibited the highest corrosion current density of 3811.94 μA·cm−2.

Table 2.
Corrosion properties of the alloys.
3.3. Corrosion Mechanism
Compared with as-cast and annealed alloys, the microstructure of extruded E561 is more homogeneous, so it was chosen for the study of the corrosion mechanism.
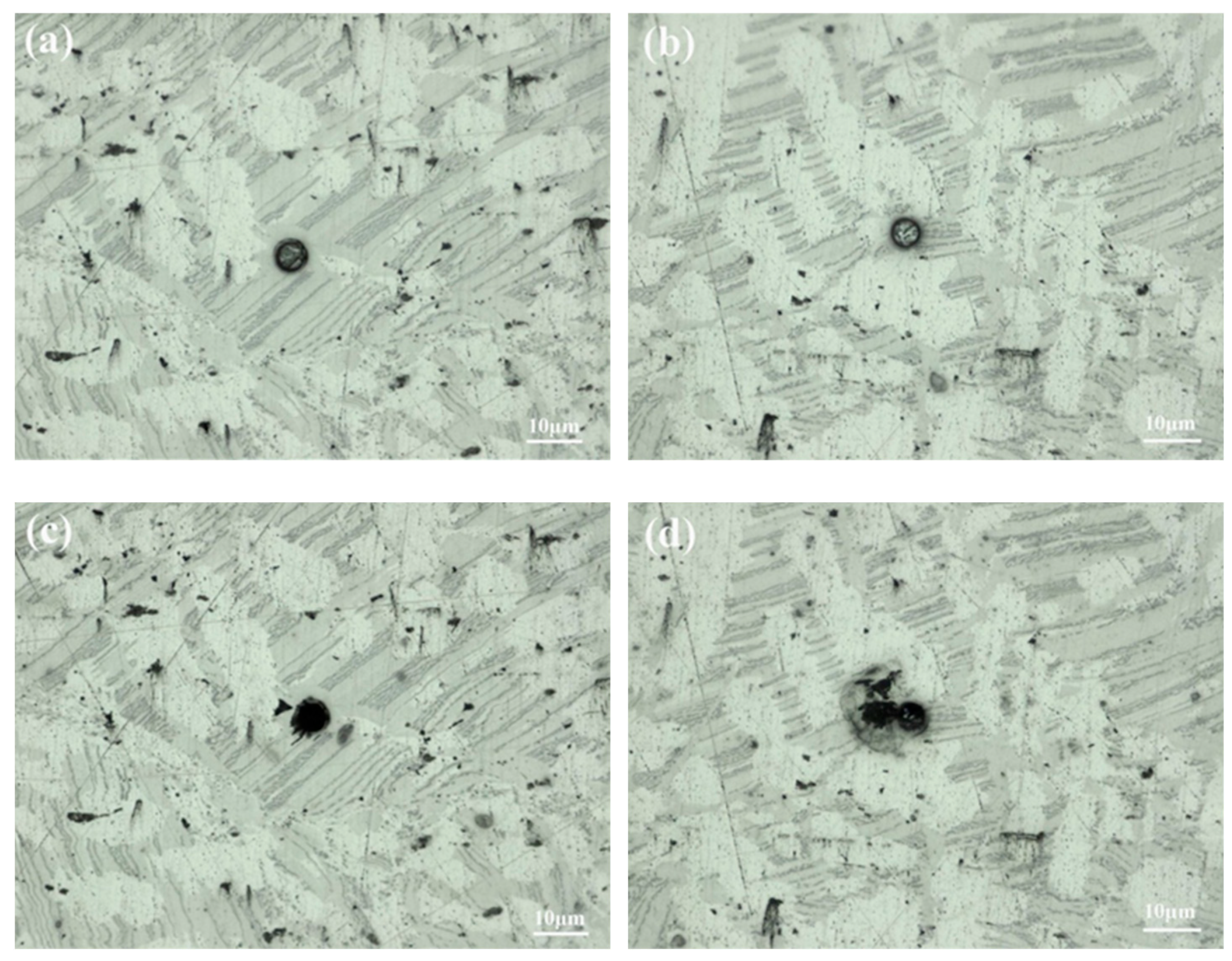
Figure 11 shows the microstructure of the investigated area. From Figure 11b, it can be seen that pitting corrosion begins to appear on the surface of the sample after 60 s of exposure. The pronounced corrosion affecting certain α-Mg regions is particularly notice-able, evident as extensive blackened areas denoting severe corrosion. After 120 s, the cor-rosion intensifies, presenting varied corrosion susceptibilities among the constituent phases. In Figure 11c, it can be seen that the α-Mg region expands into adjacent areas. For the massive LPSO phase and lamellar ES, only minor instances of pitting corrosion occur in some areas.

