Effect of Solution Treatment on Microstructure Evolution of a Powder Metallurgy Nickel Based Superalloy with Incomplete Dynamic Recrystallization Microstructure
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Initial Microstructure
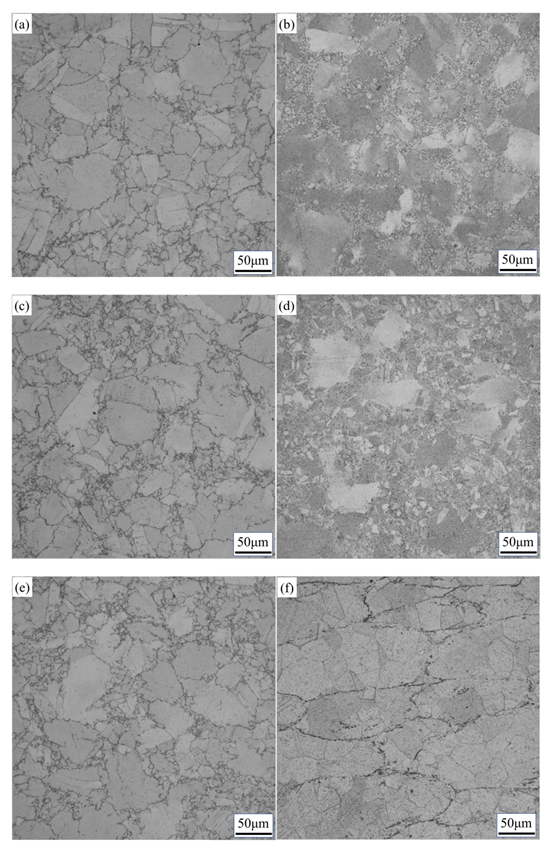
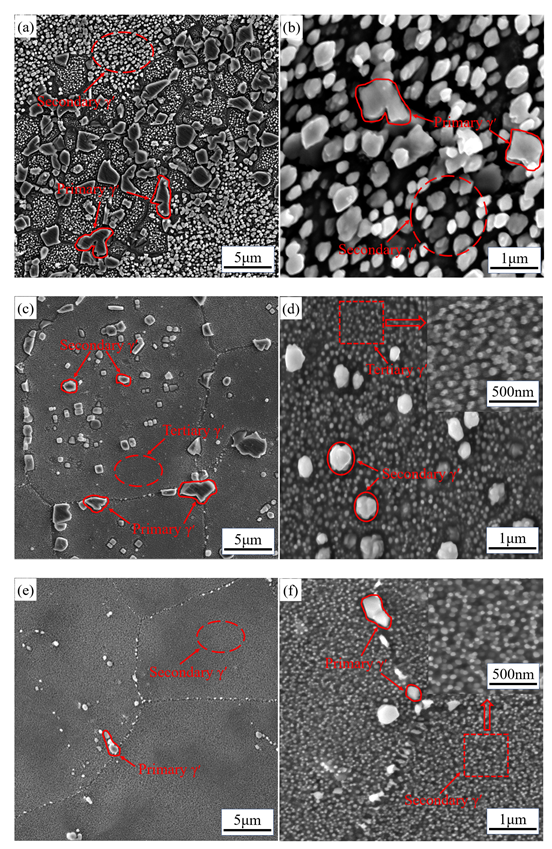
3.2. Effect of Solution Temperature on Microstructure and Precipitates
3.3. Influence of Cooling Mode on Precipitates
3.4. Influence of Holding Time on Precipitates
4. Conclusions
- With the increase of solution temperature, the recrystallization degree of the incomplete dynamic recrystallization structure gradually increases, and the average grain size first decreases and then increases when the temperature is increased 1060 °C to 1100 °C. With the increasing degree of redissolution of γ′ precipitates on the grain boundary, the pinning effect to grain boundary becomes weaker, and the size of the secondary γ′ precipitates within the grain becomes smaller and the volume fraction becomes larger.
- The reprecipitated secondary γ′ precipitates are mainly spherical under AC and have a bimodal distribution under FC. The morphology transforms from spherical to near-spherical, cuboids, octets, petaloid, and dendrites.