Figure 11.
Optical morphologies of in-situ corrosion test. (a) 0 s, (b) 60 s, (c) 120 s.
A comprehensive observation of the entire corrosion process reveals varying degrees of susceptibility to corrosion among different components. Specifically, α-Mg exhibits heightened sensitivity, with pitting corrosion rapidly developing and connecting into patches, forming a large area of corrosion and continuously expanding outward. It is inferred that there is a potential difference between α-Mg and other second phases, which results in galvanic corrosion. The potential difference between the LPSO and ES phases is smaller, so the corrosion rate is slower. During the experiment, the serious corrosion of α-Mg is the main reason for the high dissolution rate of the material.
In addition, the network structure, comprising the LPSO and ES phases in the extruded alloy, exhibits a relatively dense configuration. This network acts as a barrier, moderately impeding the spread of the corrosion process. This barrier effect notably contributes to the significant decrease in material dissolution rate observed after extrusion treatment [21].
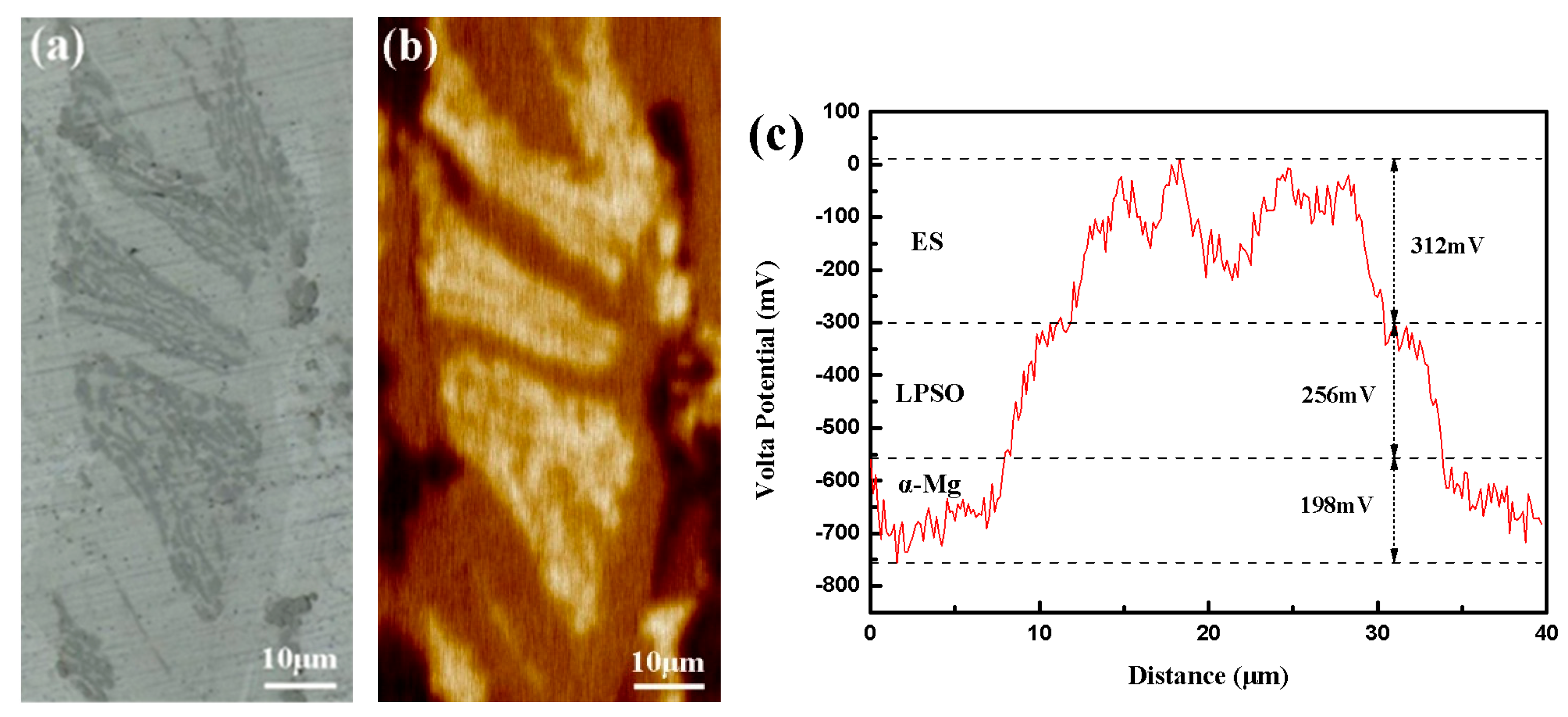