- The γ′ precipitates have a small size and large volume fraction due to high nucleation density and high cooling rate with the extension of holding time under a higher solution temperature (1100 °C).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, J.C.; Starke, E.A. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Pollock, T.M.; Tin, S. Nickel-Based Superalloys for Advanced Turbine Engines: Chemistry, Microstructure and Properties. J. Propuls. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Hu, L.X.; Feng, X.Y. The research and development of powder metallurgy superalloy. Powder Metall. Ind. 2018, 28, 1–7. (In Chinese) [Google Scholar]
- Zhang, X.; Chen, Y.; Hu, J. Recent advances in the development of aerospace materials. Prog. Aerosp. Sci. 2018, 97, 22–34. [Google Scholar] [CrossRef]
- Pollock, T.M. Alloy design for aircraft engines. Nat. Mater. 2016, 15, 809–815. [Google Scholar] [CrossRef]
- Guo, W.M.; Dong, J.X.; Wu, J.T.; Zhang, F.G.; Chen, G.S.; Chen, S.D. Microstructure and Properties of PM Superalloys FGH96. J. Iron Steel Res. 2005, 17, 59–63. [Google Scholar]
- Ning, Y.; Yao, Z.; Fu, M.W.; Guo, H. Dynamic recrystallization of the hot isostatically pressed P/M superalloy FGH4096 in hot working process. Mater. Sci. Eng. A 2010, 527, 6968–6974. [Google Scholar] [CrossRef]
- Li, F.; Fu, R.; Yin, F.; Feng, D.; Wang, H.; Du, G.; Feng, Y. Impact of γ′(Ni3(Al,Ti)) phase on dynamic recrystallization of a Ni-based disk superalloy during isothermal compression. J. Alloys Compd. 2017, 693, 1076–1082. [Google Scholar] [CrossRef]
- Liu, X.T.; Ding, H.H.; Yang, C.; Liu, F.; Huang, L.; Jiang, L. Microstructure and mechanical properties of hot extruded FGH96 powder metallurgy superalloy. Trans. Nonferrous Met. Soc. China 2016, 26, 354–364. [Google Scholar]
- Jia, C.L.; Ge, C.C.; Yan, Q.Z. Microstructure evolution and mechanical properties of disk superalloy under multiplex heat treatment. Mater. Sci. Eng. A 2016, 659, 287–294. [Google Scholar] [CrossRef]
- Kim, I.S.; Choi, B.G.; Jung, J.E.; Do, J.; Seok, W.Y.; Lee, Y.H.; Jeong, I.Y. Effect of heat treatment on microstructural evolution and creep behaviors of a conventionally cast nickel-based superalloy. Mater. Charact. 2020, 165, 110378. [Google Scholar] [CrossRef]
- Qiao, S.; Wang, Y.; Lv, L.; Huang, Z.; Tan, G. Normal and abnormal grain growth in a FGH96 superalloy during thermomechanical treatment. J. Mater. Res. Technol. 2021, 15, 7033–7049. [Google Scholar] [CrossRef]
- Kumar, S.S.; Raghu, T.; Bhattacharjee, P.P.; Rao, G.A.; Borah, U. Evolution of microstructure and micro texture during hot deformation in an advanced P/M nickel base superalloy. Mater. Charact. 2018, 146, 217–236. [Google Scholar] [CrossRef]
- Qiu, C.; Wu, X.; Mei, J.; Andrews, P.; Voice, W. Influence of heat treatment on microstructure and tensile behavior of a hot isostatically pressed nickel-based superalloy. J. Alloys Compd. 2013, 578, 454–464. [Google Scholar] [CrossRef]
- Tian, T.; Hao, Z.; Li, X.; Jia, C.; Peng, S.; Zhu, Q.; Ge, C. Influence of aging treatment on microstructure and properties of a novel spray formed powder metallurgy superalloy FGH100L. J. Alloys Compd. 2020, 830, 154699. [Google Scholar] [CrossRef]
- Ding, H.H.; He, G.A.; Xin, W.; Feng, L.; Huang, L.; Jiang, L. Effect of cooling rate on microstructure and tensile properties of powder metallurgy Ni-based superalloy. Trans. Nonferrous Met. Soc. China 2018, 28, 451–460. [Google Scholar] [CrossRef]
- Huang, H.; Liu, G.; Wang, H.; Wang, Z.; Zhang, H.; Shao, Y.; Hu, B. Effect of cooling rate and resulting microstructure on tensile properties and deformation mechanisms of an advanced PM nickel-based superalloy. J. Alloys Compd. 2019, 805, 1254–1259. [Google Scholar] [CrossRef]
- Han, Z.Y.; Zhang, P.X.; Song, J.M.; Wang, Q.X.; Liang, S.J.; Tang, B.; Lai, Y.J. Effect of Heat Treatment on Microstructure and Mechanical Properties of P/M Inconel 718 Superalloy. Rare Met. Mater. Eng. 2021, 50, 693–698. (In Chinese) [Google Scholar]
- Zhang, L.; Li, D.; Qu, X.; Qin, M.; He, X.; Li, Z. Microstructure and tensile properties optimization of MIM418 superalloy by heat treatment. J. Mater. Process. Technol. 2016, 227, 71–79. [Google Scholar] [CrossRef]
- Hao, Z.; Ge, C.; Li, X.; Tian, T.; Jia, C. Effect of Heat Treatment on Microstructure and Mechanical Properties of Nickel-Based Powder Metallurgy Superalloy Processed by Selective Laser Melting. Acta Metall. Sin. 2020, 56, 1133–1143. (In Chinese) [Google Scholar]
- Tian, G.; Jia, C.; Liu, J.; Hu, B. Experimental and simulation on the grain growth of P/M nickel-base superalloy during the heat treatment process. Mater. Des. 2009, 30, 433–439. [Google Scholar] [CrossRef]
- Liu, J.T.; Tao, Y.; Zhang, Y.W. Microstructure and mechanical property of FGH96 alloy dual property disk. Trans. Mater. Heat Treat. 2010, 31, 71–74. [Google Scholar]
- Gabb, T.P.; Kantzos, P.T.; Telesman, J.; Gayda, J.; Sudbrack, C.K.; Palsa, B. Fatigue resistance of the grain size transition zone in a dual microstructure superalloy disk. Int. J. Fatigue 2011, 33, 414–426. [Google Scholar] [CrossRef]
- Gao, J.; Luo, J.; Li, M.Q. Advance in manufacture technology and mechanism of aero-engine dual property disk. J. Aeronaut. Mater. 2012, 32, 37–43. (In Chinese) [Google Scholar]
- Wu, H.; Zhuang, X.; Nie, Y.; Li, Y.; Jiang, L. Effect of heat treatment on mechanical property and microstructure of a powder metallurgy nickel-based superalloy. Mater. Sci. Eng. A 2019, 754, 29–37. [Google Scholar] [CrossRef]
- Wan, Z.; Hu, L.; Sun, Y.; Wang, T.; Li, Z.; Zhang, Y. Effect of solution treatment on microstructure and tensile properties of a U720LI Ni-based superalloy. Vacuum 2018, 156, 248–255. [Google Scholar] [CrossRef]
- Jiang, R.; Song, Y.D.; Reed, P.A. Fatigue crack growth mechanisms in powder metallurgy Ni-based superalloys—A review. Int. J. Fatigue 2020, 141, 105887. [Google Scholar] [CrossRef]
- Mao, J.; Chang, K.M.; Yang, W.; Furrer, D.U.; Ray, K.; Vaze, S.P. Cooling precipitation and strengthening study in powder metallurgy superalloy Rene88DT. Mater. Sci. Eng. A 2002, 332, 318–329. [Google Scholar] [CrossRef]
- Tian, G.F.; Jia, C.C.; Wen, Y.J.; Liu, G.Q.; Hu, B.F. Cooling γ′ precipitation behavior and strengthening in powder metallurgy superalloy FGH4096. Rare Met. 2008, 27, 410–417. [Google Scholar] [CrossRef]
- Li, C.; Chen, L.L.; Qu, Z.H.; Lai, Y.J.; Liang, S.J. Measurement of complete dissolution temperature of γ’ phase in nickel-based P/M superalloy FGH4096. Heat Treat. Met. 2021, 46, 174–177. (In Chinese) [Google Scholar]