In order to further examine the role of the LPSO phase and ES on the corrosion mech-anism of magnesium alloy, the volta potential distributions of the alloy are shown in Fig-ure 12. This visualization aids in determining the cathodic or anodic behavior of the alloy during the corrosion process. Figure 12a shows the microstructure of the scanning area. In the surface potential map of Figure 12b, darker regions represent lower potentials. It can be seen that the α-Mg region with the darkest color has the lowest potential among the three constituent phases. Therefore, in the electrochemical system where corrosion oc-curs, α-Mg with the lowest potential acts as an anode to accelerated dissolution. From the potential distribution on the profile line in Figure 12b, the maximum potential difference between the three phases reached 766 mV. Such a large potential difference will cause serious galvanic corrosion in the dissolution process. This result highlights the significant potential differences and their direct correlation with the observed galvanic corrosion, supporting our earlier hypotheses.
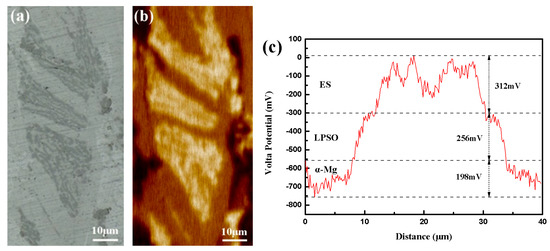
Figure 12.
The target area for immersion experiments. (a) Optical morphology of SKPFM testing area, (b) SKPFM surface potential map, (c) SKPFM potential line profile.
Following the determination of potential levels of the ES and LPSO phase, different electrochemical systems during the corrosion process were individually investigated through micro-electrochemical characterization.
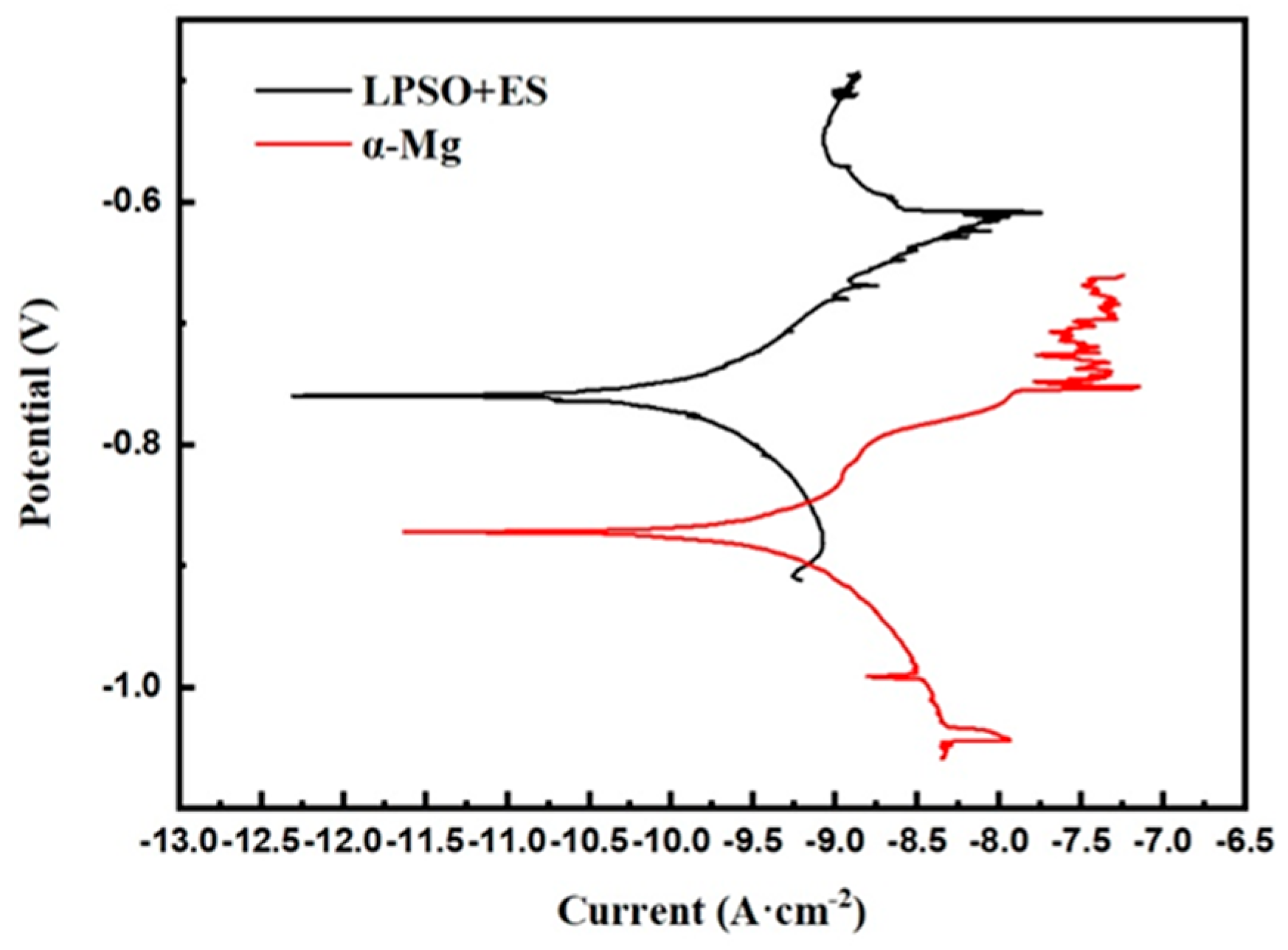
The mixed structure comprising the LPSO phase and ES is shown in Figure 13a, while Figure 13b shows the area mainly composed of the α-Mg. A comparison between the pre-experimental and post-experimental conditions of the test sites reveals extensive corrosion in these regions. According to Tafel curve extrapolation in Figure 14, the corrosion potentials of the aforementioned areas were −0.481 VAg/AgCl and −1.394 VAg/AgCl, respectively, with the corrosion current densities measuring 2.07 × 10−8 mA·cm−2 and 5.41 × 10−8 mA·cm−2 respectively. Even with a limited presence of the LPSO phase and ES, the electrochemical system comprising these phases along with α-Mg showed a corrosion current density approximately 2.7 times higher than its original value. These results indicate that the regions with three mixed phases exhibit a lower potential and a higher corrosion current density during the corrosion process, which means that these regions had a faster corrosion rate and significantly contributed to the total corrosion rate.
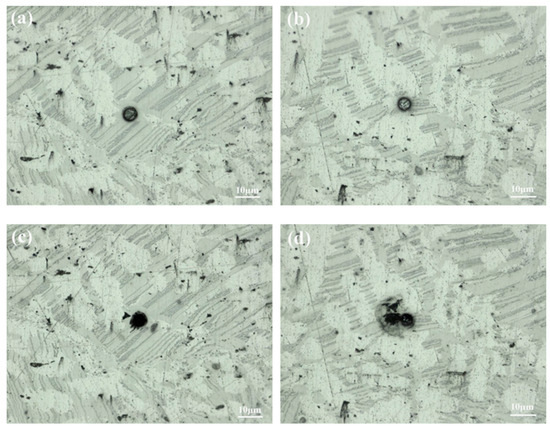
Figure 13.
Optical morphologies of the sample before (a,b) and after (c,d) electrochemical test.
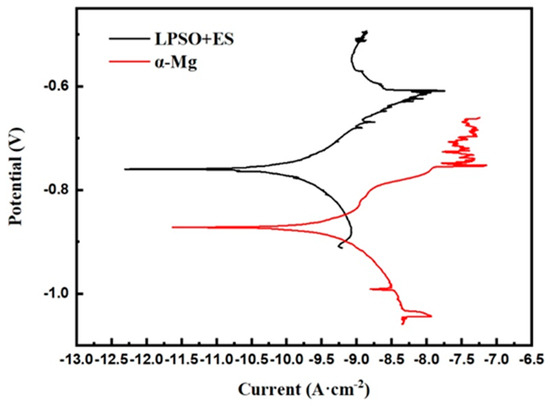
Figure 14.