- Lan, J.; Huang, H.; Mao, H.J.; Hua, L. Phase transformation and grain growth behaviors of superalloy IN718 during heat treatment. Mater. Today Commun. 2020, 24, 101347. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Preuss, M.; Tin, S.; Hardy, M.C. The influence of cooling rate from temperatures above the γ′ solvus on morphology, mismatch and hardness in advanced polycrystalline nickel-base superalloys. Mater. Sci. Eng. A 2008, 473, 158–165. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.; Zhang, B.; Ning, Y.; Fu, M.W. Re-precipitation mechanisms of the γ′ phase with sphere, near-sphere, cubic, octets and finally-dendrite in as-cast Ni-based superalloys. J. Alloys Compd. 2021, 876, 160104. [Google Scholar] [CrossRef]
- Masoumi, F.; Shahriari, D.; Jahazi, M.; Cormier, J.; Devaux, A. Kinetics and Mechanisms of γ′ Reprecipitation in a Ni-based Superalloy. Sci. Rep. 2016, 6, 28650. [Google Scholar] [CrossRef]
- Yoo, Y.S. Morphological instability of spherical γ′ precipitates in a nickel base superalloy. Scr. Mater. 2005, 53, 81–85. [Google Scholar] [CrossRef]
- Fan, X.; Guo, Z.; Wang, X.; Yang, J.; Zou, J. Morphology evolution of γ′ precipitates in a powder metallurgy Ni-base superalloy. Mater. Charact. 2018, 139, 382–389. [Google Scholar] [CrossRef]
- Breidi, A.; Allen, J.; Mottura, A. First-principles modeling of superlattice intrinsic stacking fault energies in Ni3Al based alloys. Acta Mater. 2018, 145, 97–108. [Google Scholar] [CrossRef]
- Qiu, C.L.; Andrews, P. On the formation of irregular-shaped gamma prime and serrated grain boundaries in a nickel-based superalloy during continuous cooling. Mater. Charact. 2013, 76, 28–34. [Google Scholar] [CrossRef]
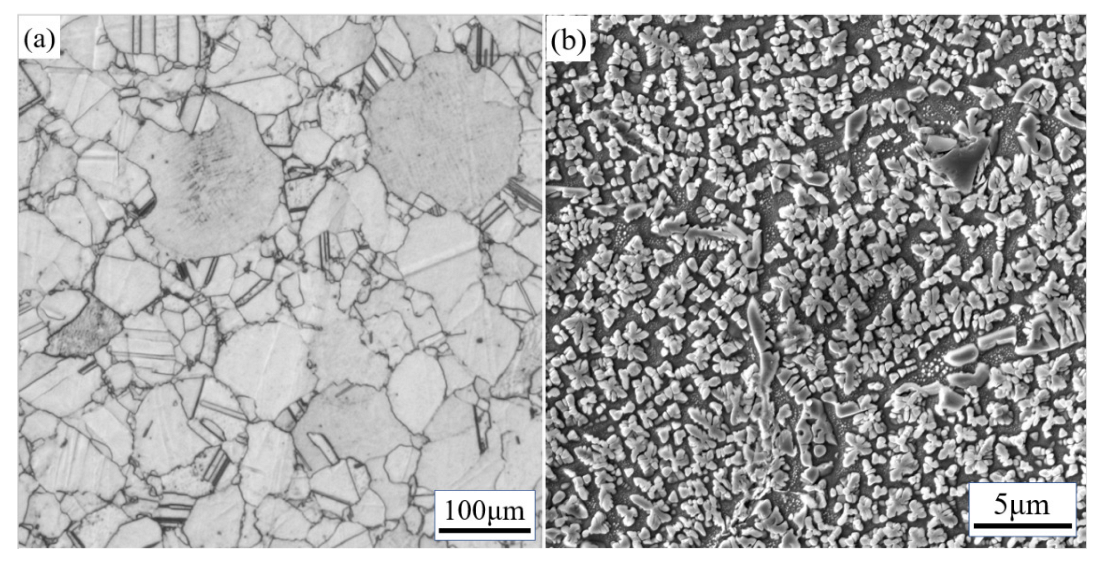


| C | Al | W | Nb | Mo | Ti |
| <0.030 | 2.321 | 4.063 | 0.832 | 3.973 | 3.721 |
| Cr | Co | Fe | B | Mn | Zr |
| 16.213 | 13.142 | <0.510 | <0.015 | <0.150 | 0.036 |
| Si | P | O | H | N | Ni |
| <0.200 | <0.015 | <0.005 | <0.001 | <0.005 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, M.; Sun, P.; Yang, G.; Song, W.; Wang, X. Effect of Solution Treatment on Microstructure Evolution of a Powder Metallurgy Nickel Based Superalloy with Incomplete Dynamic Recrystallization Microstructure. Metals 2023, 13, 239. https://doi.org/10.3390/met13020239
Liu Y, Wang M, Sun P, Yang G, Song W, Wang X. Effect of Solution Treatment on Microstructure Evolution of a Powder Metallurgy Nickel Based Superalloy with Incomplete Dynamic Recrystallization Microstructure. Metals. 2023; 13(2):239. https://doi.org/10.3390/met13020239
Chicago/Turabian StyleLiu, Yanhui, Miao Wang, Pengwei Sun, Guang Yang, Wenjie Song, and Xiaofeng Wang. 2023. "Effect of Solution Treatment on Microstructure Evolution of a Powder Metallurgy Nickel Based Superalloy with Incomplete Dynamic Recrystallization Microstructure" Metals 13, no. 2: 239. https://doi.org/10.3390/met13020239
APA StyleLiu, Y., Wang, M., Sun, P., Yang, G., Song, W., & Wang, X. (2023). Effect of Solution Treatment on Microstructure Evolution of a Powder Metallurgy Nickel Based Superalloy with Incomplete Dynamic Recrystallization Microstructure. Metals, 13(2), 239. https://doi.org/10.3390/met13020239