Micro-electrochemical potentiodynamic polarization curves.
This micro-electrochemical analysis underscores the distinct electrochemical behavior observed in regions with different alloy phases during the corrosion process, emphasizing the substantial impact of mixed-phase regions on corrosion kinetics and the resultant overall corrosion rate.
3.4. General Discussion
Based on the experimental findings above, the phase composition of the alloy re-mained unchanged after annealing. However, a notable reduction in the dissolution rate, compared to the as-cast alloy, was observed. Previous studies indicated an increased dis-solution rate after annealing [21,28]. They suggested that the increase in corrosion rate after annealing is partly due to the decomposition of the network structure formed by the LPSO phase, which cannot continue to serve as a barrier to hinder the spread of corrosion. Furthermore, certain LPSO phases with the 18R structure transformed into lamellar 14H structures after annealing, providing additional corrosion propagation pathways. How-ever, our investigation presented a contrary outcome.
In our research, the as-cast alloy has already exhibited a 14H LPSO phase character-ized by a lamellar structure. Therefore, the increase in the corrosion rate cannot be at-tributed to the increasing corrosion propagation pathways. According to Figure 9 and Figure 11, a severe corrosion attack was observed in the α-Mg phase area, indicating that the network structure in our research did indeed serve as a barrier, but cannot effectively prevent the spread of corrosion. Compared to the extruded alloy, the network structure in the as-cast alloy is more porous. This increases the contact area between different phases, thus promoting the galvanic corrosion process. Analysis through SKPFM (Figure 12) revealed that the potential difference between the second phases and the substrate reaches over 700 mV, indicating a strong galvanic corrosion tendency among this network structure. The micro-electrochemical system comprised of three phases exhibited a lower corrosion potential and higher corrosion current density, as shown in Figure 14, indicating that the dominance of the galvanic corrosion effect significantly influences the corrosion rate. After annealing, the content of the second phases in the alloy decreased by around 36%, significantly weakening the galvanic corrosion effect and reducing the corrosion rate.
4. Conclusions
The Mg-5Gd-6Ni-1Y alloy mainly comprises α-Mg, a LPSO phase, and ES (Eutectic structure formed by α-Mg, LPSO phase, and Mg2Ni). These secondary phases are intricately interwoven within the substrate, forming a network structure. Upon annealing, this network structure underwent decomposition, transforming into an island-like configuration.
At room temperature in 3.5 wt.% NaCl, the as-cast alloy exhibited an exceptionally high dissolution rate, measured at 55.85 ± 13 mg/h−1·cm−2. With the Subsequent rise in temperature, the dissolution rate exhibited a further increase. However, after annealing, the reduction in the content of the second phase correlated with a decline in the dissolution rate. Furthermore, after extrusion, the network structure of the second phase became denser, to some extent impeding the diffusion of the corrosion.
Regarding electrochemical behavior, the potential level among the three constituent phases reveals ES > LPSO phase > α-Mg, where α-Mg is prone to accelerated dissolution due to the galvanic effect. Notably, the electrochemical system comprising α-Mg, ES, and an LPSO phase demonstrated a higher corrosion current density, serving as the primary contributing factor to the comparatively larger dissolution rate exhibited by the Mg-5Gd-6Ni-1Y alloy.
Author Contributions
Conceptualization, L.W. and X.R.; investigation, J.Z. and Y.L.; data analysis, J.Z.; literature search, M.L.; data curation, M.L.; validation, M.L.; supervision, Y.L and X.R.; project administration, Y.A., L.W. and X.R.; methodology, L.W.; writing—original draft preparation, writing—review and editing, L.W. and X.R.; resources, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Key Research and Development Program of China (2021YFA1601103) and National Natural Science Foundation of China (U21B2053).
Data Availability Statement
Data is available on reasonable request.
Conflicts of Interest
Author Mingxing Li was employed by the company Oil and Gas Technology Research Institute of Changqing Oilfield Branch of CNPC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Joost, W.J.; Krajewski, P.E. Towards magnesium alloys for high-volume automotive applications. J. Scr. Mater. 2017, 128, 107–112. [Google Scholar] [CrossRef]
- Wang, C.J.; Kang, J.W.; Deng, K.K.; Nie, K.B.; Li, W.G. Microstructure and mechanical properties of Mg-4Zn-xGd (x=0, 0.5, 1, 2) alloys. J. Magnes. Alloys 2020, 8, 441–451. [Google Scholar] [CrossRef]
- Pollock, T.M. Materials science. Weight loss with magnesium alloys. J. Sci. 2010, 328, 986–987. [Google Scholar]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koc, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Song, J.F.; She, J.; Chen, D.L.; Pan, F.S. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloys 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Jafari, S.; Raman, R.K.S.; Davies, C.H.J. Corrosion fatigue of a magnesium alloy in modified simulated body fluid. J. Eng. Fract. Mech. 2015, 137, 2–11. [Google Scholar] [CrossRef]
- Su, C.; Wang, J.F.; Hu, H.; Wen, Y.L.; Liu, S.J.; Ma, K. Enhanced strength and corrosion resistant of Mg-Gd-Y-Al alloys by LPSO phases with different Al content. J. Alloys Compd. 2021, 885, 160557. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Feng, Y.; Zhang, J.H.; Liu, S.J.; Yang, Q.; Liu, Z.; Li, R.G.; Meng, J.; Wu, R.Z. Development of extruded Mg-6Er-3Y-1.5Zn-0.4Mn (wt.%) alloy with high strength at elevated temperature. J. Mater. Sci. Technol. 2019, 35, 2365–2374. [Google Scholar] [CrossRef]
- Luo, Z.P.; Song, D.Y.; Zhang, S.Q. Strengthening effects of rare earths on wrought Mg-Zn-Zr-RE alloys. J. Alloys Compd. 1995, 230, 109–114. [Google Scholar] [CrossRef]
- Kishida, K.; Nagai, K.; Matsumoto, A.; Inui, H. Data in support of crystal structures of highly-ordered long-period stacking-ordered phases with 18R, 14H and 10H-type stacking sequences in the Mg–Zn–Y system. J. Data in Brief. 2015, 5, 314–320. [Google Scholar] [CrossRef]
- Song, W.J.; Dong, H.P.; Zhang, G.; Liu, J.; Yang, G.; Liu, Y.H.; Li, Y.Z.; Li, J.S.; Shen, J.H.; Chen, Y.X.; et al. Enhanced hydrogen absorption kinetics by introducing fine eutectic and long-period stacking ordered structure in ternary eutectic Mg–Ni–Y alloy. J. Alloys Compd. 2020, 820, 153187. [Google Scholar] [CrossRef]
- Itoi, T.; Seimiya, T.; Kawamura, Y.; Hirohashi, M. Long period stacking structures observed in Mg97Zn1Y2 alloy. J. Scr. Mater. 2004, 51, 107–111. [Google Scholar] [CrossRef]
- Kawamura, Y.; Kasahara, T.; Izumi, S.; Yamasaki, M. Elevated temperature Mg97Y2Cu1 alloy with long period ordered structure. J. Scr. Mater. 2006, 55, 453–456. [Google Scholar] [CrossRef]
- Yamasaki, M.; Anan, T.; Yoshimoto, S.; Kawamura, Y. Mechanical properties of warm-extruded Mg–Zn–Gd alloy with coherent 14H long periodic stacking ordered structure precipitate. J. Scr. Mater. 2005, 53, 799–803. [Google Scholar] [CrossRef]
- Wang, L.S.; Jiang, J.H.; Liu, H.; Saleh, B.; Ma, A.B. Microstructure characterization and corrosion behavior of Mg–Y–Zn alloys with different long period stacking ordered structures. J. Magnes. Alloys 2020, 8, 1208–1220. [Google Scholar] [CrossRef]
- Wang, D.D.; Zhang, W.B.; Zong, X.M.; Nie, K.B.; Xu, C.X.; Zhang, J.S. Abundant long period stacking ordered structure induced by Ni addition into Mg–Gd–Zn alloy. J. Mater. Sci. Eng. A 2014, 618, 355–358. [Google Scholar] [CrossRef]
- Liu, H.; Xue, F.; Bai, J.; Zhou, J.; Liu, X.D. Effect of substitution of 1 at% Ni for Zn on the microstructure and mechanical properties of Mg94Y4Zn2 alloy. J. Mater. Sci. Eng. A 2013, 585, 387–395. [Google Scholar] [CrossRef]
- Garces, G.; Perez, P.; Cabeza, S.; Lin, H.K.; Kim, S.; Gan, W.; Adeva, P. Reverse tension/compression asymmetry of a Mg–Y–Zn alloys containing LPSO phases. J. Mater. Sci. Eng. A 2015, 647, 287–293. [Google Scholar] [CrossRef]
- Niu, H.Y.; Deng, K.K.; Nie, K.B.; Cao, F.F.; Zhang, X.C.; Li, W.G. Microstructure, mechanical properties and corrosion properties of Mg-4Zn-xNi alloys for degradable fracturing ball applications. J. Alloys Compd. 2019, 787, 1290–1300. [Google Scholar] [CrossRef]
- Ma, K.; Wang, J.F.; Peng, Y.H.; Dai, C.H.; Pan, Y.L.; Wang, Y.; Wang, D.Q.; Wang, J.X.; Ma, Y.L.; Pan, F.S. Achieving high strength and rapid degradation in Mg-Gd-Ni alloys by regulating LPSO phase morphology combined with extrusion. J. Magnes. Alloys 2022, in press. [CrossRef]
- Dai, C.N.; Wang, J.F.; Pan, Y.L.; Ma, K.; Peng, Y.H.; Ren, J.; Wang, Y.; Wang, D.Q.; Wang, J.X.; Ma, Y.L. Tailoring the microstructural characteristic and improving the corrosion rate of Mg-Gd-Ni alloy by heat treatment with different volume fraction of LPSO phase. J. Corros. Sci. 2023, 210, 110806. [Google Scholar] [CrossRef]
- Ma, K.; Wang, J.F.; Peng, Y.H.; Dai, C.N.; Pan, Y.L.; Wang, D.Q.; Wang, Y.; Pei, S.L.; Ma, Y.L. Enhanced degradation properties of Mg-Gd-Ni alloys by regulating LPSO morphology. J. Phys. Chem. Solids 2022, 171, 110974. [Google Scholar] [CrossRef]
- Jin, Y.; Lai, Z.G.; Bi, P.; Yan, S.T.; Wen, L.; Wang, Y.C.; Pan, J.S.; Leygraf, C. Combining lithography and capillary techniques for local electrochemical property measurements. J. Electrochem. Commun. 2018, 87, 53–57. [Google Scholar] [CrossRef]
- Lai, Z.G.; Bi, P.; Wen, L.; Xue, Y.P.; Jin, Y. Local electrochemical properties of fusion boundary region in SA508-309L/308L overlay welded joint. J. Corros. Sci. 2019, 155, 75–85. [Google Scholar] [CrossRef]
- Lai, Z.G.; Zou, Y.; Zhao, Z.Y.; Huang, F.F.; Liu, P.; Lai, T.X.; Jin, Y. An Automated Test Platform for High-Throughput Micro-Electrochemical Characterization of Metallic Materials and Its Application on a Fe–Cr–Ni Combinatorial Materials Chip. J. Electrochem. Soc. 2021, 168, 091501. [Google Scholar] [CrossRef]
- Hao, L.Y.; Yang, X.; Lv, S.L.; Fang, X.G.; Wu, S.S. Influence of squeeze casting pressure and heat treatment on microstructure and mechanical properties of Mg94Ni2Y4 alloy with LPSO structure. J. Mater. Sci. Eng. A 2017, 707, 280–286. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, S.; Bi, Y.D.; Li, H.X.; Ren, Y.P.; Qin, G.W. Phase equilibria of the long-period stacking ordered phase in the Mg–Ni–Y system. J. Intermetallics. 2015, 57, 127–132. [Google Scholar] [CrossRef]
- Ma, K.; Wang, J.F.; Ren, J.; Dai, C.N.; Liu, S.J.; Peng, Y.H.; Pan, Y.L. Enhanced degradation properties of Mg-Y-Ni alloys by tailoring the LPSO morphology for fracturing tools applications. J. Mater. Charact. 2021, 181, 111489. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).